Abstract
Background/Aim: The aim of the present study was to evaluate a multimodal approach for the treatment of canine malignant mammary gland neoplasms, including surgery, chemotherapy, thalidomide, and metronomic chemotherapy (MC). Materials and Methods: Fifty-eight female dogs were submitted to four different treatments: surgery; surgery with chemotherapy; surgery with chemotherapy and thalidomide; and surgery with chemotherapy and metronomic chemotherapy and overall survival was evaluated. Results: No statistical difference was found in the proliferative index and microvessel density of primary neoplasms and distant metastases following thalidomide treatment. Diffuse intense inflammatory infiltrate was predominant in primary tumors and diffuse moderate inflammatory infiltrate in metastatic lesions. No statistically significant difference was observed in median survival time (MST) between treatment groups when including all clinical stages (p=0.3177). However, animals diagnosed with distant metastasis treated with surgery and chemotherapy associated with thalidomide or MC presented longer MST when compared to animals treated only with surgery or surgery and chemotherapy (p<0.0001). Conclusion: The proposed multimodal therapy protocols including antiangiogenic and immunomodulatory therapies demonstrated a clinical benefit for patients in advanced clinical stages.
Keywords: Canine, mammary gland, metastasis, thalidomide, metronomic chemotherapy
Many mammalian species present mammary gland neoplasms similar to breast cancer in women, and the high incidence of spontaneous disease in domestic dogs and cats characterize them as pertinent comparative models (1). Human and canine breast cancer incidence present similar proportional distribution by age and sex (2), and resemblance in type, molecular transformative abnormalities, and behavior. This enables comparative studies involving carcinogenesis and the efficacy of novel therapies in mammary neoplasms in pets (1).
Mammary gland neoplasms are the most common neoplasms that affect female dogs (3). Although the optimal extent remains unclear (4), surgery remains the most effective treatment for the disease. Adjuvant therapies may be required for high-risk, undifferentiated and advanced neoplasms (5,6). Single-agent treatments have been considered less efficacious than combination therapies (7), and in solid tumors, angiogenesis has clearly been associated with metastasis and disease progression (8), and the combination of antiangiogenic strategies with conventional chemotherapy protocols will likely benefit the treatment of malignant tumors (9).
In the late 1950s, thalidomide was marketed worldwide as a sedative and a hypnotic, and as an anti-emetic drug in early pregnancy (10). Thalidomide was associated with congenital deformities and peripheral neuropathy, and the drug was withdrawn in the early 1960s (10,11). Since then, thalidomide has been used experimentally in various diseases (12,13) due to its antiangiogenic and immunomodulatory properties (8). In 2000, metronomic regimens of cytotoxic drugs were established as promising antiangiogenic therapies (14). Although metronomic chemotherapy (MC) lacks significant direct cytotoxic properties against neoplastic cells, the reduction of the interval between chemotherapy administrations limits cellular repair and replication and alters the tumor microenvironment (9).
The aim of the present study was to evaluate a multimodal approach of thalidomide added to surgery and chemotherapy for the treatment of canine malignant mammary gland neoplasms, as well as to compare its clinical benefit to surgery; surgery and chemotherapy; and surgery, chemotherapy and MC protocols.
Materials and Methods
Fifty-eight female dogs admitted to the Veterinary Hospital of the Federal University of Minas Gerais, Brazil, and diagnosed with malignant mammary gland neoplasms were evaluated in a prospective manner. The animals were divided into four groups: i) Surgery, submitted solely to surgical treatment. ii) Surgery with chemotherapy (SCT), consisting of surgical excision followed by four cycles of carboplatin at the dose of 300 mg/m2, given intravenously every 21 days. iii) Surgery with chemotherapy and thalidomide (SCTT), comprising surgical excision and carboplatin as described above, followed by thalidomide administration, initiated at 20 mg/kg, given orally every 24 hours at night time for 3 months, followed by 10 mg/kg every 24 hours at night time for 3 months; the 10 mg/kg dose of thalidomide was maintained without discontinuation in patients diagnosed with distant metastasis. iv) Surgery with chemotherapy and MC (SCTMC), including surgical excision and carboplatin as described above, followed by MC administration initiated with cyclophosphamide at 15 mg/m2, given orally every 24 hours in the morning and firocoxib at 5 mg/kg, given orally every 24 hours, for 6 months; the two drugs were maintained without discontinuation in patients diagnosed with distant metastasis. The treatment protocol chosen for each case was determined by the pet owner, following a detailed explanation on the expected adverse events and clinical benefits.
The surgical technique chosen for the excision of the primary mammary gland neoplasms aimed to be extensive enough to completely remove the tumors, and included simple mastectomy, regional mastectomy, and unilateral chain mastectomy (4). All patients underwent clinical evaluation, thoracic radiographs, abdominal ultrasound prior to the surgical procedure and every 3 months for follow-up. Clinical stage was obtained from a modified version of the original staging system established by the World Health Organization for canine mammary tumors, which evaluates tumor size, regional lymph node metastasis, and distant metastasis; classifying the neoplasms as stage I-V (4).
Tumors were classified according to the veterinary histological criteria established by the World Health Organization (15) with modifications proposed by Cassali et al. (16). The primary neoplasm of worst prognosis was chosen for analysis in patients presenting multiple tumors. Animals included in the study presented advanced clinical staging or presented histological types associated with poor prognosis, i.e. solid carcinomas, micropapillary carcinomas, anaplastic carcinomas and carcinosarcomas (16).
The neoplasms of the SCTT group were further submitted to histological grade, morphometric analysis of the inflammatory infiltrate and immunohistochemistry in an attempt to better understand the possible therapeutic properties of thalidomide in canines. When authorized by the pet owner, animals were submitted to post-mortem evaluation and pulmonary metastases were collected for morphological and immunohistochemical analysis.
Histological grade of all invasive carcinomas was established according to the Nottingham system (17), which evaluates tubule formation index, nuclear pleomorphism and mitotic count, classifying the carcinomas as grade I-III. The analysis of the inflammatory infiltrate was performed on hematoxylin and eosin-stained sections, with distribution classified as focal, multifocal, and diffuse; intensity classified as discrete, moderate, and intense; and lymphocytic infiltrate intensity divided into discrete/moderate and intense (18).
Immunohistochemistry was performed for anti-Ki-67 (MIB-1 clone, 1:50 dilution) and anti-CD31 (JC70A clone, 1:100 dilution) (Dako, Carpinteria, CA, USA). Antigen retrieval was performed with pressure chamber treatment in citrate buffer, and slides were incubated for 16 hours at 4˚C, followed by a polymeric-based detection system (Novolink Polymer Detection System, Novocastra, Newcastle, UK), and 3,3’-diaminobenzidine as the chromogen. Slides were subsequently counterstained using Harris’s hematoxylin. Sections from a canine mammary carcinoma known to express Ki-67 and CD31 were used as positive controls; both antibodies have been widely used in veterinary medicine and canine species reactivity has been described (19). Negative controls were assessed using normal serum as the primary antibody. The proliferative index and the intratumoral microvessel density were obtained as described by Dutra et al. (20) and Weidner (21). A Ki-67 cut-off value of 20% was used to stratify high-risk patients (22).
Statistical analysis was performed with D’Agostino and Pearson omnibus normality test, Student’s t-test and Mann–Whitney U-test. Survival time was defined as the period between the surgical removal of the tumor and death caused by the disease. Animals that died from unknown causes or causes unrelated to the tumor were censored. Median survival time (MST) curves were calculated by the Kaplan–Meier method and compared by the log-rank test. Results were considered significant when p≤0.05.
All procedures were performed with the approval of the Ethics Committee for Animal Experimentation of the Federal University of Minas Gerais (protocol number 132/2011) and the Ethics Committee for Animal Experimentation of the São Paulo State University (Jaboticabal Campus) (protocol number 021846/14), and informed owner consent was obtained.
Results
Fifty-eight bitches were evaluated, with an average age at diagnosis of 10.61±2.72 years. Forty patients presented advanced clinical staging, 32/54 (59.26%) stage IV and 8/54 (14.81%) stage V, while 3/54 (5.56%) were classified as stage I, 3/54 (5.56%) stage II, and 8/54 (14.81%) stage III. Neoplasm size was smaller than 3 cm in 11/53 (20.75%), 3-5 cm in 12/53 (22.64%), and larger than 5 cm in 30/53 (56.61%). Regarding the different treatment groups, 11/58 (18.97%) animals were submitted solely to surgery, 15/58 (25.86%) to SCT, 23/58 (39.65%) to SCTT, and 9/58 (15.52%) to SCTMC.
The primary neoplasms were diagnosed as 15/58 (25.86%) carcinomas in mixed tumors, 15/58 (25.86%) solid carcinomas, 8/58 (13.80%) micropapillary carcinomas, 8/58 (13.80%) carcinosarcomas, 4/58 (6.90%) tubular carcinomas, 2/58 (3.45%) papillary carcinomas, 2/58 (3.45%) malignant adenomyoepitheliomas, 1/58 (1.72%) lipid-rich carcinoma, 1/58 (1.72%) pleomorphic lobular carcinoma, 1/58 (1.72%) osteosarcoma, and 1/58 (1.72%) sarcoma in mixed tumor.
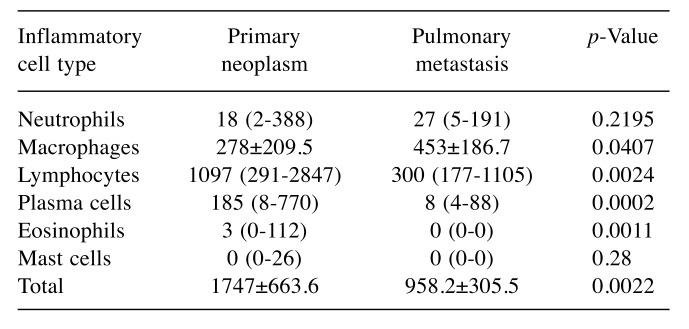
Necropsy was performed in 10 animals of the SCTT group, and 9/10 (90%) were found to have died due to the progression of the disease and presented pulmonary metastases. The mean proliferative index was 26.61%±23.08% in primary tumors and 10.05%±8.33% in pulmonary metastases. Median microvessel density (microvessels per 200× field) was 65.5 (range=33-179) and 86 (range=61-145) in primary tumors and pulmonary metastases, respectively. No statistical significant difference was found for Ki-67 and CD31 immunolabeling between primary and metastatic neoplasms (p=0.0859 and p=0.1695, respectively). MST analysis of low Ki-67 immunostaining of the primary neoplasm of patients treated with SCTT showed a significantly longer survival (MST=1052 days) when compared to neoplasms with high Ki-67 index (MST=276 days) (p=0.0029; Figure 1). A diffuse inflammatory infiltrate prevailed in 60% of primary and in 44.45% of metastatic lesions. There was an absence of discrete inflammatory reactions in primary and metastatic lesions, with a prevalence of intense reactions in 95% of primary neoplasms and moderate reactions in 55.55% of metastatic neoplasms. Intense lymphocytic infiltrate prevailed in 70% of primary neoplasms, while discrete/moderate lymphocytic infiltrate prevailed in 88.89% of metastatic neoplasms. The distribution of the inflammatory cells is demonstrated in Table I.
Figure 1. Overall survival of patients treated with surgery,chemotherapy and thalidomide according to Ki-67 immunostaining.Kaplan–Meier survival curve of 13 bitches diagnosed with high (≥20%;n=6) and low (<20%; n=7) Ki-67 immunostaining of malignantmammary gland neoplasms treated with surgery, chemotherapy andthalidomide. The median survival time of the group with low Ki-67index was significantly longer (1052 days) when compared to that withhigh Ki-67 index (276 days) (p=0.0029).
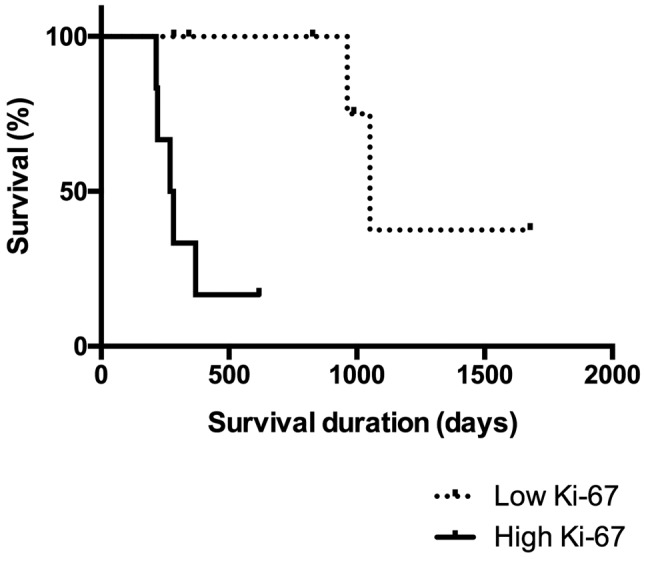
Table I. Median number (range) of inflammatory cells per eight fields(100×) of the inflammatory infiltrate in 20 primary neoplasms and 10pulmonary metastases from animals treated with surgery, chemotherapyand thalidomide.
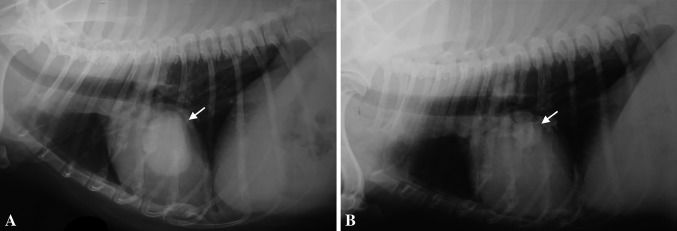
Although no statistically significant difference was observed in MST among the four treatment groups when including all clinical stages (p=0.3177), patients treated with SCTT presented a better outcome (MST=845 days), followed by patients treated with SCTMC (MST=431 days), when compared to surgery as an only treatment (MST=245 days) and SCT (MST 242 days) (Figure 2). However, when analyzing the MST of the four treatment groups including only animals that presented distant metastases before or during treatment, animals treated with SCTT (MST=463 days) or SCTMC (MST=376.5 days) presented a significantly longer survival when compared to animals treated solely with surgery (MST=150 days) and SCT (MST=148 days) (p<0.0001, Figure 3). Therapeutic evidence of thalidomide administration was also demonstrated by the evaluation of pulmonary metastases through thoracic radiographs. The metastatic lesions were mostly classified as stable, demonstrated by a slow progression of the lung metastasis. One patient presented a partial response of the metastatic lesion during thalidomide administration (Figure 4).
Figure 2. Overall survival according to the four studied treatmentprotocols. Kaplan–Meier survival curve of 58 bitches diagnosed withmalignant mammary gland neoplasms treated with surgery (n=11),surgery and chemotherapy (SCT; n=15), surgery, chemotherapy andthalidomide (SCTT; n=23), and surgery, chemotherapy and metronomicchemotherapy (SCTMC; n=9). Patients treated with SCTT and SCTMCpresented a longer median survival time (845 and 431 days,respectively) when compared to surgery as the only treatment (245 days)and SCT (242 days) (p=0.3177).
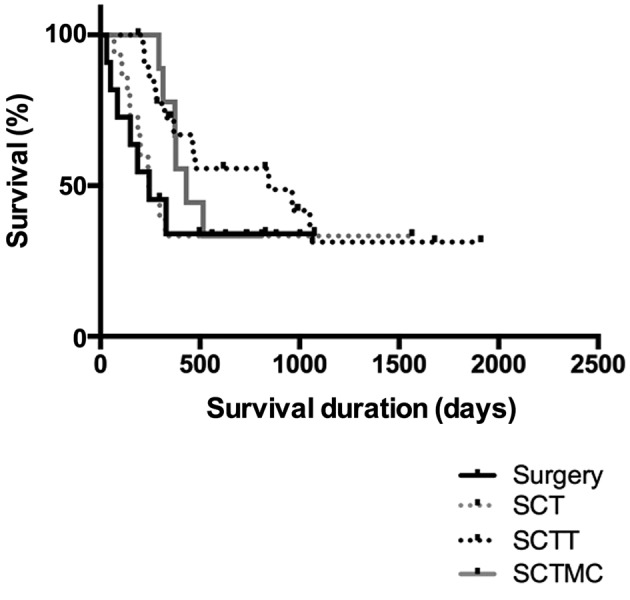
Figure 3. Overall survival according to the four studied treatment protocolsof patients with advanced clinical staging. Kaplan–Meier survival curve of27 bitches diagnosed with malignant mammary gland neoplasms presentingdistant metastasis treated with surgery (n=5), surgery and chemotherapy(SCT; n=3), surgery, chemotherapy and thalidomide (SCTT; n=13), andsurgery, chemotherapy and metronomic chemotherapy (SCTMC; n=6).Patients treated with SCTT and SCTMC (463 and 376.5 days, respectively)presented a significantly longer survival when compared to animals treatedsolely with surgery (150 days) and SCT (148 days) (p<0.0001).
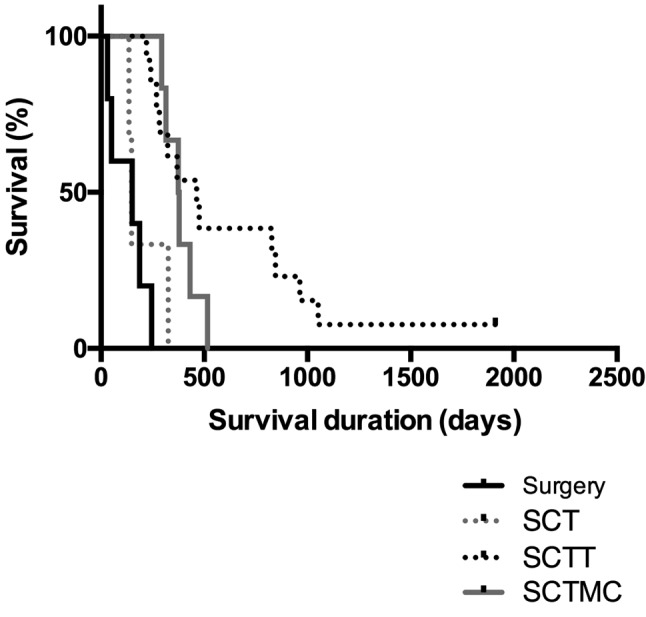
Figure 4. Decrease in distant metastasis following thalidomide treatment. Right lateral recumbency thoracic radiographs demonstrating a partial response of metastatic nodules during thalidomide treatment (arrow). A: At the beginning of thalidomide administration; B: Radiograph obtained after the patient completed 14 months of treatment with thalidomide.
All treatments were well tolerated. The 20-mg/kg dose of thalidomide was mainly associated with an excessive somnolence in some patients, with symptom improvement following dose reduction to 10 mg/kg. MC was associated with cyclophosphamide-induced sterile hemorrhagic cystitis in 4/9 (44.44%) patients and was interrupted promptly, followed by treatment with prednisone at the dose of 1 mg/kg, given orally for 10 days, which was enough to improve all symptoms and MC was resumed.
Discussion
Although there are no standard guidelines for the treatment of canine mammary gland neoplasms, large tumors with regional metastasis and aggressive histology, which prevailed in the present study, are not treated effectively with surgery alone (4). Shorter MSTs were described for animals with micropapillary carcinomas, solid carcinomas, tubular carcinomas, and carcinosarcomas when compared to those with carcinomas in mixed tumors and papillary carcinomas (23,24). The high frequency of carcinomas in mixed tumors was expected due to its high prevalence in canine mammary gland neoplasms (24,25).
Although the available evidence concerning the efficacy of adjuvant chemotherapy is limited, chemotherapy is often suggested for mammary gland neoplasms that present higher metastatic or recurrence risks (4,6). Cassali et al. recommend chemotherapy when the histotype of the primary neoplasm is associated with poor prognosis or when patients present metastasis (16). Chemotherapy causes DNA damage, interfering with DNA replication in proliferating cells (14), resulting in antiproliferative and cytotoxic actions (7). Maximum tolerated dose chemotherapy protocols aim to achieve the maximum cytotoxicity possible for tumor cells and requires an interval for patient recovery. These protocols are frequently initially efficacious and result in tumor regression or stabilization, prolonged survival, and remission. However, responses may be short-lived, with relapses resulting in resistance to the cytotoxic drug and more aggressive cancer (14). The present study was unable to demonstrate clinical benefit of adjuvant chemotherapy without the association of antiangiogenic therapy, possibly due to a tumor response that was not maintained.
Folkman proposed that tumor cells need the perfusion induced by new capillaries in order to exceed a diameter of 2-3 mm. If angiogenesis is inhibited, the tumor remains in a dormant state, hindering metastasis and increasing the susceptibility of tumor cells to cell-mediated immunologic attack (26). Acceptance of immunomodulatory therapies in the clinic may be improved if they are combined with other therapies of proven clinical utility. However, possible combinations should consider that conventional antitumor therapies may suppress host antitumor defense mechanisms and may therefore not be effective (7).
Thalidomide and its analogs inhibit several cytokines, e.g. interleukin-6, tumor necrosis factor-α, vascular endothelial growth factor and basic fibroblast growth factor, resulting in inhibition of tumor cell growth, survival, migration, drug resistance, and angiogenesis. Furthermore, interleukin-2 and interferon-γ may be stimulated, promoting antitumor immunity (8). Although the evaluation of samples of distant metastases without thalidomide treatment for comparison was not possible, such findings suggests the antitumor properties of thalidomide and are sustained by our observation of predominantly slow progression of the pulmonary metastases during thalidomide treatment. Furthermore, the proliferative index of the primary neoplasm was found to be correlated to the therapeutic response to thalidomide and influenced patient MST, and should be further evaluated as a predictive marker for thalidomide response.
Intense lymphocytic infiltrate was described as an independent prognostic factor associated with lower survival rates in canine mammary gland neoplasms (18) and the thalidomide treatment proposed here may be associated with a reduction in the intensity of lymphocytic infiltrates. Tumor-associated macrophages may be associated with tumor-promoting functions or contribute towards the efficacy of anticancer strategies (27). In mouse models, thalidomide was found to potentially reduce the pro-inflammatory effect of alveolar macrophages in pneumonia (28), inhibit polarization of M2 macrophages in allergic asthma (29), and demonstrated potent anti-inflammatory properties in an acute lung infection (30). Thalidomide was responsible for the inhibition of leukocyte recruitment in murine mammary 4T1 primary tumor and pulmonary metastatic tumors (31). The anti-inflammatory and macrophage increase in the present study may be partially responsible for the observed clinical benefit associated with the thalidomide treatment. Additional studies including macrophage phenotyping will contribute towards a better understanding of immunomodulatory effects of thalidomide.
Microvessel density has been described as a prognostic factor for various neoplasms, and that its decrease is observed during treatment with antiangiogenic therapy may suggest treatment activity (32). In the present study, no statistical difference was observed between the CD31 immunolabeling in primary neoplasms and pulmonary metastases. However, microvessel density is not directly related to angiogenic activity or dependence of a tumor, and has been described as ineffective as a predictive or therapeutic marker of antiangiogenic treatments (32).
In canines, the safety profile of thalidomide has been evaluated in healthy beagles (33). We previously reported the adverse events of thalidomide therapy in canine patients diagnosed with mammary gland neoplasms in advanced clinical staging. The drug was considered well tolerated, and 10 mg/kg and 20 mg/kg doses did not impair the activities of daily living. However, the lower dose may be considered when excessive somnolence is found (34). Two clinical trials started to evaluate thalidomide treatment in canine hemangiosarcomas (35) and various malignant neoplasms in dogs (36) but were not completed. A dog diagnosed with a malignant and metastatic Leydig cell tumor was reported to have been treated with surgery, MC and thalidomide (37), and the association of MC and thalidomide with conventional chemotherapy was reported to increase survival in canine hemangiosarcomas (38). In felines, a multimodal approach including thalidomide was proposed for the treatment of unresectable head and neck squamous cell carcinoma (39).
Recently, a multimodal protocol including toceranib, piroxicam and thalidomide with hypofractionated radiation therapy demonstrated clinical benefit in dogs with inflammatory mammary carcinoma (40); clinical benefit was also described in dogs with advanced primary lung carcinoma treated by metronomic cyclophosphamide, piroxicam and thalidomide (41). The addition of continuous thalidomide after surgical excision of splenic hemangiosarcoma in dogs was considered beneficial and suggested as a relevant therapy protocol for the disease (42).
Since thalidomide was withdrawn from the market, several countries have established regulations for the prescription and disposal of thalidomide for humans, e.g. the THALIDOMID REMS™ program of the United States of America (43). A legal and reliable source of thalidomide for veterinarians worldwide is a challenge. Therefore, MC represents an available and affordable alternative treatment to thalidomide.
In humans, few clinical trials have evaluated thalidomide as a single agent or in association with MC in breast cancer in humans (44-46). Although the trials failed to demonstrate clinical benefit of thalidomide, Baidas et al. do not exclude the possibility of thalidomide in combination with other therapeutic modalities or in micro-metastatic breast cancer (45). Thalidomide was only administered in advanced and heavily pretreated patients in these studies. Eisen et al. reported a significant improvement in patients’ sleeping and appetite (46). Colleoni et al. did, however, consider an MC protocol with low-dose oral cyclophosphamide and methotrexate effective and minimally toxic (44).
The continuous treatment schedule of MC protocols are appealing because they are generally well tolerated, and present low toxicity, easy administration, and low cost (9,47). MC alters the approach to therapy, not emphasizing the neoplastic cells, and, therefore, offers potential for the treatment of refractory neoplasms with drugs that have previously failed or the use of drugs that were considered ineffective (14). Metronomic cyclophosphamide has also been associated with an increase in antitumor immunity in animals (7). Several clinical trials for various malignant neoplasms have been performed involving MC (48-53), which may be combined with other drugs, including non-steroidal anti-inflammatory drugs and tyrosine kinase inhibitors (9). In the present study, firocoxib, a selective cyclo-oxygenase-2 (COX2) inhibitor, was chosen. Carvalho et al. demonstrated an association between COX2 expression and aggressive biological behavior, including shorter survival, metastasis, and angiogenesis, emphasizing the usefulness of selective COX2 inhibitors in the treatment for canine mammary tumors (54).
The present study failed to observe a statistically significant difference in MST among the four treatment groups composed of patients with different clinical stages. However, the addition of thalidomide was associated with an MST approximately four times longer than for those treated with surgery, and SCT, and twice as long than that of the patients treated with SCTMC.
A significantly longer MST was observed when analyzing patients that presented distant metastasis before or during the study in the groups treated with SCTT and SCTMC when compared to the groups treated with surgery and SCT, demonstrating a clear clinical benefit in the addition of antiangiogenic and immunomodulatory therapies for these patients. Survival rates for animals presenting distant metastasis was described as 13.6% at 1 year after mastectomy (55). MST for both the MC and thalidomide group in this study was more than 1 year, while MST for groups not treated with antiangiogenic therapies was approximately 150 days. Therefore, the proposed multimodal therapy protocols of surgery and chemotherapy with thalidomide or MC increased the survival time for patients presenting malignant mammary gland neoplasms with distant metastasis.
Acknowledgements
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant 2014/01329-9) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Grant 302449/2013-2).
References
- 1.Munson L, Moresco A. Comparative pathology of mammary gland cancers in domestic and wild animals. Breast Dis. 2007;28:7–21. doi: 10.3233/bd-2007-28102. [DOI] [PubMed] [Google Scholar]
- 2.Schneider R. Comparison of age, sex, and incidence rates in human and canine breast cancer. Cancer. 1970;26:419–426. doi: 10.1002/1097-0142(197008)26:2<419::aid-cncr2820260225>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Brodey RS, Goldschmidt MH, Roszel JR. Canine mammary gland neoplasms. J Am Anim Hospl Assoc. 1983;19:61–90. [Google Scholar]
- 4.Sorenmo KU, Worley DR, Goldschmidt MH. Tumors of the Mammary Gland. Withrow & MacEwen’s Small Animal Clinical Oncology. Withrow SJ, Vail DM and Page RL (eds.). . Saint Louis, Elsevier Saunders . 2013;5th ed.:538–556. [Google Scholar]
- 5.Novosad CA. Principles of treatment for mammary gland tumors. Clin Tech Small Anim Pract. 2003;18:107–109. doi: 10.1053/svms.2003.36625. [DOI] [PubMed] [Google Scholar]
- 6.Sorenmo KU. Canine mammary gland tumors. Vet Clin North Am Small Anim Pract. 2003;33:573–596. doi: 10.1016/s0195-5616(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 7.Ehrke MJ. Immunomodulation in cancer therapeutics. Int Immunopharmacol. 2003;3:1105–1119. doi: 10.1016/S1567-5769(03)00021-3. [DOI] [PubMed] [Google Scholar]
- 8.Raje N, Anderson KC. Thalidomide and immunomodulatory drugs as cancer therapy. Curr Opin Oncol. 2002;14:635–640. doi: 10.1097/00001622-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Pierini A, Bocci G, Giorgi M, Owen H, Marchetti V. From humans to dogs and back: The translational lesson of metronomic chemotherapy. Am J Anim Vet Sci. 2012;7:198–212. [Google Scholar]
- 10.McBride WG. Thalidomide embryopathy. Teratology. 1977;16:79–82. doi: 10.1002/tera.1420160113. [DOI] [PubMed] [Google Scholar]
- 11.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38:203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 12.Adlard JW. Thalidomide in the treatment of cancer. Anti-Cancer Drug. 2000;11:787–791. doi: 10.1097/00001813-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J. 2005;7:14–19. doi: 10.1208/aapsj070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misdorp W, Else RW, Hellmén E, Lipscomb TP. Histological Classification of Mammary Tumors of the Dog and the Cat. Armed Forces Institute of Pathology. American Registry of Pathology and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology, 1999 [Google Scholar]
- 16.Cassali GD, Lavalle GE, Ferreira E, Estrela-Lima A, Nardi AB De, Ghever C, Sobral RA, Amorim RL, Oliveira LO, Sueiro FAR, Beserra HEO, Bertagnolli AC, Gamba CO, Damasceno KA, Campos CB, Araujo MR, Campos LC, Monteiro LN, Nunes FC, Horta RS, Reis DC, Luvizotto MCR, Magalhaes GM, Raposo JB, Ferreira AMR, Tanaka NM, Grandi F, Ubukata R, Batschinski K, Terra EM, Salvador RCL, Jark PC, Delecrodi JER, Nascimento NA, Silva DN, Silva LP, Ferreira KCRS, Frehse MS, Santis GW, Silva EO, Guim TN, Kerr B, Cintra PP, Silva FBF, Leite JS, Mello MFV, Ferreira MLG, Fukumasu H, Salgado B, Torres R. Consensus for the diagnosis, prognosis and treatment of canine mammary tumors-2013. Braz J Vet Pathol. 2014;7:38–69. [Google Scholar]
- 17.Elston CW, Ellis IO. Assessment of histological grade. In: Systemic Pathology: The Breast. Third Edition. Elston CW and Ellis IO (eds.) London, Churchill Livingstone. 1998:pp. 365–384. [Google Scholar]
- 18.Estrela-Lima A, Araujo MS, Costa-Neto JM, Teixeira-Carvalho A, Barrouin-Melo SM, Cardoso SV, Martins-Filho OA, Serakides R, Cassali GD. Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates. BMC Cancer. 2010;10:256–269. doi: 10.1186/1471-2407-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa S, Nagaike M, Ozaki K. Databases for technical aspects of immunohistochemistry. J Toxicol Pathol. 2017;30:79–107. doi: 10.1293/tox.2016-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutra AP, Azevedo Júnior GM, Schmitt FC, Cassali GD. Assessment of cell proliferation and prognostic factors in canine mammary gland tumors. Arq Bras Med Vet Zootec. 2008;60:1403–1412. [Google Scholar]
- 21.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 22.Bustreo S, Osella-abate S, Cassoni P, Donadio M, Airoldi M, Pedani F, Papotti M, Sapino A, Castellano I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res Treat. 2016;157:363–371. doi: 10.1007/s10549-016-3817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes FC. Diagnóstico, prognóstico e tratamento dos carcinomas de glândulas mamárias de cadelas atendidas no hospital veterinário da UFMG - Estudo retrospectivo. 2015 [Google Scholar]
- 24.Nunes FC, Gamba CO, Damasceno KA, Campos CB, Horta RS, Araujo MR, Monteiro LN, Lavalle GE, Ferreira E, Cassali GD. Analysis of clinico-pathological data, therapeutical conduct and overall survival of canine mammary lesions attended at the veterinary hospital of the Federal University of Minas Gerais (UFMG) Braz J Vet Pathol. 2014;7:122–126. [Google Scholar]
- 25.Toríbio JMDML, Lima AE, Martins Filho EF, Ribeiro LGR, D’Assis MJMH, Teixeira RG, Damasceno KA, Cassali GD, Costa Neto JM Da. Clinical characterization, histopathologic diagnosis and geoprocessing of mammary tumours in bitches from the city of Salvador, Bahia State. Rev Ceres. 2012;59:427–433. [Google Scholar]
- 26.Folkman J. Tumor angiogenesis: Therapeutic implications. New Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435–445. doi: 10.1084/jem.20150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V, Harjai K, Chhibber S. Thalidomide treatment modulates macrophage pro-inflammatory function and cytokine levels in Klebsiella pneumoniae B5055 induced pneumonia in BALB/c mice. Int Immunopharmacol. 2010;10:777–783. doi: 10.1016/j.intimp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Lee HS, Kwon HS, Park DE, Woo YD, Kim HY, Kim HR, Cho SH, Min KU, Kang HR, Chang YS. Thalidomide inhibits alternative activation of macrophages in vivo and in vitro: A potential mechanism of anti-asthmatic effect of thalidomide. PLoS ONE. 2015;10:1–15. doi: 10.1371/journal.pone.0123094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar V, Chhibber S. Anti-inflammatory effect of thalidomide alone or in combination with augmentin in Klebsiella pneumoniae B5055 induced acute lung infection in BALB/c mice. Eur J Pharmacol. 2008;592:146–150. doi: 10.1016/j.ejphar.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 31.De Souza CM, Fonseca de Carvalho L, da Silva Vieira T, Cândida Araújo e Silva A, Teresa Paz Lopes M, Alves Neves Diniz Ferreira M, Passos Andrade S, Dantas Cassali G. Thalidomide attenuates mammary cancer associated-inflammation, angiogenesis and tumor growth in mice. Biomed Pharmacother. 2012;66:491–498. doi: 10.1016/j.biopha.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: Microvessel density, what it does and doesn’t tell us. J NatI Cancer Inst. 2002;94:883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 33.Teo SK, Evans MG, Brockman MJ, Ehrhart J, Morgan JM, Stirling DI, Thomas SD. Safety profile of thalidomide after 53 weeks of oral administration in beagle dogs. Toxicol Sci. 2001;59:160–168. doi: 10.1093/toxsci/59.1.160. [DOI] [PubMed] [Google Scholar]
- 34.de Campos CB, Lavalle GE, Ligorio SF, Nunes FC, Carneiro RA, L AR, Cassali GD. Absence of significant adverse events following thalidomide administration in bitches diagnosed with mammary gland carcinomas. Vet Rec. 2016;179:514–519. doi: 10.1136/vr.103764. [DOI] [PubMed] [Google Scholar]
- 35.Woods JP, Mathews KA, Binnington AG. Thalidomide for the treatment of hemangiosarcoma in dogs. Vet Comp Oncol. 2004;2:108–109. [Google Scholar]
- 36.Jankowski M, Fulton L, Sheafor S, Prescott D, Khanna C. Ongoing evaluation of single agent thalidomide in dogs with measurable cancer. Veterinary Cancer Society 19th Annual Meeting. Wood’s Hole, MA. 1999;In [Google Scholar]
- 37.Togni A, Rütten M, Bley CR, Hurter K. Metastasized Leydig cell tumor in a dog. Schweizer Archiv für Tierheilkunde. 2015;157:111–115. doi: 10.17236/sat00010. [DOI] [PubMed] [Google Scholar]
- 38.Finotello R, Henriques J, Sabattini S, Stefanello D, Felisberto R, Pizzoni S, Ferrari R, Marconato L. A retrospective analysis of chemotherapy switch suggests improved outcome in surgically removed, biologically aggressive canine haemangiosarcoma. Vet Comp Oncol. 2016;15:493–503. doi: 10.1111/vco.12193. [DOI] [PubMed] [Google Scholar]
- 39.Marconato L, Buchholz J, Keller M, Bettini G, Valenti P, Kaser-Hotz B. Multimodal therapeutic approach and interdisciplinary challenge for the treatment of unresectable head and neck squamous cell carcinoma in six cats: A pilot study. Vet Comp Oncol. 2013;11:101–112. doi: 10.1111/j.1476-5829.2011.00304.x. [DOI] [PubMed] [Google Scholar]
- 40.Rossi F, Sabattini S, Vascellari M, Marconato L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet Comp Oncol. 2018 doi: 10.1111/vco.12407. [DOI] [PubMed] [Google Scholar]
- 41.Polton G, Finotello R, Sabattini S, Rossi F, Laganga P, Vasconi ME, Barbanera A, Stiborova K, Rohrer Bley C, Marconato L. Survival analysis of dogs with advanced primary lung carcinoma treated by metronomic cyclophosphamide, piroxicam and thalidomide. Vet Comp Oncol. 2018 doi: 10.1111/vco.12393. [DOI] [PubMed] [Google Scholar]
- 42.Bray JP, Orbell G, Cave N, Munday JS. Does thalidomide prolong survival in dogs with splenic haemangiosarcoma. J Small Anim Pract. 2018;59:85–91. doi: 10.1111/jsap.12796. [DOI] [PubMed] [Google Scholar]
- 43.Celgene: Thalidomid REMS. 2015 Available from:http://www.thalomidrems.com [last accessed October 5, 2016] [Google Scholar]
- 44.Colleoni M, Orlando L, Sanna G, Rocca A, Maisonneuve P, Peruzzotti G, Ghisini R, Sandri MT, Zorzino L, Nolè F, Viale G, Goldhirsch A. Metronomic low-dose oral cyclophosphamide and methotrexate plus or minus thalidomide in metastatic breast cancer: Antitumor activity and biological effects. Ann Oncol. 2006;17:232–238. doi: 10.1093/annonc/mdj066. [DOI] [PubMed] [Google Scholar]
- 45.Baidas BSM, Winer EP, Fleming GF, Harris L, Pluda JM, Crawford JG, Yamauchi H, Isaacs C, Hanfelt J, Tefft M, Flockhart D, Johnson MD, Hawkins MJ, Lippman ME, Hayes DF. Phase II evaluation of thalidomide in patients with metastatic breast cancer. J Clin Oncol. 2000;18:2710–2717. doi: 10.1200/JCO.2000.18.14.2710. [DOI] [PubMed] [Google Scholar]
- 46.Eisen T, Boshoff C, Mak I, Sapunar F, Vaughan MM, Pyle L, Johnston SRD, Ahern R, Smith IE, Gore ME. Continuous low-dose thalidomide: A phase II study in advanced melanoma, renal cell, ovarian and breast cancer. Br J Cancer. 2000;82:812–817. doi: 10.1054/bjoc.1999.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mutsaers AJ. Antiangiogenic and Metronomic Therapy, Withrow & MacEwen’s Small Animal Clinical Oncology. Fifth Edition. Withrow SJ and Vail DM (eds.) Philadelphia, W. B. Saunders Company. 2013:229–237. [Google Scholar]
- 48.Tripp CD, Fidel J, Anderson CL, Patrick M, Pratt C, Sellon R, Bryan JN. Tolerability of metronomic administration of lomustine in dogs with cancer. J Vet Intern Med. 2011;25:278–284. doi: 10.1111/j.1939-1676.2011.0684.x. [DOI] [PubMed] [Google Scholar]
- 49.Leach TN, Childress MO, Greene SN, Mohamed S, Moore GE, Schrempp DR, Lahrman SR, Knapp DW. Prospective trial of metronomic chlorambucil chemotherapy in dogs with naturally occurring cancer. Vet Comp Oncol. 2012;10:102–112. doi: 10.1111/j.1476-5829.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 50.Schrempp DR, Childress MO, Stewart JC, Leach TN, Tan KM, Abbo AH, de Gortari AE, Bonney PL, Knapp DW. Metronomic administration of chlorambucil for treatment of dogs with urinary bladder transitional cell carcinoma. J Am Vet Med Assoc. 2013;242:1534–1538. doi: 10.2460/javma.242.11.1534. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell L, Thamm DH, Biller BJ. Clinical and immuno-modulatory effects of toceranib combined with low-dose cyclo-phosphamide in dogs with cancer. J Vet Intern Med. 2012;26:355–362. doi: 10.1111/j.1939-1676.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 52.Lana S, Lance U, Plaza S, Elmslie R, Gustafson D, Morley P, Dow S. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007;21:764–769. doi: 10.1892/0891-6640(2007)21[764:clocfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Elmslie RE, Glawe P, Dow SW. Metronomic therapy with cyclophosphamide and piroxicam effectively delays tumor recurrence in dogs with incompletely resected soft tissue sarcomas. J Vet Intern Med. 2008;22:1373–1379. doi: 10.1111/j.1939-1676.2008.0179.x. [DOI] [PubMed] [Google Scholar]
- 54.Carvalho MI, Pires I, Prada J, Raposo TP, Gregório H, Lobo L, Queiroga FL. High COX-2 expression is associated with increased angiogenesis, proliferation and tumoural inflammatory infiltrate in canine malignant mammary tumours: A multivariate survival study. Vet Comp Oncol. 2017;15(2):619–631. doi: 10.1111/vco.12206. [DOI] [PubMed] [Google Scholar]
- 55.Yamagami T, Kobayashi T, Takahashi K, Sugiyama M. Prognosis for canine malignant mammary tumors based on TNM and histologic classification. J Vet Med Sci. 1996;58:1079–1083. doi: 10.1292/jvms.58.11_1079. [DOI] [PubMed] [Google Scholar]