Abstract
Weight gain is prevalent among people with traumatic brain injury (TBI) and may be attributable to environmental or injury-specific factors such as mobility impairment, endocrine dysfunction, behavioral and emotional disorders, and sensory loss. Few weight management programs exist to meet the unique needs of this population. Researchers modified a nationally recognized, evidence-based weight-loss program, Group Lifestyle Balance™ (GLB), to address the needs of over-weight and obese people post TBI (GLB-TBI). This current randomized controlled trial (RCT) examines the efficacy of the GLB-TBI on weight and secondary outcomes compared to an attention control educational support group. Furthermore, researchers have developed a mobile technology app to further engage participants in the program. This RCT will enroll and randomize 66 participants over a two-year period. It is anticipated that findings from this current RCT will contribute to the knowledge and evidence for an effective weight-loss intervention among this underserved population, with a goal of achieving full recognition by the Centers for Disease Control and Prevention-National Diabetes Prevention Program and subsequent Center for Medicare and Medicaid Services reimbursement for participation.
Keywords: Physical activity, Nutrition, Weight loss, Rehabilitation, Intervention
1. Introduction
Weight gain is prevalent among people with traumatic brain injury (TBI) [[1], [2], [3]]. Cross-sectional data from the TBI Model Systems longitudinal database in the U.S. including 7287 people with TBI indicated that 56.8% of the sample were overweight/obese (Body Mass Index [BMI] >25) at 1, 2, 5, 10, 15, 20, and 25 year follow-ups post injury [1]. Weight gain in people with TBI can often be attributed to environmental (e.g., accessibility; social support) and injury-specific factors such as impaired mobility, neurological dysfunction, medications, and changes in metabolic processes [[4], [5], [6], [7]]. Subsequently, weight gain increases the risk of chronic diseases such as diabetes, metabolic syndrome, pulmonary and heart disease [[8], [9], [10]]. Effective approaches to weight-loss are lacking, yet necessary, due to the unique physiological and cognitive needs of persons with TBI [11,12]. Evidence suggests that interventions that improve physical activity and healthy eating behaviors concurrently offer greatest potential for weight-loss [13,14]. The Group Lifestyle Balance (GLB) intervention is a 12-month, evidence-based physical activity and healthy eating program that has been used extensively with the general population [[15], [16], [17]], but not with people with TBI. Researchers modified the program [18] to meet the needs of people with a TBI (GLB-TBI) and pilot results with 18 individuals with TBI demonstrated that participation resulted in 5% weight-loss (10.2 ± 13lbs) over 12-months [19]. To generate strong evidence of the GLB-TBI efficacy for promoting weight loss, this protocol paper describes a randomized controlled trial of the GLB-TBI Intervention compared to an attention-matched Support Group-based Educational Intervention. Researchers supplemented the GLB-TBI program with a mobile app after 94% of pilot participants indicated that text messaging to support weight-loss would have been “very helpful” to boost motivation. This protocol paper followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist (Appendix) to report relevant clinical trial details as recommended by the EQUATOR Network (Enhancing the Quality and Transparency of Health Research).
1.1. Objectives and aims
Aim 1: To examine the efficacy of the GLB-TBI compared to an attention control at 3, 6, 12, and 18 months from baseline using a randomized controlled trial. Hypothesis: The GLB-TBI group will result in improvements in primary (weight) and secondary outcomes (HbA1C; waist/arm circumference; blood pressure; 8-year diabetes risk; 10 MWT; 6MWT; step count; social support; quality of life) when compared to an attention control group at 3, 6, 12, and 18 months.
Aim 2.1: To determine participant compliance with specific components of the GLB-TBI, including: (1) session attendance (2) self-monitoring of dietary and activity behaviors, and (3) activity tracking of step count. Hypotheses: GLB-TBI participants will (1) attend 80% or more of sessions, and (2) complete and submit 85% or more of dietary and activity tracking sheets during the core sessions.
Aim 2.2: Determine if compliance with the GLB-TBI is associated with improvement in primary (weight) and secondary outcomes. Hypothesis: Participants who attend 80% or more of sessions, complete 85% or more of their dietary tracking sheets during the core sessions, and increase their step count by 25% or more over the 12-month program will have improved primary and secondary outcomes.
Aim 3.1: Determine compliance with GLB-TBI and Attention Control App to answer daily, weekly, and monthly questions. Hypotheses: Participants will (1) complete 70% or more of delivered app questions within each time period (daily, weekly, and monthly questions).
Aim 3.2: To determine usability of the GLB-TBI and Attention Control App. Hypotheses: Participants will endorse scores of ≥ 3 out of 5 on all subscales of the usability measure.
2. Methods
2.1. Study design
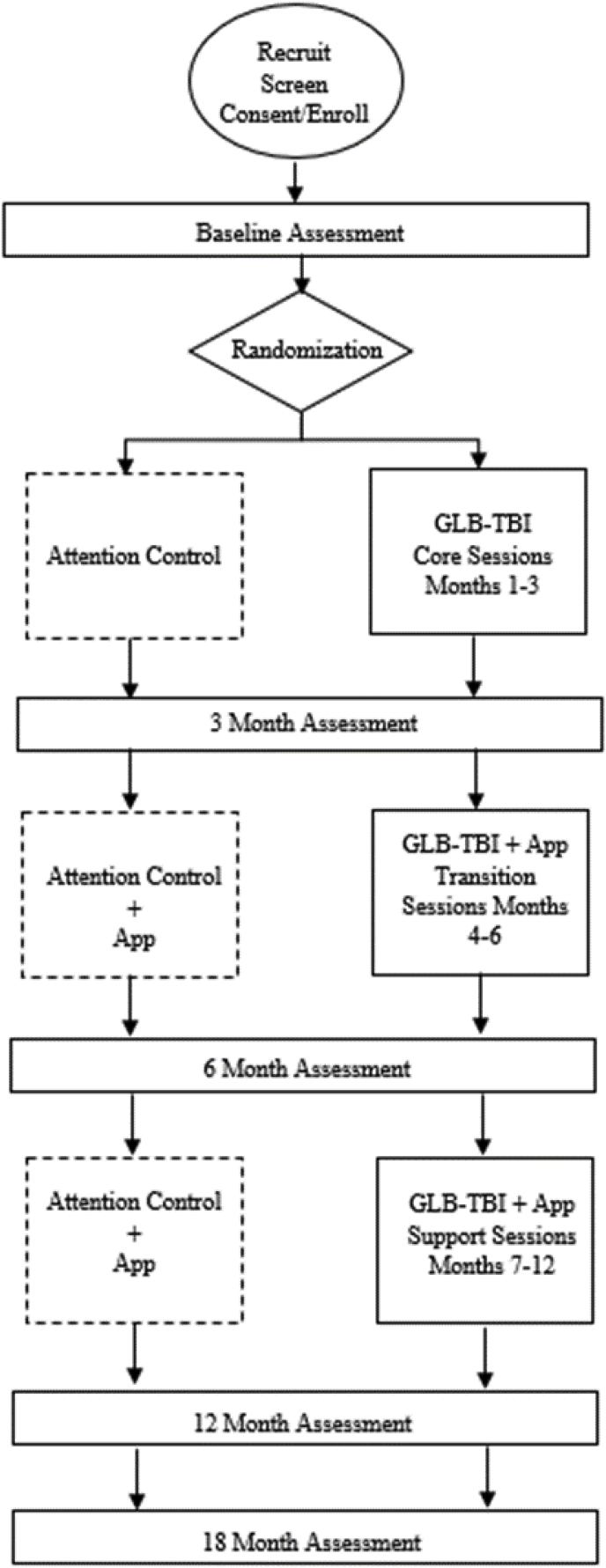
This study is a single phase, assessor-blinded, parallel-group randomized controlled trial. This study has been approved by the Baylor Scott and White Research Institute Institutional Review Board (IRB) and is prospectively registered on clinicaltrials.gov (NCT03594734). A summary of the intervention design and assessment schedule is shown in Fig. 1.
Fig. 1.
Enrollment and assessment schedule.
2.2. Study setting
All study procedures will take place at Baylor Scott and White Institute for Rehabilitation (BSW Institute for Rehabilitation), an inpatient rehabilitation hospital in an urban setting in the Southwestern United States.
2.3. Participants/recruitment
People with TBI treated at BSW Institute for Rehabilitation, the University of Texas Southwestern Medical Center clinics, or community agencies serving people with TBI in the Dallas Fort-Worth metroplex will be recruited through in person visits, flyers, calls, and emails. Recruitment will take place over a 4-month time period to ensure the target sample size is reached. Eligible individuals will contact the study team by phone or email to learn more about the study and undergo telephone screening. A snowball technique will be used for further recruitment, and interested participants will be invited to ask other individuals with TBI that they know to contact the study team about participation.
2.4. Eligibility
To determine eligibility, interested participants will contact the research team using information provided on IRB-approved study fliers and complete an eligibility screener over the phone. Eligibility criteria and rationale are summarized in Table 1. Eligible participants must also obtain medical clearance from their physician to participate in the GLB-TBI. Informed consent will be obtained by trained research personnel in a private setting at BSWIR prior to any research procedures taking place.
Table 1.
Summary of eligibility criteria and rationale.
| Inclusion Criteria | Rationale |
|---|---|
| 18–64 years of age | Younger and older individuals are excluded as there is a separate GLB curriculum for those age groups and the national physical activity guidelines are different. |
| ≥6 months post-TBI | This will allow resolution of acute consequences of TBI (e.g., hospitalizations, early neurorecovery). |
| Moderate to severe TBI at time of injury | Severity of TBI will be determined by administering the Ohio State University Traumatic Brain Injury Identification Method (OSU TBI-ID) [20] questionnaire during screening procedures. Severity scores range from 0 (no TBI) to 5 (Severe TBI). |
| BMI ≥25 kg/m2 | BMI ≥25 kg/m2 is the definition of overweight or obese by the World Health Organization [21], and places people at greater risk for pre-diabetes or diabetes. |
| Physician approval to participate in weight-loss program. | Signed approval from participant's usual physician will be required, to ensure safety of the individual based upon cardiac and other risk factors. Physicians will receive a description of the GLB-TBI intervention to help them assess their patient's risk. |
| Has smartphone/tablet or willing to use one if provided |
Participants must be willing to use their own smartphone, or a smartphone provided by the study, as the study apps will require data usage, which may incur charges, depending upon participant's plan. |
|
Exclusion Criteria |
Rationale |
| Conditions in which physical activity is contraindicated | Uncontrolled hypertension, unstable angina, severe joint disease, uncontrolled vertigo/dizziness. |
| Not fluent in the English language | GLB-TBI has been delivered in English only, and hence its efficacy in other languages is unknown at this time. |
| Low cognitive function | Low cognitive function will be defined as a score <10 on the Cognistat [22]. This is required so participants are able to understand and comply with the adapted GLB-TBI written program materials. |
| Residing in a hospital, acute rehab setting or skilled nursing facility | The intervention is intended to impact lifestyle behaviors (e.g., modified diet; increased activity) which are challenging to control/unlikely to occur in these settings. |
| Taking diabetes medication | Diabetes medications can result in weight-loss, which would confound findings from the GLB-TBI intervention. |
| Pregnancy | Pregnancy is associated with weight gain and may not allow participants to comply with the calorie and weight-loss goals or complete the 18-month program. |
| Past Participants | Participants who have previously taken the GLB-TBI pilot program will not be eligible for participation in this RCT. |
| Pre-existing diagnosis of an eating disorder | History of psychological diagnoses for eating disorders (e.g., bulimia, anorexia) require specific medical and nutrition management, which are beyond the scope of GLB-TBI. |
2.5. Intervention
2.5.1. Group Lifestyle Balance intervention
The Group Lifestyle Balance (GLB) program is a self-management intervention shown to result in weight-loss (5–7%) and reduce the risk for Type 2 diabetes through increased physical activity and healthy eating behaviors in the general population. [[23], [24], [25], [26], [27]] The GLB is grounded in the Social Cognitive Theory [28] and the Healthy Belief Model [29] and promotes participants’ engagement in health behavior change. The GLB is a direct adaptation of the Diabetes Prevention Program [23,[30], [31], [32], [33]], both developed at the Diabetes Prevention and Support Center at the University of Pittsburgh. The GLB is designed for delivery in a group-based, community setting [25], and has resulted in weight-loss in a variety of settings, such as community centers, churches, worksites, and healthcare systems [15,25,[34], [35], [36], [37], [38], [39], [40]]. The goal of the GLB program is to help the participant achieve and maintain a 5–7% weight-loss using a two pronged approach:
-
1.
Physical activity: This is based upon recommendations by the American Heart Association and the American College of Sports Medicine (ACSM) to achieve 150 min of moderate intensity activity each week. Activity is increased at a safe and slow rate each week for an ultimate goal of 150 min.
-
2.
Healthy eating: Based on United States Department of Agriculture guidelines, the GLB emphasizes healthy eating patterns and tracking dietary intake. Key recommendations include individuals consuming (1) a variety of vegetables, (2) whole fruits, (3) whole grains, (4) fat-free or low-fat dairy, (5) a variety of lean proteins, and (6) oils at every meal.
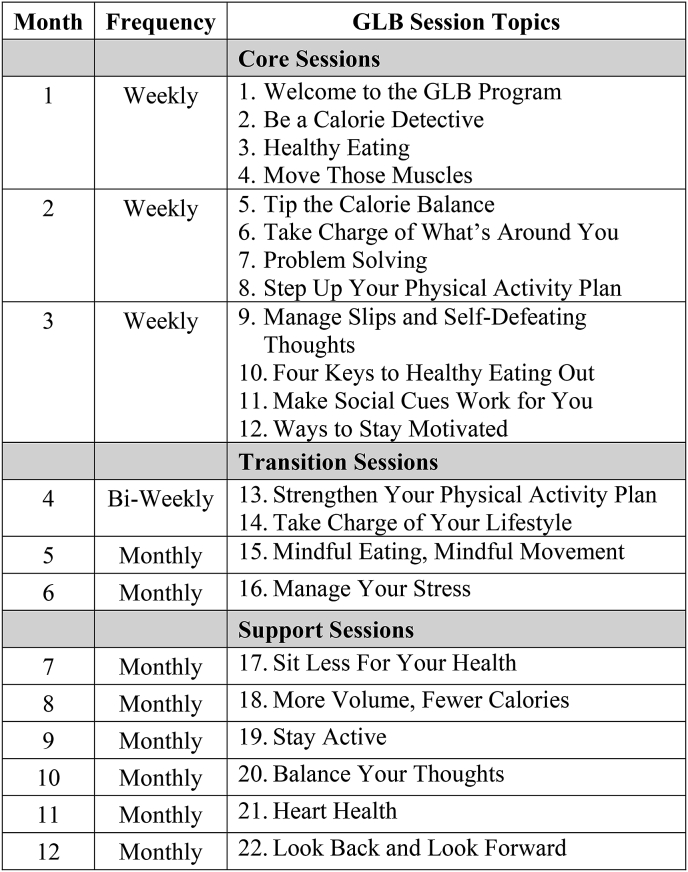
The GLB (see Fig. 2 for curriculum) is a one-year program with 22 sessions. It begins with 12 weekly sessions called the Core Program, followed by a Transition phase consisting of 2 bi-weekly and 2 monthly sessions, and a Support Phase consisting of 6 monthly sessions. The program materials are publicly available under the Creative Commons licensing agreement.
Fig. 2.
Group lifestyle balance curriculum.
The GLB curriculum was revised by researchers at BSW Institute for Rehabilitation with specific adaptations for individuals with TBI (GLB-TBI). Further information on these modifications are described elsewhere [18], but general changes included 1) reducing the volume of content to focus on 2–3 main points at each session, 2) caregiver involvement in the sessions, and 3) TBI-specific handouts on weight-loss barriers and healthy lifestyle importance. All modifications and adaptations were reviewed by the Diabetes Prevention and Support Center at the University of Pittsburgh to ensure the evidence-based educational and behavioral components of the original program were not altered.
| Month |
Frequency |
GLB Session Topics |
|---|---|---|
| Core Sessions | ||
| 1 | Weekly |
|
| 2 | Weekly |
|
| 3 |
Weekly |
|
|
Transition Sessions |
||
| 4 | Bi-Weekly |
|
| 5 | Monthly |
|
| 6 |
Monthly |
|
|
Support Sessions |
||
| 7 | Monthly |
|
| 8 | Monthly |
|
| 9 | Monthly |
|
| 10 | Monthly |
|
| 11 | Monthly |
|
| 12 | Monthly |
|
In-person sessions will take place at BSW Institute for Rehabilitation and be taught by trained lifestyle coaches. Caregivers and partners will be invited to join these sessions to speak, answer questions, and provide peer support. In person sessions may include adapted cooking demonstrations and guided exercise by an exercise specialist with experience training individuals with TBI. Participants will be provided the session materials before each session. In line with the GLB curriculum, participants will track their daily calorie intake and physical activity and submit their logs to their lifestyle coach on a regular basis. To increase feasibility of this component, particularly for individuals with impaired finger function, participants will be given the option to complete either paper or electronic logs or use the MyFitness Pal calorie counting application. Study staff will periodically review logs and deliver written comments to the participant via mail or email.
Participants will also be given Garmin Vivofit activity trackers. The Vivofit is a commercially available, arm-based activity tracker that has a visual display. Participants will be informed that the reliability of these activity trackers has not been estimated for people with TBI, but that their purpose is to provide participants with feedback to gradually increase their physical activity according to the program recommendations. Participants will be asked to regularly synchronize their arm-bands to their phone or computer so that they and the lifestyle coaches can review their activity and heart rate reports.
2.5.2. Attention control – educational support group
There are potential adverse effects of being randomized to a no-treatment or wait-list control alone (e.g., participants on wait-list finding alternatives for treatment) [41]. To address this, we will use an attention control group, in which participants will receive approximate contact or “attention” as the GLB-TBI group, but not the GLB-TBI intervention itself. The attention control group will function as an educational support group and meet at the same frequency as the GLB-TBI group (i.e., weekly for 3-months; bi-monthly for 1 month; once/month for 8 months). Topics covered in the educational support will include content from the TBI Model Systems Knowledge Translation Center's factsheets (https://msktc.org/tbi/factsheets), as well as topics recommended by people with TBI involved in the project development such as (1) healthy brain and effects of TBI on cognitive, emotional, and behavioral functioning, (2) expectations for recovery, (3) preventive and management strategies for common TBI sequela (e.g., irritability, impulsivity), (4) stress management strategies (identifying signs, relaxation techniques, reassuring thinking), (5) signs and symptoms of depression, and (6) strategies for effective communication. The educational support group will not receive any education on weight-loss strategies. The primary educational support group facilitators are speech-language pathologists who have the requisite training and experience to deliver the TBI specific content and manage the group discussion. Caregivers and partners will also be invited to attend these sessions to speak, answer questions, and provide support. Participants will be provided with materials and factsheets prior to each session.
2.5.3. Technology/app
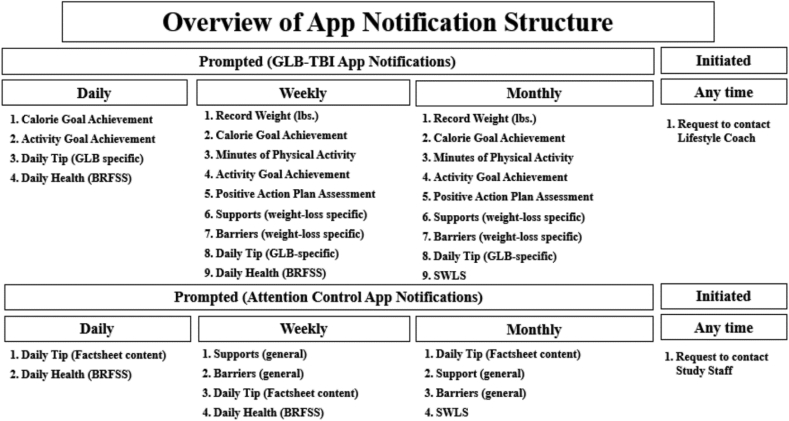
The use of mobile technologies to support clinical assessment and intervention is rapidly developing and members of our research team have demonstrated that daily app-based assessment of mood was feasible and satisfactory among individuals with TBI [42]. However, recent systematic reviews on mobile technology use for suicide prevention and in sports concussion raised concerns around the unregulated telehealth market and point to a clear a need for evidence-based apps [43,44]. In support of Aim 3, we created our smartphone app using a HIPAA-compliant native app platform. The app will provide access to content to both the GLB-TBI (e.g., activity and dietary tracking; daily GLB-TBI related tips; goal setting; self-reported health) and attention control support group (e.g., daily TBI-related tips; self-reported health). Content for the apps are structured from the GLB-TBI curriculum or from topics covered during the attention control group sessions.
The app will be introduced during week 10 and begin collecting data in week 12, which coincides with the decreased group contact from weekly to monthly. Participants will be prompted by notifications pushed directly to their smartphones to complete daily, weekly, and monthly responses that take approximately 1–3 min to complete. The content of the apps is detailed in Fig. 3. Participants will be notified through the GLB-TBI app to log activity, weight, and goal progress and for both apps to rate well-being and quality of life using ordinal scales and to receive daily tips developed around group topics. Participants will also have the option to contact their GLB-TBI lifestyle coach or support group facilitator if they need further support or app troubleshooting.
Fig. 3.
Content and structure of GLB-TBI and attention control app.
Based on positive findings from our pilot work [45], participants in both groups will also have the option to join a closed Facebook page to facilitate group cohesion and peer support, ask each other questions, share challenges and successes, provide encouragement, and share resources.
2.6. Outcome measures
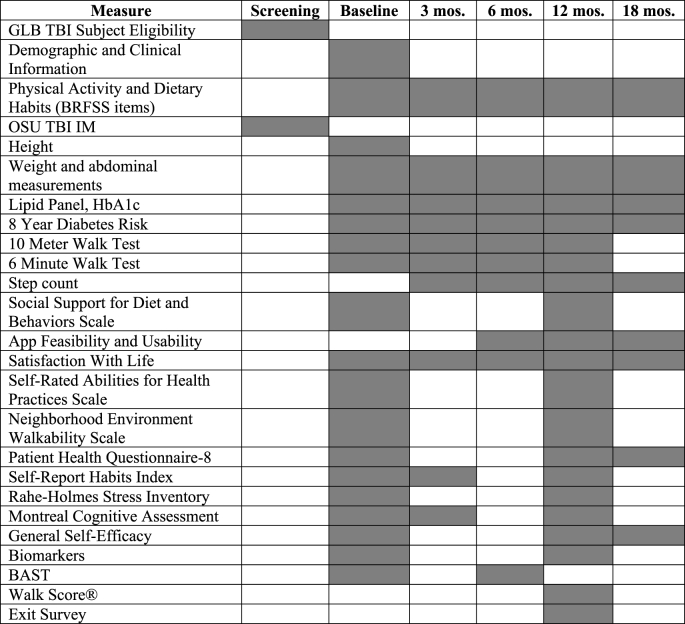
The following demographic data will be collected at the baseline assessment: severity of TBI (Ohio State University Traumatic Brain Injury Identification Method) [20]; current age and at injury; sex; parental history of diabetes; race and ethnicity; education level; pre-morbid history of mental illness; marital/relationship status; diagnosed medical conditions; previous/present smoking and cigarettes/day; alcohol consumption and drinks/week; residence status; neighborhood walk score; annual household income category; insurance type; employment status; resting metabolic rate; pre-injury weight, activity, history of weight-loss attempts. Outcome measures are summarized in Table 2 and the assessment schedule in Table 3. The outcome measures were selected due to the suitability to identifying physiologic, physical and cognitive function, and psychosocial health. Participants will be compensated $25 for their participation in each assessment time point.
Table 2.
Outcome measures for GLB-TBI project.
| Measure | Properties and Approach |
|---|---|
| Primary Outcome | |
| Weight | Obtained over the study period using the same scale that is accessible to people with and without a mobility device (e.g., walker; wheelchair). |
| Secondary Outcome | |
| HbA1C, fasting blood glucose, lipid panel | Fasting venous sample will be obtained for blood glucose, HDL/LDL cholesterol, and triglyceride level. Coordinators are trained phlebotomists. Samples will be collected at BSW Institute for Rehabilitation and analyzed by an approved lab. |
| Biomarkers | Samples will also be analyzed to examine biomarkers of brain health and recovery including Thyroid Stimulating Hormone, Cortisol, Tumor Necrosis Factor-alpha, Brain Derived Neurotropic Factor, Insulin-like Growth Factor, and Vascular Endothelial Growth Factor. |
| Circumference | Waist circumference measured at the umbilicus and mid-upper arm circumference following ACSM guidelines [46]. |
| Blood pressure | Using an automatic cuff (average of three readings, patient seated) diastolic and systolic scores will be recorded |
| MedGem® | This is an FDA-cleared and validated indirect calorimetry device. It is handheld and measures oxygen consumption (V02) to determine resting metabolic rate (RMR) [47]. |
| 8-year Diabetes Risk | The Framingham Heart Study diabetes risk score [48] will be calculated using predictors including age, gender, fasting glucose, BMI, HDL cholesterol and triglyceride levels, blood pressure, and parental history. Risk score calculator and regression model are free and used in GLB weight-loss trials [27,48,49]. |
| 10 Meter Walk Test (10MWT)a | Assesses walking speed in (m/s) which is correlated to mobility in the community, capacity to perform ADLs, risk of falls, re-hospitalization, and risk of cognitive decline [50]. For persons with TBI, a change of >0.15–0.25 m/s is considered to exceed minimally clinically important difference (MCID) [51] and between day test reliability is excellent (ICC = 0.95) [51]. |
| 6 Minute Walk Test (6MWT)b | Assesses distance walked (rolled for wheelchair users) over 6 min as a sub-maximal test of aerobic capacity. Endurance is essential to participate in community based activities. The 6MWT has excellent test-retest reliability (ICC = 0.94–96) [51,52] for people with TBI and normative gender-specific reference equations are available for comparison [53]: Men: = (7.57 × height cm) – (5.02 × age) – (1.76 × weight kg) −309; Women: = (2.11 × height cm) – (2.29 × weight kg) – (5.78 × age) + 667 m. |
| Step Countc | The Garmin Vivofit will be worn for the study duration to measure physical activity data. The Garmin device is water resistant at 1 m for 30 min and Bluetooth enabled and data will be transferred to the research team during assessment visits. It can store up to 180 days of data and the battery lasts over 12-months and does not require charging. Compliance with the Garmin during the GLB-TBI pilot study was 100% with zero lost devices. Participants will enter daily step count in the GLB-TBI app, beginning at Session 10, with app-based prompts. The device is worn on the participant's non-involved arm. |
| Social Support for Diet and Exercise Behaviors Scale | 23-item survey including four subscales: support for healthy eating (5 items); support for physical activity (11 items); social undermining for healthy eating (5 items) and physical activity (2 items) [54]. Each item is rated on a scale of 1–5 (1 none; 5 very often), with respondents asked to rate support from family, friends, and coworkers. Higher scores represent greater support and internal consistency ranged from Cronbach's α 0.72–0.76. |
| Behavioral Risk Factor Surveillance System (BRFSS) | The BRFSS is a state-based system of health surveys that collects information on health risk behaviors, preventative health practices, and health care access primarily related to chronic disease and injury. The GLB-TBI uses the two subscales of Healthy Eating and Physical Activity from the 2017 version of the BRFSS. It consists of 14 items [55]. |
| App Feasibility and Usability | The Feasibility and Usability survey includes 14 items that assesses the participant's subjective experience with the App, level of difficulty, prompting sequence, length, and understanding. Scores above 3 on the scale (1 [totally disagree] – 5 [totally agree]) indicate that the App was easy to use. |
| Patient Health Questionnaire-8 item | The PHQ-8 is a brief self-report measure of major depressive disorder, derived from the PHQ-9 by removing the last question regarding suicide assessment [56]. It is considered a valid measure of depression for population-based studies and clinical populations, and has been used in studies of patients with physical injury [56,57]. Frequency of symptoms during the last 2 weeks is assessed on a 0 (not at all) to 3 (nearly every day) scale. A cut-off score of 10 or greater is considered diagnostic for current depression. |
| Satisfaction with Life Scale (SWLS) | The Satisfaction with Life Scale (SWLS) [58] is a 5-item scale designed to measure global cognitive judgements of one's life satisfaction. Participants indicate how much they agree or disagree with each of the 5 items using a 7-point scale that ranges from 7-strongly agree to 1-strongly disagree. Scores are added together and a total score is calculated, wither higher scores signifying higher satisfaction with life and lower scores signifying lower satisfaction with life. |
| Walk Score® | Walk Score® is publicly available and measures the walkability of any address using a patented system. For each address, Walk Score ® analyzes hundreds of walking routes to nearby amenities and awards points based on distance to each amenity. Walk Score® also measures pedestrian friendliness by analyzing population density and road metrics such a block length and intersection density. Scores are given on a scale of 0–100 [59]. |
| Neighborhood Environment Walkability Scale (NEWS) | NEWS assesses residents' perception of neighborhood design features related to physical activity, including residential density, land use mix (including both indices of proximity and accessibility), street connectivity, infrastructure for walking/cycling, neighborhood aesthetics, traffic and crime safety, and neighborhood satisfaction [60]. |
| Self-Rated Abilities for Health Practice scale | Measure includes 28 items that assess health practices among people with disabilities and yields a total Health Practices score plus 4 subscales scores regarding Exercise, Nutrition, Health Practices, and Psychological Well Being. Items are rated on a 5-point scale from 0 ‘not at all’ to 4 ‘completely.’ Scores range from 0 to 28 with higher scores indicating higher exercise self-efficacy [61]. |
| Montreal Cognitive Assessment (MOCA) | The MOCA is a brief, 8-section assessment of various cognitive domains including executive function, memory, language, attention, concentration, orientation, and working memory in neurologic populations [62]. The MOCA has also been used in the TBI population [63]. Each item on the MOCA is allocated a set of points adding up to 30. |
| Holmes and Rahe Stress Inventory | This inventory consists of 40 life events and asks the participant to recall if any of the events happened within the previous year (e.g., death of spouse; personal illness; change in sleep). Endorsement of these events are totaled and higher scores indicate a greater amount of stressful life events. Point values for the Holmes and Rahe Stress Inventory were weighted and summed for each individual based on scoring instructions [64]. Individuals who scored 150 points or less were categorized as low susceptibility to a health breakdown in the next two years, 151–300 points were 50% chance of health breakdown, and 301 points or more were 80% chance of health breakdown. |
| Behavioral Assessment Screening Tool (BAST)d | The BAST is a 46 item, validated survey of behavioral and emotional symptoms for community-based adults with TBI. This is a self-report measure and is meant to be taken privately, without an assessor present. This assessment is a shortened version of the validated 77-item survey [65], with questions related to environmental stressors removed due to repetitiveness to other questions in survey packet. |
| General Self-Efficacy | The ten items from the General Self-Efficacy Scale (GSE) are designed to examine goal-setting, effort investment, persistence in face of barriers and recovery from setbacks as constructs of perceived self-efficacy [66]. The total score is the sum ranging from 10 to 40 and the instrument has been normed against the U.S. Adult population with a mean score of 29.48. |
| Self-Report Habit Index (SRHI) | This assessment measures the self-reported perceptions of habit strength for an identified behavior.112 It consists of 12 items for each selected behavior and uses a 7-point Likert scale from “completely disagree” to “completely agree.” Higher totals represent greater perception of habit strength. The SRHI showed high internal reliability across four studies with alphas of .89, .92, .89, .94, .95, .94, and .85 [67]. |
We anticipate that 10% of our sample (n = 6–7) will be wheelchair users based on our GLB-TBI pilot (n = 2 wheelchair users). The 10MWT will not be appropriate for this subgroup.
We anticipate that 5% of our sample (3–4) will be power chair users based on our GLB-TBI pilot (n = 1 power chair user). The 6MWT will not be appropriate for this subgroup.
Step count data will not be collected for power chair users (anticipated 5% of sample).
We anticipate that 70% of the surveys will be returned at each time point.
Table 3.
Schedule of measures Table.
2.7. Sample size
Analysis of pilot data determined a required sample size of 66 participants (33 per group) to detect a 5% reduction in weight (GLB-TBI program goal), with a power of 0.8 and assuming a 15% attrition rate (observed during our pilot study). These estimates are based upon the GLB-TBI weight-loss data which included weight at baseline (212 ± 35lbs) and weight-loss after 12 months (10.2 ± 13lbs) [3,19].
2.8. Allocation
Patients will be allocated to the experimental GLB-TBI or attention control groups using computer-generated random numbers in Microsoft Excel. To ensure an even distribution between groups, block randomization will be used with blocks of size 4 and 6. Randomly mixing block sizes will reduce the study coordinator's ability to predict the last assignment of each block. The randomization list will be generated by the statistician with results contained in sealed envelopes labeled with study identification numbers. After a participant is enrolled in the study, a study coordinator will select the assigned envelope to reveal the participant's group. Due to the nature of the intervention, it is not possible to blind study participants to group assignment. However, to reduce assessor bias, all participant assessments will be completed by a separate coordinator who is blinded to group assignment.
2.9. Data management, quality assurance, exclusion of bias
All paper source documents will be kept in a double locked storage cabinet in the BSW Institute for Rehabilitation research office. All case report forms and outcomes data will be entered into REDcap, a HIPAA-compliant (21 CFR Part 11) secure web application, by trained study staff. All electronic data will be kept on a secure server and data will be maintained for two years after study termination per federal guidelines, at which point it will be disposed of in accordance to current policy.
Data management functions will occur on a quarterly basis and will include data quality checks and verification, as well as internal edit and logic checks (e.g., out of range values, internal inconsistencies). Ten percent of charts will be audited for source document and data entry review. Cross tabulation checks using SAS will also be used. Data will be stored and backed-up periodically on the biostatistician's space on the secure server. Descriptive statistics will be at calculated and included into quarterly reports to ensure the quality of data and progress of the study. The principal investigator will oversee all data entry and proper data monitoring and audit procedures.
2.10. Statistical methods
Aim 1: All analysis will be performed using SAS 9.4[68] with a significance level of 0.05. Evaluation of the primary and secondary outcomes will be performed using general or generalized linear mixed effects models [69,70] for the continuous outcomes including change in weight from baseline, step count data, waist circumference, blood pressure, Hba1C and lipid panel, functional measures, quality of life, and Framingham 8-year diabetes risk score. A separate model will be run for each outcome. The distribution of each outcome will be assessed to determine if a general linear model will be utilized, or if a generalized linear model with an alternative distribution and link function, such as the gamma distribution with a log link, will be more appropriate. Fixed effects included in each model will be time (3, 6, 12, and 18 months), group (experimental or control), time by group interaction, and demographic variables, particularly if they are imbalanced after randomization, thereby providing more accurate estimates of the intervention impacts. A random patient effect with an unstructured covariance matrix will be included to account for the correlation among repeated measures.
Initial analysis will include missing observations due to either attrition or non-response. To determine if the results would change with complete data, sensitivity analysis will be performed using iterative Monte Carlo Markov Chain [71] multiple imputation to predict the primary and secondary outcomes that are missing at follow-up time points. All available demographic and outcome variables will be used for the imputation process, which allows for greater recovery of the missing data [72]. The multiple imputed datasets will then be analyzed using the same mixed models as for the initial analysis. The final model for the imputed data will be determined by pooling the estimates produced by the analysis of each imputed dataset.
Aim 2: Compliance with session attendance, self-monitoring of dietary behaviors, and activity tracking will be summarized and tested against the hypothesized values. One sample proportions tests will be used to determine if the overall attendance rate was ≥80%, if ≥ 85% of daily tracking sheets were completed and submitted, and if the average change in step count was at least 25% from baseline to 12-months.
To determine if study compliance is associated with improvement in our primary and secondary outcomes, we will analyze the data in two ways at 3, 6, 12, and 18 months. First, we will define a new group variable with 3 categories to represent control, intervention compliant, and intervention non-compliant. The compliant group will include participants who met all of the compliance goals (i.e., ≥80% session attendance; ≥ 85% tracking sheets completed; ≥25% increase step count). The general/generalized linear mixed effects models described in the Aim 1 analysis will be used with the new group variable. Second, we will include each participant's compliance measures (attendance rate, percentage of completed tracking sheets, and percentage change in average step count from baseline) as a fixed effect variable in the regression models. This analysis will include only the experimental group participants and will allow us to determine the extent to which each area of compliance is associated with primary and secondary outcomes.
Aim 3: Feasibility of the GLB-TBI app is defined as compliance (total number of completed daily, weekly, and monthly questions divided by the total number of possible questions, calculated separately) and usability (measured by the feasibility survey). Overall compliance of 70% or greater within each time period will be considered good and tested using a one sample proportions test. Usability will be defined as an average score of >3 on the usability survey. We will compare compliant and non-compliant participants using t-tests, Mann Whitney U tests, or Chi Square tests, as appropriate, to identify factors associated with compliance with the GLB-TBI app.
3. Discussion
Traumatic brain injury continues to be the leading cause of injury-related death and disability in the United States (US) [73]. More than 5.3 million people in the US currently live with TBI-related disability [74] and increasingly TBI is considered a chronic health condition rather than a single event [75,76]. Individuals with TBI also experience related secondary conditions such as weakness, impaired mobility, sensory loss, impaired cognition, and emotional or behavioral disorders, often then leading to further chronic conditions such as diabetes, heart disease, and hypertension [77]. Though evidence exists to support the impact of a healthy lifestyle on weight loss and reduction of secondary conditions such as diabetes, heart disease and hypertension, few studies exist to promote a healthy lifestyle post TBI [[78], [79], [80]]. Therefore, there is a critical need to address these barriers and modify evidence-based weight loss interventions to meet the unique needs of people with TBI.
The GLB-TBI program was adapted from the original evidence-based curriculum to specifically meet the unique weight-loss needs of people with TBI. Through a revised curriculum, inclusion of care partners, modified exercise and nutrition plans, discussion on barriers to weight-loss specific to people with a TBI, and the introduction of a mobile application to prompt engagement, researchers hope to address the unique needs of people with TBI. It is anticipated that findings from this current RCT will establish a strong evidence-based approach to weight-loss among this underserved population that is translatable into community settings nationally.
After demonstration of the efficacy of the GLB-TBI through this RCT, all materials will be made available to the public, free of cost through the Diabetes Prevention and Support Center at the University of Pittsburgh. The GLB program is recognized by the Centers for Disease Control and Prevention-National Diabetes Prevention Program (CDC-DRPP) which has developed guidelines to recognize evidence-based programs to ensure high-quality standards for effective implementation and evaluation of lifestyle programs. A report by the Centers for Medicare and Medicaid Services (CMS) found that the GLB would reduce net Medicare spending by $2650 per enrollee as it reduced participant weight by 4.7%. Hence, in 2018, the GLB became eligible for expansion into the Medicare payment program so individuals can be reimbursed for participation [81]. The ultimate goal of this RCT is to achieve full recognition by the CDC-DRPP and result in CMS reimbursement for people with a TBI living in the community.
Conflicts of interest
The principal investigator and study team members have no competing or financial interests to declare.
Authors contributions
SD was responsible for project inception, design, oversight, and the first draft of the manuscript. SJ, KB, and RD provided project oversight and contributed to the design of the study. SJ was responsible for the creation and design of the GLB apps. MB provided sample size calculations and statistical support for the project. EM was responsible for study operations and recruitment. All authors have read and contributed to this manuscript.
Funding
The contents of this publication were developed under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant number 90DPTB0013-01-00). NIDILRR is a Center within the Administration for Community Living (ACL), Department of Health and Human Services (HHS). The contents of this publication do not necessarily represent the policy of NIDILRR, ACL, or HHS, and you should not assume endorsement by the Federal Government.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100328.
Contributor Information
Simon Driver, Email: sjdriver@bswrehab.com.
Shannon Juengst, Email: shannon.juengst@utsouthwestern.edu.
Evan Elizabeth McShan, Email: evan.mcshan@bswhealth.org.
Monica Bennett, Email: monica.bennett@bswhealth.org.
Kathleen Bell, Email: kathleen.bell@utsouthwestern.edu.
Rosemary Dubiel, Email: rdubiel@bswrehab.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Dreer L.E., Ketchum J.M., Novack T.A. Obesity and overweight problems among individuals 1 to 25 Years following acute rehabilitation for traumatic brain injury: a NIDILRR traumatic brain injury model systems study. J. Head Trauma Rehabil. 2018;33(4):246–256. doi: 10.1097/HTR.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 2.Brown R.M., Tang X., Dreer L.E. Change in body mass index within the first-year post-injury: a VA Traumatic Brain Injury (TBI) model systems study. Brain Inj. 2018;32(8):986–993. doi: 10.1080/02699052.2018.1468575. [DOI] [PubMed] [Google Scholar]
- 3.Driver S., Reynolds M., Douglas M., Bennett M. Weight loss and physical activity history of individuals with traumatic brain injury prior to participation in a lifestyle change program. J. Head Trauma Rehabil. 2018;33(1):E36–E43. doi: 10.1097/HTR.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 4.Crenn P., Hamchaoui S., Bourget-Massari A., Hanachi M., Melchior J.C., Azouvi P. Changes in weight after traumatic brain injury in adult patients: a longitudinal study. Clin. Nutr. 2014;33(2):348–353. doi: 10.1016/j.clnu.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Goverover Y., Genova H., Smith A., Chiaravalloti N., Lengenfelder J. Changes in activity participation following traumatic brain injury. Neuropsychol. Rehabil. 2016:1–14. doi: 10.1080/09602011.2016.1168746. [DOI] [PubMed] [Google Scholar]
- 6.Jones T.M., Dean C.M., Hush J.M., Dear B.F., Titov N. A systematic review of the efficacy of self-management programs for increasing physical activity in community-dwelling adults with acquired brain injury (ABI) Syst. Rev. 2015;4 doi: 10.1186/s13643-015-0039-x. 51-5015-0039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Self M., Driver S., Stevens L., Warren A.M. Physical activity experiences of individuals living with a traumatic brain injury: a qualitative research exploration. Adapt. Phys. Act. Q. (APAQ) : Adapt. Phys. Act. Q. (APAQ) 2013;30(1):20–39. doi: 10.1123/apaq.30.1.20. [DOI] [PubMed] [Google Scholar]
- 8.Corrigan J.D., Horn S.D., Barrett R.S. Effects of patient preinjury and injury characteristics on acute rehabilitation outcomes for traumatic brain injury. Arch. Phys. Med. Rehabil. 2015;96(8):S209–S221. doi: 10.1016/j.apmr.2015.03.026. e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bistrian B.R., Askew W., Erdman J.W., Jr., Oria M.P. Nutrition and traumatic brain injury: a perspective from the Institute of Medicine report. JPEN - J. Parenter. Enter. Nutr. 2011;35(5):556–559. doi: 10.1177/0148607111416122. [DOI] [PubMed] [Google Scholar]
- 10.Lee-anne S.C., Lithander F.E., Gruen R.L., Williams L.T. Nutrition therapy in the optimisation of health outcomes in adult patients with moderate to severe traumatic brain injury: findings from a scoping review. Injury. 2014;45(12):1834–1841. doi: 10.1016/j.injury.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Pawlowski J., Dixon-Ibarra A., Driver S. Review of the status of physical activity research for individuals with traumatic brain injury. Arch. Phys. Med. Rehabil. 2013;94(6):1184–1189. doi: 10.1016/j.apmr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton M., Khan M., Clark R., Williams G., Bryant A. Predictors of physical activity levels of individuals following traumatic brain injury remain unclear: a systematic review. Brain Inj. 2016;30(7):819–828. doi: 10.3109/02699052.2016.1146962. [DOI] [PubMed] [Google Scholar]
- 13.Gidding S.S., Lichtenstein A.H., Faith M.S. Implementing American Heart Association pediatric and adult nutrition guidelines. Circulation. 2009;119(8):1161–1175. doi: 10.1161/CIRCULATIONAHA.109.191856. [DOI] [PubMed] [Google Scholar]
- 14.Eckel R.H., Jakicic J.M., Ard J.D. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. J. Am. Coll. Cardiol. 2013;63(25 Part B):2960–2984. doi: 10.1016/j.jacc.2013.11.003. 2014. [DOI] [PubMed] [Google Scholar]
- 15.Kramer M.K., Kriska A.M., Venditti E.M. A novel approach to diabetes prevention: evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Res. Clin. Pract. 2010;90(3):e60–e63. doi: 10.1016/j.diabres.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Kramer M.K., McWilliams J.R., Chen H.Y., Siminerio L.M. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educat. 2011;37(5):659–668. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- 17.Greenwood D.A., Kramer M.K., Hankins A.I., Parise C.A., Fox A., Buss K.A. Adapting the group lifestyle balance program for weight management within a large health care system diabetes education program. Diabetes Educat. 2014;40(3):299–307. doi: 10.1177/0145721714524281. [DOI] [PubMed] [Google Scholar]
- 18.Driver S., Reynolds M., Kramer K. Modifying an evidence-based lifestyle programme for individuals with traumatic brain injury. Brain Inj. 2017;31(12):1612–1616. doi: 10.1080/02699052.2017.1346286. [DOI] [PubMed] [Google Scholar]
- 19.Driver S., Reynolds M., Woolsey A. Impact of a community-based healthy lifestyle program on individuals with traumatic brain injury. J. Head Trauma Rehabil. 2018;33(6):E49–E58. doi: 10.1097/HTR.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 20.Corrigan J.D., Bogner J. Initial reliability and validity of the Ohio State University TBI identification method. J. Head Trauma Rehabil. 2007;22(6):318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- 21.Organization W.H. World Health Organization; 2000. Obesity: Preventing and Managing the Global Epidemic. [PubMed] [Google Scholar]
- 22.Kiernan R.J., Mueller J., LANGSTON J.W., Van Dyke C. The Neurobehavioral Cognitive Status Examination: a brief but differentiated approach to cognitive assessment. Ann. Intern. Med. 1987;107(4):481–485. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 23.Diabetes Prevention Program Research Group The diabetes prevention program. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer M.K., McWilliams J.R., Chen H.-Y., Siminerio L.M. A community-based diabetes prevention program evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educat. 2011;37(5):659–668. doi: 10.1177/0145721711411930. [DOI] [PubMed] [Google Scholar]
- 25.Kramer M.K., Kriska A.M., Venditti E.M. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am. J. Prev. Med. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Kramer M., Molenaar D., Arena V. Improving employee health: evaluation of a worksite lifestyle change program to decrease risk factors for diabetes and cardiovascular disease. J. Occup. Environ. Med./Am. Coll. Occup. Environ.l Med. 2015;57(3):284. doi: 10.1097/JOM.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J., Yank V., Xiao L. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Internal Medicine. 2013;173(2):113–121. doi: 10.1001/2013.jamainternmed.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandura A. Human agency in social cognitive theory. Am. Psychol. 1989;44(9):1175. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 29.Hochbaum G., Kegels S., Rosenstock I. United States Public Health Service; 1952. Health belief model. [Google Scholar]
- 30.Hamman R.F., Wing R.R., Edelstein S.L. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriska A. Can a physically active lifestyle prevent type 2 diabetes? Exerc. Sport Sci. Rev. 2003;31(3):132–137. doi: 10.1097/00003677-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Diabetes Prevention Program Research Group Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes. Res. 2004;12(9):1426. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kriska A.M., Edelstein S.L., Hamman R.F. Physical activity in individuals at risk for diabetes: diabetes Prevention Program. Med. Sci. Sports Exerc. 2006;38(5):826. doi: 10.1249/01.mss.0000218138.91812.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer S.F., Cumming T., Churilov L., Bernhardt J. Measuring activity levels at an acute stroke ward: comparing observations to a device. Biomed. Res. Inter. 2013:460482. doi: 10.1155/2013/460482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis-Smith Y.M., Davis-Smith M., Boltri J.M. Implementing a diabetes prevention program in a rural African-American church. J. Natl. Med. Assoc. 2007;99(4):440. [PMC free article] [PubMed] [Google Scholar]
- 36.Amundson H.A., Butcher M.K., Gohdes D. Translating the diabetes prevention program into practice in the general community findings from the Montana cardiovascular disease and diabetes prevention program. Diabetes Educat. 2009;35(2):209–223. doi: 10.1177/0145721709333269. [DOI] [PubMed] [Google Scholar]
- 37.Ackermann R.T., Finch E.A., Brizendine E., Zhou H., Marrero D.G. Translating the diabetes prevention program into the community: the DEPLOY pilot study. Am. J. Prev. Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vadheim L.M., Brewer K.A., Kassner D.R. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J. Rural Health. 2010;26(3):266–272. doi: 10.1111/j.1748-0361.2010.00288.x. [DOI] [PubMed] [Google Scholar]
- 39.Katula J.A., Vitolins M.Z., Morgan T.M. The Healthy Living Partnerships to Prevent Diabetes study: 2-year outcomes of a randomized controlled trial. Am. J. Prev. Med. 2013;44(4):S324–S332. doi: 10.1016/j.amepre.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katula J.A., Vitolins M.Z., Rosenberger E.L. One-year results of a community-based translation of the diabetes prevention program. Diabetes Care. 2011;34(7):1451–1457. doi: 10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohr D.C., Spring B., Freedland K.E. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother. Psychosom. 2009;78(5):275–284. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- 42.Juengst S.B., Graham K.M., Pulantara I.W. Pilot feasibility of an mHealth system for conducting ecological momentary assessment of mood-related symptoms following traumatic brain injury. Brain Inj. 2015;29(11):1351–1361. doi: 10.3109/02699052.2015.1045031. [DOI] [PubMed] [Google Scholar]
- 43.Luxton D.D., June J.D., Kinn J.T. Technology-based suicide prevention: current applications and future directions. Telemedicine and e-Health. 2011;17(1):50–54. doi: 10.1089/tmj.2010.0091. [DOI] [PubMed] [Google Scholar]
- 44.Lee H., Sullivan S.J., Schneiders A.G. Smartphone and tablet apps for concussion road warriors (team clinicians): a systematic review for practical users. Br. J. Sports Med. 2014;49(8):499–505. doi: 10.1136/bjsports-2013-092930. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds M., Driver S., Bennett M. The social network–using social media to support individuals with traumatic brain injury participating in a pilot study weight-loss program. Brain Inj. 2018:1–5. doi: 10.1080/02699052.2018.1496480. [DOI] [PubMed] [Google Scholar]
- 46.Palmer-McLean K., Harbst K. Stroke and brain injury. In: Durstine J.L., Moore G.E., editors. ACSM's Exercise Management for Persons with Chronic Diseases and Disabilities. second ed. Human Kinetics; Champaign, IL: 2003. pp. 238–246. [Google Scholar]
- 47.Nieman D.C., Trone G.A., Austin M.D. A new handheld device for measuring resting metabolic rate and oxygen consumption. J. Am. Diet Assoc. 2003;103(5):588–593. doi: 10.1053/jada.2003.50116. [DOI] [PubMed] [Google Scholar]
- 48.Wilson P.W., Meigs J.B., Sullivan L., Fox C.S., Nathan D.M., D'Agostino R.B. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch. Intern. Med. 2007;167(10):1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 49.Block G., Azar K.M., Romanelli R.J. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J. Med. Internet Res. 2015;17(10) doi: 10.2196/jmir.4897. e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Middleton A., Fritz S.L., Lusardi M. Walking speed: the functional vital sign. J. Aging Phys. Activ. 2015;23(2):314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Loo M., Moseley A., Bosman J., De Bie R., Hassett L. Test–re-test reliability of walking speed, step length and step width measurement after traumatic brain injury: a pilot study. Brain Inj. 2004;18(10):1041–1048. doi: 10.1080/02699050410001672314. [DOI] [PubMed] [Google Scholar]
- 52.Mossberg K.A. Reliability of a timed walk test in persons with acquired brain injury. Am. J. Phys. Med. Rehab. 2003;82(5):385–390. doi: 10.1097/01.PHM.0000052589.96202.BE. [DOI] [PubMed] [Google Scholar]
- 53.Enright P.L., Sherrill D.L. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 1998;158(5):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 54.Sallis J.F., Grossman R.M., Pinski R.B., Patterson T.L., Nader P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 55.Control CfD, Prevention Behavioral risk factor surveillance system. http://appsnccdcdcgov/brfss/indexasphttp://appsnccdcdcgov/brfss/Trends/TrendDataasp.2015
- 56.Kroenke K., Strine T.W., Spitzer R.L., Williams J.B., Berry J.T., Mokdad A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 57.Shih R.A., Schell T.L., Hambarsoomian K., Marshall G.N., Belzberg H. Prevalence of PTSD and major depression following trauma-center hospitalization. J Trauma Acute Care Surg. 2010;69(6):1560. doi: 10.1097/TA.0b013e3181e59c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diener E., Emmons R.A., Larsen R.J., Griffin S. The satisfaction with life scale. J. Pers. Assess. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 59.Brown S.C., Pantin H., Lombard J. Walk Score®: associations with purposive walking in recent Cuban immigrants. Am. J. Prev. Med. 2013;45(2):202–206. doi: 10.1016/j.amepre.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cerin E., Conway T.L., Saelens B.E., Frank L.D., Sallis J.F. Cross-validation of the factorial structure of the neighborhood environment walkability scale (NEWS) and its abbreviated form (NEWS-A) Int. J. Behav. Nutr. Phys. Activ. 2009;6(1):32. doi: 10.1186/1479-5868-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker H., Stuifbergen A., Oh H.S., Hall S. Self-rated abilities for health practices: a health self-efficacy measure. Health Values. The Journal of Health Behavior, Education & Promotion. 1993 https://psycnet.apa.org/record/1994-08112-001 [Google Scholar]
- 62.Nasreddine Z.S., Phillips N.A., Bédirian V. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 63.De Guise E., Alturki A.Y., LeBlanc J. The Montreal Cognitive Assessment in persons with traumatic brain injury. Appl. Neuropsychol.: Adultspan. 2014;21(2):128–135. doi: 10.1080/09084282.2013.778260. [DOI] [PubMed] [Google Scholar]
- 64.Holmes T.H., Rahe R.H. The social readjustment rating scale. J. Psychosom. Res. 1967;11(2):213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 65.Juengst S.B., Terhorst L., Dicianno B.E., Niemeier J.P., Wagner A.K. Development and content validity of the behavioral assessment screening tool (BASTβ) Disabil. Rehabil. 2018:1–7. doi: 10.1080/09638288.2017.1423403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwarzer R., Jerusalem M. The general self-efficacy scale (GSE) Hist. Philos. Logic. 2010;12:329–345. [Google Scholar]
- 67.Verplanken B., Orbell S. Reflections on past behavior: a self‐report index of habit strength 1. J. Appl. Soc. Psychol. 2003;33(6):1313–1330. [Google Scholar]
- 68.SAS Institute . SAS; Cary, NC: 2002-2010. SAS/STAT 9.3 User's Guide. [Google Scholar]
- 69.Raudenbush S.W., Bryk A.S. Sage Publications; Thousand Oaks, CA: 2002. Hierarchical Linear Models: Applications and Data Analysis Methods. [Google Scholar]
- 70.Singer J.D., Willett J.B. Oxford University Press; New York, NY: 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. [Google Scholar]
- 71.Enders C.K. Guilford Press; New York, NY: 2010. Applied Missing Data Analysis. [Google Scholar]
- 72.Schafer J.L., Graham J.W. Missing data: out view of the state of the art. Psychol. Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 73.Faul M., Xu L., Wald M.M., Coronado V.G. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 74.Centers for Disease C, Prevention . October. 2016. (TBI: Get the Facts). [Google Scholar]
- 75.Masel B.E., DeWitt D.S. Traumatic brain injury: a disease process, not an event. J. Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 76.Corrigan J.D., Hammond F.M. Traumatic brain injury as a chronic health condition. Arch. PM&R (Phys. Med. Rehabil.) 2013;94(6):1199–1201. doi: 10.1016/j.apmr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 77.Selassie A.W., Cao Y., Church E.C., Saunders L.L., Krause J. Accelerated death rate in population-based cohort of persons with traumatic brain injury. J. Head Trauma Rehabil. 2014;29(3):E8–E19. doi: 10.1097/HTR.0b013e3182976ad3. [DOI] [PubMed] [Google Scholar]
- 78.Brenner L.A., Braden C.A., Bates M. A health and wellness intervention for those with moderate to severe traumatic brain injury: a randomized controlled trial. J. Head Trauma Rehabil. 2012;27(6):E57–E68. doi: 10.1097/HTR.0b013e318273414c. [DOI] [PubMed] [Google Scholar]
- 79.Tomlinson M., Swartz L., Officer A., Chan K.Y., Rudan I., Saxena S. Research priorities for health of people with disabilities: an expert opinion exercise. Lancet. 2009;374(9704):1857–1862. doi: 10.1016/S0140-6736(09)61910-3. [DOI] [PubMed] [Google Scholar]
- 80.Rimmer J.H., Vanderbom K.A., Bandini L.G. GRAIDs: a framework for closing the gap in the availability of health promotion programs and interventions for people with disabilities. Implement. Sci. 2014;9(1):100. doi: 10.1186/s13012-014-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hinnant L., Razi S., Lewis R. Research Triangle Park, NC: Research Triangle Institute (RTI) International. 2015. Evaluation of the health care innovation awards: community resource planning, prevention and monitoring, annual report 2015.https://innovation.cms.gov/Files/reports/hcia-ymcadpp-evalrpt.pdf Retrieved from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.