Graphical abstract
Keywords: Melanoma, Superparamagnetic iron oxide nanoparticles, Radiosensitization
Abstract
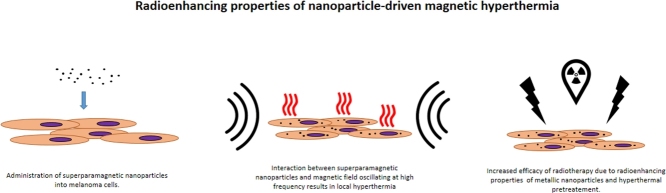
Melanoma is responsible for the majority of deaths related to skin cancer. Worryingly, prognoses show an increasing number of melanoma cases each year worldwide. Radiotherapy, which is a cornerstone of cancer treatment, has proved to be useful but insufficient in melanoma management due to exceptionally high radioresistance of melanoma cells. This problem could be overcome by superparamagnetic iron oxide nanoparticles (SPIONs) used as heat mediators in magnetic hyperthermia, which not only enhance radiosensitivity, but also enable precise targeting by exploitation of their magnetic properties.
1. Melanoma
Melanoma is a type of cancer developed from pigment-containing cells known as melanocytes, which are predominantly located in the skin and eye pupil. In the human skin, melanocytes reside within the basal layer of epidermis and in the hair follicles. Homeostasis of the cutaneous melanocytes is governed by epidermal keratinocytes. Upon exposition to ultraviolet (UV) radiation, keratinocytes produce and secrete factors stimulating melanocytes’ proliferation, differentiation, motility and melanin production. Melanin is a pigment that, due to its chemical and optical properties, plays a key role in skin protection from the detrimental effects of UV-radiation and prevents skin cancer development.
An estimated 76,380 new cases of invasive melanoma will be diagnosed in the US in 2016, from which 10,130 people will die.1 During their lifetime, 1 in 50 men and women will be diagnosed with melanoma of the skin.2 Melanoma is also one of only three cancers with an increasing mortality rate for men.3 About 86% of melanomas can be attributed to exposure to UV-radiation from the sun, the primary risk factor for cutaneous melanoma.4 The most frequently occurring type of the disease is aforementioned cutaneous melanoma, which is responsible for the majority of deaths related to skin cancer (75%).5 Survival rates in patients with melanoma (all forms) have shown considerable discrepancies between countries in Europe, ranging between <50% in Eastern Europe to >90% in Northern and Central Europe for 5-year survival after primary diagnosis.6 Overall, these statistics indicate that melanoma is a significant cause of deaths worldwide. Therefore, melanoma treatment requires major improvement in order to overcome these perilous trends.
Multifactorial disturbance of the homeostatic equilibrium between melanocytes and their microenvironment leads to carcinogenic transformation and melanoma development. Wide spectrum of genetic mutations found in melanomas renders them highly resistant to chemo- and radiotherapy treatment. Interestingly, despite clear UV radiation dependence, mutations found in melanomas are not limited to the ones caused by UV light.7, 8 Metastasis in melanoma is highly frequent and occurs usually at the late stage of the disease development; however, it can also take place during the primary tumor formation. Clinically evident metastasis is a very poor prognostic factor. The 5-year survival rate in patients with metastatic disease is less than 15%. While almost every organ can be potentially affected, the most common sites for metastasis development are the liver, bone and brain.9, 10, 11, 12
2. Radiotherapy in melanoma treatment
Although modality of choice for melanoma treatment is surgery, radiotherapy plays an important role in the disease management and offers a great alternative. Despite being perceived as a significantly radioresistant, a growing number of evidence indicates that radiotherapy treatment of melanoma gives positive results. The proportion of patients benefiting from radiotherapy at some point in their illness was calculated at 23% of all melanoma cases.13 Modes of radiotherapy utilized in melanoma can be divided into four groups: upfront radiotherapy (as a substitute to surgery), adjuvant therapy (post-surgical treatment), elective and palliative radiotherapy. Response of melanoma to irradiation depends on tumor volume, radiotherapy dose and fraction size.14 Moreover, in vitro studies have shown that sublethal doses of ionizing radiation may increase the risk of metastasis.15 Therefore, hypofractionated regimens (high dose of ionizing radiation per fraction) are seemingly more advantageous than hyperfractionated ones (high number of low dose irradiations).16 However, the former regimens introduce a high risk of side effects caused by the concomitant irradiation of normal tissue.17, 18 The degree of radiation injury is related to the total radiation dose, area and volume of tissues irradiated, and the time interval between irradiations. The normal tissue irradiation effects may be classified as acute, consequential, or late effects, according to the time before the appearance of symptoms.19 Regardless of the type of injury, patients’ quality of life is usually significantly diminished. In the worst case scenario it may result in the second primary tumor development (therapy-related cancer that arises as a consequence of the treatment) or even death.20, 21 Therefore, keeping the subtle balance between radiotoxicity and treatment efficacy is the major factor limiting utilization of radiotherapy in melanoma management. In order to obtain more favorable outcome, this equilibrium can be shifted by application of radiosensitizers.
3. Radiosensitization
The main concept behind radiosensitization is based on enhancing the susceptibility of tumor tissue to radiation, resulting in increased efficiency of radiotherapy treatment and decreased radiation dose along with concomitant side effects.22 Basic radiobiological mechanisms of interaction between the cell and radiation involve the creation of the DNA lesions, which may subsequently lead to cell death or senescence. Irradiation may interact with DNA directly or indirectly through reactive oxygen species (ROS) generated in the process of water radiolysis. In brief, an incident beam hits water molecule and causes its dissociation. In a series of chemical reactions, products of dissociation produce highly active compounds, such as hydrogen radical H•, hydroxyl radical OH•, superoxides O2− and charged water species, such as H2O+ and H2O2+.23, 24 In the next step, produced radicals interact with intracellular environment creating a wide variety of lesions including DNA damage.25
3.1. Nanoparticles as radiosensitizers
Application of nanoparticles as radiosensitizers becomes a field that draws steadily increasing attention.26 Nanoparticles are defined as particles whose size does not exceed 100 nm in diameter. Such small dimensions result in drastically improved tissue penetration and cellular uptake due to the enhanced permeability and retention (EPR) effect, providing a preferential accumulation within tumors. This selective bio distribution facilitates the acquiring of the high NPs concentration within tumor, minimizing the risk of involvement of the normal tissue.27, 28 Moreover, physicochemical properties of NPs can be tailored in detail by size and shape modification, creation of core/shell structures and functionalization of their surface, according to specific needs.29, 30, 31, 32
Due to specific interactions with ionizing radiation, metallic NPs are in the majority of NPs considered as potent radiosensitizers. The mechanism of radioenhancement depends on the beam energy and the type of metallic particle. The most important physical effects occurring as a result of X-ray/nanoparticle interaction are: photon scattering, photoelectron emission, Compton scattering, Auger emission and fluorescent photon emission. In principle, the incident radiation wave transfers its energy to an electron within the atom. If the transferred energy is higher than the binding energy, the electron is ejected from its orbital with a kinetic energy equal to the initial wave energy minus the binding energy. The emitted electron is called photoelectron. Most photoelectric interactions occur in the K shell because the density of the electron cloud is greater in this region and there is a higher probability of interaction. In the case of Compton scattering, the radiation wave energy is lower than the binding energy of an electron. When the radiation hits, an incident electron recoils and deflects the wave decreasing its energy. Production of the Auger electrons or fluorescent photons occurs as a result of electron ejection. Generated orbital deficiency causes higher energy electrons to fill the vacancy. The excess of energy caused by this replacement may either be released as fluorescent photons or transferred to another electron, leading to secondary electron ejection (Auger electrons). The derivative biological effects appear as the result of previously described ROS generation and DNA damage introduction.33
Efficiency of photoelectric effect is determined by (Z/E)3 formula where Z is the atomic number and E is the energy of the incoming photon. Due to the high Z number and good biocompatibility, it is gold nanoparticles (GNPs)34, 35 that are the most widely studied. However, to obtain significant dose enhancement, relatively large doses of GNPs are needed, presenting a challenge to biodistribution and delivery. To overcome this obstacle and expand the applicability, subsequent functionalization with targeting molecules is required.36 Although iron Z number is lower in comparison to gold (iron Z = 56, gold Z = 79), the utility of superparamagnetic iron oxide nanoparticles (SPIONs) as a potent radioenhancer has been proved.37, 38 Moreover, SPIONs provide a wide variety of additional properties, which make them a very promising tool in radioenhancement. Their unique physicochemical and magnetic characteristics have been already exploited in many applications, for example, in MRI as a contrast agent, in targeted drug delivery, in hyperthermia for cancer treatment, etc.31, 32, 35, 39, 40, 41, 42, 43
4. Magnetic hyperthermia
Magnetic hyperthermia in cancer treatment has been widely investigated for over a decade. Magnetic hyperthermia has been clinically tested and proved efficient in combination with radiotherapy as a treatment of recurring glioblastoma multiforme and recurring breast cancer.44, 45, 46 Magnetic hyperthermia is generated by magnetic nanoparticles placed in an alternating magnetic field (AMF) operating at high frequencies. Heat production occurs via three distinct mechanisms: Brownian relaxation, Néel relaxation and hysteresis loss. In Brownian relaxation, thermal energy is generated by rotation of the nanoparticle and shear stress induced in the environment. In Néel relaxation, the particle position remains fixed, while magnetic moment rotates. Thermal energy dissipates due to the rearrangement of atomic dipol moments within the crystal lattice. Hysteresis loss occurs mainly in particles with multiple magnetic domains or in large single domain particles (>100 nm). From the perspective of the 15 nm SPIONs, the dominant modes of heat generation will be Brownian and Néel relaxation. The efficiency of heat generation is described by specific absorption rate [W/g] (SAR). SAR depends on many factors such as: magnetic field amplitude and frequency, nanoparticles’ size, shape, crystallinity, concentration, aggregation, environment viscosity, etc. Therefore, theoretical predictions of SAR values require experimental verification.47 Effects of magnetic hyperthermia depend on applied thermal dose. Mild hyperthermia (∼40 °C) affects cellular components altering the structure of phospholipids, proteins and nucleic acids, which hinders their ability to perform functions and ultimately leads to cell death. Increasing temperature above 45 °C leads to protein denaturation and thermal ablation. Interestingly, cytotoxic effects have been observed without noticeable increase in temperature.48, 49 Magnetic hyperthermia has been shown to elevate production of ROS, which also contributes to cytotoxic effects.50 Considering magnetic hyperthermia at the tissue level, studies showed that the application of magnetic hyperthermia significantly increased oxygenation of cancer cells, which directly contributes to enhancement of radiosensitivity. Taking into account all biological aspects of magnetic hyperthermia, there is a very high probability for the combinatorial approach to be successful.
5. Superparamagnetic iron oxide nanoparticles
5.1. Synthesis
The synthesis of SPIONs could be done using several methods: co-precipitation, hydrothermal method and thermal decomposition. Co-precipitation is a simple, high-yield and, therefore, popular method, in which an aqueous solution mixture containing ferric or ferrous slats is precipitated using a base of pH 8–14 at elevated temperature of 70–90 °C in the absence of oxygen. The functionalization of particles after synthesis is not needed, because of their hydrophilic character. Although because of an inability to properly separate nucleation and growth, the size distribution of the particles is rather wide.51 The nanoparticles synthesized with this method also lack crystallinity.52 On the other hand, the hydrothermal method involves dissolving iron precursors with capping agents in an aqueous solution and placing the mixture in an autoclave in high temperature and high pressure conditions. In order to obtain metal oxides addition of NaOH, urea or ammonia is usually needed.53 Size, shape and magnetic properties can be adjusted by manipulation of temperature, reaction time and the precursor/capping agent ratio. This time-consuming method yields moderate crystallinity of obtained SPIONs.51 The most sophisticated method allowing synthesis of highly crystalline, monodispersed, uniform in size and shape nanoparticles is thermal decomposition. It involves the decomposition of organic iron compounds, e.g. iron oleate or iron acetyloacetonate with a non-polar solvent in the presence of a capping agent acting as a stabilizing factor. The iron salt is heated up to the boiling temperature of the solvent and kept in this temperature for a desired amount of time. Because of perfect separation of nucleation and growth processes, thermal decomposition of iron salts enables fine size tuning, narrow size-distribution and scalability. Simple one-pot, cost-efficient synthesis allows to obtain gram amounts of monodispersed NPs.54 SPIONs obtained using thermal decomposition are hydrophilic, although they can be converted into water-soluble particles by either ligand exchange or bilayer surfactant stabilization methods.51
5.2. Physicochemical properties
Magnetic properties of SPIONs depend strongly on their composition and size. Magnetite (Fe3O4) and maghemite (γ-Fe2O3) have superior magnetic properties in comparison to wüstite (FeO) and hematite (α-Fe2O3), thus being the most desirable constituents of SPIONs. Superparamagnetism is a phenomenon defined as a magnetization upon application of an external magnetic field. In the presence of magnetic field, SPIONs become magnetized and act as a ferromagnetic material. When the field is removed, no residual magnetization is observed. This property is size-dependent and can be observed in particles of up to 20 nm in diameter. Below this size, each particle acts as a single domain or a single spin. In comparison, bulk magnets contain many domains with spins aligned in one direction. This phenomenon provides exceptionally high magnetic susceptibility.55, 56
Surface chemistry of SPIONs provides a broad spectrum of modifications that can be easily implemented and utilized in many applications. Surface design allows for fine tuning of such crucial parameters as bioavailability, toxicity, cellular uptake and retention, biodistribution, stability, etc. There is a plethora of scientific articles providing protocols and in-depth analyses of many different coatings, for example, dextran, polyethylene glycol (PEG), polyvinyl alcohol (PVA), or functional groups, such as thiols, amines or carboxyls.57, 58, 59, 60
5.3. Biological properties
Toxicity and cytotoxicity of SPIONs is strongly dependent on their size, type of coating and concentration.61 In several studies, a wide range of surface coatings have been tested to prove that toxic effects of SPIONs can be diminished to a great extent.62 The mechanism of cellular uptake is determined by particles’ size and coating. The most frequently postulated internalization pathway is endocytosis.63, 64 However, the exact mechanism has been shown to be cell-type dependent.65, 66, 67 SPIONs that are in the size range between 10 and 100 nm are considered to be optimal for intravenous administration, whereas particles >200 nm and <10 nm are sequestered by the spleen or removed through renal clearance, respectively.68 After intravenous administration in rats, the particles were cleared out by the mononuclear phagocyte system. Internalized NPs were subsequently degraded in the lysosomal compartment. The highest concentrations of SPIONs can be observed in the Kupffer cells, the liver and reticuloendothelial system of the spleen.69, 70 Iron ions released by NPs decomposition are added to the physiological iron pool and utilized in the production of hemoglobin and other metabolic processes. Nonetheless, the intracellular increase of unbound iron leads to the increased levels of ROS and may result in cell injury or death. Moreover, high levels of Fe may also trigger pro-inflammatory response, epigenetic events and DNA damage.63
6. Summary and outlook
Despite many postulated adverse effects, magnetic properties of SPIONs allow a very precise targeting, thus limiting undesirable activity to minimum. Moreover, cutaneous localization of melanoma enables direct administration of SPIONs, diminishing the risk of side effects even further. Interestingly, cells loaded with SPIONs have shown substantially decreased motility, which may, in theory, reduce the risk of metastasis.71, 72
In summary, combination of SPIONs-mediated magnetic hyperthermia and radiotherapy may provide an innovative tool for human cutaneous melanoma treatment. Bimodal treatment utilizing SPION driven hyperthermia and radiotherapy stands as yet unexplored path for melanoma therapy. Synergistic effect of the proposed treatment may provide a new option for cancer management extending beyond melanoma. Moreover, surface chemistry of SPION allows for further development of this strategy by additional functionalization with therapeutic agents.
Conflict of interest
None declared.
Financial disclosure
This work was supported by UMO-2016/23/B/NZ7/01288 grant from National Science Centre (Poland).
Acknowledgments
The author would like to thank Adam Mieloch, Dr. Wiktoria Suchorska and Prof. Dr. Michael Giersig for their advice and comments. This work was supported by UMO-2016/23/B/NZ7/01288 grant from National Science Centre (Poland).
Footnotes
Article from the Special Issue on Nanoparticle and Immunotherapy.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.SEER . National Cancer Institute; 2011. Cancer statistics review 1975–2009; pp. 1992–2009. [Google Scholar]
- 3.Jemal A., Siegel R., Xu J. Cancer statistics, 2010. CA Cancer J Clin. 2009;59:1–25. [Google Scholar]
- 4.Parkin D.M. 10. Cancers attributable to exposure to hormones in the UK in 2010. Br J Cancer. 2011;105(Suppl):S42–S48. doi: 10.1038/bjc.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schadendorf D., Hauschild A. Melanoma in 2013: Melanoma—the run of success continues. Nat Rev Clin Oncol. 2014;11:75–76. doi: 10.1038/nrclinonc.2013.246. [DOI] [PubMed] [Google Scholar]
- 6.De Angelis R., Sant M., Coleman M.P. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 7.Satyamoorthy K., Herlyn M. Cellular and molecular biology of human melanoma. Cancer Biol Ther. 2002;1:14–17. doi: 10.4161/cbt.1.1.32. [DOI] [PubMed] [Google Scholar]
- 8.TCGA Genomic Classification of Cutaneous Melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damsky W.E., Theodosakis N., Bosenberg M. Melanoma metastasis: new concepts and evolving paradigms. Oncogene. 2014;33:2413–2422. doi: 10.1038/onc.2013.194. [DOI] [PubMed] [Google Scholar]
- 10.Tas F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J Oncol. 2012;2012:1–9. doi: 10.1155/2012/647684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowak-Sadzikowska J., Walasek T., Jakubowicz J. Current treatment options of brain metastases and outcomes in patients with malignant melanoma. Rep Pract Oncol Radiother. 2016;21:271–277. doi: 10.1016/j.rpor.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kycler W., Grodecka-Gazdecka S., Bręborowicz J. Prognostic factors in melanoma. Rep Pract Oncol Radiother. 2006;11:39–48. [Google Scholar]
- 13.Delaney G., Barton M., Jacob S. Estimation of an optimal radiotherapy utilization rate for gastrointestinal carcinoma: a review of the evidence. Cancer. 2004;101:657–670. doi: 10.1002/cncr.20443. [DOI] [PubMed] [Google Scholar]
- 14.Strojan P. Role of radiotherapy in melanoma management. Radiol Oncol. 2010;44:1–12. doi: 10.2478/v10019-010-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rofstad E.K., Mathiesen B., Galappathi K. Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res. 2004;64:13–18. doi: 10.1158/0008-5472.can-03-2658. [DOI] [PubMed] [Google Scholar]
- 16.Stevens G., Thompson J.F., Firth I. Locally advanced melanoma: results of postoperative hypofractionated radiation therapy. Cancer. 2000;88:88–94. doi: 10.1002/(sici)1097-0142(20000101)88:1<88::aid-cncr13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez R.J., Kudchadkar R., Rao N.G. Adjuvant immunotherapy and radiation in the management of high-risk resected melanoma. Ochsner J. 2011;10:108–116. [PMC free article] [PubMed] [Google Scholar]
- 18.Piotrowski I., Kulcenty K., Suchorska W.M. Carcinogenesis induced by low-dose radiation. Radiol Oncol. 2017;51:369–377. doi: 10.1515/raon-2017-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodemann H.P., Blaese M.A. Responses of normal cells to ionizing radiation. Semin Radiat Oncol. 2007;17:81–88. doi: 10.1016/j.semradonc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Bentzen S.M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 21.Allan J.M., Travis L.B. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5:943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 22.Muschel R.J., Soto D.E., McKenna W.G. Radiosensitization and apoptosis. Oncogene. 1998;17:3359–3363. doi: 10.1038/sj.onc.1202580. [DOI] [PubMed] [Google Scholar]
- 23.Laverne J.A., Laverne J.A. OH radicals and oxidizing products in the gamma radiolysis of water. Radiat Res. 2000;153:196–200. doi: 10.1667/0033-7587(2000)153[0196:oraopi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Mieloch A.A., Suchorska W.M. The concept of radiation-enhanced stem cell differentiation. Radiol Oncol. 2015;49:209–216. doi: 10.1515/raon-2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desouky O., Din N., Zhou G. Targeted and non-targeted effects of ionizing radiation. J Radiat Res Appl Sci. 2015;8:1–8. [Google Scholar]
- 26.Babaei M., Ganjalikhani M. The potential effectiveness of nanoparticles as radio sensitizers for radiotherapy. BioImpacts. 2014;4:15–20. doi: 10.5681/bi.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agents Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 28.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164:138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 29.Sun T., Zhang Y.S., Pang B. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 30.Haracz S., Hilgendorff M., Rybka J.D. Effect of surfactant for magnetic properties of iron oxide nanoparticles. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms. 2015;364 [Google Scholar]
- 31.Mieloch A.A., Krȩcisz M., Rybka J.D. The influence of ligand charge and length on the assembly of Brome mosaic virus derived virus-like particles with magnetic core. AIP Adv. 2018;8 [Google Scholar]
- 32.Wierzbinski K.R., Szymanski T., Rozwadowska N. Potential use of superparamagnetic iron oxide nanoparticles for in vitro and in vivo bioimaging of human myoblasts. Sci Rep. 2018:8. doi: 10.1038/s41598-018-22018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwatra D., Venugopal A., Anant S. Nanoparticles in radiation therapy: a summary of various approaches to enhance radiosensitization in cancer. Transl Cancer Res. 2013;2:330–342. [Google Scholar]
- 34.Hainfeld J.F., Slatkin D.N., Smilowitz H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 35.Mesbahi A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Rep Pract Oncol Radiother. 2010;15:176–180. doi: 10.1016/j.rpor.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper D.R., Bekah D., Nadeau J.L. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front Chem. 2014;2:1–13. doi: 10.3389/fchem.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roeske J.C., Nunez L., Hoggarth M. Characterization of the theoretical radiation dose enhancement from nanoparticles. Technol Cancer Res Treat. 2007;6:395–401. doi: 10.1177/153303460700600504. [DOI] [PubMed] [Google Scholar]
- 38.Klein S., Sommer A., Distel L.V.R. Superparamagnetic iron oxide nanoparticles as radiosensitizer via enhanced reactive oxygen species formation. Biochem Biophys Res Commun. 2012;425:393–397. doi: 10.1016/j.bbrc.2012.07.108. [DOI] [PubMed] [Google Scholar]
- 39.Li L., Jiang W., Luo K. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3:595–615. doi: 10.7150/thno.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun C., Lee J., Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laurent S., Dutz S., Häfeli U.O. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23. doi: 10.1016/j.cis.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Tran N., Webster T.J. Magnetic nanoparticles: biomedical applications and challenges. J Mater Chem. 2010;20:8760. [Google Scholar]
- 43.Giersig M., Hilgendorff M. Magnetic nanoparticle superstructures. Eur J Inorg Chem. 2005:3571–3583. [Google Scholar]
- 44.Frank K.M., Dirk U. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodis S. Hyperthermia and radiotherapy in loco-regional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol • Biol • Phys. 2016 doi: 10.1016/j.ijrobp.2015.12.361. [DOI] [PubMed] [Google Scholar]
- 46.Bañobre-López M., Teijeiro A., Rivas J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep Pract Oncol Radiother. 2013;18:397–400. doi: 10.1016/j.rpor.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deatsch A.E., Evans B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J Magn Magn Mater. 2014;354:163–172. [Google Scholar]
- 48.Torres-lugo M., Rinaldi C., Creixell M. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano. 2011:7124–7129. doi: 10.1021/nn201822b. [DOI] [PubMed] [Google Scholar]
- 49.Hilger I. In vivo applications of magnetic nanoparticle hyperthermia. Int J Hyperthermia. 2013;6736:828–834. doi: 10.3109/02656736.2013.832815. [DOI] [PubMed] [Google Scholar]
- 50.Wydra R.J., Rychahou P.G., Evers B.M. The role of ROS generation from magnetic nanoparticles in an alternating magnetic field on cytotoxicity. Acta Biomater. 2016:284–290. doi: 10.1016/j.actbio.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kandasamy G., Maity D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int J Pharm. 2015;496:191–218. doi: 10.1016/j.ijpharm.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 52.Marinin A. 2012. Synthesis and Characterization of Superparamagnetic Iron Oxide Nanoparticles Coated With Silica. [Google Scholar]
- 53.Liang H., Chen W., Yao Y. Hydrothermal synthesis, self-assembly and electrochemical performance of α-Fe2O3 microspheres for lithium ion batteries. Ceram Int. 2014;40:10283–10290. [Google Scholar]
- 54.Hufschmid R., Arami H., Ferguson R.M. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale. 2015;7:11142–11154. doi: 10.1039/c5nr01651g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–3471. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haracz S., Mrõz B., Rybka J.D. Magnetic behaviour of non-interacting colloidal iron oxide nanoparticles in physiological solutions. Cryst Res Technol. 2015:50. [Google Scholar]
- 57.Petri-Fink A., Chastellain M., Juillerat-Jeanneret L. Development of functionalized superparamagnetic iron oxide nanoparticles for interaction with human cancer cells. Biomaterials. 2005;26:2685–2694. doi: 10.1016/j.biomaterials.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 58.Fresnais J., Yan M., Courtois J. Poly(acrylic acid)-coated iron oxide nanoparticles: quantitative evaluation of the coating properties and applications for the removal of a pollutant dye. J Colloid Interface Sci. 2013;395:24–30. doi: 10.1016/j.jcis.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Tassa C., Shaw S.Y., Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011;44:842–852. doi: 10.1021/ar200084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y.J., Tao J., Xiong F. Characterization and in vitro cellular uptake of PEG coated iron oxide nanoparticles as MRI contrast agent. Pharmazie. 2010;65:481–486. [PubMed] [Google Scholar]
- 61.Krȩcisz M., Rybka J.D., Strugała A.J. Interactions between magnetic nanoparticles and model lipid bilayers – Fourier transformed infrared spectroscopy (FTIR) studies of the molecular basis of nanotoxicity. J Appl Phys. 2016:120. [Google Scholar]
- 62.Gupta K., Curtis S. Surface modified superparamagnetic nanoparticles for drug delivery: interaction studies with human fibroblasts in culture. J Mater Sci Mater Med. 2004;15:493–496. doi: 10.1023/b:jmsm.0000021126.32934.20. [DOI] [PubMed] [Google Scholar]
- 63.Singh N., Jenkins G.J.S., Asadi R. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION) Nano Rev. 2010;1:1–16. doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luther E.M., Petters C., Bulcke F. Endocytotic uptake of iron oxide nanoparticles by cultured brain microglial cells. Acta Biomater. 2013;9:8454–8465. doi: 10.1016/j.actbio.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 65.Petters C., Irrsack E., Koch M. Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem Res. 2014:1648–1660. doi: 10.1007/s11064-014-1380-5. [DOI] [PubMed] [Google Scholar]
- 66.Gu J.L., Xu H.F., Han Y.H. The internalization pathway, metabolic fate and biological effect of superparamagnetic iron oxide nanoparticles in the macrophage-like RAW264.7 cell. Sci China Life Sci. 2011;54:793–805. doi: 10.1007/s11427-011-4215-5. [DOI] [PubMed] [Google Scholar]
- 67.Xu H., Dai W., Han Y. Differential internalization of superparamagnetic iron oxide nanoparticles in different types of cells. J Nanosci Nanotechnol. 2010;10:7406–7410. doi: 10.1166/jnn.2010.2830. [DOI] [PubMed] [Google Scholar]
- 68.Elias A., Tsourkas A. Imaging circulating cells and lymphoid tissues with iron oxide nanoparticles. Hematol Am Soc Hematol Educ Program. 2009:720–726. doi: 10.1182/asheducation-2009.1.720. [DOI] [PubMed] [Google Scholar]
- 69.Induces P., Stress O., Human I.N. Iron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolism. Science (80-) 2002;33:1527–1533. [Google Scholar]
- 70.Briley-Saebo K., Bjørnerud A., Grant D. Hepatic cellular distribution and degradation of iron oxide nanoparticles following single intravenous injection in rats: implications for magnetic resonance imaging. Cell Tissue Res. 2004;316:315–323. doi: 10.1007/s00441-004-0884-8. [DOI] [PubMed] [Google Scholar]
- 71.Diana V., Bossolasco P., Moscatelli D. Dose dependent side effect of superparamagnetic iron oxide nanoparticle labeling on cell motility in two fetal stem cell populations. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0078435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cromer Berman S.M., Kshitiz, Wang C.J. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn Reson Med. 2013;69:255–262. doi: 10.1002/mrm.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]