Abstract
Background/Aim: Polio is predominantly an enteric viral infection that was progressively eradicated in the United States after the introduction of polio vaccine in the early 1950s. U.S. colorectal cancer rates have dropped steadily for individuals born between 1890 and 1950, but have been increasing for every generation born since 1950. Moreover, the lowest worldwide age adjusted rates of colorectal cancer in 2012 were in sub-Saharan Africa, Gambia and Mozambique, where polio has not been eradicated. In the current study, poliomyelitis incidence in US states before the introduction of polio vaccine was analyzed. Materials and Methods: Reported cases of poliomyelitis per 100,000 population by state 1932-1951 were from Centers for Disease Control. Colorectal cancer deaths per 100,000 in men (2005-2009) by US State are from the American Cancer Society. US state overweight and obesity data are from the Centers for Disease Control and Prevention (CDC). Smoking data are from the CDC. Results: By US state, colorectal cancer incidence per 100,000 in men for 2005-2009 was inversely correlated with reported cases of poliomyelitis per 100,000 for 1932-1951 (r=−0.311, p=0.032). Colorectal cancer deaths per 100,000 in men in 2005-2009 were also inversely correlated with reported cases of poliomyelitis per 100,000 by state for 1932-1951 (r=−0.493, p<0.001). The relationship between colorectal cancer deaths and polio incidence was significant (β=−0.196, p=0.028) and independent of the effects of smoking (β=0.289, p=0.012) and overweight (β=0.547, p<0.001). The relationship in females with colorectal cancer was identical. Conclusion: Polio virus infection of cells of the colon may induce some degree of resistance to the development of colon cancer decades later. The effect of polio virus infection seems to be especially potent in reducing the rate of death from colon cancer.
Keywords: Polio virus, colorectal cancer, the Cancer Genome Atlas, PVR gene
Colorectal cancer rates have dropped steadily for individuals born between 1890 and 1950, but have been increasing for every generation born since 1950. Adults born in 1990 have twice the risk of colon cancer and four times the risk of rectal cancer compared to adults born in 1950 of comparable age. A total of 16,450 new cases of colon or rectal cancer will be diagnosed in 2018 in Americans under 50 years of age, according to the American Cancer Society. In 2014, 43% of colorectal cancer cases in those under 50 years old were in individuals aged 45 to 49 years, with a rise in colorectal cancer in adults as young as their 20s and 30s (1).
No one knows what may be driving the rise in cases. Lifestyle, environmental or genetic factors, obesity, cigarette smoking, a diet high in red or processed meats and lack of physical activity have been tied to increased risk (2). Other possible causes include prolonged use of antibiotics during adulthood, which raise the risk of developing precancerous polyps, possibly because antibiotics can alter gut bacteria (3).
Polio, predominantly an enteric viral infection, was progressively eradicated in the United States after the introduction of polio vaccine in the early 1950s. In the current study, we analyzed poliomyelitis incidence in US states before the introduction of polio vaccine. The Cancer Genome Atlas (TCGA) was used to examine the relationship of the polio virus cellular receptor (PVR) to colon cancer incidence.
Materials and Methods
Reported cases of poliomyelitis per 100,000 population by state for 1932-1951 were from (4). Colorectal cancer deaths per 100,000 in men for 2005-2009 by US state were from (5). US state overweight and obesity data are from the Centers for Disease Control and Prevention (CDC; http://www.cdc.gov/obesity/data/adult.html). Smoking data are from the CDC (https://www.cdc.gov/ tobacco/data_statistics). Data from Alaska and Hawaii were not included because they were not U.S. states in 1951.
Data from The Cancer Genome Atlas, Colon Cancer cohort (COAD) (551 samples) were analyzed. Tumor gene regulatory network visualization was from cBioportal (6). Tumor copy number segment analysis and microsatellite instability were from UCSC Xena browser (https://xenabrowser.net) (7).
Microsatellite instability (MSI) was evaluated by two measures. The older measure segregated data by presence or absence of MSI (8). The newer method segregated data into three groups: MSI-high, MSI-low, MS-stable (9). Data analysis was by multivariate linear regression, t-test, one-way analysis of variance and Tukey’s post-hoc test.
Results
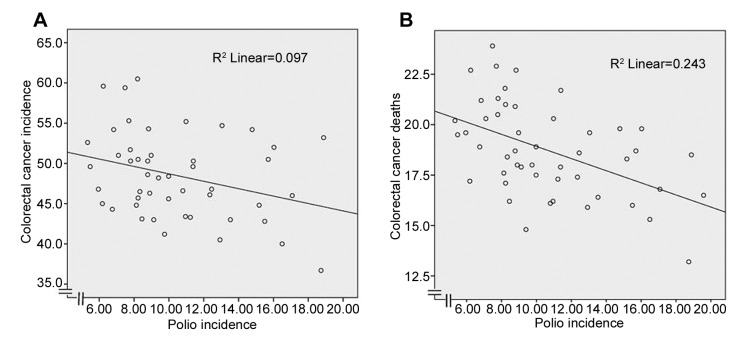
Colorectal cancer incidence per 100,000 in men in 2005-2009 versus reported cases of poliomyelitis per 100,000 population in 1932-1951 is shown in Figure 1A. The correlation was significant (r=−0.311, p=0.032).
Figure 1. Colorectal cancer incidence (A) and deaths (B) per 100,000 in men in 2005-2009 versus reported cases of poliomyelitis per 100,000population in 1932-1951 by US state (r=−0.311, p=0.032 and r=−0.493, p<0.001, respectively). Polio data from 48 states are included. Hawaiiand Alaska were not states in 1951.
Colorectal cancer deaths per 100,000 in men in 2005-2009 versus reported cases of poliomyelitis per 100,000 population by state in 1932-1951 are shown in Figure 1B. The correlation was significant (r=−0.493, p<0.001). The relationship for females with colorectal cancer was identical.
Since obesity and cigarette smoking are risk factors for colorectal cancer, multivariate linear regression was performed with colorectal cancer deaths as the dependent variable, and polio incidence, overweight rates and smoking rates as independent variables. The relationship between colorectal cancer deaths and polio incidence was significant (β=−0.196, p=0.028) and independent of the effects of smoking (β=0.289, p=0.012) and overweight (β=0.547, p<0.001).
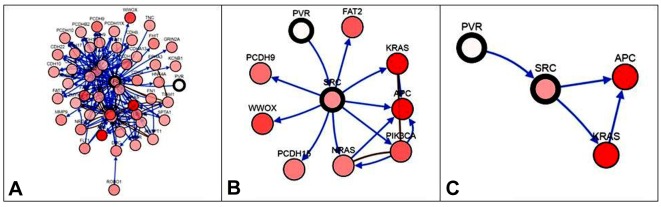
Gene regulatory network with PVR is shown in Figure 2. This network was derived from TCGA colon cancer data (10). PVR was not expressed, while SRC proto-oncogene, non-receptor tyrosine kinase (SRC); adenopolyposis coli (APC); and Kirsten rat sarcoma virus (KRAS) were expressed.
Figure 2. Gene regulatory network (The Cancer Genome Atlas colorectal cancer from cBioportal) with SRC proto-oncogene, non-receptor tyrosine kinase(SRC) in the center (SRC is highly similar to the v-src gene of Rous sarcoma virus.), polio virus receptor (PVR) nearby, neighbors filtered by percentagealteration: A: No alteration, B: 10.3% alteration, C: 32.5% alteration. SRC, adenopolyposis coli (APC) and Kirsten rat sarcoma virus (KRAS) are allinvolved in colorectal cancer. Note that in C, APC and KRAS are strongly expressed (red), SRC less so (pink), and PVR not at all (white).
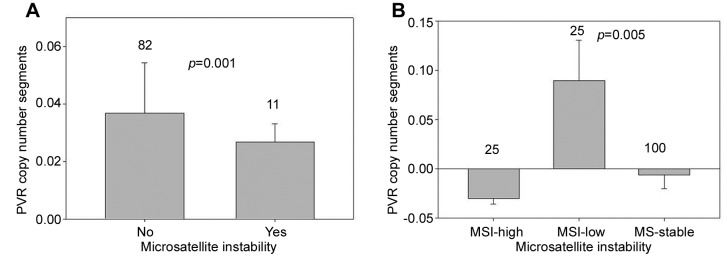
Because MSI lends distinctive features to colorectal cancer, the association of MSI with PVR in the Cancer Genome Atlas TCGA Colon Cancer cohort (COAD) was examined (551 samples). MSI differed significantly in relation to PVR copy number segments (Figure 3).
Figure 3. Polio virus receptor (PVR) copy number segments log2 (tumor/normal, mean + SEM) according to microsatellite instability (MSI) incolorectal cancer as a dichotomized variable (A) and as class (B). Note that in A, lack of MSI (82 tumors) was associated with significantly increasedPVR copy number segments compared to microsatellite instability (11 tumors). In B, MSI-low (100 tumors) status was associated with significantlyincreased PVR copy number segments compared to MS-stable (25 tumors) and MSI-high (25 tumors) status (p=0.005). Tukey’s post hoc test showedthat PVR copy number segments for those with MSI-high/MS-stable status were both significantly different from those with MSI-low status (p=0.01).PVR copy number segments for those with MSI-high and MS-stable colorectal cancer were not significantly different from one another (p=0.76).
Discussion
Poliovirus enters the body through the mouth, infecting the first cells with which it comes in contact, those of the pharynx and intestinal mucosa. It enters by binding to an immunoglobulin-like receptor, the poliovirus receptor CD155 on the cell membrane (11). Poliovirus replicates within gastrointestinal cells for about 7 days, whence it spreads to the follicular dendritic cells in the tonsillar germinal centers, the intestinal lymphoid tissue (M cells of Peyer's patches) and the deep cervical and mesenteric lymph nodes, where it replicates prolifically. The virus then enters the bloodstream (12).
Cluster of differentiation 155 (CD155) is a protein that in humans is encoded by the PVR gene (13). Activation of endothelial PVR with anti-PVR or interaction with its ligand DNAX accessory molecule-1 results in recruitment of the tyrosine-protein phosphatase non-receptor type 11 (SHP2) and this process is dependent on SRC kinases (14). SRC is highly similar to the v-src gene of Rous sarcoma virus. The PVR gene interacts within a network (Figure 2) containing SRC, Kirsten rat sarcoma virus (KRAS) and adenopolyposis coli (APC), which are implicated in colorectal cancer (15).
As was noted, MSI varied significantly in relation to PVR copy number segments. MSI is a result of loss of DNA mismatch-repair activity. MSI is found in 15% of colorectal cancer cases; 3% have Lynch syndrome (hereditary nonpolyposis colorectal cancer) and the other 12% are sporadic. Colorectal tumors with MSI have specific features, including a propensity to arise in the proximal colon, lymphocytic infiltrate and a poorly differentiated, mucinous or signet ring appearance. They have a slightly better prognosis than colorectal tumors without MSI and respond to Programmed cell death protein 1 checkpoint inhibitors such as pembrolizumab and nivolumab (16). Therefore, PVR expression might be a marker for tumor sensitivity to pembrolizumab or nivolumab.
Prior to the 20th century, polio infected mostly children 6 months to 4 years of age. Young children who contract polio generally suffer only mild symptoms but as a result they become permanently immune to the disease. In developed countries during the late 19th and early 20th centuries, improved sewage disposal, clean water supplies and better hygiene prevented infants and young children from encountering and developing immunity to polio. Exposure to poliovirus was therefore delayed until late childhood or adult life, when it was more likely to take the paralytic form (17). In 1% of infections, poliovirus spreads along nerve fiber pathways, preferentially replicating in and destroying motor neurons within the spinal cord, brain stem, or motor cortex. The destruction leads to the development of paralytic poliomyelitis, the various forms of which (spinal, bulbar and bulbospinal) differ only in the amount of neuronal damage and inflammation that occurs and the region of the CNS affected.
The data presented above suggest that polio virus infection of the cells of the colon may induce some degree of resistance to the development of colon cancer decades later. The incidence of colon cancer has begun to increase in adults who were vaccinated against polio as children, possibly because the cells of their colon were never infected by polio virus. The effect of polio virus infection seems to be especially potent in reducing the rate of death from colon cancer, perhaps mediated by CD155 and PVR.
Recombinant oncolytic poliovirus, PVSRIPO, has potent cytotoxic and innate inflammatory effects, mediating therapy in human breast and prostate cancer xenograft models (18); it may be a treatment for malignant glioma (19).
Oral attenuated live virus (Sabin) polio vaccine is no longer administered in the US because it can result in vaccine-associated paralytic poliomyelitis (in about three cases per million doses) (17). In the Democratic Republic of the Congo, a mutant of the type 2 virus in the oral vaccine has regained its virulence, spread and paralyzed 29 children (20). Public health officials fear that the virus may run rampant in Africa. Of interest is the fact that the lowest worldwide age-adjusted rates of colorectal cancer in 2012 were in sub-Saharan Africa, Gambia and Mozambique, where polio has not been eradicated (21).
It would be interesting to know if the live virus Sabin vaccine provided any protection against colorectal cancer. Was the vaccine-induced infection too limited, or does the Sabin vaccine lack something that is present in the wild-type polio virus? If the Sabin vaccine was protective, we should see a greater rate of colon cancer in patients vaccinated with the Salk killed virus vaccine. In order to determine whether Sabin oral polio vaccine might reduce the chance of colorectal cancer in persons at high risk, further investigation is warranted.
References
- 1.Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–281. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 2.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Inte J Cancer. 2009;125:171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, Nguyen LH, Izard J, Fuchs CS, Garrett WS, Huttenhower C, Ogino S, Giovannucci EL, Chan AT. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672–678. doi: 10.1136/gutjnl-2016-313413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serfling Re, Sherman Il. Poliomyelitis distribution in the United States. Public Health Rep. 1953;68:453–466. [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society: Cancer Facts and Figures. . J Clin. 2013:1–68. [Google Scholar]
- 6.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldman M, Craft B, Brooks AN, Zhu J, Haussler D. The UCSC Xena Platform for cancer genomics data visualization and interpretation. bioRxiv. 2018 [Google Scholar]
- 8.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 9.Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nacu S, Critchley-Thorne R, Lee P, Holmes S. Gene expression network analysis and applications to immunology. Bioinformatics. 2007;23:850–858. doi: 10.1093/bioinformatics/btm019. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Mueller S, Chipman PR, Bator CM, Peng X, Bowman VD, Mukhopadhyay S, Wimmer E, Kuhn RJ, Rossmann MG. Complexes of poliovirus serotypes with their common cellular receptor, CD155. J Virol. 2003;77:4827–4835. doi: 10.1128/JVI.77.8.4827-4835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuthill TJ, Bubeck D, Rowlands DJ, Hogle JM. Characterization of early steps in the poliovirus infection process: receptor-decorated liposomes induce conversion of the virus to membrane-anchored entry-intermediate particles. J Virol. 2006;80:172–180. doi: 10.1128/JVI.80.1.172-180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan DP, Seidman MA, Muller WA. Poliovirus receptor (CD155) regulates a step in transendothelial migration between PECAM and CD99. Am J Pathol. 2013;182:1031–1042. doi: 10.1016/j.ajpath.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong HL, Wah Ng LP, Koh SP, Chi Chan LW, Kwan Wong EY, Xue VW andy Tsang HF, Ching Chan AK, Chiu KY, Cheuk W, Cesar Wong SC. Hotspot KRAS exon 2 mutations in CD166 positive colorectal cancer and colorectal adenoma cells. Oncotarget. 2018;9:20426–20438. doi: 10.18632/oncotarget.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshinsky DM. Polio: An American story. Oxford University Press. 2005 [Google Scholar]
- 18.Holl EK, Brown MC, Boczkowski D, McNamara MA, George DJ, Bigner DD, Gromeier M, Nair SK. Recombinant oncolytic poliovirus, PVSRIPO, has potent cytotoxic and innate inflammatory effects, mediating therapy in human breast and prostate cancer xenograft models. Oncotarget. 2016;7:79828–79841. doi: 10.18632/oncotarget.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent Glioblastoma Treated with Recombinant Poliovirus. New England Journal of Medicine. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts L. Polio outbreaks in the DRC threaten eradication effort. Science. 2018;361:10. doi: 10.1126/science.361.6397.10. [DOI] [PubMed] [Google Scholar]
- 21.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]