Abstract
Introduction
Kluyvera ascorbata is a gram-negative, catalase-positive, oxidase-negative, aerobic fermentative bacterium with flagella. This organism colonizes in the human body and its pathogenicity is extremely low; few clinical cases of K. ascorbata infection have been reported.
Presentation of case
We report on a patient who experienced severe sepsis and acute cholangitis due to K. ascorbata bacteremia and was treated with levofloxacin following antibiotic susceptibility testing. To our knowledge, this is the first case report of third-generation cephalosporins resistant K. ascorbata infection in Japan.
Discussion
Although this pathogen produces innate CTX-M type β-lactamases and is generally resistant to first- and second-generation penicillins and cephalosporins, multi-drug resistant K. ascorbata infection, including ceftriaxone resistant infection has seldom been reported.
Conclusion
The increase of drug-resistant pathogens is of concern; in such cases, rapid microbial identification and appropriate antibiotic selection are crucial for successful treatment.
Keywords: Kluyvera ascorbata, Cholangitis, Bacteremia, CTX-M type β-lactamase
Introduction
We report the case of a 78-year-old male patient with severe sepsis and acute cholangitis due to third-generation cephalosporins resistant Kluyvera ascorbata bacteremia. This pathogen is believed to be benign and colonizes the human gastro-intestinal tract. There has been a recent increase in the number of case reports of patients with K. ascorbata infection as a causative pathogen; moreover, K. ascorbata has innate resistance to ampicillin and first-generation cephalosporins. In this case report, we describe the clinical course of a patient with the first case of third-generation cephalosporins resistant K. ascorbata cholangitis. We also review the literature on Kluyvera species.
Case report
A 78-year-old man with a history of hypertension, stroke, and cholangiocarcinoma visited the emergency room (ER), and presented with diarrhea, nausea, fatigue, and fever, lasting a few days. He additionally developed appetite loss and abdominal pain that did not improve with non-steroidal, anti-inflammatory drug administration. His regular medications included a proton pump inhibitor, aspirin, and an antihistamine. Two months prior to admission, he underwent administration of his first course of gemcitabine plus cisplatin combination chemotherapy for the cholangiocarcinoma. One month prior to admission, he underwent endoscopic retrograde biliary drainage (ERBD) tube insertion due to cholestasis. In the ER, His body temperature was 36.8 °C, blood pressure was 87/44 mmHg, pulse was 66 bpm and regular, respiratory rate was 16 breaths/min, and the percutaneous oxygen saturation was 96% (room air). Chest examination revealed normal S1 and S2 heart sounds plus normal breath sounds. Upon blood examination, there was a marked increase in aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels (1449 U/L, 546 U/L, and 1509 U/L, respectively). His total white cell count was 3,300/μL, and C-reactive protein level was 0.67 mg/dL. Although chest radiography and computed tomography were performed on admission, initially both examinations did not reveal any abnormal findings, nor was there evidence of ERBD tube obstruction and gallbladder enlargement. Severe sepsis with cholangitis was suspected based on his symptoms and clinical course. He was therefore admitted to the emergency department and administration of piperacillin/tazobactam was initiated after drawing two sets of blood culture samples. Although his abdominal pain and serum liver enzyme levels had slightly improved, on day 4, gram-negative rods were documented in his blood culture taken on admission (Fig. 1). The following day, K. ascorbata was confirmed and antibiotic susceptibility testing revealed resistance to amoxicillin, piperacillin, cefazolin, and ceftriaxone; conversely, the organism was susceptible to ampicillin/sulbactam, piperacillin/tazobactam, aztreonam, levofloxacin, and meropenem as determined via a Micro Scan Walk Away 96 plus microbiology analyzer (Beckman Coulter, Brea, CA, USA) (Table 1). Based on these results, piperacillin/tazobactam was replaced by intravenous levofloxacin and then switched to oral levofloxacin. On day 11, after his symptoms improved, the ERBD tube was replaced due to a suspected ERBD tube obstruction. Consequently, his liver enzyme levels returned to normal. After 14 days of antibiotic treatment, his symptoms fully improved, and he was discharged from the hospital. Afterwards, the pathogen was identified as K. ascorbatawith a score value of 2.17 via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker, Billerica, MA, USA).
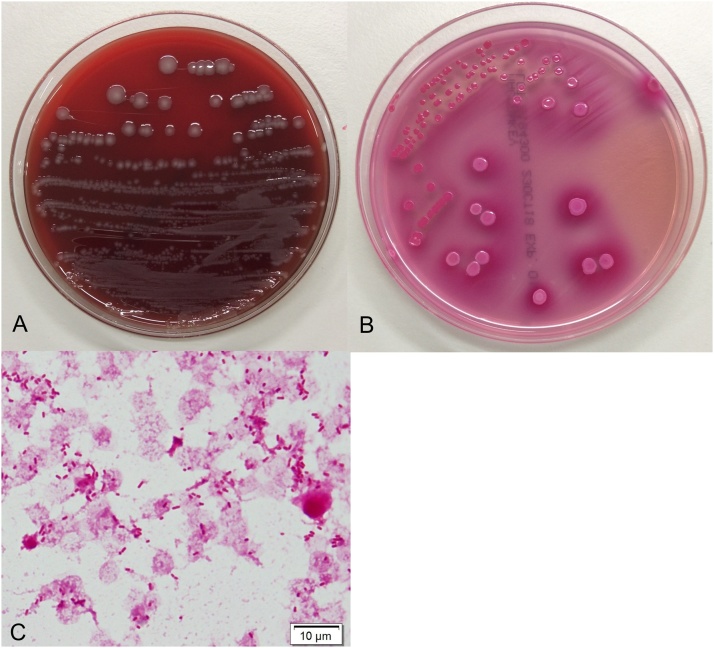
Fig. 1.
(A) Growth of Kluyvera ascorbata was circular, convex, greyish, and smooth in 8% sheep blood agar. (B) Pink, round-shaped colonies were noted in MacConkey agar. (C) Gram stain: gram-negative barrel-shaped microorganism is found.
Table 1.
The antibiotic susceptibility pattern of isolated Kluyvera ascorbata.
| MIC (μg/mL) | Susceptibility | |
|---|---|---|
| Aztreonam | ≦4 | S |
| Ampicillin | >16 | R |
| Piperacillin | >64 | R |
| Ampicillin-sulbactam | ≦8 | S |
| Piperacillin-tazobactam | ≦16 | S |
| Cefazolin | >16 | R |
| Cefotiam | 16 | I |
| Ceftriaxone | >2 | R |
| Cefepime | ≦2 | S |
| Amikacin | ≦4 | S |
| Gentamicin | ≦2 | S |
Antibiotic susceptibility profile of K. ascorbata isolated from blood culture.
Abbreviations: MIC, minimal inhibitory concentration; S, susceptible; I, intermediately susceptible; R, resistant.
Discussion
K. ascorbata is a gram-negative, oxidase-negative, fermentative bacterium with flagellated rods, which was identified by Asai et al. in the 1950s [1]. Initially, it was included in the genus Escherichia, however; a new genus for this group of bacteria was proposed in 1981 [2]. At present, the Kluyvera genus is classified into four species: K. ascorbata, K. cryocrescens, K. georgiana, and K. cochleae. These species are found in environments such as soil and sewage systems, and occasionally in mollusks such as snails and slugs [3,4]. Isolation from the human body has been documented in the gastrointestinal system and urinary tract; however, understanding of the pathogenicity of Kluyvera species is incomplete and its localization remains uncertain. Generally, K. ascorbata is benign, and not present in numbers high enough to be pathogenic in the human body. To date, few cases of infection caused by this pathogen have been reported, including urinary tract infection, bacteremia, soft tissue infection, and mediastinitis, with varying severity and a wide range in patient age [[5], [6], [7], [8]]. Zoonotic infection in Egyptian fruit bats has also been reported [9].
Only two cases of biliary tract infections due to K. ascorbata have been reported [10,11] (Table 2). One of a 67-year-old woman with acute cholecystitis, and another of a 23-year-old man with liver cirrhosis due to hepatitis B infection. Both pathogens were susceptible for amoxicillin/clavulanic acid, fluoroquinolones, aminoglycoside, and third-generation cephalosporins.
Table 2.
Previous reports of biliary tract infections caused by Kluyvera ascorbata.
| Article | Age | Sex | Symptom | Underlying diseases | Diagnosis | Specimen | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Batista N, 2002 [10] | 67 | F | Abdominal pain, Vomiting, | None | Acute cholecystitis | Gall bladder fluid | Cholecystectomy Gentamycin |
Cured |
| Oteo J, 1998 [11] | 23 | M | Fever, Abdominal pain, Nausea, Vomiting |
Chronic hepatitis B | Acute cholecystitis | Blood | Cefoxitin Netilmicin |
Cured |
Kluyvera spp. have an innate antibiotic resistant mechanism and may likely be a progenitor of the CTX-M type β-lactamase. CTX-M belongs to extended spectrum β-lactamase (ESBL), with its antimicrobial resistant mechanism clustered into five main subgroups, CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25, as per their amino acid sequences [12]. K. ascorbata is referred to contain the natural progenitor of genes encoding CTX-M-1 and CTX-M-2 subgroups, and K. georgiana have been correlated with the CTX-M-8, CTX-M-9, and CTX-M-25 subgroups [13]. These enzymes hydrolyze cefotaxime more readily than ceftazidime [14]. Recently, CTX-M type β-lactamase is described as the most prevalent variant of ESBLs [12]. Moreover, the mobilization of chromosomal blaCTX-M gene is occasionally reported; plasmid encoding CTX-M genes could cause emerging multi-drug resistant pathogen [15].
To our knowledge, we report herein the first definitive case of third-generation cephalosporins resistant K. ascorbata cholangitis. Generally, K. ascorbata has atypical susceptibility pattern, that is, low-level resistance to penicillins, cephalothin, and cefuroxime; while, this pathogen has potentiation effect on the reversal of resistance by clavulanate [16]. Thus, ceftriaxone, fluoroquinolones, and aminoglycosides are generally considered to be active against this pathogen [3]. On the other hand, exposure to cefotaxime, ceftazidime, and piperacillin enhances transposition frequency to other Enterobacteriaceae and could cause the spread of antimicrobial resistance [17].
To date, there is only one case of multi-drug (including third-generation cephalosporins resistant) K. ascorbata infection isolated from the blood [18]. This was in a 64-year-old African-American male resident of a nursing home with an indwelling suprapubic catheter due to prostate cancer and recurrent urinary tract infections. The organism was only susceptible to amikacin, tobramycin, and imipenem, and resistant to ceftriaxone, cefotaxime, and levofloxacin. He was therefore successfully treated with meropenem.
Although the risk factors for K. ascorbata infection are still unknown, patients in previous reports had various underlying diseases such as malignancy, liver cirrhosis, chronic kidney diseases, or congenital heart diseases. As our patient also had a cholangiocarcinoma and was receiving chemotherapy, it seems that this organism may be pathogenic in patients with immune dysfunctions.
Presently, there is little consensus regarding first line antibiotic treatment of K. ascorbata, and previous reports administered antibiotics based on their resistance and susceptibility profiles. In this case described here, we initially administered piperacillin/tazobactam and subsequently de-escalated to levofloxacin after considering the susceptibility characteristics. Since fluoroquinolone also has an oral administration route and could be a viable choice of treatment, we administered levofloxacin and were able to complete the treatment.
Our patient had no history of frequent hospitalizations and was not previously exposed to a broad range of antibiotics, but the pathogen had already acquired resistance to ceftriaxone at first isolation. This suggests that emerging drug resistant organisms are of concern to public health, and K. ascorbata is certainly no exception.
In conclusion, K. ascorbata is not associated with high virulence and morbidity; however, clinicians should pay attention to its characteristic antibiotic resistant pattern, and the advent of multi-drug resistance, including resistance to third-generation cephalosporins. Careful selection of appropriate antibiotic treatment is crucial when K. ascorbata is identified in clinical specimens.
Authorship statement
All authors meet the ICMJE authorship criteria.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Writing and design: Yoshikazu Mutoh.
Chief physician and advice of clinical course: Tomohisa Kobe and Tomoya Hirano.
Modification of article: Toshihiko Ichihara.
Assistance in the preparation and improvement of the article: Hiroyuki Takenaka, Takuro Niinomi, and Takumi Umemura.
Approval and grammar check: Masanori Kuroiwa.
All authors checked and reviewed this article in detail and approved the final version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations of interest
None.
References
- 1.Asai T., Okumura S., Tsumoda T. On a new genus, Kluyvera. Proc Jpn Acad. 1956;32:488–493. [Google Scholar]
- 2.Farmer J.J., Fanning G.R., Huntley-Carter G.P., Holmes B., Hickman F.W., Richard C. Kluyvera, a new (redefined) genus in the family Enterobacteriaceae: identification of Kluyvera ascorbata sp. nov. and Kluyvera cryocrescens sp. nov. in clinical specimens. J Clin Microbiol. 1981;13:919–933. doi: 10.1128/jcm.13.5.919-933.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarria J.C., Vidal A.M., Kimbrough R.C., 3rd. Infections caused by Kluyvera species in humans. Clin Infect Dis. 2001;33:E69–74. doi: 10.1086/322686. [DOI] [PubMed] [Google Scholar]
- 4.Carter J.E., Evans T.N. Clinically significant Kluyvera infections: a report of seven cases. Am J Clin Pathol. 2005;123:334–338. doi: 10.1309/61XP-4KTL-JYWM-5H35. [DOI] [PubMed] [Google Scholar]
- 5.Isozaki A., Shirai K., Mimura S., Takahashi M., Furushima W., Kawano Y. A case of urinary tract infection caused by Kluyvera ascorbata in an infant: case report and review of the literature. J Infect. 2010;16:436–438. doi: 10.1007/s10156-010-0067-3. [DOI] [PubMed] [Google Scholar]
- 6.Lin J.C., Chen C.H., Siu L.K., Chang F.Y. Nosocomial outbreak of Kluyvera cryocrescens bacteremia. Infect Control Hosp Epidemiol. 2002;23:62–64. doi: 10.1086/503456. [DOI] [PubMed] [Google Scholar]
- 7.West B.C., Vijayan H., Shekar R. Kluyvera cryocrescens finger infection: case report and review of eighteen Kluyvera infections in human beings. Diag Microbiol Infect Dis. 1998;32:237–241. doi: 10.1016/s0732-8893(98)00087-x. [DOI] [PubMed] [Google Scholar]
- 8.Sierra-Madero J., Pratt K., Hall G.S., Stewart R.W., Scerbo J.J., Longworth D.L. Kluyvera mediastinitis following open-heart surgery: a case report. J Clin Microbiol. 1990;28:2848–2849. doi: 10.1128/jcm.28.12.2848-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J.E., Gomez D.K., Kim J.H., Choresca C.H., Jr, Shin S.P., Park S.C. Isolation of a zoonotic pathogen Kluyvera ascorbata from Egyptian fruit-bat Rousettus aegyptiacus. J Vet Med Sci. 2010;72:85–87. doi: 10.1292/jvms.08-0342. [DOI] [PubMed] [Google Scholar]
- 10.Batista N., Diez O., Moreno A., Ode J. Acute cholecystitis due to Kluyvera ascorbata [Spanish] Enferm Infec Microbiol Clin. 2002;20:370–371. doi: 10.1016/s0213-005x(02)72819-x. [DOI] [PubMed] [Google Scholar]
- 11.Oteo J., Gomez-Garces J.L., Alos J.I. Acute cholecystitis and bacteremia caused by Kluyvera ascorbata in a cirrhotic patient. Clin Microbiol Infect. 1998;4:113–115. doi: 10.1111/j.1469-0691.1998.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 12.Cantón R., Coque T.M. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Nordmann P., Lartigue M.F., Poirel L. Beta-lactam induction of ISEcp1B-mediated mobilization of the naturally occurring bla(CTX-M) beta-lactamase gene of Kluyvera ascorbata. FEMS Microbiol Lett. 2008;288:247–249. doi: 10.1111/j.1574-6968.2008.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bush K., Jacoby G.A. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernández-García M., León-Sampedro R., Pérez-Viso B., Morosini M.I., López-Fresneña N., Díaz-Agero C. First report of an OXA-48- and CTX-M-213-producing Kluyvera species clone recovered from patients admitted in a University Hospital in Madrid, Spain. Antimicrob Agents Chemother. 2018;62(11) doi: 10.1128/AAC.01238-18. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humeniuk C., Arlet G., Gautier V., Grimont P., Labia R., Philippon A. β-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrob Agents Chemother. 2002;46:3045–3049. doi: 10.1128/AAC.46.9.3045-3049.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lartigue M.F., Poirel L., Aubert D., Nordmann P. In vitro analysis of ISEcp1B-mediated mobilization of naturally occurring beta-lactamase gene blaCTX-M of Kluyvera ascorbata. Antimicrob Agents Chemother. 2006;50:1282–1286. doi: 10.1128/AAC.50.4.1282-1286.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moonah S., Deonarine K., Freeman C. Multidrug resistant Kluyvera ascorbata septicemia in an adult patient: a case report. J Med Case Rep. 2010;4:197. doi: 10.1186/1752-1947-4-197. [DOI] [PMC free article] [PubMed] [Google Scholar]