Abstract
Climate change is increasingly exposing populations to rare and novel environmental conditions. Theory suggests that extreme conditions will expose cryptic phenotypes, with a concomitant increase in trait variation. Although some empirical support for this exists, it is also well established that physiological mechanisms (e.g. heat shock protein expression) change when organisms are exposed to constant versus fluctuating temperatures. To determine the effect of common, rare and novel temperatures on the release of hidden variation, we exposed fathead minnows, Pimephales promelas, to five fluctuating and four constant temperature regimes (constant treatments: 23.5, 25, 28.5 and 31°C; all fluctuating treatments shared a minimum temperature of 22°C at 00.00 and a maximum of 25, 28, 31, 34 or 37°C at 12.00). We measured each individual's length weekly over 60 days, critical thermal maximum (CTmax), five morphometric traits (eye anterior–posterior distance, pelvic fin length, pectoral fin length, pelvic fin ray count and pectoral fin ray count) and fluctuating asymmetry (FA, absolute difference between left and right morphometric measurements; FA is typically associated with stress). Length-at-age in both constant and fluctuating conditions decreased with temperature, and this trait's variance decreased with temperature under fluctuating conditions but increased and then decreased in constant temperatures. CTmax in both treatments increased with increasing water temperature, while its variance decreased in warmer waters. No consistent pattern in mean or variance was found across morphometric traits or FA. Our results suggest that, for fathead minnows, variance can decrease in important traits (e.g. length-at-age and CTmax) as the environment becomes more stressful, so it may be difficult to establish comprehensive rules for the effects of rarer or stressful environments on trait variation.
This article is part of the theme issue ‘The role of plasticity in phenotypic adaptation to rapid environmental change’.
Keywords: cryptic, hidden, temperature, fathead minnow
1. Introduction
Earth is experiencing vast changes in both spatial and temporal patterns of climate variables such as temperature, wind and precipitation. The mean combined land and ocean surface temperature has increased 0.85°C between 1880 and 2012 [1]. In addition, precipitation [2], sea level [3], ice cover [4] and frequency of severe weather [5] are all changing at an unprecedented rate.
These changes are, in turn, having widespread effects in ecosystems, mediated by the effect of climatic variables on the physiology of organisms [6]. Geographical range [7] and migration pattern shifts [8], increased variability in population abundance through time [9] and modification of species interactions [10] are only some of the observed consequences. For these reasons, the ability to cope with future environmental changes will be crucial to the survival of many species [11,12]. In particular, how species respond to changes in temperature, one of the most consequential abiotic variables (especially for ectotherms), will, in large part, determine their fate [13].
Although climate change is often discussed in terms of averages (e.g. increase in projected mean temperature), the increasing prevalence of extreme temperatures is also quite apparent. Increases in variance have already been observed and are predicted to become further exacerbated [1]. In fact, a complete departure from current temperature regimes is projected to have happened by 2047 (assuming a stay-the-course scenario; [14]). Periods of extreme temperature can have a disproportionate impact on the mortality and morbidity of plants and animals, yet are not necessarily well accounted for with means [15–17]. Thus, many populations will be increasingly challenged by rare and novel environmental conditions [18–20].
Some theory suggests that natural selection acts to canalize phenotypes in commonly faced environments [21,22], but rare and novel conditions could expose hidden phenotypes, with a concomitant increase in trait variation [22–24]. Variation is, of course, a critical component of the adaptive capacity of a population, as the rate of evolution is proportional to the additive genetic variance [25]. Increased trait variation can lead to significant ecological [26–28] and evolutionary changes [29–33], and the release of this variation in extreme environments has been suggested to be an important evolutionary process [34–36]. In yellow dung flies, for instance, exposure to heat stress led to an increase in variance in the number of sperm storage organs in females [37]. The expression of cryptic variation has also been shown to increase in experimental brown trout populations exposed to novel pH levels [38], and previously unencountered diet conditions are likely to have released variation and driven the evolutionary transition from omnivory to carnivory in spadefoot toad tadpoles [30]. More examples are summarized in [35,39].
However, it is unclear whether the theoretical prediction of increased variance in novel environments occurs consistently (i.e. whether reaction norms really ‘fan out’ as predicted; [40–42]). For instance, if optimal conditions are required to produce a fully expressed phenotype, then stressful environments will only induce a subset of potential phenotypes, thereby decreasing variation [40]. Studies on Drosophila revealed increases in the variance of some traits and decreases in others when exposed to stressful environments [43]. In a survey of 247 studies, Hollander & Bourdeau [44] found that organisms exposed to native predators (thus experiencing a ‘common’ environment) showed significantly more plasticity than those exposed to introduced (novel) ones. Yet another recent meta-analysis arrived at a similar conclusion, finding mixed evidence for the release of variation in rare or novel environments (increased coefficients of variation for life-history traits but not for morphological traits under highly stressful environments; [45]).
Additionally, almost all work on phenotypic variation in novel conditions is conducted under constant environments (but see [46]). Although simpler from an experimental standpoint, important differences exist when individuals are subjected to fluctuating conditions (reviewed in [47,48]). Growth, development and thermal tolerance in many ectotherms can differ significantly when exposed to daily thermal fluctuations versus a constant environment with the same mean temperature (e.g. [49,50]). For example, daily fluctuating temperatures can delay embryonic development in a longhorned beetle [51] and can increase the upper thermal tolerance limit in zebrafish [52] relative to that of constant temperatures. Not surprisingly, failing to incorporate thermal variability can seriously bias predictions of species' responses to climate change [47,53].
Here, we use the fathead minnow, Pimephales promelas, to explore the interaction of these two phenomena in thermal biology: the effects of novel temperatures and of daily thermal variation on life-history and morphological traits. We reared these fish under constant (23.5, 25, 28.5 and 31°C) and fluctuating (22–25, 22–28, 22–31, 22–34 and 22–37°C) temperature environments that ranged from common to exceptionally hot. We then quantified variation in length-at-age, critical thermal maximum (CTmax), meristic and morphometric characters, and fluctuating asymmetry (FA). We hypothesized that fluctuating temperatures will result in improved performance at more stressful temperatures (when compared with fish in constant conditions), and that fluctuating thermal regimes will result in higher levels of phenotypic variation, as the underlying physiology is likely to be different.
2. Material and methods
(a). Model system
The fathead minnow is a cyprinid endemic to large parts of North America. Its range stretches from the Northwest Territories of Canada to northern Mexico, as well as from New York to Nevada and into parts of California in the continental USA [54]. Fathead minnows can tolerate a wide range of temperatures; when acclimated to 22°C, they exhibit a critical thermal maximum of 36.4 ± 0.75 (mean ± s.d.) [55]. Fathead minnows are used for a variety of purposes, including mosquito population control [56], bait [54,56] and toxicology studies [57].
(b). Temperature set-up
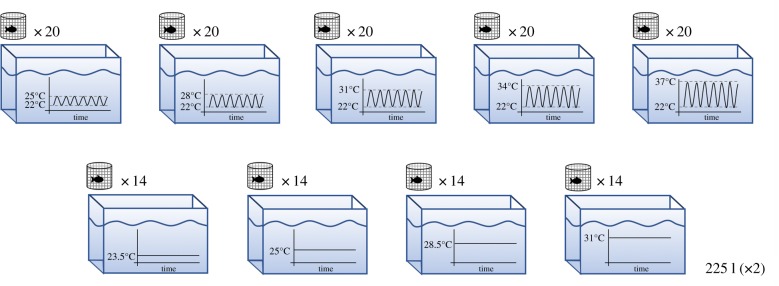
One-day-old P. promelas were shipped to our laboratory on 1 July 2017 from the US Environmental Protection Agency's Mid-Continent Ecology Division Laboratory. We allowed them to acclimate for 24 h at 25°C (temperature at which they were spawned) and then moved them to one of the temperature treatments. For the first 11 days, larvae were kept in groups of approximately 10 fish. Thereafter, fish were reared in individual chambers inside 225 l tanks. Individual growth chambers consisted of plastic Petri dish bottoms surrounded by cylinders of 762 µm mesh.
In all treatments, temperatures were controlled by tank-specific APEX Jr controllers (Neptune Systems, Morgan Hill, CA, USA) on an hourly basis and actual temperature was within ±0.2°C of the nominal treatment temperature. In all fluctuating treatments, the lowest temperature was 22°C at 00.00. Daily temperatures increased linearly to one of five maximum temperatures: 25 (mean daily temperature: 23.5°C), 28 (mean 25.2), 31 (mean 26.5), 34 (mean 28.4) or 37°C (mean 29.5) (figure 1). Twenty fish were grown at each of the fluctuating treatments. Controllers were checked daily for consistency/accuracy. For comparison, there were four constant temperature treatments: 23.5, 25, 28.5 and 31°C (±0.2°C), with 14 individuals per treatment (figure 1). Fish were kept under these conditions for the duration of the experiment (60 days). We replicated each temperature treatment in two 225 l tanks. For reference, water temperature at the Lake Superior National Estuarine Research Reserve (Superior, WI, USA) can reach 27–28°C during summer days, and water can remain greater than 27°C for 10 h these days (summer mean temperature: 20.6°C; electronic supplementary material, figure S1). Our temperature treatments were selected based on this water temperature data.
Figure 1.
Schematic of the temperature treatments. (Online version in colour.)
(c). Husbandry
Water was filtered and checked weekly to ensure appropriate quality. We fed fish 1-day-old Artemia nauplii (San Francisco Bay Brand, Newark, CA, USA) for the first 11 days and then switched to TetraMin flake food (Tetra Spectrum Brands, Blacksburg, VA, USA). All fish were fed ad libitum three times daily, and the light cycle was maintained at a 16 L : 8 D cycle for the duration of the experiment. This rearing protocol is similar to the standard for these fish [58].
(d). Measurements
(i). Length-at-age
Starting with 13-day-old larvae, we measured length every week for a total of eight measurements per fish. We photographed each fish from above at a standard height of 65 cm, while the fish remained in 1 cm of water (within its chamber, to minimize stress). A Nikon D7200 camera with an AF-S Micro Nikkor 105 mm macro lens was used. Photos were measured using ImageJ 1.50i (NIH, Bethesda, MD, USA).
(ii). Critical thermal maximum
CTmax, defined as the temperature at which locomotion becomes disorganized [59], was measured at age 54–56 days. Fish were transferred from their experimental table to the test chamber and allowed to acclimate for 10 min. Water temperature was raised 1°C every 2 min until visual inspection indicated that the fish were unable to maintain equilibrium (uncontrolled swimming) for approximately 2 s. The CTmax assay was performed on all fish at their peak daily temperature.
(iii). Meristic/morphometric traits and fluctuating asymmetry
We measured five traits on the left and right side of individuals: eye anterior–posterior distance, pelvic fin length, pectoral fin length, pelvic fin ray count and pectoral fin ray count. Fish were sacrificed and then photographed; measurements were obtained electronically on ImageJ. These measurements were also used to calculate FA (i.e. the absolute difference between left and right measurements; a common approach to evaluate stress in organisms; [60]). The characters measured are all commonly used in studies of asymmetry [60].
(e). Statistical analysis
To assess trends with temperature in the mean and variance of different traits, we fitted a series of general linear and additive models. The goals of these analyses are to elucidate the main patterns, rather than test specific hypotheses. Specifically, we wished to know whether there was a trend with temperature and whether or not the trend was linear. Thus, to allow full flexibility for the trends, we considered linear models, piecewise linear models with two segments and penalized B-spline models. Model selection was done using Akaike's information criterion (AIC). However, the B-spline model was never the ‘best’ model for any trait and we did not consider it further. Thus, our model comparison was restricted to continuous models with one or two linear segments. These two-segment models are occasionally referred to in the literature as ‘breakpoint regressions', and the value of the independent variable at which the slope changes is referred to as the ‘breakpoint’.
For the mean, we fitted the raw data for each individual using a Gaussian likelihood. To determine a trend with temperature in the variance for each trait, we used squared deviations from the mean for each temperature, i.e. z = [y − m(x)]2, as the input data [61]. To see why this works, note that if the raw data are normally distributed conditional on the independent variable, i.e. y|x ∼ N(m(x),v(x)), then the likelihood for z is Gamma(3/2, 1/[2v(x)]) for which E(z|x) = v(x). Since regression minimizes the distance between the model estimate and E(z|x), it should provide an adequate description of the trend in variance.
In addition, because the collection of traits measured on a given individual are unlikely to be independent, we also conducted MANOVA analyses testing for differences between constant and fluctuating treatments, using mean temperature in each treatment as a covariate. This analysis was performed on (i) all of the traits combined, and (ii) traits grouped according to their correlation structure. Specifically, group A consisted of all of the length-at-age traits, group B included all of the morphometric and meristic traits and group C the asymmetry traits. Analysing the independent blocks separately allows somewhat greater interpretability without loss of information. We adopted a maximum-likelihood approach to performing the MANOVA using likelihood ratio tests to evaluate the effect of fluctuations, temperature and their interaction. All analyses were carried out in Matlab 2017a.
3. Results
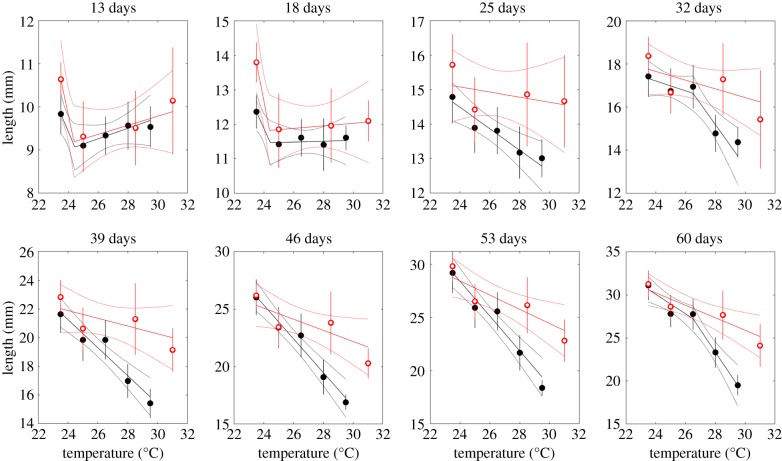
Mean length-at-age for both constant and fluctuating conditions tended to decrease with temperature in all ages measured (figure 2). Sixty-day-old fathead minnows were 10.6 mm shorter in fluctuating 22–37°C than in fluctuating 22–25°C water, and 7.1 mm shorter at constant 31°C than at constant 23.5°C. Under fluctuating conditions, the relationship between length and temperature was linear over the temperatures measured at early ages, yet the oldest ages were better modelled by a two-segment regression (electronic supplementary material, table S1). The break in the regression occurred between the fluctuating 22–31°C (mean 26.5°C) and fluctuating 22–34°C (mean 28°C) treatments. It is important to note that the exact break point in the regression is approximate, and indicates only that the response changes direction somewhere in the vicinity of the temperature indicated by the break.
Figure 2.
Mean length-at-age versus temperature under constant (open red circles) and fluctuating (closed black circles) temperature treatments. Measurements were taken when fish were 13, 18, 25, 32, 39, 46, 53 and 60 days old. Depending on model selection results, regressions are shown with either one or two segments. Means and regression lines shown with 2 standard error confidence bounds.
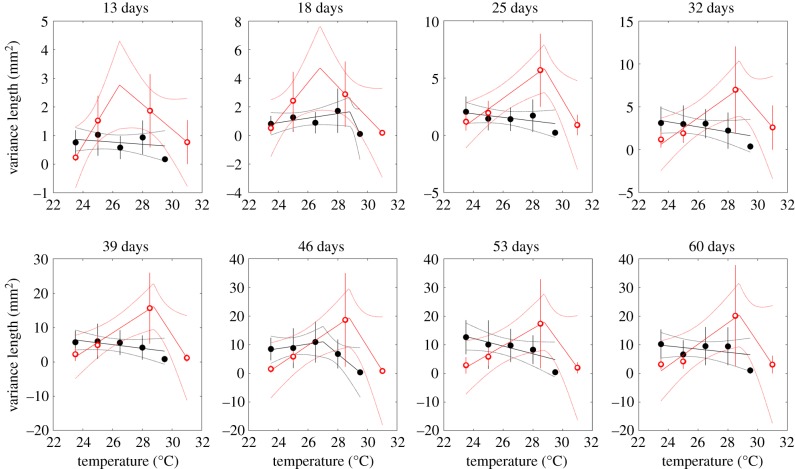
Variance in length, on the other hand, exhibited a more complicated pattern (figure 3). Interestingly, it decreased consistently with temperature under fluctuating conditions, especially at older ages (figure 3). Under constant thermal environments, over the last three ages measured (46, 53 and 60 days old), variance increases as water warms up to a point (between 25 and 28.5°C) and then decreases at very high temperatures (as evidenced by AIC preferring the linear model with two segments; electronic supplementary material, table S2).
Figure 3.
Variance in length-at-age versus temperature under constant (open red circles) and fluctuating (closed black circles, graphed on the x-axis at the corresponding mean temperature) temperature treatments. Depending on model selection results, regressions are shown with either one or two segments. Means and regression lines shown with 2 standard error confidence bounds.
As temperature increased, so did mean CTmax for fish in both constant and fluctuating conditions (figure 4). Differences in CTmax between fish at the lowest and highest temperature treatments were 4.6°C (fluctuating) and 4.5°C (constant). Two-segment models outperformed their one-segment counterparts (electronic supplementary material, table S1), though the break was at a higher temperature in the constant environments. Variance in CTmax decreased as a function of temperature in both thermal treatments (figure 4).
Figure 4.
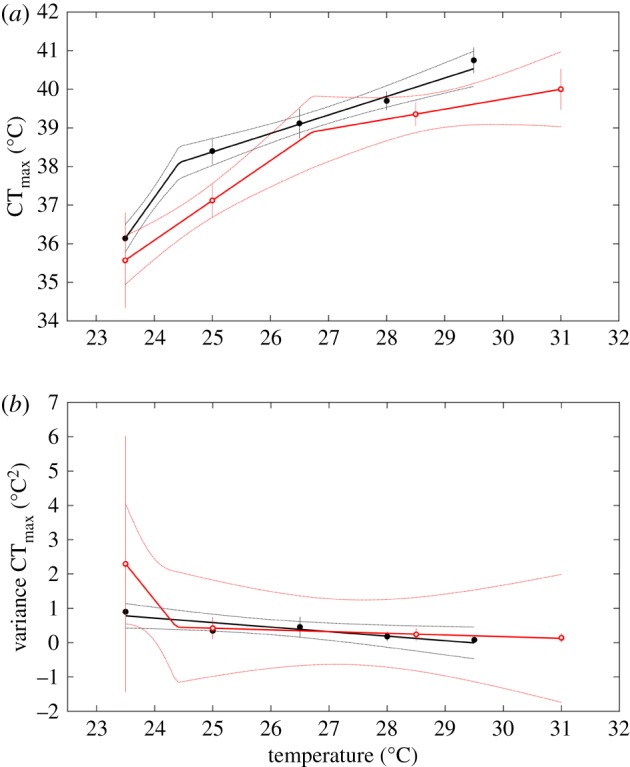
Mean (a) and variance (b) critical thermal maximum (CTmax) versus temperature under constant (open red circles) and fluctuating (closed black circles) temperature treatments. Depending on model selection results, regressions are shown with either one or two segments. Means and regression lines shown with 2 standard error confidence bounds.
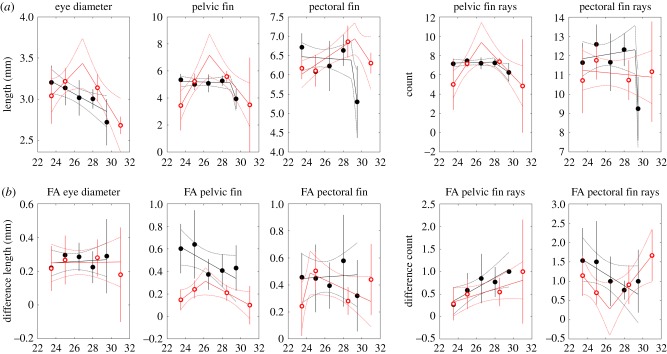
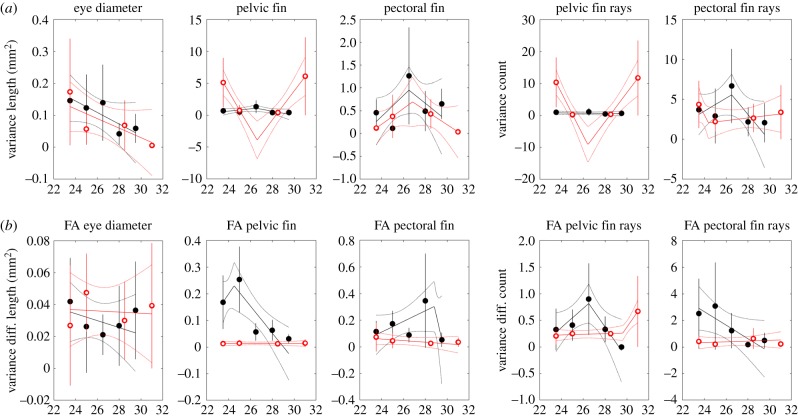
FA in pelvic and pectoral fin length was generally smaller under constant environments, but this was not the case for eye diameter or pelvic and pectoral fin ray counts (figure 5). Variance in pelvic fin length and pelvic fin ray count was high at cooler and warmer temperatures in the constant treatment; under fluctuating conditions, there was no trend with temperature (figure 6).
Figure 5.
Mean morphometric trait values (a) and mean FA values (b) versus temperature under constant (open red circles) and fluctuating (closed black circles) temperature treatments. Depending on model selection results, regressions are shown with either one or two segments. Means and regression lines shown with 2 standard error confidence bounds.
Figure 6.
Variance in morphometric trait value (a) and variance in FA values (b) versus temperature under constant (open red circles) and fluctuating (closed black circles) temperature treatments. Depending on model selection results, regressions are shown with either one or two segments. Means and regression lines shown with 2 standard error confidence bounds.
Integrating all traits into a single MANOVA indicated a significant effect of temperature, temperature treatment (constant versus fluctuating) and their interaction (electronic supplementary material, table S3).
Survival in both treatments was depressed at higher temperatures: fluctuating 22–25°C = 0.95, fluctuating 22–28°C = 0.95, fluctuating 22–31°C = 0.95, fluctuating 22–34°C = 0.80, fluctuating 22–37°C = 0.55, constant 23.5°C = 0.57, constant 25°C = 1.00, constant 28.5°C = 1.00 and constant 31°C = 0.36.
4. Discussion
The importance of hidden phenotypic variation is underscored by recent calls for a mechanistic (i.e. physiological) understanding of climate change impacts [13,62]. We must understand how populations react to rare/novel environments if we are to accurately predict their fates over short and long time scales [63]. Consistent with earlier results on the temperature-size rule [64], we found that mean length-at-age in older fish decreases as temperatures increase. This was true in constant and fluctuating conditions, suggesting that our treatments were in the decreasing part of their thermal performance curve. In addition, the decrease with temperature was more extreme when temperatures fluctuated than when they were constant.
Although we were unable to fit nonlinear reaction norms to our data, this result is consistent with the idea that the reaction norm for the highest temperatures is convex (i.e. the reaction norm must accelerate downward in those temperatures that the fish in the fluctuating treatments experienced for which we have no constant temperature data). Specifically, because the growth trajectories are nearly linear, we can imagine that growth in length is approximately given by dL/dt = g(T) + σ(T)ɛ, where g(T) is the mean growth rate and σ(T)ɛ is white noise with zero mean and temperature-dependent variance and σ2(T). The mean size at age t is given by the integral . To see the effect of convexity in g, we can approximate this integral around the mean temperature as and hence provided that , which is Jensen's inequality [65,66]. Note also that this simple model predicts that the effect of varying temperature on the mean size of fish should increase with age as it does in our data.
Variance in length-at-age (in older fish) showed an analogous pattern with temperature in the two treatments: fish in waters whose temperatures fluctuated daily were less variable at the more extreme thermal conditions, while fish exposed to constant temperatures exhibited higher variability at intermediate temperatures (25 and 28.5°C). Under the simple model above, the variance in length is given by , so in the constant temperature treatments, . Based on figure 3, this suggests that there is a unimodal relationship between the noise variance and temperature. Again, we expect the variation across temperature treatments to be more extreme in the older fish. In addition, the variance in the fluctuating temperature treatment will be less than in the constant temperature treatment when the noise magnitude is a convex function of temperature, i.e. , which is—at least broadly—consistent with what was observed in the constant temperature treatments. This is analogous to Jensen's inequality for variance (see also [67]).
Mean critical thermal maxima (upper thermal tolerance limits) for fish in both treatments increased with increasing water temperature, as is repeatedly observed [68]. CTmax was higher in fish reared under fluctuating conditions (comparison of black and red data points in figure 4 under similar mean temperatures). Hormesis (i.e. mild exposure to a stressful temperature having positive effects on an organism; [69]) appears to be at work here. Mechanistically, extreme temperatures influence the kinetic induction of heat shock proteins such as Hsp70, Hsp90, Hsp27 and Hsp22, which have differing transcriptional responses when exposed to fluctuating versus constant temperature regimes [70]. Fathead minnows are also able to ramp up heat shock protein production in the spring as water temperatures increase [71]. Owing to the different magnitude and timing of induction, these proteins could play a role in mediating thermal tolerance, although the frequency of the exposure (i.e. daily versus seasonal versus episodic events like heat waves) could also be important [70,72]. It is possible, then, that exposing fathead minnows to higher temperatures daily could trigger upregulation of these chaperone genes. Interestingly, in the most extreme fluctuating treatments, the daily excursions to high temperatures provided a beneficial mild exposure for CTmax but a detrimental one when it came to growth, as was reported for zebrafish [52] and three Australian frogs [73]. This suggests that a trade-off may exist between growth and thermal tolerance [74], an important consideration when making predictions related to climate change. Variance in CTmax—like in length-at-age—decreased in warmer waters.
No consistent pattern in mean or variance was found across morphometric traits or FA, although variance in FA tended to be lower in constant temperatures than in fluctuating temperatures for pelvic and pectoral fin lengths.
Our results add to a growing body of evidence, suggesting that variance can decrease as the environment becomes more stressful [41,43] (but see [75,76]). This could be a result of strong genetic correlations between benign and rarer/more stressful environments, so that trait values at the extreme are determined in large part by the shape of the reaction norm in common environments [77,78]. We note, however, that sometimes different genes are upregulated based on the severity of the thermal stress [79], and it is unclear how the expression of these genes is correlated across environments. Furthermore, an organism's response to constant stress, as opposed to stress under fluctuating conditions, can be quite different. Fluctuating temperatures have been shown to cause differential gene expression [70] and to possibly call on different responses: constant temperature regimes appear to involve modification of a large part of the transcriptome while (non-stressful) fluctuations induce smaller changes in gene expression, but ones that are different and independent [80].
If stress responses are somewhat similar regardless of stressor [81], then we would expect less variation when exposing organisms to any number of stressful environments. Hoover et al. [82] reared fathead minnows under four salinity treatments and found more variation in reproductive traits (e.g. egg number, egg fertilization rate and time spent caring for nests), not less as we found for length-at-age and CTmax. Different stressors have been shown to have disparate effects on a given trait of wild mustard [83], and even a single stressor can have different effects on variance in multiple traits (e.g. low nutrient conditions reduced phenotypic variance in leaf length and number, among others, but increased it in seedling height [83]). Given the existence of several other examples (e.g. [43,83–85], establishing comprehensive rules for the effects of rarer environments on trait variation will be a challenge.
Most work to date has been focused on identifying the mean thermal limit of a population (see [86] for a historical perspective), and evolutionary forecasts have been attempted based on these population-average values [9,87–89]. Yet, many aspects of a population's thermal biology can be traced not to mean temperature measures, but to fluctuations, extremes and episodic events (e.g. [90–92]). Despite the difficulties, it is clear that we need to incorporate these into predictions of species responses to climate change.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the guest editors for the invitation to contribute to the special issue and three anonymous referees for constructive comments on an earlier version of the manuscript. We are also grateful to Kathleen Jensen (Mid-Continent Ecology Division, National Health and Environmental Effects Research Laboratory, EPA) for generously providing us with fathead minnows and Dr Zach Hoover for sharing data with us. We thank Forrest Duddles, Dave Saxman, Carl Paul and the rest of the FacMan crew for putting our fish room together, and Dr Jack Bley for the copy stand.
Ethics
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Kalamazoo College.
Data accessibility
The dataset supporting this article is available as part of the electronic supplementary material.
Authors' contributions
S.S., S.E.I., C.L.S. and S.Q.G. designed the experiments. S.E.I., C.L.S. and S.Q.G. led experiment set-up and data collection efforts. S.S. and S.B.M. analysed data and drafted the manuscript. All authors revised it critically and approved its content.
Competing interests
We have no competing interests.
Funding
S.E.I., C.L.S. and S.Q.G. were supported by Batts Fellowships.
References
- 1.IPCC. 2013. Climate change 2013—the physical science basis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Trenberth KE. 2011. Changes in precipitation with climate change. Clim. Res. 47, 123–138. ( 10.3354/cr00953) [DOI] [Google Scholar]
- 3.Church JA, White NJ. 2011. Sea-level rise from the late 19th to the early 21st century. Surv. Geophys. 32, 585–602. ( 10.1007/s10712-011-9119-1) [DOI] [Google Scholar]
- 4.Comiso JC, Parkinson CL, Gersten R, Stock L. 2008. Accelerated decline in the Arctic sea ice cover. Geophys. Res. Lett. 35, 413–416. ( 10.1029/2007GL031972) [DOI] [Google Scholar]
- 5.Rosenzweig C, Iglesias A, Yang XB, Epstein PR, Chivian E. 2001. Climate change and extreme weather events: implications for food production, plant diseases, and pests. Global Change Hum. Health 2, 90–104. ( 10.1023/A:1015086831467) [DOI] [Google Scholar]
- 6.Roessig JM, Woodley CM, Cech JJ Jr, Hansen LJ. 2004. Effects of global climate change on marine and estuarine fishes and fisheries. Rev. Fish Biol. Fisher. 14, 251–275. ( 10.1007/s11160-004-6749-0) [DOI] [Google Scholar]
- 7.Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. ( 10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- 8.McLeman R, Smit B. 2006. Migration as an adaptation to climate change. Clim. Change 76, 31–53. ( 10.1007/s10584-005-9000-7) [DOI] [Google Scholar]
- 9.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 10.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 11.Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. 2011. Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53–58. ( 10.1126/science.1200303) [DOI] [PubMed] [Google Scholar]
- 12.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. ( 10.1111/j.1461-0248.2011.01736.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ Jr, Stenseth NC, Pertoldi C. 2010. Adapting to climate change: a perspective from evolutionary physiology. Clim. Res. 43, 3–15. ( 10.3354/cr00879) [DOI] [Google Scholar]
- 14.Mora C, et al. 2013. The projected timing of climate departure from recent variability. Nature 502, 183–187. ( 10.1038/nature12540) [DOI] [PubMed] [Google Scholar]
- 15.Mearns LO, Katz RW, Schneider SH. 1984. Extreme high-temperature events: changes in their probabilities with changes in mean temperature. J. Appl. Meteorol. Clim. 23, 1601–1613. () [DOI] [Google Scholar]
- 16.Bailey LD, van de Pol M. 2015. Tackling extremes: challenges for ecological and evolutionary research on extreme climatic events. J. Anim. Ecol. 85, 85–96. ( 10.1111/1365-2656.12451) [DOI] [PubMed] [Google Scholar]
- 17.Moreno J, Møller AP. 2011. Extreme climatic events in relation to global change and their impact on life histories. Curr. Zool. 57, 375–389. ( 10.1093/czoolo/57.3.375) [DOI] [Google Scholar]
- 18.Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742. ( 10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobbs RJ, Higgs E, Harris JA. 2009. Novel ecosystems: implications for conservation and restoration. Trends Ecol. Evol. 24, 599–605. ( 10.1016/j.tree.2009.05.012) [DOI] [PubMed] [Google Scholar]
- 20.Garcia RA, Cabeza M, Rahbek C, Araujo MB. 2014. Multiple dimensions of climate change and their implications for biodiversity. Science 344, 1247579 ( 10.1126/science.1247579) [DOI] [PubMed] [Google Scholar]
- 21.Rutherford SL. 2000. From genotype to phenotype: buffering mechanisms and the storage of genetic information. BioEssays 22, 1095–1105. () [DOI] [PubMed] [Google Scholar]
- 22.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 23.Schlichting CD. 2008. Hidden reaction norms, cryptic genetic variation, and evolvability. Ann. N Y Acad. Sci. 1133, 187–203. ( 10.1196/annals.1438.010) [DOI] [PubMed] [Google Scholar]
- 24.Paaby AB, Rockman MV. 2014. Cryptic genetic variation: evolution's hidden substrate. Nat. Rev. Genet. 15, 247–258. ( 10.1038/nrg3688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 26.Aubret F, Shine R. 2009. Genetic assimilation and the postcolonization erosion of phenotypic plasticity in island tiger snakes. Curr. Biol. 19, 1932–1936. ( 10.1016/j.cub.2009.09.061) [DOI] [PubMed] [Google Scholar]
- 27.Otaki JM, Hiyama A, Iwata M, Kudo T. 2010. Phenotypic plasticity in the range-margin population of the lycaenid butterfly Zizeeria maha. BMC Evol. Biol. 10, 252 ( 10.1186/1471-2148-10-252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsman A. 2014. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 276–284. ( 10.1038/hdy.2014.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauter N, Doebley J. 2002. Genetic variation for phenotypically invariant traits detected in teosinte: implications for the evolution of novel forms. Genetics 160, 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ledon-Rettig CC, Pfennig DW, Crespi EJ. 2010. Diet and hormonal manipulation reveal cryptic genetic variation: implications for the evolution of novel feeding strategies. Proc. R. Soc. B 277, 3569–3578. ( 10.1098/rspb.2010.0877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. 2010. Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol. Evol. 25, 459–467. ( 10.1016/j.tree.2010.05.006) [DOI] [PubMed] [Google Scholar]
- 32.McGuigan K, Nishimura N, Currey M, Hurwit D, Cresko WA. 2011. Cryptic genetic variation and body size evolution in threespine stickleback. Evolution 65, 1203–1211. ( 10.1111/j.1558-5646.2010.01195.x) [DOI] [PubMed] [Google Scholar]
- 33.Nonaka E, Svanbäck R, Thibert-Plante X, Englund G, Brännström Å. 2015. Mechanisms by which phenotypic plasticity affects adaptive divergence and ecological speciation. Am. Nat. 186, E126–E143. ( 10.1086/683231) [DOI] [PubMed] [Google Scholar]
- 34.Pigliucci M, Murren CJ. 2003. Genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution 57, 1455–1464. ( 10.2307/3448748) [DOI] [PubMed] [Google Scholar]
- 35.Badyaev AV. 2005. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R. Soc. B 272, 877–886. ( 10.1098/rspb.2004.3045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider RF, Meyer A. 2016. How plasticity, genetic assimilation and cryptic genetic variation may contribute to adaptive radiations. Mol. Ecol. 26, 330–350. ( 10.1111/mec.13880) [DOI] [PubMed] [Google Scholar]
- 37.Berger D, Bauerfeind SS, Blanckenhorn WU, Schäfer MA. 2011. High temperatures reveal cryptic genetic variation in a polymorphic female sperm storage organ. Evolution 65, 2830–2842. ( 10.1111/j.1558-5646.2011.01392.x) [DOI] [PubMed] [Google Scholar]
- 38.Purchase CF, Moreau DTR. 2012. Stressful environments induce novel phenotypic variation: hierarchical reaction norms for sperm performance of a pervasive invader. Ecol. Evol. 2, 2567–2576. ( 10.1002/ece3.364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann AA, Parsons PA. 1997. Extreme environmental change and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 40.Hoffmann AA, Merilä J. 1999. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 14, 96–101. ( 10.1016/S0169-5347(99)01595-5) [DOI] [PubMed] [Google Scholar]
- 41.McGuigan K, Sgrò CM. 2009. Evolutionary consequences of cryptic genetic variation. Trends Ecol. Evol. 24, 305–311. ( 10.1016/j.tree.2009.02.001) [DOI] [PubMed] [Google Scholar]
- 42.Ledon-Rettig CC, Pfennig DW, Chunco AJ, Dworkin I. 2014. Cryptic genetic variation in natural populations: a predictive framework. Integr. Comp. Biol. 54, 783–793. ( 10.1093/icb/icu077) [DOI] [PubMed] [Google Scholar]
- 43.Woods RE, Sgrò CM, Hercus MJ, Hoffmann AA. 1999. The association between fluctuating asymmetry, trait variability, trait heritability, and stress: a multiply replicated experiment on combined stresses in Drosophila melanogaster. Evolution 53, 493–505. ( 10.1111/j.1558-5646.1999.tb03784.x) [DOI] [PubMed] [Google Scholar]
- 44.Hollander J, Bourdeau PE. 2016. Evidence of weaker phenotypic plasticity by prey to novel cues from non-native predators. Ecol. Evol. 6, 5358–5365. ( 10.1002/ece3.2271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rowiński PK, Rogell B. 2017. Environmental stress correlates with increases in both genetic and residual variances: a meta-analysis of animal studies. Evolution 71, 1339–1351. ( 10.1111/evo.13201) [DOI] [PubMed] [Google Scholar]
- 46.Bozinovic F, Bastías DA, Boher F, Clavijo-Baquet S, Estay SA, Angilletta MJ Jr. 2011. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol. Biochem. Zool. 84, 543–552. ( 10.1086/662551) [DOI] [PubMed] [Google Scholar]
- 47.Colinet H, Sinclair BJ, Vernon P, Renault D. 2015. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 60, 123–140. ( 10.1146/annurev-ento-010814-021017) [DOI] [PubMed] [Google Scholar]
- 48.Brakefield PM, Mazzotta V. 1995. Matching field and laboratory environments: effects of neglecting daily temperature variation on insect reaction norms. J. Evol. Biol. 8, 559–573. ( 10.1046/j.1420-9101.1995.8050559.x) [DOI] [Google Scholar]
- 49.Kingsolver JG, Ragland GJ, Diamond SE. 2009. Evolution in a constant environment: thermal fluctuations and thermal sensitivity of laboratory and field populations of Manduca sexta. Evolution 63, 537–541. ( 10.1111/j.1558-5646.2008.00568.x) [DOI] [PubMed] [Google Scholar]
- 50.Folguera G, Bastías DA, Caers J, Rojas JM, Piulachs M-D, Bellés X, Bozinovic F. 2011. An experimental test of the role of environmental temperature variability on ectotherm molecular, physiological and life-history traits: implications for global warming. Comp. Biochem. Phys. A 159, 242–246. ( 10.1016/j.cbpa.2011.03.002) [DOI] [PubMed] [Google Scholar]
- 51.García-Ruiz E, Marco V, Pérez-Moreno I. 2011. Effects of variable and constant temperatures on the embryonic development and survival of a new grape pest, Xylotrechus arvicola (Coleoptera: Cerambycidae). Environ. Entomol. 40, 939–947. ( 10.1603/EN11080) [DOI] [PubMed] [Google Scholar]
- 52.Schaefer J, Ryan A. 2006. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish Biol. 69, 722–734. ( 10.1111/j.1095-8649.2006.01145.x) [DOI] [Google Scholar]
- 53.Bernhardt JR, Sunday JM, Thompson PL, O'Connor MI. 2018. Nonlinear averaging of thermal experience predicts population growth rates in a thermally variable environment. Proc. R. Soc. B 285, 20181076 ( 10.1098/rspb.2018.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker GC. 1983. Fishes of Wisconsin. Madison, WI: University of Wisconsin Press. [Google Scholar]
- 55.Richards VL, Beitinger TL. 1995. Reciprocal influences of temperature and copper on survival of fathead minnows, Pimephales promelas. Bull. Environ. Contam. Toxicol. 55, 230–236. ( 10.1007/BF00203014) [DOI] [PubMed] [Google Scholar]
- 56.Eddy S, Surber T. 1974. Northern fishes: with special reference to the upper Mississippi valley. Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- 57.Ankley GT, Villeneuve DL. 2006. The fathead minnow in aquatic toxicology: past, present and future. Aquat. Toxicol. 78, 91–102. ( 10.1016/j.aquatox.2006.01.018) [DOI] [PubMed] [Google Scholar]
- 58.U.S. Environmental Protection Agency. 2006. Culturing of fathead minnows (Pimephales promelas). Report EPA-833-C-06-001. Washington, DC: US Environmental Protection Agency. [Google Scholar]
- 59.Cowles RB, Bogert CM. 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus. Nat. Hist. 83, 261–296. [Google Scholar]
- 60.Allenbach DM. 2010. Fluctuating asymmetry and exogenous stress in fishes: a review. Rev. Fish Biol. Fisher. 21, 355–376. ( 10.1007/s11160-010-9178-2) [DOI] [Google Scholar]
- 61.Diggle PJ, Verbyla AP. 1998. Nonparametric estimation of covariance structure in longitudinal data. Biometrics 54, 401–415. ( 10.2307/3109751) [DOI] [PubMed] [Google Scholar]
- 62.Helmuth B, Kingsolver JG, Carrington E. 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 67, 177–201. ( 10.1146/annurev.physiol.67.040403.105027) [DOI] [PubMed] [Google Scholar]
- 63.Sheldon KS, Dillon ME. 2016. Beyond the mean: biological impacts of cryptic temperature change. Integr. Comp. Biol. 56, 110–119. ( 10.1093/icb/icw005) [DOI] [PubMed] [Google Scholar]
- 64.Angilletta MJ., Jr 2009. Thermal adaptation: a theoretical and empirical synthesis. New York: NY: Oxford University Press. [Google Scholar]
- 65.Ruel J, Ayres M. 1999. Jensen's inequality predicts effects of environmental variation. Trends Ecol. Evol. 14, 361–366. ( 10.1016/S0169-5347(99)01664-X) [DOI] [PubMed] [Google Scholar]
- 66.Denny M. 2017. The fallacy of the average: on the ubiquity, utility and continuing novelty of Jensen's inequality. J. Exp. Biol. 220, 139–146. ( 10.1242/jeb.140368) [DOI] [PubMed] [Google Scholar]
- 67.Dowd WW, King FA, Denny MW. 2015. Thermal variation, thermal extremes and the physiological performance of individuals. J. Exp. Biol. 218, 1956–1967. ( 10.1242/jeb.114926) [DOI] [PubMed] [Google Scholar]
- 68.Beitinger TL, Bennett WA. 2000. Quantification of the role of acclimation temperature in temperature tolerance of fishes. Environ. Biol. Fish. 58, 277–288. ( 10.1023/A:1007618927527) [DOI] [Google Scholar]
- 69.Costantini D. 2014. Does hormesis foster organism resistance to extreme events? Front. Ecol. Environ. 12, 209–210. ( 10.1890/14.WB.005) [DOI] [Google Scholar]
- 70.Podrabsky JE, Somero GN. 2004. Changes in gene expression associated with acclimation to constant temperatures and fluctuating daily temperatures in an annual killifish Austrofundulus limnaeus. J. Exp. Biol. 207, 2237–2254. ( 10.1242/jeb.01016) [DOI] [PubMed] [Google Scholar]
- 71.Fader SC, Yu Z, Spotila JR. 1994. Seasonal variation in heat shock proteins (hsp 70) in stream fish under natural conditions. J. Therm. Biol. 19, 335–341. ( 10.1016/0306-4565(94)90070-1) [DOI] [Google Scholar]
- 72.Sørensen JG, Kristensen TN, Loeschcke V. 2003. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. ( 10.1046/j.1461-0248.2003.00528.x) [DOI] [Google Scholar]
- 73.Kern P, Cramp RL, Franklin CE. 2015. Physiological responses of ectotherms to daily temperature variation. J. Exp. Biol. 218, 3068–3076. ( 10.1242/jeb.123166) [DOI] [PubMed] [Google Scholar]
- 74.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 75.Imasheva AG, Bosenko DV, Bubli OA. 1999. Variation in morphological traits of Drosophila melanogaster (fruit fly) under nutritional stress. Heredity 82, 187–192. ( 10.1038/sj.hdy.6884660) [DOI] [PubMed] [Google Scholar]
- 76.Sisodia S, Singh BN. 2009. Variations in morphological and life-history traits under extreme temperatures in Drosophila ananassae. J. Biosci. 34, 263–274. ( 10.1007/s12038-009-0030-6) [DOI] [PubMed] [Google Scholar]
- 77.De Jong G. 2005. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytol. 166, 101–118. ( 10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- 78.Chevin L-M, Hoffmann AA. 2017. Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 372, 20160138 ( 10.1098/rstb.2016.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Logan CA, Somero GN. 2011. Effects of thermal acclimation on transcriptional responses to acute heat stress in the eurythermal fish Gillichthys mirabilis (Cooper). Am. J. Physiol. Reg. I. 300, R1373–R1383. ( 10.1152/ajpregu.00689.2010) [DOI] [PubMed] [Google Scholar]
- 80.Sørensen JG, Schou MF, Kristensen TN, Loeschcke V. 2016. Thermal fluctuations affect the transcriptome through mechanisms independent of average temperature. Sci. Rep. 6, 2068–2011. ( 10.1038/srep30975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barton BA, Iwama GK. 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1, 3–26. ( 10.1016/0959-8030(91)90019-G) [DOI] [Google Scholar]
- 82.Hoover Z, Weisgerber JN, Pollock MS, Chivers DP, Ferrari MCO. 2013. Sub-lethal increases in salinity affect reproduction in fathead minnows. Sci. Total Environ. 463–464, 334–339. ( 10.1016/j.scitotenv.2013.06.046) [DOI] [PubMed] [Google Scholar]
- 83.Stanton ML, Roy BA, Thiede DA. 2000. Evolution in stressful environments. I. Phenotypic variability, phenotypic selection, and response to selection in five distinct environmental stresses. Evolution 54, 93–111. ( 10.1111/j.0014-3820.2000.tb00011.x) [DOI] [PubMed] [Google Scholar]
- 84.Bennington CC, McGraw JB. 1996. Environment-dependence of quantitative genetic parameters in Impatiens pallida. Evolution 50, 1083–1097. ( 10.2307/2410649) [DOI] [PubMed] [Google Scholar]
- 85.Charmantier A, Garant D. 2005. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B 272, 1415–1425. ( 10.1098/rspb.2005.3117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574. ( 10.1139/z97-783) [DOI] [Google Scholar]
- 87.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 88.Shaw RG, Etterson JR. 2012. Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol. 195, 752–765. ( 10.1111/j.1469-8137.2012.04230.x) [DOI] [PubMed] [Google Scholar]
- 89.Walters RJ, Blanckenhorn WU, Berger D. 2012. Forecasting extinction risk of ectotherms under climate warming: an evolutionary perspective. Funct. Ecol. 26, 1324–1338. ( 10.1111/j.1365-2435.2012.02045.x) [DOI] [Google Scholar]
- 90.Hoffmann AA. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880. ( 10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 91.Denny MW, Dowd WW. 2012. Biophysics, environmental stochasticity, and the evolution of thermal safety margins in intertidal limpets. J. Exp. Biol. 215, 934–947. ( 10.1242/jeb.058958) [DOI] [PubMed] [Google Scholar]
- 92.Ketola T, Kristensen TN. 2017. Experimental approaches for testing if tolerance curves are useful for predicting fitness in fluctuating environments. Front. Ecol. Evol. 5, 541–546. ( 10.3389/fevo.2017.00129) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article is available as part of the electronic supplementary material.