Abstract
Background/Aim: Our aim was to determine serumTLR-9 levels in sepsis and evaluate the relationship betweensepsis and serum TLR-9 levels. Materials and Methods: Thestudy group consisted of 80 consecutive patients with sepsisand 100 healthy individuals. The demographic characteristics,co-morbidities and hemodynamic data of all patients wererecorded. Results: TLR-9 serum levels in sepsis werestatistically significantly lower compared to the control group.It was also seen that when the lactate level was >5 mmol/l inpatients in the sepsis group, the serum TLR-9 levels weresubstantially higher. Conclusion: There is a relationshipbetween sepsis-induced immunosuppression and serum TLR-9 levels. The host immunity system can be activated by meansof TLR-9-related systems, while hyperlactatemia may play astimulating role in the re-activation of the immune system
Keywords: Sepsis, TLR9, immunosuppression, toll-like receptors
Sepsis is a serious clinical problem worldwide (1-3). Severesepsis and septic shock lead to significant morbidity andmortality in critically-ill patients despite improvements inintensive care and treatment methods (4). Sepsis ischaracterized by an uncontrolled systemic inflammatoryresponse in the immune system as a result of complexinteractions between host and infectious agents (5). Sepsis,serious sepsis, and septic shock represent increasingly severedegrees of systemic inflammatory responses to infection (6).Increased uncontrolled inflammatory response leads toincreased mortality from sepsis.Toll-like receptors (TLRs) have been recognized as acomponent of the innate immune system. TLRs have animportant role in the innate immune system to recognizemany pathogens and create a host immune response throughproduction of necrosis factor-α, interleukin (IL)-1, IL6 andother pro-inflammatory cytokines (7,8). In our study, serumTLR9 levels in sepsis were analyzed in relation to clinicaland prognostic parameters.
Patients and Methods
This prospective study was approved by the Ethics Committee ofthe Istanbul University, Turkey (Approval number 1341). All theprocedures followed in the study were in accordance with theDeclaration of Helsinki. Informed consent was obtained from allindividual participants included in the study. A total of 180 patientswere enrolled in the study. The study group consisted of 80consecutive patients with diagnosis of sepsis at the intensive careunit. In each case, diagnoses were established with histologicalexamination. The control group comprised 100 healthy individuals.The demographic characteristics, co-morbidities and hemodynamicdata of all patients were recorded. Blood gas samples and laboratorydata of the cases were collected. Microbial cultures of blood,sputum, a wound swab, abscess, and urine from each patient wereperformed. Blood samples were collected in EDTA-coated tubes bystandard venipuncture method. The serum samples of theparticipants were stored at −20˚C until analysis. TLR9 levels weredetermined by ELISA technique (Uscn Life Science Inc, Wuhan,Hubei, PRC; Lot No: L150309211).Statistical analyses. Statistical analyses, using SPSS softwareversion 21.0 (Armonk, NY, USA, Armonk, NY, USA), includedthe Chi-square test. Student’s t-test, one-way ANOVA and Mann–Whitney U-tests were used to compare the demographic databetween the groups. Whenever an expected cell value was less thanfive, Fisher’s exact test was used. A p-value of less than 0.05 wasregarded as statistically significant.
Results
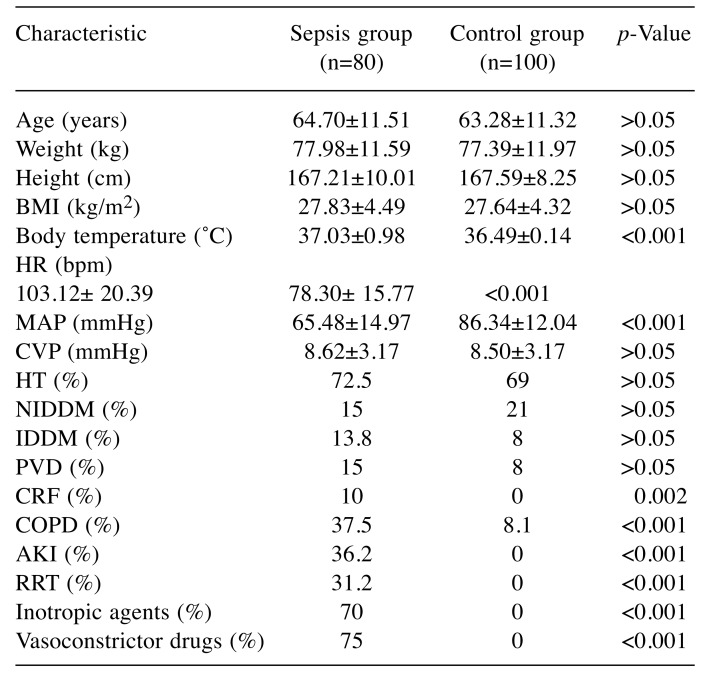
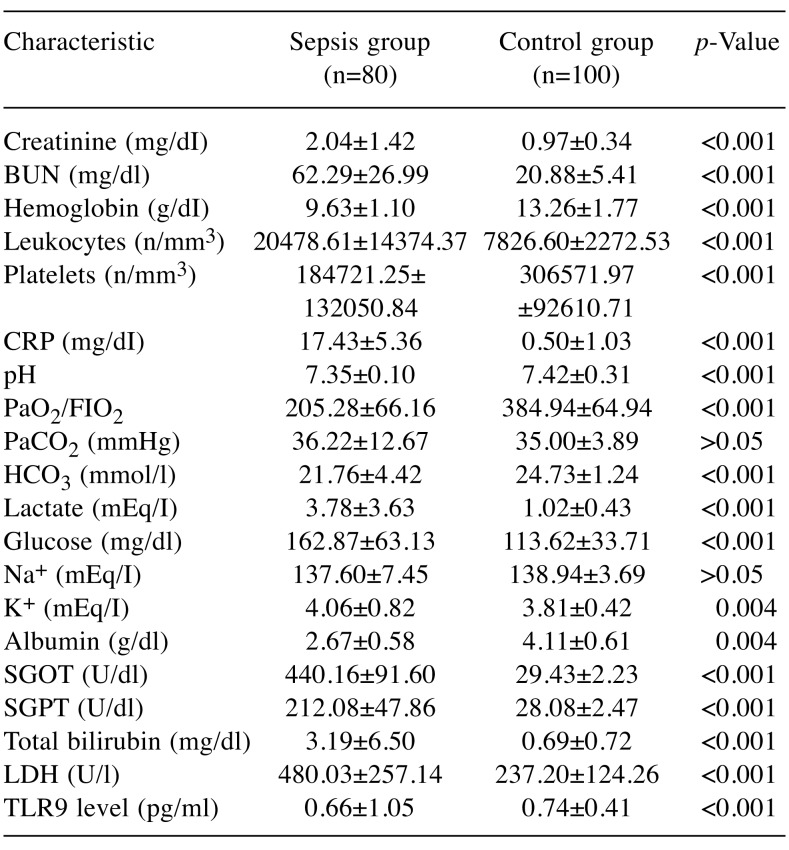
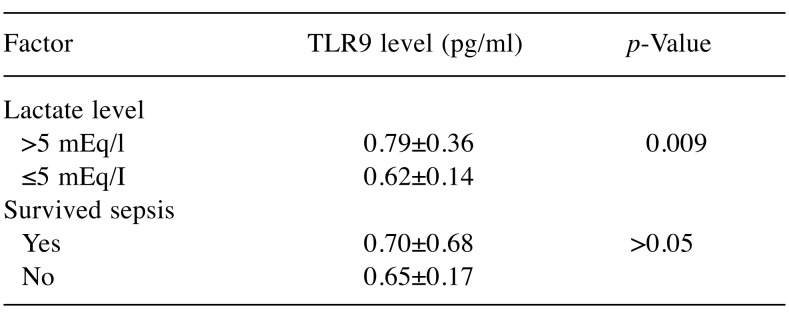
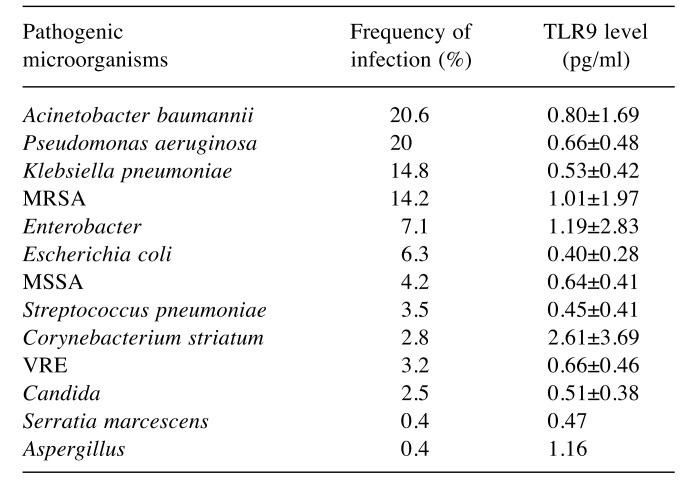
The demographic characteristics, biochemistry andlaboratory data are presented in Table I and Table II. Nodemographic differences were noted between the two patientgroups. However, the number of patients with chronic renalfailure, chronic obstructive pulmonary disease, acute kidneyinjury and those requiring continuous renal replacementtherapy was statistically higher in the sepsis group comparedto the control group. Acute kidney injury developed in 36.2%and renal replacement therapy was needed in 31.2% ofpatients with sepsis.The mean number of leukocytes, and levels of serumC-reactive protein, lactate, glucose, serum creatinine, bloodurea nitrogen, plasma potassium, serum glutamic pyruvictransaminase, serum glutamic oxaloacetic transaminase,lactate dehydrogenase, total bilirubin and body temperaturewere all statistically significantly higher in the sepsis groupcompared to the control group. However, arterial blood gaspH and levels of HCO3, blood hemoglobin and serumsodium and the number of platelets and the PaO2/FIO2 ratiowere statistically significantly lower in the group ofpatients with sepsis. In the study, mean arterial pressure ofour sepsis cases was also significantly lower, while heartrate values, inotropic agent and vasoconstrictor drug usagerates were found to be significantly higher than those of thecontrol group. Mortality was found to be 93.8% in thesepsis group.The mean serum level of TLR9 in the sepsis group wasstatistically significantly lower than that of the control group(Table II). Although without statistical significance, the serumTLR9 level was lower in the patients who died than in thepatients who survived in the sepsis group. Nonetheless, whenwe grouped the patients in the sepsis group according to theirlactate level (≤5 mEq/l and >5 mEq/l), it was observed that theTLR9 level was substantially and statistically higher in thepatients with an increased lactate level (Table III). The serumlevel of TLR9 according to the type of causative pathogenmicroorganisms in the sepsis group are shown in Table IV.Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiellapneumoniae and methicillin-resistant Staphylococcus aureuswere the organisms most frequently detected by microbialculture. The results of microbial cultures show that some of thesepsis cases were infected with more than one pathogenicmicroorganism. Although the number of infected individualswas insufficient for statistical evaluation, the serum TLR9 levelwas found to be highest in the presence of Corynebacteriumstriatum infection and the lowest in Escherichia coli infection.
Table I. Demographic data of our study groups. Data are presented as mean±SD or proportions.
BMI: Body mass index, HR: heart rate, BPM: beat per minute, MAP:mean arterial pressure, CVP: central venous pressure, HT: hypertension,NIDDM: non-insulin-dependent diabetes mellitus, IDDM: insulindependentdiabetes mellitus, PVD: peripheral vascular disease, COPD:cronic obstructive pulmonary disease, CRF: chronic renal failure, AKI:acute kidney injury, RRT: renal replacement therapy.
Table II. Biochemistry and laboratory data of study groups. Data are presented as mean±SD or proportions.
BUN: Blood urea nitrogen, CRP: C-reactive protein, PaCO2: arterial partial CO2 pressure, HCO3: bicarbonate, SGOT: serum glutamic oxaloacetic transaminase, SGPT: serum glutamic pyruvic transaminase, LDH: lactate dehydrogenase, PaO2/FIO2: the ratio of partial arterial O2 pressure and fraction of inspired oxygen.
Table III. Serum toll-like receptor 9 (TLR9) levels (mean±SD) in patients with sepsis.
Table IV. Serum toll-like receptor 9 (TLR9) level (mean±SD) in the sepsis group according to pathogen.
MRSA: Methicillin-resistant Staphylococcus aureus, MSSA: methicillinsensitiveS. aureus, VRE: vancomycin-resistant Enterococcus.
Discussion
Today, despite all research, the usage of modern antibiotics andadvanced resuscitation methods in intensive-care units, sepsisis still seen at a high frequency and is one of the mostimportant causes of death in hospitals and intensive care units(9-11). The pathogenesis of the sepsis process is tremendouslycomplex and involves a dynamic interplay between thepathogen and the host immune system. In order to reducesepsis-induced morbidity and mortality, it is essential tounderstand the molecular mechanisms associated withdevelopment of sepsis and sepsis-linked organ injury (7,8).The immune system consists of two parts: natural andacquired. The innate immune system is an essential form of hostdefense against infections. TLRs are recognized as a componentof the natural immune system. TLRs are transmembraneproteins. They have a considerable role in host immune systemby both creating a host natural immune response against manypathogens and activating the gained immune response (12,13).The stimulation of TLR receptors by pathogenic microbialsubstances leads to the activation of many inflammatorycytokines and downstream inflammatory pathways (14,15).This is the host immune inflammatory response. Additionally, TLR9 is required for optimal activation ofbactericidal activity. It carries the pattern of recognition formicrobial DNA. TLR9 is essential for the uptake andintracellular killing of the bacteria during infection with K.pneumoniae (16). Some studies defined the role of TLR9 asrecruiting and activating leukocytes, including dendritic cellsand macrophages (17-18). Similar results for Streptococcuspneumoniae, Haemophilus influenzae and Neisseriameningitidis have been published in other studies, whereTLR9 has critical functions in the generation of theIL12/interferon-γ response (19-21).There is a hyperinflammatory response during the initialphase of sepsis. During this period, the complement systemis activated and TLRs are highly expressed on monocytesand macrophages (22). Termination of thishyperinflammatory response and restoration of the hostimmune system are mediated by compensatory antiinflammatorycytokines after control of infection. Thiscompensatory anti-inflammatory response syndrome appearsin the majority of patients with sepsis (14). Indeed, the hostimmune system produces two types of responses against thesepsis. These two immunological responses are consecutiveand overlapping phases. If the compensatory antiinflammatoryresponse is ineffective, the inflammatoryreaction can result in destruction of the host cells andbecame injurious to the body overall. This may influencesepsis-related organ injury or dysfunction and eventualmortality (23-25). On the other hand, deterioration in theregulation of control mechanisms during sepsis may lead toloss of control of inflammation due to excessive activationor extreme suppression of the inflammatory response. Forinstance, as a result of the long duration of infection or theinability for it to be controlled by antibiotics and othertreatment methods, it is possible to switch to the antiinflammatorystate. This is the period of immuno-paralysisor immuno-suppression and is a hallmark of sepsis. Thesetwo extreme immune responses in the fight against infection, inflammatory and anti-inflammatory processes, canultimately harm the patient (26).It is known that sepsis affects immune responses leading toimmunosuppression. Studies demonstrated that sepsis impairshost immune system homeostasis (27,28). This is an importantpathological mechanism in sepsis and is one of the importantbreakpoints in sepsis-related death (29,30). In patients,secondary infection or herpes virus reactivation may developduring the sepsis-associated immunosuppressive stage (31). Inour study, infection due to multiple pathogenic microorganismswas detected at a high rate in patients with sepsis.In a septic-shock study, investigators found that 35% ofpatients had a down-regulation of TLR gene expressioncompared to their baseline values (32). We are of the opinionthat the low levels of serum TLR9 detected in our study werebecause of down-regulation in these sepsis cases. Thedecrease in the serum level of TLR9 in the sepsis group inour study can be explained by the fact that the patients hadsevere sepsis. Again, to stress the point, TLR9 levels in ourstudy were measured only in patients with serious sepsis andseptic shock. TLR9 levels may be reduced due tocompensatory anti-inflammatory condition in an advancedstage in severe cases of sepsis. Additionally, there was alsoa statistically significant relationship between these lowvalues and mortality in our study.Myocardial functions are also affected by variousmechanisms in sepsis. Cardiovascular dysfunction is aserious complication in about 40% of patients with sepsisand increases mortality by 20-70%. TLR2 and TLR4particularly contribute to cardiac dysfunction (33,34).Nevertheless there is little information on the role of TLR9in myocardium, but TLR9 expression in the heart has beenshown (35). It is also known that TLR9 stimulation leads tonuclear factor ĸB activation in different tissues (36). In onestudy, it was found that bacterial DNA increased myocardialcytokine production via TLR9 and caused depletion ofcardiomyocyte contractile ability (37).In addition to hyperinflammation, severe sepsiscomplicated by hypoperfusion and organ dysfunction due tothis cardiovascular dysfunction develops. One of the bestindicators in diagnosis of severe sepsis is the level of lactate(38). Our study shows that there is a relationship betweenserious sepsis progressing to septic shock and increasedserum TLR9 level, particularly in those cases where thelactate level increased to >5 mmol/l due to tissue hypoperfusion.Our conclusion is that there is a decrease in serumTLR9 level in serious sepsis through its down-regulation,while there is an increase in serum TLR9 level, perhaps inconnection with TLR9 re-expression, in the cases in whichtissue perfusion deteriorates, leading to high values of lactate.Recurrent cycles of hyper- and hypoinflammatory conditionmay occur in sepsis (39). It is not clear which clinicalconditions trigger or cause this change. An increase in serumlactate level may play a crucial role as an immunostimulantor an agent that triggers this change in the TLR9 pathway.In fact, both inflammatory and anti-inflammatoryprocesses reflect not only uncontrolled systemic response butalso survival efforts of the patients with sepsis. Currentinflammatory status determination and appropriatemanagement are important points for sepsis treatment.Targeted therapies for inflammatory cytokines in a patientduring the hyperinflammatory phase may be performed.However, in clinical trials, these therapies have shown noclinical benefit or, in some cases, have worsened survival(40,41). Furthermore, compensatory anti-inflammatoryresponse to counter-regulate the host immune response insepsis may result in immunosuppression, whereasimmunostimulants may be more beneficial in these patients(42-44). The immunological state of each patient may differ.For this reason, while one patient may benefit from targetedtherapy, another may suffer as a result of the same therapy.Targeted immune therapies designed according to the patient’scurrent inflammatory condition should be planned. Thereforethe immunological status of the patient should be welldetermined for sepsis-appropriate management and treatment.Ideally, specific biomarkers should be used to helpmanagement of sepsis (45). The importance of TLRs in thistopic will be determined by future studies.This study has some limitations; the most important ofthem is that the current clinic study had a relatively smallsample size, it was single-institutional and serum TLR9levels were analyzed only once. Repetitive segmentaldeterminations would make the evaluations and progress ofpatients more understandable. We believe that our results areencouraging, but they need to be supported by study in alarger sample and perhaps in different phases of sepsis.Sepsis is characterized by a hyperinflammatory responsein the early stage, but it may be followed by animmunosuppressive phase. This immunosuppression isbelieved to be the potent factor which is tightly associatedwith high mortality in sepsis. Modulation of thisimmunosuppression may help to develop new therapeuticstrategies. TLRs have an important role in sepsispathophysiology. However, the role of TLRs in thedevelopment of sepsis and in the creation of tissue damageand sepsis-related organ injury is not clear. Targeting TLRsis an exciting and promising area for inflammation controland sepsis management (46).Actually, developing sepsis-specific TLR-targetedtherapies might not be easy. Blocking a single mediator oractivating a single channel will not be enough to intervenein sepsis (47,48). Further experimental studies are requiredto understand the underlying mechanism of infectionpathogenesis. Animal sepsis models can provide valuableinformation about the role of TLRs and the underlyingmechanism of infection pathogenesis. A better understanding of TLR biology and the change ofthe TLR9 level in sepsis may unveil novel therapeuticapproaches for sepsis. It may be possible to detect thosepatients who are on the cusp of septic shock, which wouldinfer higher mortality, and specific alterations in treatmentmight be considered. Studies have shown that not onlymicrobial products but also some endogenous molecules canstimulate TLRs (49). The host immune system can beactivated by means of TLR9- related systems. Hypoperfusionand hyperlactatemia may play an effective stimulatory rolein reactivation of the immune system.
Funding
This study was supported by the Istanbul University Scientific Research Projects Unit (Project No: 38207).
Conflicts of Interest
None of the Authors has any conflict of interest to declare.
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis andorgan failure and guidelines for the use of innovative therapiesin sepsis. The ACCP/SCCM Consensus Conference Commitee.American College of Chest Physicians/Society of Critical CareMedicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC. 2001 SCC/ESICM/ACCP/ATS/SIS International sepsis definitions conferance. CritCare Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology:Sampling, selection, and society. Crit Care. 2004;8:222–226. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC, Surviving Sepsis Campaign The Surviving SepsisCampaign: Results of an international guideline-basedperformance improvement program targeting severe sepsis. CritCare Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 5.Oberholzer A, Oberholzer C, Moldawer LL. Sepsissyndromes: Understanding the role of innate and acquiredimmunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Rittirsch D, Flieri MA, Ward PA. Harmful molecularmechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne EP. Regulation of B-cell responses by toll-likereceptors. Immunology. 2012;136:370–379. doi: 10.1111/j.1365-2567.2012.03587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA inthe lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 9.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapidincrease in hospitalization and mortality rates for severe sepsisin the United States: A trend analysis from 1993 to 2003. CritCare Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein B, Giroir B, Randolph A. International pediatricsepsis consensus conference: Definitions for sepsis and organdysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 12.Opal SM. Phylogenetic and functional relationships betweencoagulation and the innate immune response. Crit Care Med. 2000;28:77–80. doi: 10.1097/00003246-200009001-00017. [DOI] [PubMed] [Google Scholar]
- 13.Tenover FC. Rapid detection and identification of bacterialpathogens using novel molecular technologies: Infection controland beyond. Clin Infect Dis. 2007;44:418–423. doi: 10.1086/510684. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 15.Underhill DM, Ozinsky A. Toll-like receptors: key mediatorsof microbe detection. Curr Opin Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 16.Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going onthe offense with a strong defense. Microbiol Mol Rev. 2016;80(3):629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence againstpneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, Zeng X, Moore TA, McMillan TR, Krieg AM, Akira S, Standiford TJ. TLR9 is required for protective innate immunity in Gramnegativebacterial pneumonia: role of dendritic cells. J Immunol. 2007;179:3937–3946. doi: 10.4049/jimmunol.179.6.3937. [DOI] [PubMed] [Google Scholar]
- 19.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA: Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 20.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. LiveStreptococcus pneumoniae, Haemophilus influenzae, andNeisseria meningitidis activate the inflammatory responsethrough toll-like receptors 2, 4, and 9 in species-specificpatterns. J Leukoc Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- 21.Sjölinder H, Mogensen TH, Kilian M, Jonsson AB, Paludan SR. Important role for toll-like receptor 9 in host defense againstmeningococcal sepsis. Infect Immun. 2008;76:5421–5428. doi: 10.1128/IAI.00615-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Ma S. The cytokine storm and factors determiningthe sequence and severity of organ dysfunction in multiple organdysfunction syndrome. Am J Emerg Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Utahaisangsook S, Day NK, Bahna SL, Good RA, Haraguchi S. Innate immunity and its role against infections. Ann Allergy Asthma Immunol. 2002;88:253–265. doi: 10.1016/S1081-1206(10)62005-4. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 25.Beutler B, Poltorak A. Sepsis and evolution of the innateimmune response. Crit Care Med. 2001;29:2–6. doi: 10.1097/00003246-200107001-00002. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood ER, Hotchkiss RS. BTLA as a biomarker andmediator of sepsis-induced immunosuppression. Crit Care. 2013;17:1022. doi: 10.1186/cc13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Cardinal JS, Bahar R, Evankovich J, Huang H, Nace G, Billiar TR, Rosengart MR, Pan P, Tsung A. İnterferonregulatory factor-1 regulates the autophagic response in LPSstimulatedmacrophages through nitric oxide. Mol Med. 2012;18:201–208. doi: 10.2119/molmed.2011.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Yuhang AI, Tsung A. Clinical application:Restoration of immune homeostasis by autophagy as a potentialtherapeutic target in sepsis. Exp Ther Med. 2016;11:1159–1167. doi: 10.3892/etm.2016.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Karl IE. The Pathophysiology and treatmentof sepsis. N Engl J Med. 2003;348/2:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 30.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 31.Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F, Meisner C, Jahn G, Koenigsrainer A, Unertl K, Hamprecht K. Cytomegalovirus reactivation and associated outcome ofcritically ill patients with severe sepsis. Crit Care. 2011;15:R77. doi: 10.1186/cc10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piazza O, Pulcrano G, Fiori PL, Tufano R, Lonardo M, Rossano F, Catania MR. Toll-like receptor kinetics in septic shockpatients: A preliminary study. Int J Immunopathol Pharmacol. 2012;25:425–433. doi: 10.1177/039463201202500212. [DOI] [PubMed] [Google Scholar]
- 33.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, Carabello BA, Mann DL, Vallejo JG. CD14-deficient mice are protected against lipopolysaccharide-inducedcardiac inflammation and left ventricular dysfunction. Circulation. 2002;106:2608–2615. doi: 10.1161/01.cir.0000038110.69369.4c. [DOI] [PubMed] [Google Scholar]
- 34.Knuefermann P, Sakata Y, Baker JS, Huang Ch, Sekiguchi K, Hardarson HS, Takeuchi O, Akira S, Vallejo JG. Toll-likereceptor 2 mediates Staphylococcus aureus-induced myocardialdysfunction and cytokine production in the heart. Circulation. 2004;110:3693–3698. doi: 10.1161/01.CIR.0000143081.13042.04. [DOI] [PubMed] [Google Scholar]
- 35.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Tolllikereceptor stimulation in cardiomyoctes decreases contractilityand initiates an NF-kappaB-dependent inflammatory response. Cardiovasc Res. 2006;72:384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces selfand cross-hyporesponsiveness of RAW264.7 cells in response toCpG DNA and lipopolysaccharide: alterations in IL-1 receptorassociatedkinase expression. J Immunol. 2003;170:1052–1061. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- 37.Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rğdiger M, Boehm O, Fink K, Dreiner U, Grohe C, Hoeft A, Baumgarten G, Koch A, Zacharowski K, Meyer R. BacterialDNA induces myocardial inflammation and reducescardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res. 2008;78:26–35. doi: 10.1093/cvr/cvn011. [DOI] [PubMed] [Google Scholar]
- 38.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate isassociated with mortality in severe sepsis independent of organfailure and shock. Critical Care Medicine. 2009;5:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 39.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costsin patients with ventilator-associated pneumonia attributed topotentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134:281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 40.Echtenacher B, Urbaschek R, Weigl K, Freudenberg MA, Mannel DN. Treatment of experimental sepsis-induced immunoparalysis with TNF. Immunobiology. 2003;208:381–389. doi: 10.1078/0171-2985-00282. [DOI] [PubMed] [Google Scholar]
- 41.Mathias B, Szpila BE, Moore FA, Efron PA, Moldawer LL. Areview of GM-CSF therapy in sepsis. Medicine. 2015;94:e2044. doi: 10.1097/MD.0000000000002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kox WJ, Volk T, Kox SN, Volk HD. Immunomodulatorytherapies in sepsis. Intensive Care Med 26(Supp. 2000;1):124–128. [Google Scholar]
- 43.Volk HD, Reinke P, Döcke WD. Clinical aspects: Fromsystemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–177. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 44.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, Hotchkiss RS. Characterization andmodulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78(4):1582–1592. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer M. Biomarkers in sepsis. Curr Opin Pulm Med. 2013;19:305–309. doi: 10.1097/MCP.0b013e32835f1b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angus DC. The search for effective therapy for sepsis: Back tothe drawing board. JAMA. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 47.Cohen J, Opal S, Calandra T. Sepsis studies need newdirection. Lancet Infect Dis. 2012;12:503–505. doi: 10.1016/S1473-3099(12)70136-6. [DOI] [PubMed] [Google Scholar]
- 48.Tse MT. Trial watch: Sepsis study failure highlights need fortrial design rethink. Nat Rev Drug Discov. 2013;12:334. doi: 10.1038/nrd4016. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of toll-likereceptor-mediated inflammatory response by complement invivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]