Brettanomyces bruxellensis is one of the most important wine spoilage microorganisms, with the use of sulfite being the major method to control spoilage. However, this species displays a wide intraspecies distribution in sulfite tolerance, with some strains capable of tolerating high concentrations of SO2, with relatively high concentrations of this antimicrobial needed for their control. Although SO2 tolerance has been studied in several organisms and particularly in S. cerevisiae, little is known about the mechanisms that confer SO2 tolerance in B. bruxellensis. Here, we confirmed the functionality of the sulfite efflux pump encoded by BbSSU1 and determined the efficiencies of four different BbSSU1 haplotypes. Gene expression analysis showed greater expression of the haplotype conferring greater SO2 tolerance. Our results suggest that a combination of BbSSU1 haplotype efficiency, copy number, and haplotype expression levels likely contributes to the diverse SO2 tolerances observed for different B. bruxellensis strains.
KEYWORDS: SO2, Ssu1p, allele specific expression, transcriptome, wine, yeast
ABSTRACT
The addition of SO2 is practiced in the wine industry to mitigate the risk of microbial spoilage and to extend wine shelf-life. Generally, this strategy does not interfere with primary alcoholic fermentation, as wine strains of Saccharomyces cerevisiae exhibit significant SO2 tolerance, largely driven by the efflux pump Ssu1p. One of the key yeast species responsible for wine spoilage is Brettanomyces bruxellensis, which also exhibits strain-dependent SO2 tolerance, although this occurs via unknown mechanisms. To evaluate the factors responsible for the differential sulfite tolerance observed in B. bruxellensis strains, we employed a multifaceted approach to examine both expression and allelic differences in the BbSSU1 gene. Transcriptomic analysis following exposure to SO2 highlighted different inducible responses in two B. bruxellensis strains. It also revealed disproportionate transcription of one putative BbSSU1 haplotype in both genetic backgrounds. Here, we confirm the functionality of BbSSU1 by complementation of a null mutant in a S. cerevisiae wine strain. The expression of four distinct BbSSU1 haplotypes in the S. cerevisiae ΔSSU1 mutant revealed up to a 3-fold difference in conferred SO2 tolerance. Substitution of key amino acids distinguishing the encoded proteins was performed to evaluate their relative contribution to SO2 tolerance. Protein modeling of two haplotypes which differed in two amino acid residues suggested that these substitutions affect the binding of Ssu1p ligands near the channel opening. Taken together, preferential transcription of a BbSSU1 allele that encodes a more efficient Ssu1p transporter may represent one mechanism that contributes to differences in sulfite tolerances between B. bruxellensis strains.
IMPORTANCE Brettanomyces bruxellensis is one of the most important wine spoilage microorganisms, with the use of sulfite being the major method to control spoilage. However, this species displays a wide intraspecies distribution in sulfite tolerance, with some strains capable of tolerating high concentrations of SO2, with relatively high concentrations of this antimicrobial needed for their control. Although SO2 tolerance has been studied in several organisms and particularly in S. cerevisiae, little is known about the mechanisms that confer SO2 tolerance in B. bruxellensis. Here, we confirmed the functionality of the sulfite efflux pump encoded by BbSSU1 and determined the efficiencies of four different BbSSU1 haplotypes. Gene expression analysis showed greater expression of the haplotype conferring greater SO2 tolerance. Our results suggest that a combination of BbSSU1 haplotype efficiency, copy number, and haplotype expression levels likely contributes to the diverse SO2 tolerances observed for different B. bruxellensis strains.
INTRODUCTION
Sulfur dioxide is used extensively in the wine industry due to its antimicrobial and antioxidant properties. While the addition of SO2 before fermentation is mainly used as a tool to reduce the number of indigenous microorganisms present in the grape must, its addition postfermentation protects wine from oxidation and prevents the growth of spoilage microorganisms which can impart off-flavors in the final wine. One of the most important wine spoilage microorganisms is the yeast Brettanomyces bruxellensis.
B. bruxellensis was first identified in brewing (1), but since then, it has been isolated from a wide range of fermentative products, including cider (2), kombucha (3), bioethanol (4), kefir (5), and wine (6). B. bruxellensis exhibits key physiological traits which enable it to grow in wine, including high ethanol tolerance (up to 15% [vol/vol]) (7); high acid tolerance, with 94% of B. bruxellensis strains from a global collection able to grow at pH 2.0 (8); an ability to assimilate a wide range of carbon sources, including cellobiose (9) and, potentially, chitin (10); and varied capacity to utilize nitrate as a nitrogen source (8). In wine, B. bruxellensis converts hydroxycinnamic acids into 4-ethylphenol and 4-ethylguaiacol (11). These compounds impart an aroma described as “barnyard,” “earthy,” “Band-Aid,” and “medicinal” and can also affect wine flavor, contributing to a “metallic aftertaste” (12).
Tolerance to SO2 has been shown to vary widely for B. bruxellensis isolates (8). Curtin et al. (13) reported a correlation between genotype and SO2 tolerance when screening Australian B. bruxellensis wine isolates and grouping them according to amplified fragment length polymorphism (AFLP) genotype. This finding was extended recently through microsatellite analysis of more than 1,400 B. bruxellensis isolates, which revealed distinct genetic clusters defined by ploidy and substrate of isolation (14). One of these, the AWRI1499-like triploid wine group, was composed of isolates highly tolerant to SO2, while isolates from substrates other than wine were all sensitive, suggesting a link between SO2 exposure in wine and tolerance to this preservative (14, 15).
The antimicrobial activity of SO2 is due to its capacity to interfere with intracellular processes through disruption of enzymatic activity and protein function. Bacterial, yeast, and mammalian cells have evolved various mechanisms to deal with SO2 exposure, such as reduction, oxidation, sequestration via acetaldehyde production, sulfitolysis, and active efflux (16). Active efflux has been heavily studied in Saccharomyces cerevisiae, where the relative activity of the sulfite efflux pump encoded by SSU1 is a key determinant of a strain’s ability to tolerate SO2 (17, 18).
In contrast, we know little about the mechanisms that confer SO2 tolerance in B. bruxellensis. To address this gap in knowledge, we investigated the intraspecific differences in both gene expression and protein activity that may contribute to differential performance (and therefore sulfite resistance) of BbSSU1. RNA sequencing (RNA-seq) analysis of two B. bruxellensis strains exposed to differentiating concentrations of sulfite showed disproportionate allelic expression ratios for the B. bruxellensis ortholog of SSU1, suggesting that one copy of the gene was preferentially transcribed. These findings led us to characterize functionality of four different SSU1 haplotypes from three B. bruxellensis strains (AWRI2804 [haploid], AWRI1613 [diploid], and AWRI1499 [triploid]), including the impact of BbSsu1p amino acid substitutions on conferred sulfite tolerance.
RESULTS
In order to evaluate factors responsible for the differential sulfite tolerances observed between different B. bruxellensis strains, a multifaceted approach was employed to examine differences in either gene expression or allelic variation in the coding region of the BbSSU1 gene.
RNA-seq evaluation of genes involved in B. bruxellensis response to sulfite.
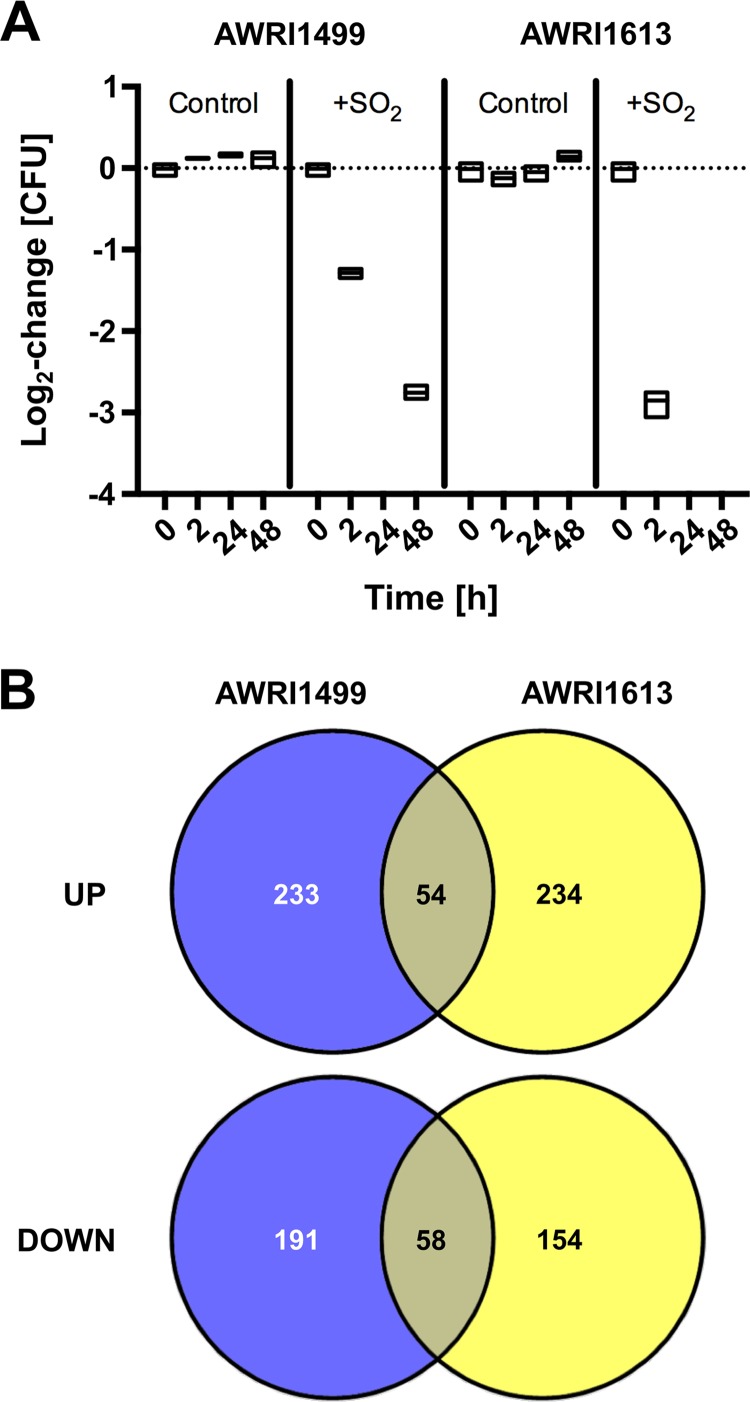
Two B. bruxellensis strains (AWRI1499 and AWRI1613) that were previously shown to differ in their capacities to tolerate SO2 (13) were grown to late-log phase in chemically defined wine medium (CDWM) under anaerobic conditions and then subjected to 0.4 mg/liter molecular SO2. Two hours after this treatment, the culturable AWRI1499 population had decreased by just over one order of magnitude, while AWRI1613 decreased by about three orders of magnitude (Fig. 1A). No culturable cells were detectable after 24 h (below the limit of detection of 10 CFU/ml) for either strain; however, for AWRI1499, a culturable population was detected at 48 h and maintained up to 358 h (data not shown). These results were consistent with previously described SO2 tolerance for these strains (13). RNA-seq was performed on samples taken 2 h after exposure to detect SO2-inducible stress responses for both B. bruxellensis strains.
FIG 1.
Population and transcriptional responses of B. bruxellensis to sulfite treatment. (A) Relative change in culturability (CFU) for triplicate cultures of strains AWRI1499 and AWRI1613 treated with SO2; boxes represent range with median. (B) Venn diagram summarizing open reading frames (ORFs) and novel transcriptionally active regions (nTARs) significantly upregulated (UP) or downregulated (DOWN) 2 h after SO2 treatment for both strains.
Similar numbers of open reading frames (ORFs) and novel transcriptionally active regions (nTARs) were differentially expressed for both strains in response to SO2 (Fig. 1B), but only 13.8% of these were in common. GO enrichment analysis of the ORFs/nTARs induced or repressed for each strain reflected this relative lack of overlap (see Tables S2 to S4 in the supplemental material). For AWRI1499, processes, such as gene expression (GO:0010467), RNA metabolism (GO:0016070), and ribosome biogenesis (GO:0042254) were induced, along with related processes (Table S2). Repressed ORFs/nTARs were overrepresented in DNA replication (GO:0006260) and lipid catabolism (GO:0016042) and underrepresented for gene expression and translation (GO:0006412) (Table S3). Among induced ORFs/nTARs for AWRI1613, the only overrepresented process among enriched GO terms was carbohydrate metabolism (GO:0005975) (Table S4), while no processes were significantly repressed.
Of the 20 loci most induced by SO2 relative to controls (Tables S5 and S6), there were four in common for the two strains (g257.t1, g2392.t1, g4321.t1, and AWRI1499_mt0051). The 823-bp AWRI1499_mt0051 region was most strongly induced in both strains and may represent a novel SO2-responsive genetic element, as it had no blast matches against the NCBI nonredundant and transcriptome shotgun assembly databases. Likewise, g257.t1 may represent an SO2-responsive element in B. bruxellensis, while its ortholog in S. cerevisiae (FUN19) has unknown function but is differentially regulated in response to a range of stress conditions. The remaining two common ORFs (g2392.t1 and g4321.t1) may both be involved in phosphate metabolism; g2392.t1 is annotated as a duf221 family protein and in blastx searches has strong homology to S. cerevisiae Phm7p, which is regulated by phosphate, and g4321.t1 encodes a putative acid phosphatase. Among the 20 loci most strongly repressed by SO2, there were two common elements (g1252.t1 and g1823.t1). These are annotated as a cyclin involved in cell cycle progression (Pcl1p) (19) and an arginase, respectively (Tables S7 and S8).
Notably absent among differentially regulated loci was BbSSU1 (g80.t1), which is predicted to encode a sulfite efflux pump (10). This result contrasts with RNA-seq data from a recent study (20), where a >2-fold induction of this ORF was observed following sulfite treatment and during subsequent recovery of culturability. Upon closer examination of the mapped data, it was apparent that the expected allelic ratios (2:1 for triploid AWRI1499 and 1:1 for diploid AWRI1613) were not observed at this locus (Fig. 2). Instead, irrespective of SO2 treatment, the median major allele frequency was >0.9 for AWRI1499 and >0.8 for AWRI1613. A similar observation could be made for data derived from a study by Capozzi et al. (20), despite the lack of equivalent genome sequence data, by comparison of BbSSU1 major allele frequency of >0.7 relative to major allele frequency across all polymorphic sites in the B. bruxellensis Unifg14 transcriptome (0.57 ± 0.08).
FIG 2.
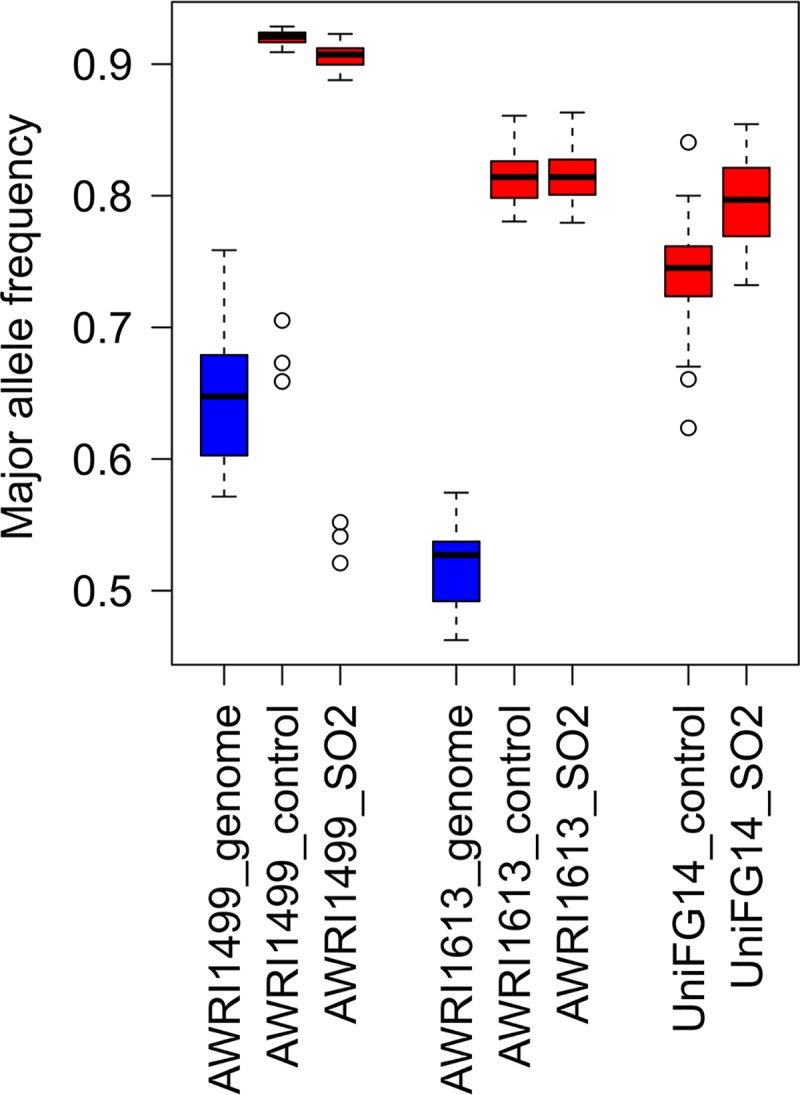
Skewed transcript abundance of BbSSU1 allele. Major allele frequency calculated at each polymorphic SNP across BbSSU1 (g80.t1) open reading frame for genomic (blue) and transcriptomic (red) data sets. Box plots represent 12 polymorphic sites for triploid AWRI1499, 11 for diploid AWRI1613, and 11 for UniFG14 (ploidy unknown) across all biological replicates (n = 3 for RNA-seq, n = 1 for genomic data).
Determination of B. bruxellensis SSU1 haplotypes.
The observation of disproportionate allele expression at BbSSU1 prompted us to consider whether certain haplotypes of this gene may encode stronger SO2 pumps and therefore make a more significant contribution to SO2 tolerance. To investigate this, an additional B. bruxellensis strain (UCD2041/AWRI2804), previously described as sulfite sensitive (21), was included for analysis. Direct comparison confirmed that AWRI2804 tolerated lower SO2 concentrations than AWRI1613 or AWRI1499 (Fig. S1A). AWRI1499 was the most tolerant strain, although no growth was observed for sulfite concentrations over 0.71 mg/liter molecular SO2. AWRI1613 was able to grow in concentrations up to 0.44 mg/liter, while AWRI2804 was the least tolerant, with no growth observed above 0.35 mg/liter molecular SO2.
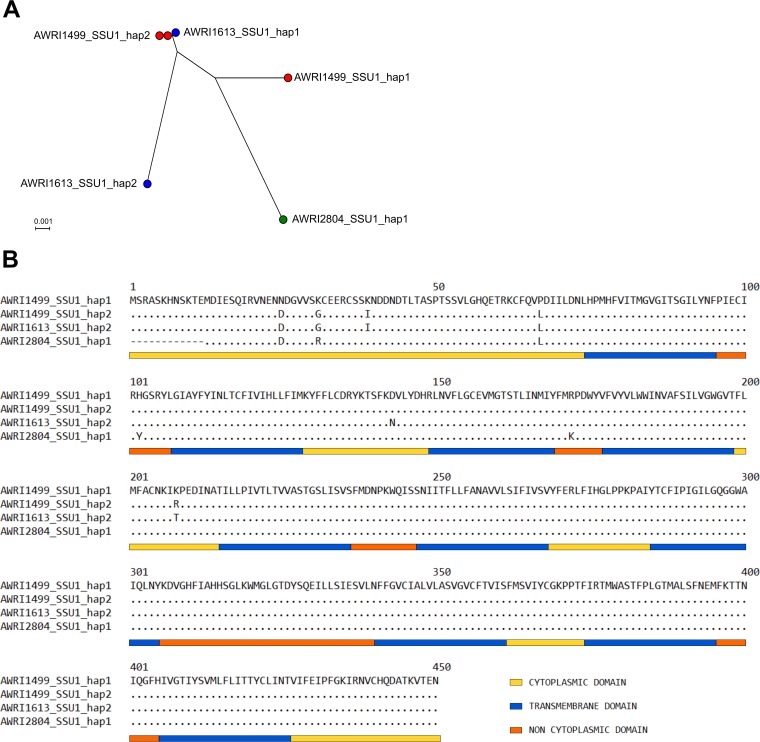
Manual phasing of single-nucleotide polymorphisms (SNPs) into haplotypes from the published triploid AWRI1499 (10, 22) and diploid AWRI1613 (22) genomic data sets revealed three SSU1 sequences, while an additional distinct sequence was identified in B. bruxellensis AWRI2804 (sequenced using the Sanger method). As shown in Fig. 3A, B. bruxellensis AWRI1499 has two haplotypes, AWRI1499_SSU1_hap1 and AWRI1499_SSU1_hap2, with AWRI1499_SSU1_hap2 being present in two copies. B. bruxellensis AWRI1613 also has two haplotypes, one identical to AWRI1499_SSU1_hap2 and a second annotated as AWRI1613_SSU1_hap2. B. bruxellensis AWRI2804 exhibits a single haplotype, AWRI2804_SSU1_hap1. At each polymorphic site, it was apparent that the observed skew in major allele frequency in RNA-seq data sets for AWRI1499 and AWRI1613 at BbSSU1 (Fig. 2) was due to preferential transcription of haplotype AWRI1499_SSU1_hap2. This haplotype was also responsible for the detected major allele at each polymorphic site for UniFG14.
FIG 3.
(A) Phylogenetic tree for B. bruxellensis SSU1 haplotypes. AWRI1499 (red) has two haplotypes, one being present in two copies; AWRI1613 (blue) has two haplotypes; and AWRI2804 (green) has only one haplotype. (B) Amino acid sequences of Ssu1 proteins encoded by the four B. bruxellensis SSU1 haplotypes. Differences in amino acid residues are shown using AWRI1499_SSU1_hap1 as the template. Cytoplasmic domains (yellow bar), transmembrane domains (blue bar), and noncytoplasmic domains (orange bar) were predicted using SPOCTOPUS.
Protein topology analysis for B. bruxellensis Ssu1p using SPOCTOPUS indicated a protein length of 438 to 450 amino acids (Fig. 3B). All four haplotypes have a long cytoplasmic domain (62 to 74 residues) at the N terminus, followed by 10 transmembrane domains interspaced with short cytoplasmic and noncytoplasmic loops. A comparison of the amino acid sequences for the four haplotypes revealed eight locations of amino acid variation between the haplotypes and a deletion of 12 amino acids at the N terminus of AWRI2804_SSU1_hap1 (summarized in Table 1). All variations were observed within the first 208 amino acids and located primarily within the cytoplasmic domains of the protein.
TABLE 1.
Summary of differences in protein sequences between Ssu1p from B. bruxellensis strains
| Position(s) | AWRI1499 SSU1_hap1 | AWRI1499 SSU1_hap2 | AWRI1613 SSU1_hap2 | AWRI2804 SSU1_hap1 |
|---|---|---|---|---|
| 1–12 | Deletion | |||
| 25 | Asn (N) | Asp (D) | Asp (D) | Asp (D) |
| 31 | Lys (K) | Gly (G) | Gly (G) | Arg (R) |
| 39 | Lys (K) | Ile (I) | Ile (I) | Lys (K) |
| 67 | Pro (P) | Leu (L) | Leu (L) | Leu (L) |
| 102 | His (H) | His (H) | His (H) | Tyr (Y) |
| 143 | Asp (D) | Asp (D) | Asn (N) | Asp (D) |
| 173 | Arg (R) | Arg (R) | Arg (R) | Lys (K) |
| 208 | Lys (K) | Arg (R) | Thr (T) | Lys (K) |
Other sulfite transporters from the tellurite-resistance/dicarboxylate transporter (TDT) family also have 10 transmembrane domains; however, BbSsu1p showed more similarity to S. cerevisiae Ssu1p than to other members of the family (27% identity) (Fig. S2A). For fungal sulfite transporters in the TDT family, two potential signature sequences, located in the 3rd and 7th transmembrane domains, have been identified (23) (Fig. S2B). These motifs are located in the same transmembrane domains for BbSsu1p, with all four B. bruxellensis haplotypes exhibiting the same sequence.
Expression of B. bruxellensis SSU1 haplotypes in S. cerevisiae.
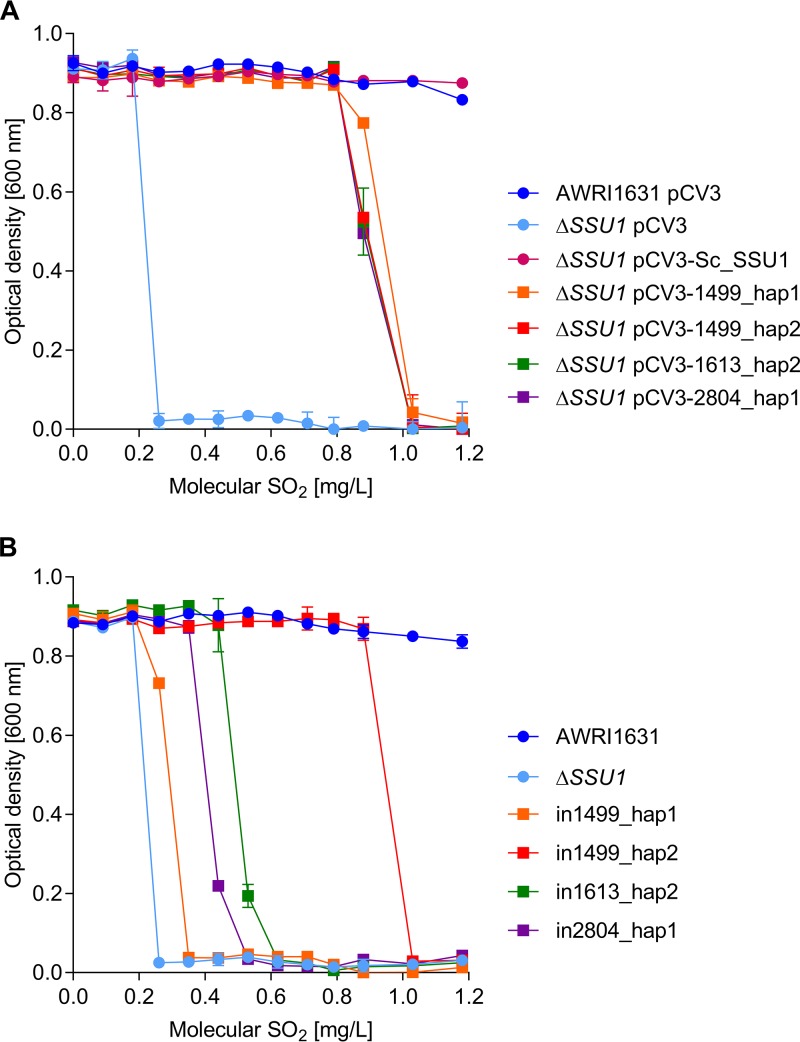
In order to assess the function of putative BbSSU1 haplotypes, a S. cerevisiae wine yeast strain (AWRI1631 haploid) lacking SSU1 was constructed. Compared to the wild type, which could tolerate up 2.1 mg/liter molecular SO2, the deletion strain was unable to grow when sulfite concentrations reached 0.26 mg/liter (Fig. S1B). Complementation was first assessed by introducing each BbSSU1 haplotype into S. cerevisiae AWRI1631 ΔSSU1 via a multicopy expression vector (pCV3), under the control of a strong yeast promoter. When expressed in high copy number, all B. bruxellensis SSU1 haplotypes conferred similar SO2 tolerance in S. cerevisiae (Fig. 4A), partially complementing the native gene deletion. Introduction of the vector had no effect on the maximum optical density at 600 nm (OD600) for all strains tested. When pCV3-Sc_SSU1, carrying the S. cerevisiae SSU1 gene, was introduced into S. cerevisiae AWRI1631 ΔSSU1, sulfite tolerance was fully restored, with growth not significantly different from that of S. cerevisiae AWRI1631/pCV3 or S. cerevisiae AWRI1631 (Fig. 4A). S. cerevisiae AWRI1631 ΔSSU1/pCV3-1499_hap1 showed higher growth than S. cerevisiae AWRI1631 ΔSSU1/pCV3-1499_hap2, S. cerevisiae AWRI1631 ΔSSU1/pCV3-1613_hap2, and S. cerevisiae AWRI1631 ΔSSU1/pCV3-2804_hap1 at 0.88 mg/liter of molecular SO2. Nevertheless, none of these strains were able to grow when sulfite concentrations reached 1.03 mg/liter.
FIG 4.
(A) Multicopy expression of B. bruxellensis SSU1 haplotypes in S. cerevisiae. SSU1 haplotypes were inserted in the multicopy vector pCV3 and expressed in S. cerevisiae strain AWRI1631 ΔSSU1. The results shown represent the average of the results from three replicates per strain repeated twice as independent experiments. (B) Single-copy expression of B. bruxellensis SSU1 haplotypes in S. cerevisiae. SSU1 haplotypes were expressed in S. cerevisiae strain AWRI1631 ΔSSU1 under the control of the native S. cerevisiae promoter. Fisher’s LSD test was used to determine significant differences (P < 0.05).
To directly compare whether the B. bruxellensis SSU1 haplotypes were able to confer similar degrees of sulfite tolerance, each was introduced as a single copy in S. cerevisiae AWRI1631, replacing the native SSU1 ORF in the chromosome. Thus, each B. bruxellensis haplotype remained under the control of the S. cerevisiae native SSU1 promoter. As shown in Fig. 4B, the four haplotypes conferred significantly different tolerances to sulfite. Gene replacement had no effect on the maximum OD600 on all strains in the absence of sulfite. As described above, S. cerevisiae AWRI1631 ΔSSU1 exhibited no growth when sulfite concentrations reached 0.26 mg/liter. AWRI1499_SSU1_hap1 conferred the least sulfite tolerance, with no growth observed at 0.35 mg/liter. AWRI1499_SSU1_hap2 was the strongest haplotype, providing growth up to 0.88 mg/liter sulfite. AWRI1613_SSU1_hap2 conferred tolerance up to 0.53 mg/liter SO2, while AWRI2804_SSU1_hap1 provided yeast growth until concentrations reached 0.44 mg/liter. Therefore, in order of relative sulfite tolerance when expressed in S. cerevisiae, AWRI1499_SSU1_hap2 was the strongest haplotype, followed by AWRI1613_SSU1_hap2, AWRI2804_SSU1_hap1, and finally, AWRI1499_SSU1_hap1.
Effect of amino acid substitutions in B. bruxellensis Ssu1.
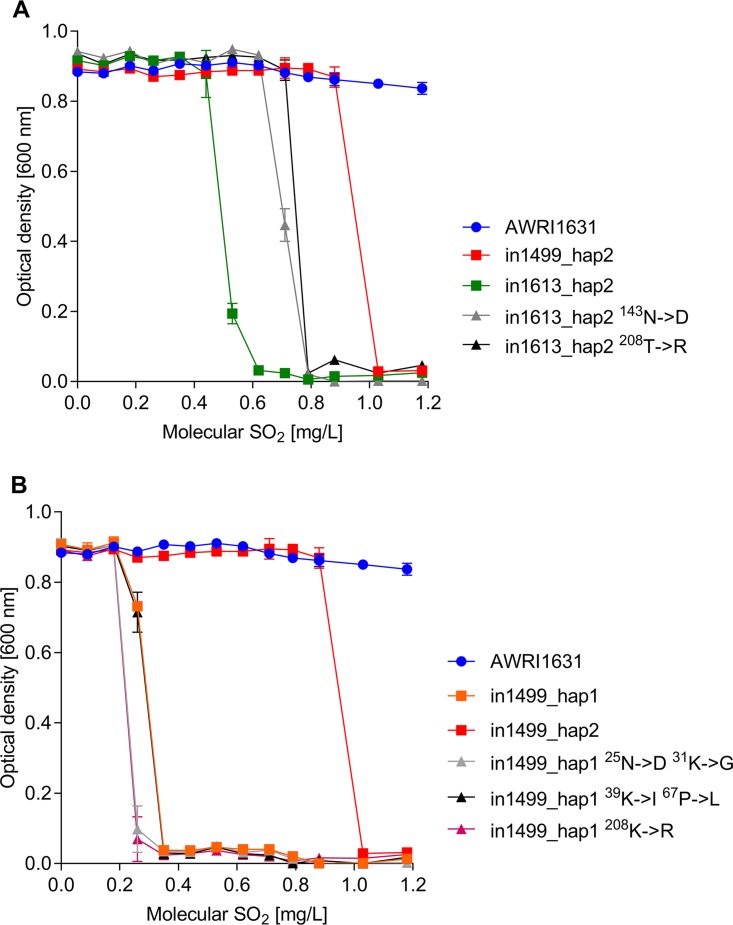
Although B. bruxellensis Ssu1 proteins showed only 8 locations where the amino acid sequence differed, these variations conferred a wide range in sulfite tolerance when expressed in S. cerevisiae. Therefore, we evaluated the effect of some of the amino acid substitution present in B. bruxellensis Ssu1p to identify key amino acids conferring sulfite tolerance. As described above, AWRI1613_SSU1_hap2 conferred a maximum sulfite tolerance to 0.53 mg/liter, whereas AWRI1499_SSU1_hap2 provided yeast growth up to 0.88 mg/liter of SO2 (Fig. 5A). There are two amino acid changes between these haplotypes, Asn143Asp and Thr208Arg (Table 1). We performed single-amino-acid substitutions in AWRI1613_SSU1_hap2 and evaluated the resulting change in sulfite tolerance (Fig. 5A). Amino acid substitution had no impact on maximum OD600, but both Asn143Asp and Thr208Arg caused increased sulfite tolerance compared to that with AWRI1613_SSU1_hap2, providing growth up to 0.71 mg/liter SO2. However, this increase in tolerance did not match the tolerance observed for AWRI1499_SSU1_hap2, indicating that both substitutions are required for maximum tolerance.
FIG 5.
(A) Evaluation of Ssu1p amino acid substitutions between AWRI1613_SSU1_hap2 and AWRI1499_SSU1_hap2. (B) Evaluation of Ssu1p amino acid substitutions between AWRI1499_SSU1_hap1 and AWRI1499_SSU1_hap2. Fisher’s LSD test was used to determine significant differences (P < 0.05).
The two SSU1 haplotypes from B. bruxellensis AWRI1499 vary greatly in their sulfite tolerances; AWRI1499_SSU1_hap1 confers tolerance up to 0.26 mg/liter, whereas AWRI1499_SSU1_hap2 confers tolerance up to 0.88 mg/liter (Fig. 5B). These two haplotypes differ in five amino acids (Table 1). Thus, we performed two double-amino-acid changes to target different sections of the first cytoplasmic domain (Asn25Asp-Lys31Gly and Lys39Ile-Pro67Leu) and a single-amino-acid change targeting the third cytoplasmic domain (Lys208Arg) in AWRI1499_SSU1_hap1. None of the substitutions performed influenced the maximum growth of the strains (Fig. 5B). The double substitution Lys39Ile-Pro67Leu had no effect on sulfite tolerance compared to AWRI1499_SSU1_hap1. However, both the single substitution Lys208Arg and the double substitution Asn25Asp-Lys31Gly decreased sulfite tolerance slightly compared to AWRI1499_SSU1_hap1. These results indicate that the substitutions may be required in conjunction to contribute to the tolerance seen in AWRI1499_SSU1_hap2.
Modeling Ssu1p structure for B. bruxellensis haplotypes.
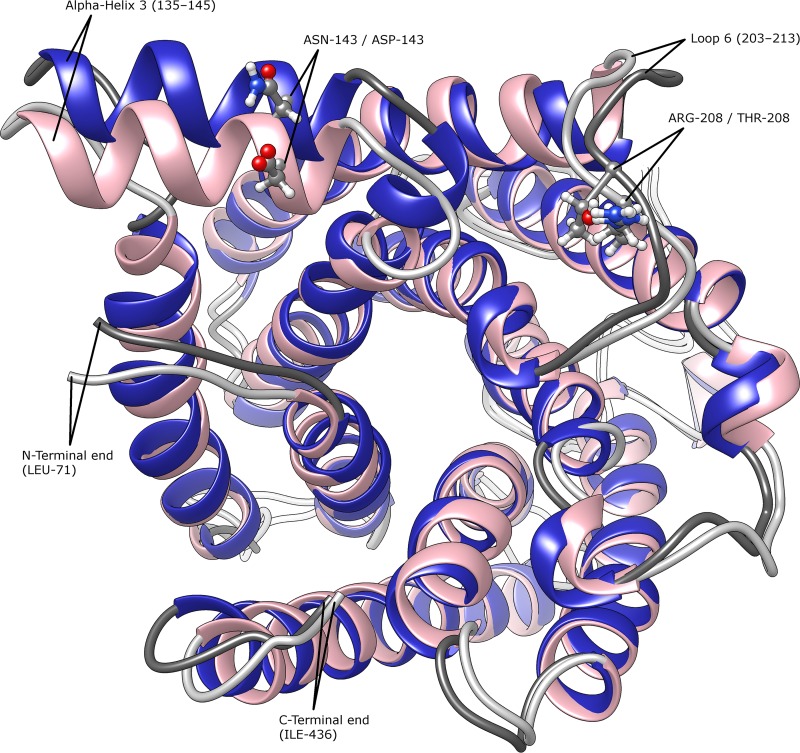
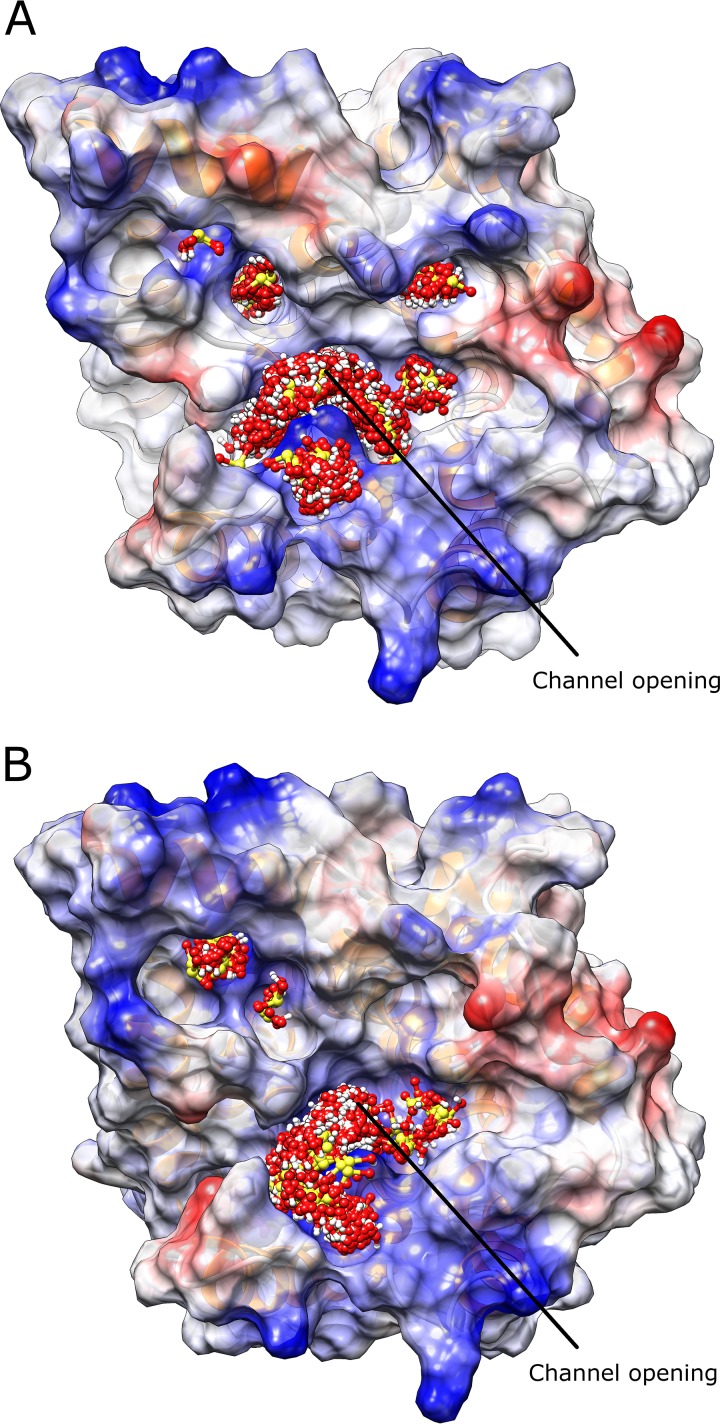
Although a template for modeling AWRI1499_SSU1_hap2 was found in the SWISS-MODEL database, this template lacked structural information for a number of residues. Thus, the homology model for AWRI1499_SSU1_hap2 did not include the N-terminal residues 1 to 70 and the C-terminal residues 367 to 450, and it lacked structural conformation for residues 307 to 346, which comprise a noncytoplasmic domain between transmembrane domains 7 and 8. Nevertheless, the model included most cytoplasmic domains and the entire channel structure. In fact, the model contained sufficient information to estimate structural differences between AWRI1499_SSU1_hap2 and AWRI1613_SSU1_hap2, which differed only by two amino acid residues. Superposition of both protein structures showed a positional shift for alpha-helix 3 (residues 135 to 145) near the active site and a conformation difference for loop 6 in the cytoplasmic region from residues 203 to 213 (Fig. 6). Ligand docking simulations for both structures showed similar binding patterns for SO2 between haplotypes, with ligand conformations clustering tightly in the channel opening, inside the channel, and in several pockets on both sides of the protein (data not shown). However, binding patterns for HSO3– differed substantially. For AWRI1499_SSU1_hap2, most of the ligand conformations are clustered around the positively charged channel opening and three smaller positively charged pockets nearby (Fig. 7A). Compared to AWRI1499_SSU1_hap2, AWRI1613_SSU1_hap2 showed a reduced cluster at the channel opening and only two of the three positively charged pockets near the channel (Fig. 7B). Thus, the Arg208Thr substitution resulted in a relatively neutral pocket avoiding HSO3– binding.
FIG 6.
Superposition of average protein structures for AWRI1499_SSU1_hap2 (pink) and AWRI1613_SSU1_hap2 (blue). Amino acid residues differing between both haplotypes and relevant protein domains are shown.
FIG 7.
HSO3− docking simulations for protein structures of AWRI1499_SSU1_hap2 (A) and AWRI1613_SSU1_hap2 (B). Protein structures are shown from the cytoplasm with protein molecular surface colored by Coulombic electrostatic potential (blue, positive charge; red, negative charge). HSO3− is colored by atom (red, oxygen; white, hydrogen; yellow, sulfur).
DISCUSSION
The addition of SO2 to wine is used to control microbial spoilage both before and after alcoholic fermentation. The ability to tolerate this preservative is an important survival strategy for food and beverage microbes; here, we sought to better understand the mechanisms conferring SO2 tolerance upon wine spoilage yeast B. bruxellensis. Thus, we used a multifaceted approach that investigated differences attributable to variations in both gene expression and protein activity.
Transcriptomic analysis revealed a dynamic response within 2 h of SO2 induced stress, with approximately 500 ORFs/nTARs differentially expressed relative to controls for both AWRI1499 and AWRI1613. This result contrasts with differential expression of only 58 ORFs for S. cerevisiae over the same time frame following SO2 treatment (24) and 30 differentially regulated ORFs for B. bruxellensis Unifg14 samples taken 20 min after SO2 treatment (20). In the study with B. bruxellensis Unifg14, RNA-seq was also performed on samples taken 20 min after removal of the SO2 stress, finding 1,634 differentially expressed ORFs at that time point. Experimental design, growth conditions, and the level of applied stress all contribute to the differences in observations across these studies. Furthermore, we observed significant differences in responses for strains with different genetic backgrounds, something also noted during a global analysis of B. bruxellensis intracellular metabolome when grown in the presence of SO2 (21). Nevertheless, some shared observations can be made. For example, Capozzi et al. (20) observed increased transcription of ORFs related to amino acid transport, and Vigentini et al. (21) found that SO2-treated cells contained higher concentrations of amino acids. Our data for B. bruxellensis AWRI1613 showed that SO2 stress significantly induced the transcription of ORFs involved in carbohydrate metabolism. Park and Hwang (24) found that for S. cerevisiae, 50% of SO2-induced genes belonged to this category, and Capozzi et al. (20) also noted upregulation of several ORFs encoding carbohydrate metabolism proteins for B. bruxellensis Unifg14 during its recovery from SO2 stress. Together, these data suggest that one strategy for yeasts to cope with SO2-induced stress may be to increase carbohydrate metabolism and, potentially as a consequence, metabolic rate, as a compensatory mechanism for ATP depletion (24).
For some strains/experimental setups, the opposite appears to be the case. Proteomic analysis of B. bruxellensis LO2E2 following exposure to SO2 revealed a decreased abundance of proteins involved in glycolysis (25). This result was interpreted as evidence for the entry of B. bruxellensis cells into a viable but nonculturable (VBNC) state under SO2 stress. While we did not observe repression of ORFs/nTARs involved in carbohydrate metabolism for AWRI1499, there was repression of functions important for population growth (e.g., DNA replication). This was also found during initial recovery from SO2-induced stress for B. bruxellensis Unifg14 (20); the authors claimed this as clear evidence for resuscitation of cells from a VBNC state. In our experiment, an ortholog of Pcl1p was among the most repressed ORFs for both AWRI1499 and AWRI1613 following SO2 treatment. In S. cerevisiae, this protein regulates cell cycle progression (19); therefore, if functionally equivalent in B. bruxellensis, this observation provides support for halted population growth as an SO2 survival mechanism.
One of the most studied mechanisms for SO2 tolerance involves the sulfite efflux pump Ssu1p. This protein is a member of the TDT family (23), and it has been mainly studied in S. cerevisiae (17, 18, 26–31). Chromosomal translocations have furnished SO2-tolerant S. cerevisiae strains with altered promoter regions for SSU1, leading to higher expression levels (30). Thus, it was somewhat surprising that AWRI1499 SSU1 transcript abundance was neither inducible nor constitutively higher than that observed for sulfite-sensitive AWRI1613. Induction of SSU1 in the data set of Capozzi et al. (20) was not discussed by the authors, presumably due to its annotation as a membrane transporter. Across all three strains, however, it was apparent that one particular haplotype of BbSSU1 was preferentially expressed.
Four SSU1 haplotypes were identified from three B. bruxellensis strains exhibiting different tolerances to sulfite. The four haplotypes encoded proteins between 438 and 450 amino acids in length and showed 8 sites with amino acid variations. Although BbSsu1p showed some features to those from other sulfite transporters from the TDT family, signature motifs for this family (23) were not evident in B. bruxellensis.
Multicopy expression in a S. cerevisiae SSU1 null mutant enabled us to confirm that each haplotype of ORF AWRI1499_0080 does encode a functional sulfite pump. On the other hand, their ability to confer sulfite tolerance when expressed in single copy varied. Different SSU1 haplotypes have also been described in S. cerevisiae (31, 32), though none of these amino acid variations have been shown to affect sulfite tolerance, nor do they correspond to the substitutions observed in B. bruxellensis haplotypes. Interestingly, when expressed in multicopy, all haplotypes conferred increased sulfite tolerance. These findings have also been observed for multicopy expression of S. cerevisiae SSU1, which increased sulfite resistance between 3 and 8 times (18).
It is worth noting that of the strains studied here, B. bruxellensis AWRI1499 showed the highest sulfite tolerance and exhibited two genomic copies of the strongest haplotype, AWRI1499_SSU1_hap2, and one copy of AWRI1499_SSU1_hap1. B. bruxellensis AWRI1613 showed moderate sulfite tolerance and had one copy each of AWRI1499_SSU1_hap2 and AWRI1613_SSU1_hap2. B. bruxellensis AWRI2804 (haploid) showed the lowest sulfite tolerance and contained a single copy of AWRI2804_SSU1_hap1. Indeed, different gene expression profiles have been observed for SSU1 as a result of variation in promoter activity (29), transcript levels (26), and gene translocations resulting in promoter sequence changes (27, 28, 30). Therefore, it is likely that a combination of haplotype efficiency, copy number, and expression levels are at least partly responsible for the diverse sulfite tolerances observed in different B. bruxellensis strains.
Since structure is key to the function of membrane transporters, we evaluated the effect of different amino acid substitutions on SSu1p structure and their potential impact on sulfite tolerance. The constructed model did not contain structural information about the N-terminal loop where most amino acid substitutions were located; therefore, we could not evaluate the importance of structure across all four haplotypes. However, the model did contain enough information to estimate structural differences between AWRI1499_SSU1_hap2 and AWRI1613_SSU1_hap2. At physiological pH, the two major forms of sulfite are SO2 and HSO3–. Conformational differences between these two haplotypes due to amino acid substitutions resulted in divergent binding patterns for HSO3–, with the Arg208Thr substitution causing the loss of a favorable binding pocket for HSO3– near the channel opening. Docking simulations showed that electrostatic interactions from positively charged residues were crucial for binding HSO3–, suggesting that substitutions which result in the loss of positively charged residues may have a similar detrimental effect on HSO3– binding. Although molecular dynamics simulations indicated that the structural stability of the protein, particularly the highly conserved transmembrane helix-barrel structure, is likely not dependent on unmodeled loop regions, unmodeled loop regions may be responsible for binding HSO3– and therefore play a role on sulfite efflux. Docking simulations for SO2 revealed several high-affinity binding pockets in both the cytoplasmic and extracellular sides of Ssu1p and through the hydrophobic channel. It is possible that after binding to Ssu1p, HSO3– is protonated to SO2 at the channel opening, which then freely moves through the channel to the extracellular space.
In summary, transcriptome data revealed significant differences in transcriptional responses of sulfite-tolerant and sulfite-sensitive strains to this preservative and favored expression of one haplotype of the BbSSU1 transcript. While all four characterized SSU1 haplotypes were confirmed to function as sulfite efflux pumps by expression in S. cerevisiae, they conferred different tolerances to sulfite. The transcriptionally favored haplotype was shown to confer greater tolerance, and through structural modeling and amino acid substitution, this difference in function can most likely be attributed to differential binding of the ligands SO2 and HSO3–. Thus, in B. bruxellensis, a combination of haplotype efficiency, copy number, and haplotype expression levels likely contribute to the diverse sulfite tolerances observed for different strains of this species.
MATERIALS AND METHODS
Yeast strains and growth media.
S. cerevisiae AWRI1631, B. bruxellensis AWRI1499, B. bruxellensis AWRI1613, and B. bruxellensis AWRI2804 were obtained from the Australian Wine Research Institute (AWRI) Wine Microorganism Culture Collection (WMCC). Cryogenically preserved (–80°C) strains were cultured and maintained on YM plates (3 g/liter malt extract, 3 g/liter yeast extract, 5 g/liter peptone, 10 g/liter glucose, 16 g/liter agar) and stored at 4°C. The wine yeast S. cerevisiae AWRI1631 was used as a parental strain for all genetically modified constructs. AWRI1631 is a stable haploid generated by sporulation of a wine yeast and deletion of the HO locus (33).
B. bruxellensis exposure to sulfite under wine-like conditions.
Chemically defined wine medium (CDWM) was prepared as described by Curtin et al. (34), according to the method of Harris et al. (35), with the exception that S. cerevisiae AWRI1631 was used in place of S. cerevisiae PDM for fermentation. Finished wine was adjusted to 12% (vol/vol) ethanol and pH 3.5 using 10 M NaOH. Residual sugar concentration was determined using a glucose-fructose enzymatic assay (Boehringer Mannheim) and then adjusted to 2.5 g/liter glucose and 2.5 g/liter fructose. Adjusted CDWM was then filter sterilized (0.22-μm pore size; Millipore).
Starter cultures of B. bruxellensis strains AWRI1499 and AWRI1613 were prepared by transferring multiple colonies from YM agar into 5 ml YPD medium (10 g/liter yeast extract, 20 g/liter peptone, 20 g/liter glucose) in sterile 14-ml tubes and then incubating at 28°C for 48 h. These starters were used to inoculate adaptation cultures (YPD with 6% ethanol), which were incubated at 28°C for 48 h. Sterile Schott bottles containing 500 ml CDWM were transferred to an anaerobic hood (Coy Laboratories Products, Inc.; 5% [vol/vol] H2, 95% N2, palladium oxygen scrubbing catalyst) maintained at 22°C. After 4 days of equilibration, these were inoculated at 1 × 105 cells/ml from AWRI1499 and AWRI1613 adaptation cultures. Fermentations were shaken daily and sampled periodically for determination of culturable populations and residual sugar concentrations. Culturable populations were quantified by standard dilution and microdrop plating onto YM agar and then incubated for 7 days at 28°C. Samples were also centrifuged and analyzed for residual sugar (glucose and fructose) using an enzymatic kit (Boehringer Mannheim). After 14 days, fermentations were homogenized and dispensed in 50-ml aliquots in sterile Schott bottles. Triplicate cultures of AWRI1499 and AWRI1613 were exposed to a dosage of SO2 known to be sublethal for AWRI1499 (0.4 mg/liter molecular SO2 added as potassium metabisulfite [PMS]) and verified using the aspiration/titration method (36). Samples were taken after 2, 24, 48, 150, and 358 h. RNA was extracted from samples taken at 2 h using the following procedure. Samples containing 5 ml of culture were mixed with 10 ml of cold RNAlater (Life Technologies, Melbourne, Australia). Tubes were then centrifuged for 10 min at 4,000 rpm and 4°C and, after discarding the supernatant, the cell pellets were frozen and stored at –80°C. RNA extraction was performed using the RiboPure-Yeast kit, as described by the manufacturer (Life Technologies). Extracted RNA was quantified using the Quant-iT RiboGreen RNA assay kit (Life Technologies).
RNA-seq analysis.
RNA sequencing (RNA-seq) libraries were prepared using the Illumina TruSeq mRNA kit and sequenced using single-ended 100-bp chemistry (AGRF, Australia, and The Ramaciotti Centre for Genomics, Australia). RNA-seq reads were first mapped to a predicted set of open reading frames (ORFs) using novoalign version 2.07 (Novocraft) using the following parameters: -F ILM1.8, --ILQ_SKIP. Determination of differentially expressed genes between conditions was then performed using cuffdiff version 2.2.1 from the cufflinks package (37), with the parameters -N -u –max-bundle-frags 1 × 107, and using a false-discovery rate cutoff of 0.01.
Pooled RNA-seq reads from all AWRI1499 samples were used to identify 1,038 transcribed regions that were outside the previously annotated open reading frames of AWRI1499 (10). These sequences were visualized and manually marked using Integrated Genome Viewer (IGV) version 2.3 and were designated novel transcriptionally active regions (nTARs). nTARs were annotated using Blast2Go version 2.5 and used to create an updated AWRI1499 annotation file (Table S1).
For each pairwise comparison, a list of statistically significant differentially expressed ORFs/nTARs (false-discovery rate [FDR], ≤0.01) was compiled with the criterion of a ≥2-fold difference in reads per kilobase per million (RPKM). GO term enrichment analyses were conducted using two-tailed Fisher’s exact test in Blast2GO version 2.5, with FDR correction (P < 0.05) and the updated B. bruxellensis AWRI1499 annotation.
Mapped RNA-seq data files (list) from the study by Capozzi et al. (20) were downloaded from BioProject PRJNA318157. SAMtools (version 1.3.1) mpileup and Varscan (version 2.3.9) were used to call SNPs and variant read depth at polymorphic loci across the transcriptome of B. bruxellensis UniFG14, from which the major allele frequency was calculated.
Molecular techniques.
The following three types of gene modifications were performed: (i) deletion of the SSU1 gene, where the ORF for SSU1 was deleted; (ii) SSU1 replacement, where the ORF for SSU1 from B. bruxellensis strains was inserted in the chromosome replacing the native S. cerevisiae SSU1 coding sequence; and (iii) multicopy expression of the B. bruxellensis SSU1 genes, where SSU1 was cloned in a plasmid under the control of a strong constitutive yeast promoter and introduced in S. cerevisiae. All yeast transformations were carried out using the lithium acetate-polyethylene glycol method (38). The strains generated for this study and their genetic modifications are described in Table 2.
TABLE 2.
Plasmids and strains used in this study
| Plasmid or strain | Description or genotypea | Source/reference |
|---|---|---|
| Plasmids | ||
| pAG25 | E. coli replicating plasmid natMX cassette, clonNATr | EURSCARF collection |
| pCV2_BB | E. coli replicating plasmid FBA1p-MCS-PGKt kanMX, construct flanked by EcoRI/SpeI 5′ end and XbaI 3′ end | This study |
| pCV2_BB-1499_hap1 | B. bruxellensis AWRI1499 SSU1 haplotype 1 for chromosomal insertion, kanMX | This study |
| pCV2_BB-1499_hap2 | B. bruxellensis AWRI1499 SSU1 haplotype 2 for chromosomal insertion, kanMX | This study |
| pCV2_BB-1613_hap2 | B. bruxellensis AWRI1613 SSU1 haplotype 2 for chromosomal insertion, kanMX | This study |
| pCV2_BB-2804_hap1 | B. bruxellensis AWRI2804 SSU1 haplotype 1 for chromosomal insertion, kanMX | This study |
| pCV3 | Shuttle vector E. coli/S. cerevisiae FBA1p-MCS-PGKt, kanMX | 49 |
| pCV3-Sc_SSU1 | S. cerevisiae SSU1 under the control of FBA1 promoter, kanMX | This study |
| pCV3-1499_hap1 | B. bruxellensis AWRI1499 SSU1 haplotype 1 under control of FBA1 promoter, kanMX | This study |
| pCV3-1499_hap2 | B. bruxellensis AWRI1499 SSU1 haplotype 2 under control of FBA1 promoter, kanMX | This study |
| pCV3-1613_hap2 | B. bruxellensis AWRI1613 SSU1 haplotype 2 under control of FBA1 promoter, kanMX | This study |
| pCV3-2804_hap1 | B. bruxellensis AWRI2804 SSU1 haplotype 1 under control of FBA1 promoter, kanMX | This study |
| Strains | ||
| AWRI1499 | B. bruxellensis, triploid | AWRI Culture Collection |
| AWRI1613 | B. bruxellensis, diploid | AWRI Culture Collection |
| AWRI2804 | B. bruxellensis, haploid | AWRI Culture Collection |
| AWRI1631 | S. cerevisiae, haploid derivative of commercial wine strain N96, MATa, HOΔ | 33 |
| AWRI1631 ΔSSU1 | ssu1Δ::natMX mutant, clonNATr | This study |
| AWRI1631 in1499_hap1 | ssu1Δ::Bb_AWRI1499_SSU1_hap1-kanMX, Geneticinr | This study |
| AWRI1631 in1499_hap2 | ssu1Δ::Bb_AWRI1499_SSU1_hap2-kanMX, Geneticinr | This study |
| AWRI1631 in1613_hap2 | ssu1Δ::Bb_AWRI1613_SSU1_hap2-kanMX, Geneticinr | This study |
| AWRI1631 in2804_hap1 | ssu1Δ::Bb_AWRI2804_SSU1_hap1-kanMX, Geneticinr | This study |
| AWRI1631 in1499_hap1 25N→D 31K→G | AWRI1631 in1499_hap1 with amino acid substitutions in positions 25 and 31, Geneticinr | This study |
| AWRI1631 in1499_hap1 39K→I 67P→L | AWRI1631 in1499_hap1 with amino acid substitutions in positions 39 and 67, Geneticinr | This study |
| AWRI1631 in1499_hap1 208K→R | AWRI1631 in1499_hap1 with amino acid substitution in position 208, Geneticinr | This study |
| AWRI1631 in1613_hap2 143N→D | AWRI1631 in1499_hap2 with amino acid substitution in position 143, Geneticinr | This study |
| AWRI1631 in1613_hap2 208T→R | AWRI1631 in1499_hap2 with amino acid substitution in position 208, Geneticinr | This study |
| AWRI1631 pCV3 | AWRI1631 carrying plasmid pCV3, Geneticinr | This study |
| AWRI1631 ΔSSU1/pCV3 | AWRI1631 ΔSSU1 carrying plasmid pCV3, Geneticinr | This study |
| AWRI1631 ΔSSU1/pCV3-Sc_SSU1 | AWRI1631 ΔSSU1 carrying plasmid pCV3, Geneticinr | This study |
| AWRI1631 ΔSSU1/pCV3-1499_hap1 | AWRI1631 ΔSSU1 carrying plasmid pCV3-1499_hap1, Geneticinr | This study |
| AWRI1631 ΔSSU1/pCV3-1499_hap2 | AWRI1631 ΔSSU1 carrying plasmid pCV3-1499_hap2, Geneticinr | This study |
| AWRI1631 ΔSSU1/pCV3-1613_hap2 | AWRI1631 ΔSSU1 carrying plasmid pCV3-1613_hap2, Geneticinr | This study |
| AWRI1631 ΔSSU1/pCV3-2804_hap1 | AWRI1631 ΔSSU1 carrying plasmid pCV3-2804_hap1, Geneticinr | This study |
clonNATr, nourseothricin resistant; MCS, multiple-cloning site; Geneticinr, Geneticin resistant.
Deletions were carried out by replacing the targeted SSU1 ORF with either a natMX cassette encoding nourseothricin (clonNAT) resistance. The cassette was PCR amplified from plasmid pAG25 (EURSCARF collection) using primers containing 50-bp flanking regions corresponding to up- and downstream regions outside the ORF. Positive S. cerevisiae transformants were confirmed by both PCR and sequencing of the corresponding gene locus.
DNA sequences for all B. bruxellensis SSU1 genes, two haplotypes from AWRI1499, one from AWRI1613 and one from AWRI2804, as well as modified sequences resulting in amino acids substitutions in Ssu1p (Table 2), were synthesized by Invitrogen GeneArt (Thermo Fisher Scientific, MA, USA). Sequences were codon optimized for their expression in S. cerevisiae and flanked with a NotI restriction site at the 5′ end and an SmaI site at the 3′ end. Synthesized fragments were digested with NotI and SmaI and cloned in pCV2_BB and pCV3 (Table 2). In both plasmids, the SSU1 gene was flanked by the strong yeast promoter FBA1p and the PGK1 terminator. To replace the native S. cerevisiae SSU1 coding sequence, constructs containing the B. bruxellensis SSU1 gene and the kanMX cassette (encoding Geneticin resistance) but not the FBA1 promoter were amplified by PCR from pCV2_BB and introduced in S. cerevisiae by transformation. These constructs were amplified with primers containing 50-bp flanking sequences corresponding to up- and downstream regions outside the S. cerevisiae SSU1 coding sequence. Positive S. cerevisiae transformants were confirmed by both PCR and sequencing of the SSU1 locus. Successful cloning of B. bruxellensis SSU1 genes in pCV3 was confirmed by sequencing. To evaluate the multicopy expression of the B. bruxellensis SSU1 genes, pCV3-derived plasmids (Table 2) were introduced in S. cerevisiae by transformation, and positive transformants were confirmed by PCR.
Sulfite tolerance assay.
A microplate method adapted from a study by Curtin et al. (13) was used to assess sulfite tolerance. For B. bruxellensis, all culturing occurred in 96-well microplates sealed with Breathe-Easy sealing membranes (Diversified Biotech, USA). A 200-μl starter culture was made in YPD medium and incubated at 28°C for 2 days; 5 μl was then subcultured into 195 μl of minimal medium containing 5 g/liter glucose and 6.7 g/liter yeast nitrogen base (YNB [pH 3.5]) with amino acids (MM5; Difco, USA) and grown for 2 days at 28°C. For the tolerance assay, 5 μl of starter culture was inoculated in a 96-well microplate containing 195 μl of MM5 supplemented with different concentrations of a sterile PMS solution (20 g/liter). Plates were incubated at 28°C and the OD600 measured after day 5 using a Tecan Infinite M200 plate reader (Austria). Each strain was replicated up to four times per plate, and experiments were repeated twice.
For S. cerevisiae, starter cultures were made in 2 ml YPD medium and incubated overnight at 28°C with shaking (120 rpm). Cultures were then inoculated into minimal medium containing 20 g/liter glucose and 6.7 g/liter YNB with amino acids (pH 3.5) (MM20) and grown overnight at 28°C with shaking (120 rpm). These cultures were then used for assessing SO2 tolerance, as follows: 5 μl of starter culture was inoculated in a 96-well microplate containing 195 μl of MM20 supplemented with different concentrations of a sterile PMS solution (20 g/liter). Microplates were sealed with a Breathe-Easy sealing membranes and incubated at 28°C. Optical density at 600 nm (OD600) was measured twice daily using a Tecan Infinite M200 plate reader (Austria). Each strain was replicated at least three times per plate, and experiments were repeated twice.
Free SO2 was measured in minimal medium supplemented with freshly prepared PMS using the aspiration/titration method (36); these values were then used to estimate the concentration of molecular SO2. Unless specified, SO2 concentrations mentioned here refer to molecular SO2.
Transmembrane topology prediction.
To estimate if differences in the amino acid sequences of Ssu1p transporters were associated with protein topology, transmembrane domains were predicted using SPOCTOPUS (39). SPOCTOPUS is a method for the combined prediction of signal peptides and membrane protein topology suitable for whole-genome annotation, which has shown the highest accuracy prediction compared to similar methods (40).
Modeling protein structures for B. bruxellensis Ssu1.
A model for AWRI1499_SSU1_hap2 was generated using the SWISS-MODEL Web server (41) and the crystal structure of the plant SLAC1 protein (PDB ID 3M76) as the template structure. The model was checked and positioned in USCF Chimera version 1.11rc (42) prior to running molecular dynamics (MD) simulations. The model was then loaded into the CHARMM-GUI Input Generator’s Membrane Builder (43, 44) to generate the files needed to run MD simulations using GROMACS (45). Membrane composition was modeled based on the lipid content reported for S. cerevisiae (46). K+ and Cl– ions were added to the simulation to neutralize the global charge at the default concentration of 0.15 M. Initial simulations were run for 25 ns, and stable equilibrium was reached after approximately 10 ns. The final frame of the initial MD simulations was exported and used to set up another two 10-ns simulations, one of the unmodified AWRI1499_SSU1_hap2 structure and one with the AWRI1613_SSU1_hap2 structure. Average structures for these two simulations were calculated using USCF Chimera version 1.11rc and used for docking simulations.
SO2 and HSO3– docking simulations.
Docking was performed using RE_DOCK6, a Rigid Exhaustive docking extension for UCSF Dock6.7 (47, 48). Average simulated structures for AWRI1499_SSU1_hap2 and AWRI1613_SSU1_hap2 were prepared for docking using USCF Chimera version 1.11rc, whereas the ligands SO2 and HSO3– were created using ChemAxon MarvinSketch 16.6.13.0. A region encompassing both the cytoplasmic and the extracellular channel openings, as well as the pore channel, was selected for docking simulations. Different orientations for both ligands were then docked to this entire region and scored for binding affinity using the default Grid score for UCSF Dock6.7. The top 10,000 scoring orientations for each ligand enabled a definition of the binding hot spots for both SO2 and HSO3–.
Statistical analysis.
Differences between growth measurements were determined using one-way analysis of variance (ANOVA) and Fisher’s LSD test with the software GraphPad Prism version 6.03. Differences were considered significant when P values were lower than 0.05.
Data availability.
Sequence data for RNA-seq analysis are available under BioProject number PRJNA489698.
Supplementary Material
ACKNOWLEDGMENTS
We thank Robyn Kievit and Toni Cordente for their technical assistance with the transcriptomic experiment.
The AWRI, a member of the Wine Innovation Cluster in Adelaide, is supported by Australia’s grape growers and winemakers through their investment body Wine Australia with matching funds from the Australian Government.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02429-18.
REFERENCES
- 1.Claussen NH. 1904. On a method for the application of Hansen's pure yeast system in the manufacturing of well‐conditioned English stock beers. J Inst Brewing 10:308–331. doi: 10.1002/j.2050-0416.1904.tb04656.x. [DOI] [Google Scholar]
- 2.Morrissey W, Davenport B, Querol A, Dobson A. 2004. The role of indigenous yeasts in traditional Irish cider fermentations. J Appl Microbiol 97:647–655. doi: 10.1111/j.1365-2672.2004.02354.x. [DOI] [PubMed] [Google Scholar]
- 3.Teoh AL, Heard G, Cox J. 2004. Yeast ecology of kombucha fermentation. Int J Food Microbiol 95:119–126. doi: 10.1016/j.ijfoodmicro.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 4.de Souza Liberal A, Basílio A, do Monte Resende A, Brasileiro B, Silva‐Filho D, De Morais J, Simões D, De Morais M. 2007. Identification of Dekkera bruxellensis as a major contaminant yeast in continuous fuel ethanol fermentation. J Appl Microbiol 102:538–547. [DOI] [PubMed] [Google Scholar]
- 5.Laureys D, De Vuyst L. 2014. Microbial species diversity, community dynamics, and metabolite kinetics of water kefir fermentation. Appl Environ Microbiol 80:2564–2572. doi: 10.1128/AEM.03978-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peynaud E, Domercq S. 1956. Sur les Brettanomyces isolés de raisins et de vins. Arch Mikrobiol 24:266–280. doi: 10.1007/BF00419012. [DOI] [PubMed] [Google Scholar]
- 7.Barata A, Caldeira J, Botelheiro R, Pagliara D, Malfeito-Ferreira M, Loureiro V. 2008. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulphur dioxide. Int J Food Microbiol 121:201–207. doi: 10.1016/j.ijfoodmicro.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Conterno L, Joseph CL, Arvik TJ, Henick-Kling T, Bisson LF. 2006. Genetic and physiological characterization of Brettanomyces bruxellensis strains isolated from wines. Am J Enol Vitic 57:139–147. [Google Scholar]
- 9.Blondin B, Ratomahenina R, Arnaud A, Galzy P. 1982. A study of cellobiose fermentation by a Dekkera strain. Biotechnol Bioeng 24:2031–2037. doi: 10.1002/bit.260240910. [DOI] [PubMed] [Google Scholar]
- 10.Curtin CD, Borneman AR, Chambers PJ, Pretorius IS. 2012. De-novo assembly and analysis of the heterozygous triploid genome of the wine spoilage yeast Dekkera bruxellensis AWRI1499. PLoS One 7:e33840. doi: 10.1371/journal.pone.0033840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatonnet P, Dubourdie D, Boidron J-N, Pons M. 1992. The origin of ethylphenols in wines. J Sci Food Agric 60:165–178. doi: 10.1002/jsfa.2740600205. [DOI] [Google Scholar]
- 12.Lattey K, Bramley B, Francis I. 2010. Consumer acceptability, sensory properties and expert quality judgements of Australian Cabernet Sauvignon and Shiraz wines. Aust J Grape Wine Res 16:189–202. doi: 10.1111/j.1755-0238.2009.00069.x. [DOI] [Google Scholar]
- 13.Curtin C, Kennedy E, Henschke PA. 2012. Genotype‐dependent sulphite tolerance of Australian Dekkera (Brettanomyces) bruxellensis wine isolates. Lett Appl Microbiol 55:56–61. doi: 10.1111/j.1472-765X.2012.03257.x. [DOI] [PubMed] [Google Scholar]
- 14.Avramova M, Cibrario A, Peltier E, Coton M, Coton E, Schacherer J, Spano G, Capozzi V, Blaiotta G, Salin F, Dols-Lafargue M, Grbin P, Curtin C, Albertin W, Masneuf-Pomarede I. 2018. Brettanomyces bruxellensis population survey reveals a diploid-triploid complex structured according to substrate of isolation and geographical distribution. Sci Rep 8:4136. doi: 10.1038/s41598-018-22580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avramova M, Vallet-Courbin A, Maupeu J, Masneuf-Pomarède I, Albertin W. 2018. Molecular diagnosis of Brettanomyces bruxellensis’ sulfur dioxide sensitivity through genotype specific method. Front Microbiol 9:1260. doi: 10.3389/fmicb.2018.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divol B, Du Toit M, Duckitt E. 2012. Surviving in the presence of sulphur dioxide: strategies developed by wine yeasts. Appl Microbiol Biotechnol 95:601–613. doi: 10.1007/s00253-012-4186-x. [DOI] [PubMed] [Google Scholar]
- 17.Avram D, Bakalinsky AT. 1997. SSU1 encodes a plasma membrane protein with a central role in a network of proteins conferring sulfite tolerance in Saccharomyces cerevisiae. J Bacteriol 179:5971–5974. doi: 10.1128/jb.179.18.5971-5974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H, Bakalinsky AT. 2000. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 16:881–888. doi:. [DOI] [PubMed] [Google Scholar]
- 19.Espinoza FH, Ogas J, Herskowitz I, Morgan DO. 1994. Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85. Science 266:1388–1391. doi: 10.1126/science.7973730. [DOI] [PubMed] [Google Scholar]
- 20.Capozzi V, Di Toro MR, Grieco F, Michelotti V, Salma M, Lamontanara A, Russo P, Orrù L, Alexandre H, Spano G. 2016. Viable but not culturable (VBNC) state of Brettanomyces bruxellensis in wine: new insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol 59:196–204. doi: 10.1016/j.fm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Vigentini I, Lucy Joseph CM, Picozzi C, Foschino R, Bisson LF. 2013. Assessment of the Brettanomyces bruxellensis metabolome during sulphur dioxide exposure. FEMS Yeast Res 13:597–608. doi: 10.1111/1567-1364.12060. [DOI] [PubMed] [Google Scholar]
- 22.Borneman AR, Zeppel R, Chambers PJ, Curtin CD. 2014. Insights into the Dekkera bruxellensis genomic landscape: comparative genomics reveals variations in ploidy and nutrient utilisation potential amongst wine isolates. PLoS Genet 10:e1004161. doi: 10.1371/journal.pgen.1004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Léchenne B, Reichard U, Zaugg C, Fratti M, Kunert J, Boulat O, Monod M. 2007. Sulphite efflux pumps in Aspergillus fumigatus and dermatophytes. Microbiology 153:905–913. doi: 10.1099/mic.0.2006/003335-0. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Hwang Y-S. 2008. Genome-wide transcriptional responses to sulfite in Saccharomyces cerevisiae. J Microbiol 46:542–548. doi: 10.1007/s12275-008-0053-y. [DOI] [PubMed] [Google Scholar]
- 25.Serpaggi V, Remize F, Recorbet G, Gaudot-Dumas E, Sequeira-Le Grand A, Alexandre H. 2012. Characterization of the “viable but nonculturable” (VBNC) state in the wine spoilage yeast Brettanomyces. Food Microbiol 30:438–447. doi: 10.1016/j.fm.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Goto-Yamamoto N, Kitano K, Shiki K, Yoshida Y, Suzuki T, Iwata T, Yamane Y, Hara S. 1998. SSU1-R, a sulfite resistance gene of wine yeast, is an allele of SSU1 with a different upstream sequence. J Ferment Bioeng 86:427–433. doi: 10.1016/S0922-338X(98)80146-3. [DOI] [Google Scholar]
- 27.Pérez-Ortın JE, Querol A, Puig S, Barrio E. 2002. Molecular characterization of a chromosomal rearrangement involved in the adaptive evolution of yeast strains. Genome Res 12:1533–1539. doi: 10.1101/gr.436602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuasa N, Nakagawa Y, Hayakawa M, Iimura Y. 2004. Distribution of the sulfite resistance gene SSU1-R and the variation in its promoter region in wine yeasts. J Biosci Bioeng 98:394–397. doi: 10.1016/S1389-1723(04)00303-2. [DOI] [PubMed] [Google Scholar]
- 29.Yuasa N, Nakagawa Y, Hayakawa M, Iimura Y. 2005. Two alleles of the sulfite resistance genes are differentially regulated in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 69:1584–1588. doi: 10.1271/bbb.69.1584. [DOI] [PubMed] [Google Scholar]
- 30.Zimmer A, Durand C, Loira N, Durrens P, Sherman DJ, Marullo P. 2014. QTL dissection of lag phase in wine fermentation reveals a new translocation responsible for Saccharomyces cerevisiae adaptation to sulfite. PLoS One 9:e86298. doi: 10.1371/journal.pone.0086298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardi T, Corich V, Giacomini A, Blondin B. 2010. A sulphite-inducible form of the sulphite efflux gene SSU1 in a Saccharomyces cerevisiae wine yeast. Microbiology 156:1686–1696. doi: 10.1099/mic.0.036723-0. [DOI] [PubMed] [Google Scholar]
- 32.Aa E, Townsend JP, Adams RI, Nielsen KM, Taylor JW. 2006. Population structure and gene evolution in Saccharomyces cerevisiae. FEMS Yeast Res 6:702–715. doi: 10.1111/j.1567-1364.2006.00059.x. [DOI] [PubMed] [Google Scholar]
- 33.Borneman AR, Forgan AH, Pretorius IS, Chambers PJ. 2008. Comparative genome analysis of a Saccharomyces cerevisiae wine strain. FEMS Yeast Res 8:1185–1195. doi: 10.1111/j.1567-1364.2008.00434.x. [DOI] [PubMed] [Google Scholar]
- 34.Curtin C, Langhans G, Henschke PA, Grbin PR. 2013. Impact of Australian Dekkera bruxellensis strains grown under oxygen-limited conditions on model wine composition and aroma. Food Microbiol 36:241–247. doi: 10.1016/j.fm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Harris V, Ford CM, Jiranek V, Grbin PR. 2008. Dekkera and Brettanomyces growth and utilisation of hydroxycinnamic acids in synthetic media. Appl Microbiol Biotechnol 78:997–1006. doi: 10.1007/s00253-007-1328-7. [DOI] [PubMed] [Google Scholar]
- 36.Rankine B, Pocock K. 1970. Alkalimetric determination of sulphur dioxide in wine. Aust Wine Brew Spirit Rev 88:40–44. [Google Scholar]
- 37.Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. 2013. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K. 1987. Current protocols in molecular biology. Wiley, New York, NY. [Google Scholar]
- 39.Viklund H, Bernsel A, Skwark M, Elofsson A. 2008. SPOCTOPUS: a combined predictor of signal peptides and membrane protein topology. Bioinformatics 24:2928–2929. doi: 10.1093/bioinformatics/btn550. [DOI] [PubMed] [Google Scholar]
- 40.Reddy A, Cho J, Ling S, Reddy V, Shlykov M, Saier MH. 2014. Reliability of nine programs of topological predictions and their application to integral membrane channel and carrier proteins. J Mol Microbiol Biotechnol 24:161–190. doi: 10.1159/000363506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T. 2014. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Cheng X, Swails JM, Yeom MS, Eastman PK, Lemkul JA, Wei S, Buckner J, Jeong JC, Qi Y, Jo S, Pande VS, Case DA, Brooks CL III, MacKerell AD, Klauda JB, Im W. 2016. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J Chem Theory Comput 12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu EL, Cheng X, Jo S, Rui H, Song KC, Dávila-Contreras EM, Qi Y, Lee J, Monje-Galvan V, Venable RM, Klauda JB, Im W. 2014. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J Comput Chem 35:1997–2004. doi: 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. 2015. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. doi: 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- 46.Zara G, Bardi L, Belviso S, Farris GA, Zara S, Budroni M. 2008. Correlation between cell lipid content, gene expression and fermentative behaviour of two Saccharomyces cerevisiae wine strains. J Appl Microbiol 104:906–914. doi: 10.1111/j.1365-2672.2007.03608.x. [DOI] [PubMed] [Google Scholar]
- 47.Roach M. 2017. mroach-awri/RE_DOCK6 a rigid exhaustive extension for UCSF dock 6. Zenodo; https://zenodo.org/record/439792#.XA82Z_lKi1s. [Google Scholar]
- 48.Allen WJ, Balius TE, Mukherjee S, Brozell SR, Moustakas DT, Lang PT, Case DA, Kuntz ID, Rizzo RC. 2015. DOCK 6: impact of new features and current docking performance. J Comput Chem 36:1132–1156. doi: 10.1002/jcc.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krömer JO, Nunez-Bernal D, Averesch NJ, Hampe J, Varela J, Varela C. 2013. Production of aromatics in Saccharomyces cerevisiae—a feasibility study. J Biotechnol 163:184–193. doi: 10.1016/j.jbiotec.2012.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data for RNA-seq analysis are available under BioProject number PRJNA489698.