Abstract
Background
Elevated serum fibroblast growth factor 23 (FGF23) is strongly associated with cardiovascular risk and mortality. Tenapanor, an inhibitor of gastrointestinal sodium/hydrogen exchanger isoform 3, decreased serum phosphate in a randomized, double-blind, placebo-controlled Phase 2 trial (ClinicalTrials.gov identifier NCT02081534) of patients receiving hemodialysis with hyperphosphatemia. Here, we report a secondary analysis of effects on serum FGF23 during that study.
Methods
After 1–3 weeks of washout of phosphate binders, 162 patients were randomized to receive 4 weeks of treatment with placebo or one of six tenapanor regimens (3 or 30 mg once daily, or 1, 3, 10 or 30 mg twice daily). Intact FGF23 concentrations were determined from serum samples collected at screening, post-washout and end of treatment, assayed in duplicate in a single batch at the end of the study.
Results
After phosphate-binder washout, serum FGF23 concentrations increased in all groups [range of geometric means: 1430–2605 pg/mL before, to 2601–6294 pg/mL after washout (P < 0.001 for all patients analyzed as a single group)]. Serum FGF23 concentrations subsequently decreased in tenapanor-treated patients (2030–3563 pg/mL), whereas they increased further in placebo-treated patients (6930 pg/mL). In an analysis of covariance, FGF23 decreased by 9.1–27.9% in tenapanor-treated patients and increased by 21.9% in placebo-treated patients (P ≤ 0.001–0.04).
Conclusions
Following a marked increase in serum FGF23 in response to withdrawal of phosphate binders, tenapanor significantly decreased serum FGF23 in patients receiving hemodialysis with hyperphosphatemia. Further studies are required to explore the long-term effects of controlling FGF23 with tenapanor.
Keywords: fibroblast growth factor 23, hyperphosphatemia, phosphate binder, sodium/hydrogen exchanger isoform 3, tenapanor
ADDITIONAL CONTENT
An author video to accompany this article is available at: https://academic.oup.com/ndt/pages/author_videos.
INTRODUCTION
Hyperphosphatemia is a common metabolic complication in patients receiving dialysis that results from impaired kidney function and inability of conventional hemodialysis to sufficiently remove the daily dietary phosphate load [1]. Fibroblast growth factor 23 (FGF23) regulates phosphate homeostasis (reviewed by Wolf [2]). It is secreted by osteocytes and osteoblasts, and stimulates urinary phosphate excretion by downregulating phosphate reabsorption in the renal proximal tubule [2]. FGF23 also suppresses circulating concentrations of 1,25-dihydroxyvitamin D by inhibiting its production in the kidney and accelerating its degradation [3, 4]. The resultant deficiency of 1,25-dihydroxyvitamin D contributes to secondary hyperparathyroidism [5]. Factors known to stimulate FGF23 production include dietary phosphate and calcium intake, elevated serum calcium concentrations, administration of 1,25-dihydroxyvitamin D or its analogs, iron deficiency and inflammation [6, 7]. Serum FGF23 concentrations increase substantially as kidney function declines such that patients with end-stage renal disease receiving dialysis can have levels that are more than 1000-fold higher than those of healthy individuals [8].
Like hyperphosphatemia [9], elevated serum FGF23 concentration is strongly associated with cardiovascular disease and mortality in patients with chronic kidney disease (CKD) Stages 2–5D [9–13]. In the Chronic Renal Insufficiency Cohort study of patients with CKD Stages 2–4, elevated FGF23 was independently associated with left ventricular hypertrophy (LVH) [10], heart failure [13], atrial fibrillation [14] and mortality [12]. Animal data support a possible causal role for FGF23 excess in the development of LVH, with FGF23 having been shown to cause pathological hypertrophy of rat cardiomyocytes in vitro and LVH in vivo [10]. In contrast, it has also been suggested that cardiac hypertrophy occurs independently of FGF23 [15]. FGF23 has also been shown to impair monocyte and neutrophil function [16, 17]. This may explain the increased risk of infection-related mortality associated with elevated FGF23 [18], although it is possible that FGF23 may be upregulated in response to infection [19]. It has also been shown that treatments that solely target FGF23 reduction, including neutralizing anti-FGF23 antibodies, precipitate severe hyperphosphatemia, and accelerate vascular calcification and mortality [20]. Collectively, these data demonstrate the need to identify therapies capable of jointly lowering both serum phosphate and FGF23 concentrations.
Dietary phosphate restriction and administration of phosphate binders are first-line treatments for managing hyperphosphatemia in patients receiving hemodialysis. However, the ability to effectively reduce dietary phosphate intake is hampered by supplementation of the food supply with inorganic phosphate-based food additives and the expanding list of common items to which phosphate has been added (e.g. flavored water and other beverages) [21]. These phosphate sources remain largely ‘hidden’ because they are not quantified on food labels and are unaccounted for by dietary data collection instruments and nutritional databases. Furthermore, the effects of phosphate binders on serum FGF23 concentrations have been inconsistent. While non-calcium-based binders variably lower serum FGF23 concentrations by up to 40%, calcium-based binders have minimal FGF23-lowering effects and may actually increase FGF23, perhaps owing to the FGF23-stimulatory effects of calcium loading (reviewed in Isakova et al. [22]).
Tenapanor is a small-molecule inhibitor of the sodium/hydrogen exchanger isoform 3. In addition to lowering gastrointestinal absorption of sodium [23–25], tenapanor also decreases gastrointestinal absorption of phosphate [24, 26] via a mechanism distinct from luminal phosphate binding, the details of which are under active investigation. We recently reported that tenapanor treatment significantly and dose-dependently decreased serum phosphate in a Phase 2 study of patients receiving hemodialysis with hyperphosphatemia [27]. Here, we describe a secondary analysis of serum FGF23 concentrations during the Phase 2 clinical trial. We tested the hypothesis that tenapanor decreases serum FGF23 in parallel with serum phosphate.
MATERIALS AND METHODS
Study design and patients
The design and methods of this randomized, double-blind, placebo-controlled, multicentre Phase 2B study (EudraCT no. 2013–004319–33; ClinicalTrials.gov identifier NCT02081534) have been previously described [27]. In brief, patients with CKD Stage 5D (receiving hemodialysis) on a stable dose of phosphate binders and with serum phosphate concentrations of 3.5–8.0 mg/dL were screened. After a 1- to 3-week washout of phosphate binders, patients with serum phosphate concentrations of 6.0 to <10 mg/dL who had an increase of at least 1.5 mg/dL from screening after 1, 2 or 3 weeks of phosphate-binder washout were randomly assigned to 4 weeks of treatment with placebo or one of six tenapanor regimens (3 or 30 mg once daily, or 1, 3, 10 or 30 mg twice daily). The study was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The protocol was approved by independent ethics committees. All participants provided written informed consent.
Study endpoints and assays
The primary efficacy endpoint of the trial was change in serum phosphate concentration from the end of the phosphate-binder washout period (baseline) to the end of treatment or early termination. FGF23 was a secondary exploratory biomarker. For the analysis of serum FGF23 concentrations, blood samples were collected before dialysis at screening, post-washout and at the end of treatment or early termination. Intact human FGF23 was measured by enzyme-linked immunosorbent assay (EMD Milipore, Billerica, MA, USA). All samples were stored at −80°C until analysis, which was conducted in one batch at the end of the study in duplicate [inter-assay and intra-assay coefficients of variation (CVs) <11%] [28]. Samples were diluted 1:20 for analysis, except for one individual’s sample that required 1:100 dilution. Validation of linear dilution was performed using test samples containing high FGF23 concentrations to confirm that the signal was independent of the dilution (i.e. there was no plasma matrix effect).
Statistical analyses
Owing to the highly right-skewed data, we calculated geometric means for all FGF23 measurements, and presented the geometric CV, along with medians, ranges and ratios of geometric means (with 95% confidence limits). All inferential analyses were performed using natural log-transformed FGF23 data.
We used an analysis of covariance (ANCOVA) to identify potential factors associated with serum FGF23 concentrations at screening. Candidate factors included age, sex, race/ethnicity, serum calcium and phosphate concentrations and types of phosphate binders (calcium- or non-calcium-based). In addition, we presented the Pearson partial correlation coefficient between serum FGF23 and each of serum calcium (adjusted for sex and phosphate based on the results of the ANCOVA) and serum phosphate (adjusted for sex and calcium based on the results of the ANCOVA) concentrations at screening.
To analyze the effect of tenapanor on serum FGF23 concentrations from post-phosphate-binder washout to the end of treatment, we performed an ANCOVA on FGF23 change at the end of treatment, with treatment as a fixed factor and post-washout serum FGF23 concentration as a covariate. Within the framework of the ANCOVA model, we performed a pairwise comparison between each tenapanor dose and placebo using a t-test with a significance level of 0.05, as described previously, unless otherwise specified [27].
To investigate the correlation between changes in serum FGF23 and serum phosphate concentrations from post-washout to the end of treatment, we plotted changes in natural log-transformed serum FGF23 against changes in serum phosphate and calculated the Pearson correlation coefficient (ρ).
RESULTS
Patient disposition and serum phosphate
Following a 1- to 3-week washout of phosphate binders, 162 patients who met the criteria to continue the study (serum phosphate 6.0 to <10 mg/dL) were randomly assigned to receive 4 weeks of treatment with either placebo or tenapanor at doses of 3 or 30 mg once daily, or 1, 3, 10 or 30 mg twice daily (seven groups in total). The mean (standard deviation) age of participants in the study population was 59.1 (13.7) years and 64% were men. A total of 115 patients (71%) completed the study. Clinical characteristics of the randomized groups have been reported previously; they were well-balanced across all demographic and clinical characteristics [27]. During the treatment period, gastrointestinal disorders were the most common adverse event type (by system organ class) in the tenapanor groups (tenapanor-treated groups, 23–76%; placebo group, 19%), with diarrhea the most frequent adverse event (tenapanor-treated groups, 18–68%; placebo group, 12%), particularly at the highest doses of tenapanor (10 mg twice daily, 48%; 30 mg once daily, 52%; 30 mg twice daily, 68%) [27]. A total of 19 patients (12%) discontinued treatment owing to diarrhea, with the highest rates of discontinuation in the two highest-dose tenapanor groups (30 mg once daily, 29%; 30 mg twice daily, 32%). Other types of adverse events were uncommon, with the adverse event profile of tenapanor otherwise similar to that of placebo [27].
At the end of the washout period, mean serum phosphate concentrations were 7.32–7.92 mg/dL in the tenapanor-treated groups and 7.87 mg/dL in the placebo-treated group. Tenapanor treatment decreased serum phosphate concentrations from the post-washout level in a dose-dependent manner (least-squares mean changes: tenapanor-treated groups, −0.47 to −1.98 mg/dL; placebo group, −0.54 mg/dL; P = 0.01, ANCOVA; F-test). The most pronounced reductions in serum phosphate from the post-washout level were observed in the tenapanor 10 and 30 mg twice daily dosing groups (each P < 0.05 versus placebo, ANCOVA; t-test) [27].
Serum FGF23 at screening and following phosphate-binder washout
Serum-intact FGF23 concentrations throughout the study are presented in Figure 1. At screening, the geometric mean serum intact FGF23 (CV, percentage) was 1996 pg/mL (274%) for all patients analyzed as a single group [n = 147 (screening samples with complete FGF23 measurements)], and the range of geometric means for all groups was 1430–2605 pg/mL. Men had significantly higher serum FGF23 concentrations at screening than women [geometric means (CV, percentage): men, 2432 pg/mL (222%); women, 1407 pg/mL (360%); P= 0.005]. Screening FGF23 concentrations correlated directly with serum concentrations of calcium and phosphate (Pearson partial correlation coefficients: calcium, 0.35; phosphate, 0.69; both P < 0.001). No significant differences were observed in serum FGF23 at screening by race, age or use of calcium-based versus non-calcium-based phosphate binders (P values all >0.05).
FIGURE 1.
Serum FGF23 concentrations at screening, post-phosphate-binder washout and at the end of study treatment with tenapanor or placebo. Geometric means (CV, percentage) at post-washout reported previously [27]. b.i.d., twice daily; EOT/ET, end of treatment/early termination; q.d., once daily.
Serum FGF23 concentrations increased for all groups from screening to post-phosphate-binder washout (Figure 1), with geometric means of FGF23 approximately doubling by the end of the washout period. For all patients analyzed as a single group [n =146 (post-washout samples with complete FGF23 measurements)], geometric mean serum FGF23 (CV, percentage) was 4201 pg/mL (245%) after phosphate-binder washout compared with 1996 pg/mL (274%) at screening (P < 0.001); the range of post-washout geometric means for all groups was 2601–6294 pg/mL [27].
Effect of tenapanor or placebo on serum FGF23
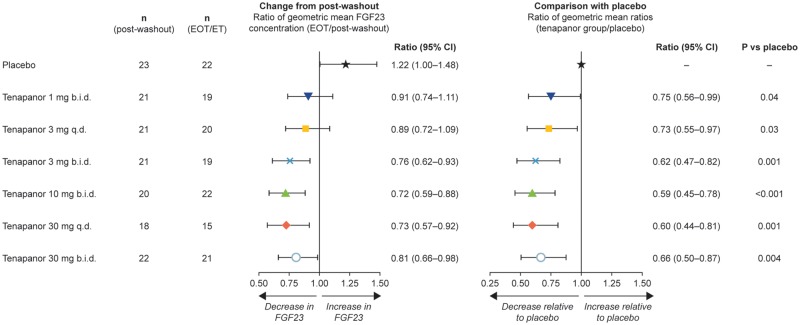
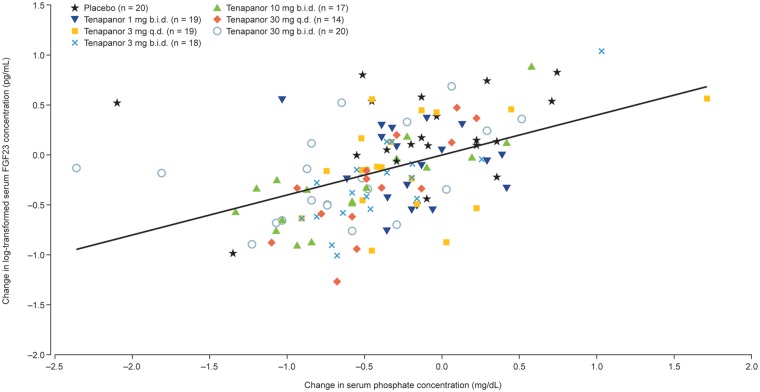
Up to 4 weeks of tenapanor treatment decreased serum FGF23 concentrations from post-phosphate-binder washout (range of geometric means for all tenapanor groups after tenapanor treatment: 2030–3563 pg/mL), whereas FGF23 continued to increase in patients receiving placebo (geometric mean: after placebo treatment, 6930 pg/mL; after phosphate-binder washout, 4937 pg/mL; Figure 1). Similar results were observed when an ANCOVA was performed on the change in FGF23 from post-washout to the end of treatment (Figure 2). There was a 21.9% increase in geometric mean FGF23 for the placebo group and a 9.1–27.9% decrease in geometric mean FGF23 for the tenapanor-treated groups. Tenapanor doses of at least 3 mg twice daily were required to decrease FGF23 concentrations substantially compared with post-washout levels (Figure 2), whereas all tenapanor doses significantly decreased FGF23 compared with placebo (P ≤ 0.001–0.04; Figure 2). At the end of the study, serum FGF23 concentrations had not completely returned to screening levels in the groups treated with tenapanor (Figure 1). When all tenapanor- and placebo-treated patients were considered, the change in serum FGF23 concentrations from post-phosphate-binder washout to the end of treatment correlated with concomitant changes in serum phosphate (ρ = 0.48, P < 0.001; Figure 3).
FIGURE 2.
Forest plots of ratios of geometric mean of serum FGF23 concentration following treatment with tenapanor or placebo. Data are presented as ratios (with 95% confidence intervals), which can be converted to percentage changes from post-washout or placebo treatment. ANCOVA was performed on data for the change from post-washout in natural log-transformed FGF23 concentration at the end of treatment/early termination, with treatment as a fixed factor and post-washout FGF23 (natural log-transformed) as a covariate. Ratios for change from post-washout reported previously [27]. b.i.d., twice daily; CI, confidence interval; EOT/ET, end of treatment/early termination; q.d., once daily.
FIGURE 3.
Scatter plot of changes in serum FGF23 (natural log transformed) and phosphate concentrations from post-phosphate-binder washout to the end of treatment/early termination for all study participants. The change in serum FGF23 concentrations from post-phosphate-binder washout to the end of treatment correlated with concomitant changes in serum phosphate (Pearson correlation coefficient, ρ = 0.48; P < 0.001). b.i.d., twice daily; q.d., once daily.
DISCUSSION
This secondary analysis of a Phase 2 placebo-controlled randomized clinical trial of tenapanor in patients receiving hemodialysis with hyperphosphatemia provides important new findings relating to the management of disordered phosphate homeostasis in patients receiving hemodialysis. The ability of non-calcium-based phosphate binders to lower elevated FGF23 concentrations in patients with CKD has been inconsistent, and calcium-based phosphate binders may actually increase FGF23 [22]. Here, we provide, to our knowledge, the first data showing that FGF23 markedly and rapidly increases following 1–3 weeks of withdrawal from maintenance phosphate-binder therapy, which provides indirect clinical evidence of a beneficial effect of phosphate binders on FGF23 control. We also report that in this study tenapanor significantly lowered FGF23 versus placebo following just 4 weeks of treatment. In aggregate, these data provide strong support for the overarching approach of targeting reduction of dietary phosphate absorption as a strategy to lower serum FGF23 concentrations in parallel with serum phosphate in patients with CKD.
Consistent with our hypothesis, the magnitude of FGF23 reduction correlated with the magnitude of change in serum phosphate. The degree of FGF23 reduction in this study compared favorably to that in other studies with similarly brief durations of treatment [22, 29, 30]. In a study of patients undergoing hemodialysis with iron deficiency [31], median intact FGF23 concentration decreased from 2000 pg/mL at baseline to 1771 pg/mL (P = 0.01) 12 weeks after the phosphate binder they were taking was changed from sevelamer hydrochloride to ferric citrate hydrate, consistent with a reduction in median intact FGF23 of ∼11% [31]. In other studies in normophosphatemic patients with CKD Stage 3 or 4, reductions in median intact FGF23 of ∼40% were achieved after 6 weeks of treatment with sevelamer hydrochloride [30], while 4 weeks of treatment with lanthanum carbonate resulted in reductions in median C-terminal FGF23 levels of 22% relative to baseline [32]. In contrast, following 1 year of randomized treatment with either calcium acetate or sevelamer hydrochloride, patients receiving hemodialysis experienced a significant reduction in median intact FGF23 from 16 478 pg/mL at baseline to 5378 pg/mL (P< 0.0001), consistent with a reduction of ∼67% [33]. However, because many patients in that study changed their dialysate calcium concentration from 3.5 to 2.5 mEq/L, and many withdrew calcitriol treatment for the entire year of investigation, it is difficult to ascertain the magnitude of FGF23 reduction that can be attributed to each of the simultaneous interventions. It is also unknown how much of the FGF23 reduction occurred within the first 4 weeks of phosphate-binder treatment. In a separate study in patients with Stage 3 nondiabetic CKD treated with sevelamer carbonate or placebo for 40 weeks, the modest yet significant reduction in FGF23 concentration in patients receiving sevelamer relative to placebo was not accompanied by a significant effect on left ventricular mass [34]. Further studies are thus needed to evaluate whether tenapanor-induced reductions in FGF23 are associated with improvements in cardiovascular parameters, such as left ventricular mass.
Although tenapanor did not decrease serum FGF23 to pre-phosphate-binder washout concentrations, it is important to acknowledge that we investigated only a 4-week course of treatment after abruptly stopping phosphate binders that patients may have been taking for years, and that FGF23 continued to increase in patients receiving placebo. These findings suggest that pharmacological lowering of elevated FGF23 in patients with CKD may require more time than that required for FGF23 to rise in the absence of treatment. It is also currently unclear how to optimally and consistently reduce FGF23, with the most effective therapies described so far being calcimimetics, which can provide sustained reductions in serum calcium and serum phosphate and subsequently FGF23. The calcimimetic cinacalcet reduced median intact FGF23 concentration from 5555 pg/mL at baseline to 2255 pg/mL after 20 weeks of treatment (P < 0.001 versus placebo) in patients receiving hemodialysis with secondary hyperparathyroidism. The magnitude of the FGF23 reduction induced by cinacalcet was associated with a reduced risk of cardiovascular events, supporting a therapeutic benefit of reducing serum FGF23 concentrations in patients receiving hemodialysis [35]. Similarly, a second-generation intravenous calcimimetic, etelcalcetide, lowered median intact FGF23 concentrations from 4032 pg/mL at baseline to 938 pg/mL after 12 weeks of treatment of patients receiving hemodialysis with secondary hyperparathyroidism [36]. Longer-term studies of tenapanor are, therefore, needed to elucidate whether progressive and lasting reductions in serum FGF23 concentrations are achievable and are associated with improved clinical outcomes.
Phosphate lowering is only one component of an effective long-term treatment strategy to reduce FGF23. A number of other factors are known to stimulate FGF23 in patients receiving dialysis including serum calcium concentration, calcium load, vitamin D, iron status and inflammation (see reviews by Wolf [2] and Francis and David [37]). Other non-pharmacological interventions may also affect FGF23 concentrations. For example, a study by the Frequent Hemodialysis Network Trial Group showed that patients who had hemodialysis six times per week had improved control of hypertension and hyperphosphatemia and a lower risk of death or increased left ventricular mass relative to patients who had dialysis three times per week [38]. Furthermore, diet was shown to influence FGF23 concentrations in a two-way crossover study of patients with advanced CKD in which patients received a 1-week meat-based diet and a 1-week vegetarian-based diet with equivalent nutritional content; serum phosphate and FGF23 concentrations were significantly higher after the meat-based period than after the vegetable-based diet period [39].
As described in the primary article [27], gastrointestinal disorders, particularly diarrhea, were the most common adverse events recorded during the treatment period of our study. The frequent occurrence of looser and less formed stools is consistent with the mechanism of action of tenapanor, which increases stool sodium and water content [24, 25]. These pharmacological effects of tenapanor were also confirmed in a study in patients receiving hemodialysis; however, the same study also showed these effects were not accompanied by detectable differences in interdialytic weight gain between patients receiving tenapanor and those receiving placebo [23]. In a recent study in patients receiving hemodialysis, the effect of tenapanor on the changes in stool form and frequency were characterized utilizing an electronic telephone diary [40]. Results from this study indicate that, although in the clinical trial setting the term diarrhea is used to characterize these stool changes, tenapanor causes softer, more frequent bowel movements that, on average, remained within the normal range with regard to both frequency and form [40]. Notwithstanding these considerations, the high rates of diarrhea observed in our study in patients receiving high doses of tenapanor represent a potential therapy limiting factor. It will be important for future studies to monitor whether stool form and frequency changes will limit the ability of tenapanor to reduce FGF23 concentrations.
In our study, we performed the key statistical analyses of FGF23 using geometric means. The geometric mean was used because FGF23 data are typically highly skewed and the natural log-transformed FGF23 data are approximately normally distributed. The central tendency of data with a log–normal distribution is best represented by the geometric mean. On the other hand, the median—included in our article as a secondary statistic—is often presented as the primary statistic when a distribution-free (non-parametric) analysis is performed, but such an analysis is in general less efficient than a parametric one if the assumed parametric distribution is true. Beyond the strengths of this randomized study, we also acknowledge certain limitations. Although collection of serum samples for analysis of biomarkers was pre-specified at the start of the study, all analyses of FGF23 data were conducted post hoc. We measured FGF23 using an intact assay. As reviewed by Smith [41], the selection of intact versus C-terminal assays for measurement of serum FGF23 is a controversial topic and assays of intact versus C-terminal FGF23 can show poor analytical agreement. Other limitations related to this clinical trial, such as its lack of tenapanor dose titration and higher proportion of men than women in all treatment groups, have been acknowledged previously [27] and are also relevant to this analysis. Additionally, the number of patients per group (∼n = 20) was modest; however, when analysing all tenapanor-treated patients as a single group as part of the analysis of FGF23 from screening to end of phosphate-binder washout, the sample size was relatively large (n = 147).
In summary, we demonstrate that serum FGF23 concentrations increase markedly and rapidly following withdrawal of maintenance phosphate-binder therapy in patients receiving hemodialysis with hyperphosphatemia. Subsequent tenapanor treatment significantly decreased serum FGF23 concentrations. Together, these data help to illustrate the importance of dietary phosphate intake and serum phosphate as important regulators of serum FGF23, and suggest that reductions in dietary phosphate absorption with tenapanor, through a mechanism distinct from direct gastrointestinal phosphate binding, may aid in the control of FGF23 in patients receiving dialysis. In future studies, we will test the hypothesis that longer-term treatment with tenapanor achieves progressive and lasting reductions in serum FGF23 concentrations [42].
ACKNOWLEDGEMENTS
We thank all the patients and study investigators. Medical writing support was provided by Tim Ellison, PhD and Steven Inglis, PhD of PharmaGenesis London, London, UK, and was funded by Ardelyx, Inc.
FUNDING
This study was funded by AstraZeneca.
AUTHORS’ CONTRIBUTIONS
G.A.B. was involved in conception, design and conduct of the study, interpretation of data, drafting and revision of the manuscript for important intellectual content and approval of the final version for submission. D.P.R. was involved in conception, design and conduct of the study, analysis and interpretation of data, drafting and revision of the manuscript for important intellectual content and approval of the final version for submission. A.Y. was involved in design and conduct of statistical analyses and interpretation of data, revision of the manuscript for important intellectual content and approval of the final version for submission. P.J.G. was involved in conception and design of the study, interpretation of data, revision of the manuscript for important intellectual content and approval of the final version for submission. G.M.C. was involved in conception and design of study, interpretation of data, drafting and revision of the manuscript for important intellectual content and approval of the final version for submission. M.W. was involved in interpretation of data, drafting and revision of the manuscript for important intellectual content and approval of the final version for submission.
CONFLICT OF INTEREST STATEMENT
Some of the results presented in this article have been published previously (Block GA, Rosenbaum DP, Leonsson-Zachrisson et al. Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 2017; 28: 1933–1942, and in abstract form) and the primary article has been duly cited; however, FGF23 was not the focus of previous publication and the data were not fully interrogated. We now provide new results from an extended analysis of the data generated in the study and put our data into the context of previous relevant studies of FGF23. G.A.B. has received consulting fees from and has ownership interest in Ardelyx, Inc. D.P.R. and A.Y. are employees of and have ownership interest in Ardelyx, Inc. P.J.G. is an employee of and has ownership interest in AstraZeneca. G.M.C. is a consultant to and has received equity ownership interest in Ardelyx, Inc. M.W. has received consulting fees from Ardelyx, Inc.
REFERENCES
- 1. Hruska KA, Mathew S, Lund R. et al. Hyperphosphatemia of chronic kidney disease. Kidney Int 2008; 74: 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int 2012; 82: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gutierrez O, Isakova T, Rhee E. et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 2005; 16: 2205–2215 [DOI] [PubMed] [Google Scholar]
- 4. Shimada T, Hasegawa H, Yamazaki Y. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004; 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 5. Shigematsu T, Kazama JJ, Yamashita T. et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 2004; 44: 250–256 [DOI] [PubMed] [Google Scholar]
- 6. David V, Martin A, Isakova T. et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 2016; 89: 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrow EG, Yu X, Summers LJ. et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA 2011; 108: E1146–E1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsson T, Nisbeth U, Ljunggren O. et al. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 2003; 64: 2272–2279 [DOI] [PubMed] [Google Scholar]
- 9. Palmer SC, Hayen A, Macaskill P. et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 2011; 305: 1119–1127 [DOI] [PubMed] [Google Scholar]
- 10. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isakova T, Xie H, Yang W. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scialla JJ, Xie H, Rahman M. et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 2014; 25: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta R, Cai X, Lee J. et al. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the chronic renal insufficiency cohort study. JAMA Cardiol 2016; 1: 548–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie J, Yoon J, An SW. et al. Soluble klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol 2015; 26: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bacchetta J, Sea JL, Chun RF. et al. Fibroblast growth factor 23 inhibits extrarenal synthesis of 1, 25-dihydroxyvitamin D in human monocytes. J Bone Miner Res 2013; 28: 46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossaint J, Oehmichen J, Van Aken H. et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 2016; 126: 962–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chonchol M, Greene T, Zhang Y. et al. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 2016; 27: 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bansal S, Friedrichs WE, Velagapudi C. et al. Spleen contributes significantly to increased circulating levels of fibroblast growth factor 23 in response to lipopolysaccharide-induced inflammation. Nephrol Dial Transplant 2017; 32: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shalhoub V, Shatzen EM, Ward SC. et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 2012; 122: 2543–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalantar-Zadeh K, Gutekunst L, Mehrotra R. et al. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5: 519–530 [DOI] [PubMed] [Google Scholar]
- 22. Isakova T, Ix JH, Sprague SM. et al. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol 2015; 26: 2328–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Block GA, Rosenbaum DP, Leonsson-Zachrisson M. et al. Effect of tenapanor on interdialytic weight gain in patients on hemodialysis. Clin J Am Soc Nephrol 2016; 11: 1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johansson S, Rosenbaum DP, Knutsson M. et al. A phase 1 study of the safety, tolerability, pharmacodynamics, and pharmacokinetics of tenapanor in healthy Japanese volunteers. Clin Exp Nephrol 2017; 21: 407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spencer AG, Labonte ED, Rosenbaum DP. et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med 2014; 6: 227–236 [DOI] [PubMed] [Google Scholar]
- 26. Labonte ED, Carreras CW, Leadbetter MR. et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26: 1138–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Block GA, Rosenbaum DP, Leonsson-Zachrisson M. et al. Effect of tenapanor on serum phosphate in patients receiving hemodialysis. J Am Soc Nephrol 2017; 28: 1933–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Merck Millipore. EZHFGF23-32K Human FGF-23 ELISA Kit http://www.merckmillipore.com/GB/en/product/Human-FGF-23-ELISA-Kit,MM_NF-EZHFGF23-32K (7 March 2018, date last accessed)
- 29. Isakova T, Barchi-Chung A, Enfield G. et al. Effects of dietary phosphate restriction and phosphate binders on FGF23 levels in CKD. Clin J Am Soc Nephrol 2013; 8: 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oliveira RB, Cancela AL, Graciolli FG. et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 2010; 5: 286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iguchi A, Kazama JJ, Yamamoto S. et al. Administration of ferric citrate hydrate decreases circulating FGF23 levels independently of serum phosphate levels in hemodialysis patients with iron deficiency. Nephron 2015; 131: 161–166 [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A. et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant 2011; 26: 2567–2571 [DOI] [PubMed] [Google Scholar]
- 33. Cancela AL, Oliveira RB, Graciolli FG. et al. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract 2011; 117: c74–c82 [DOI] [PubMed] [Google Scholar]
- 34. Chue CD, Townend JN, Moody WE. et al. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 2013; 24: 842–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moe SM, Chertow GM, Parfrey PS. et al. Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Circulation 2015; 132: 27–39 [DOI] [PubMed] [Google Scholar]
- 36. Block GA, Bushinsky DA, Cheng S. et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA 2017; 317: 156–164 [DOI] [PubMed] [Google Scholar]
- 37. Francis C, David V.. Inflammation regulates fibroblast growth factor 23 production. Curr Opin Nephrol Hypertens 2016; 25: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chertow GM, Levin NW, Beck GJ. et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med 2010; 363: 2287–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moe SM, Zidehsarai MP, Chambers MA. et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Block GA, Rosenbaum DP, Korner P. et al. Gastrointestinal tolerability of tenapanor to treat hyperphosphatemia in patients on hemodialysis [Abstract]. J Am Soc Nephrol 2017; 28: 373 [Google Scholar]
- 41. Smith ER. The use of fibroblast growth factor 23 testing in patients with kidney disease. Clin J Am Soc Nephrol 2014; 9: 1283–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ardelyx. An 8-Week, Multicenter, Randomized, Double-Blind, Parallel Group Study With A 4-Week, Placebo-Controlled, Randomized Withdrawal Period To Evaluate The Efficacy, Safety, And Tolerability Of Tenapanor To Treat Hyperphosphatemia In End-Stage Renal Disease Patients On Hemodialysis (ESRD-HD) ClinicalTrials.gov identifier: NCT02675998. https://clinicaltrials.gov/ct2/show/NCT02675998 (7 March 2018, date last accessed)