Abstract
Plasticity, both within and across generations, can shape sexual traits involved in mate choice and reproductive success, and thus direct measures of fitness. Especially, transgenerational plasticity (TGP), where parental environment influences offspring plasticity in future environments, could compensate for otherwise negative effects of environmental change on offspring sexual traits. We conducted a mate choice experiment using stickleback (Gasterosteus aculeatus) with different thermal histories (ambient 17°C or elevated 21°C) within and across generations under simulated ocean warming using outdoor mesocosms. Parentage analysis of egg clutches revealed that maternal developmental temperature and reproductive (mesocosm) environment affected egg size, with females that developed at 17°C laying smaller eggs in 21°C mesocosms, likely owing to metabolic costs at elevated temperature. Paternal developmental temperature interacted with the reproductive environment to influence mating success, particularly under simulated ocean warming, with males that developed at 21°C showing lower overall mating success compared with 17°C males, but higher mating success in 21°C mesocosms. Furthermore, mating success of males was influenced by the interaction between F1 developmental temperature and F0 parent acclimation temperature, demonstrating the potential role of both TGP and within-generation plasticity in shaping traits involved in sexual selection and mate choice, potentially facilitating rapid responses to environmental change.
This article is part of the theme issue ‘The role of plasticity in phenotypic adaptation to rapid environmental change’.
Keywords: transgenerational plasticity, mate choice, reproductive success, climate change, parentage analysis, Gasterosteus aculeatus
1. Introduction
The world's oceans are warming at unprecedented rates [1], and organisms need to respond to these fast-changing environments. Response mechanisms include migration (shifting distributions), rapid evolution (genetic tracking) and/or adaptive phenotypic plasticity (reviewed in [2]). Plasticity can occur both within a generation (genotype by environment interaction or G × E) and across generations (transgenerational plasticity or TGP). For TGP, the environment that parents experience influences offspring plasticity, manifest as a parent environment by offspring environment interaction [3]. In the face of rapid climate change, within-generation plasticity can buffer individuals from negative impacts of their immediate environment [4]. When parent and offspring environments match, TGP is likely to play an important role [5] because it is a phenotypic response that is transferred across generations, priming offspring for future conditions, and potentially buying time for slower genetic change to catch up [6,7]. The number of studies documenting TGP in response to simulated climate change scenarios has exploded over the past few years (reviewed in [8]). In many cases, acclimation of parents to rapidly changing environmental conditions resulted in compensation of offspring traits to otherwise negative effects. For example, TGP in response to climate warming, changes to salinity and ocean acidification were shown in numerous taxa spanning the tree of life [9], with benefits for offspring traits such as improved survival, development, growth, fecundity and metabolism [8]. What are currently lacking in the TGP-climate change literature, however, are studies that not only measure such proxies for fitness but also examine direct measures of fitness such as reproductive success (but see [10]). The importance of within-generation plasticity in shaping traits involved in sexual selection has recently come to light [11], but the role of TGP in mate choice and reproductive success is not well known.
Reproductive success is the ultimate measure of fitness [12]. Since reproductive success is determined not solely by the number of offspring produced, but also by the probable reproductive success of those offspring, mate choice plays an important part in this success [13]. Mate choice is the process that occurs whenever the effects of traits expressed in one sex lead to non-random mating with members of the opposite sex [14]. The topic of mate choice is broad and has gained substantial attention in the literature (reviewed in [15]). Briefly, in resource-based mating systems, the choosing sex, typically the female, selects partners based on direct benefits (i.e. resources such as food, shelter, parental care or protection). In non-resource-based systems, genetic components such as ‘good’ or ‘compatible’ genes often constitute an important factor mediating mate choice [14]. Both empirical and theoretical works suggest that within-generation plasticity (G × E) can influence variation in sexually selected traits [16]. Recently, theoretical models have also drawn attention to how TGP and other non-genetic inheritance mechanisms such as epigenetic marks can lead to adaptive (or non-adaptive) traits in offspring that influence sexual selection and mate choice [17]. Both the expression of sexual traits (e.g. body size, ornamentation, condition) and mating preferences for these traits can be altered by G × E and parental effects [18]. However, empirical studies explicitly demonstrating the interaction between parental environment, offspring environment and offspring mate choice are scarce (but see examples of sexual imprinting [19,20]).
Mate choice is expected to select for traits that reliably indicate mate quality and/or compatibility [14], but environments change in space and time, and cues that are indicative of mate quality in one environment may represent sub-optimal or maladaptive cues under changed conditions. In fast-changing environments, mate quality may be highly environment-dependent, and individuals might choose based on unreliable cues unless multiple cues or alternative signals are involved in choice [21,22]. For instance, increased water turbidity due to eutrophication reduced reliance on visual cues for mate quality in several fish species, leading to a greater investment in courtship and reliance on other (e.g. olfactory) cues [23–25]. Plasticity, and especially TGP, could buffer some of the negative consequences of rapidly changing environments on mate quality cues, since individuals that can quickly adjust their phenotype or are pre-acclimated to specific environments should have an advantage over naive individuals or those with fixed phenotypes [17,20]. Consequently, mating preference for individuals with phenotypic traits (e.g. body size, condition) optimized for specific environments via within-generation plasticity or TGP could occur, leading to mate choice based on phenotype matching (assortative mating based on similar phenotype [26,27]). Examples of phenotype matching are widespread and include mate choice based on similar body size [28–32], shape [33], symmetry [34], colour [35], behaviour [36] and complementary MHC genotype (important for parasite resistance [37,38]). Nevertheless, the potential for phenotype matching based on TGP-optimized phenotypes under climate change has not yet been investigated.
Threespine stickleback, Gasterosteus aculeatus (Linnaeus, 1758), hereafter referred to simply as stickleback, is an ideal model organism to study phenotypic plasticity in general owing to its high phenotypic diversity across environmental conditions (e.g. temperature, salinity, season length, habitats, predators) [39,40] and plasticity of mate choice specifically, as its complex mating behaviour has been extensively studied for decades [39,41–43]. At the start of the breeding season, males migrate to shallow water to establish a territory and build a nest to court females to lay their eggs. The majority of eggs are fertilized by the nest owner, but alternative reproductive tactics such as sneaking and egg thievery are common [44]. Females choose among nesting males based on visual and olfactory cues signalling male quality [45]. Males display an intense red breeding coloration that has been shown to indicate overall condition, parental ability (males care for eggs and young offspring) and parasite infection status [46,47]. Olfactory-based mate choice experiments have further demonstrated that females prefer to mate with males with an MHC (major histocompatibility complex) genotype that provides the optimum number of MHC variants (intermediate MHC diversity) in the offspring [48]. Another important visual cue is body size. Several studies found that females chose big males owing to their presumably better condition, and competitive or courting ability [49–51]. Yet, studies of stickleback species-pairs or ecotypes (e.g. benthic–limnetic, stream–lake or anadromous–freshwater) found that females preferred males of similar size, and few interspecific or between-ecotype matings occurred between fish with conspicuous size differences [29,36,52,53]. Indeed, size-matching was found to be a more important choice factor than size itself when body size was experimentally manipulated to remove confounding effects [30,32].
Previous studies of the oceanic stickleback population investigated here found that body size was highly plastic in response to environmental temperature both within and across generations. Exposure to a simulated +4°C climate change scenario during development had negative effects on growth and resulting body size [54–59]. But, when mothers were acclimated to elevated temperature during reproductive conditioning, TGP resulted in (relatively) larger offspring in the +4°C climate scenario [56,58]. The mechanism underlying better growth at elevated temperature was more efficient metabolism via the inheritance of optimized mitochondria from mothers [56]. Optimized mitochondrial function was underlain by changes to mitochondrial and other gene expression depending on the maternal and also the grand-maternal thermal environment, suggesting an epigenetic basis for TGP [60,61]. Nevertheless, fish with a history of elevated temperature across three generations were smaller than those with an ambient temperature history [57], indicating that a continued increase in climate warming will likely result in progressively smaller adult fish (see also [62]). However, bigger is not always better when environmental conditions change [63]. Smaller fish may be favoured under size-selective predation, or when larger size is associated with higher physiological demands under heat stress [64]; hence, smaller size might be more attractive under these conditions [65]. Still, when body size is removed as a choice factor by size-matching potential mates, other phenotypic signals indicating mate quality in changed environments due to within-generation plasticity or TGP benefits may become important [18,20]. In this case, individuals displaying phenotypes optimized for a specific environment may be chosen.
Here, we investigated the role of within-generation plasticity and TGP in mate choice and reproductive success of stickleback under simulated ocean warming in semi-natural conditions using large, outdoor mesocosms. We predicted that females should choose males with phenotypic signals indicating high quality in their specific environment, and removed body size per se as a choice factor by size-matching males and females from different thermal histories (ambient or elevated temperature). Specifically, we predicted that phenotype matching based on mate quality cues and sexual traits underlain by within-generation plasticity and/or TGP benefits should lead to more mating success between males and females with an ambient temperature history in ambient temperature conditions, and more mating success between males and females with an elevated temperature history in elevated temperature conditions. By estimating the reproductive success of offspring with parents from different thermal histories under changing climate conditions, we can also begin to understand the role of and adaptive significance of TGP under climate change for fitness in wild populations.
2. Material and methods
(a). Mate choice experiment
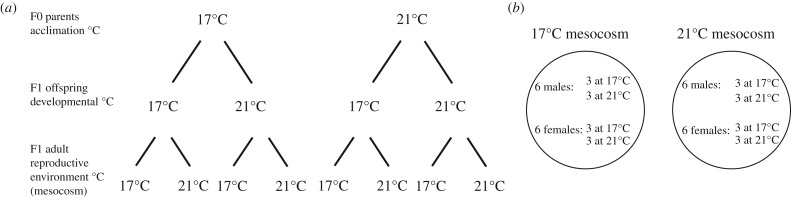
Stickleback used in the mate choice experiment originated from an oceanic population in the Sylt-Rømø Bight, Germany (55°05′ N, 8°39′ E). Wild adult fish were caught by trawling in February 2015, brought back to the laboratory and used in a TGP experiment in spring 2015. Starting on 12 March 2015, wild adult fish (F0) experienced acclimation at 17°C or 21°C for between six and eight weeks during their reproductive conditioning phase (see [59] for details). F1 offspring from those parents were raised in the laboratory until adulthood (approx. 1 year) at either 17°C or 21°C. In the current experiment, these F1 adults were further assigned to reproductive environments (mesocosms) set to either 17°C or 21°C (figure 1a). Specifically, in June 2016, 144 adult F1 individuals from a mix of genetic (family) backgrounds that showed signs of sexual maturity (72 females and 72 males) were chosen for the mating experiment. For both males and females, 36 developed at 17°C and 36 developed at 21°C (figure 1b). Of 72 F1 males, 45 (62.5%) also originated from either 17°C or 21°C F0 parents. For F1 females, 29/72 (40.3%) also had F0 parents acclimated to either 17°C or 21°C. The remaining F1 males and females had F0 parents acclimated for six to eight weeks to fluctuating temperatures, where experimental temperatures either varied weekly between 17°C and 21°C or fluctuated stochastically between 14°C and 23°C (see [59]). Although fluctuating (unpredictable) environments are not predicted to promote TGP [7], we took a conservative approach and only used F1 fish whose F0 parents were acclimated at constant 17°C and 21°C in the analysis of transgenerational effects. With this set-up, we could investigate the role of within-generation plasticity (developmental temperature) and reproductive environment (mesocosm) temperature on mate choice for the full number of F1 individuals (n = 144), while still investigating the influence of TGP (F0 acclimation temperature) and reproductive environment temperature on mate choice for a subset of fish (n = 74).
Figure 1.
Experimental design using two generations of stickleback (Gasterosteus aculeatus) to investigate the role of within-generation plasticity and transgenerational plasticity in mating success of F1 adults. Schematic depicts (a) F0 acute acclimation temperature, F1 offspring developmental temperature and F1 adult reproductive environment (mesocosm) temperature at either ambient (17°C) or simulated climate change (21°C) conditions, and (b) F1 males and females that developed at both 17°C and 21°C assigned to mesocosms set to either 17°C or 21°C (one of six mesocosm pairs is shown).
At the start of the experiment, standard length (±1 mm), sex and weight were determined for each F1 fish. The first dorsal spine of each fish was clipped and stored at −20°C for later genotyping (see below), and fish were kept individually for 2 days in 2 l aquaria with permanent flow-through of filtered seawater at their developmental temperature (17°C or 21°C) to ensure they were in good condition at the start of the experiment. Twelve mating groups were then formed, of which six were assigned to mesocosms set to 17°C and six were assigned to mesocosms set to 21°C. Each mating group contained six males (three that developed at 17°C; three that developed at 21°C) and six females (three that developed at 17°C; three that developed at 21°C; n = 144 F1 males and females in total) from mixed F1 families to ensure that potential mating partners were not related, and each mating group contained the same number of F1 families (figure 1b). A full-factorial three-way experimental design (F0 °C × F1 °C × mesocosm °C) was possible for males, with between n = 3 and n = 8 males per three-way combination, but not for females (e.g. no females in the 21°C × 21°C × 17°C combination, but with between n = 2 and n = 6 in all other three-way combinations). Since body size-matching is known to play a strong role in stickleback mate choice [32], our priority was to match sizes within each mesocosm among the 144 F1 males and females from the two developmental temperatures. F1 males and females in each mating group were size-matched within (±5 mm) and between (±2 mm) sexes.
Each mating group was transferred to one of 12 1800 l outdoor mesocosms (see [66] for mesocosm structural details) that contained a mating arena built with 5 mm mesh on a 97 × 97 cm frame positioned on a platform at 50 cm water depth. Each mating arena included six plastic trays (25 × 14 × 6 cm) filled with 1.25 kg of washed sand and 1.5 g nesting material (Wenco Nm 30/3 black sewing thread cut into 5–7 cm lengths conditioned for 2 days in seawater), so that each male could potentially build a nest and establish a territory. The experiment was conducted for 18 days, and nest building activity and adult mortality were checked daily. Temperature data from each mesocosm were measured every 30 min using installed multi-sensor probes (Hydrolab DS5X Probe, OTT Messtechnik GmbH, Kempten, Germany). At the end of the experiment, all fish were recaptured, weighed (±1 mg) and photographed (using a Canon Eos 60 D, Canon EF 100 mm f/2.8L Macro) to determine the standard length (±1 mm) and sex. Fish were then euthanized in an overdose of MS222 and stored at −20°C for later genetic analyses.
Trays with active nests were removed every third day to carefully detach egg clutches under a dissecting microscope to minimize disturbance of the nest. After adding 0.2 g of replacement nest material, trays were put back to the same position in the mesocosm. The experiment was stopped when at least six egg clutches had been collected from each mesocosm. Egg clutches were photographed under a dissecting microscope for later determination of egg size and clutch size using imaging software (LEICA QWIN, Leica Microsystems Imaging Solutions Ltd, Cambridge, UK). Mean egg size per clutch was determined by measuring the diameter of 10 randomly chosen eggs, where the outer edge of each measured egg was clearly visible and not distorted by neighbouring eggs. Clutch size was determined by summing the number of eggs in each clutch. Egg clutches were then transferred into 1000 ml glass beakers containing an air supply and filtered seawater heated to the corresponding mesocosm temperature (17°C or 21°C). Water in the beakers was changed daily until larvae began to hatch. Hatching success was estimated as the proportion of live larvae in relation to total larvae (live and dead) plus dead eggs/embryos after 2 days of hatching (i.e. unfertilized eggs were not included). At 5 days post-hatch, 24 larvae from each clutch were randomly selected, euthanized in MS222 and frozen at −20°C for later determination of larval genotypes.
(b). Genotyping and parentage analysis
All 144 adult F1 fish, as well as 16 F2 larvae from each of 72 randomly chosen clutches (six clutches from each mesocosm; n = 1152 larvae in total), were genotyped at five microsatellite loci (see [48] for loci details). DNA was extracted from spine-clips for adults and from whole 5-day-old larvae using DNeasy Blood and Tissue Kits (Qiagen, Hilden, Germany) following the manufacturer's protocol. The 20 µl multiplex polymerase chain reactions (PCRs) consisted of 10 µl Multiplex Master Mix (Qiagen, Hilden, Germany), 8 µl primer mix (forward and reverse primer of 5196 HEX, 4170 6_FAM, 1125 6_FAM, 1097 NED and 7033 NED) and 2 µl DNA. Thermal cycling started with an initial denaturation step at 95°C for 5 min followed by 30 cycles of 94°C for 1 min, 58°C for 1 min and 72°C for 1 min, and ended with a final extension step at 72°C for 10 min (as in [55]). Amplified fragments were diluted with water (1 : 20), and 1 µl of diluted PCR product was denatured in 15 µl Hi-Di Formamide containing an internal size standard (ROX500; Applied Biosystems, Foster City, CA, USA). Fragments were analysed on an ABI 3130xl sequencer. Electropherograms were manually inspected using PeakScanner 1.0 (Applied Biosystems), and Tandem 1.01 [67] was used to bin final allele sizes.
Parentage analysis was performed using colony 2.0 [68], a likelihood-based method that uses sibship reconstruction to infer genealogies using a group-wise approach. The full likelihood model was used with medium precision allowing for polygamous mating in both sexes. Each mesocosm was analysed separately, with males and females (six potential fathers and mothers each) representing the potential fathers and mothers for the respective clutches collected in that mesocosm (as in [69]). Only ‘best maximum-likelihood (ML) configuration’ assignments with the ML obtained at the end of the computation were used for subsequent analyses. The colony analysis implements a full-pedigree likelihood approach that considers the likelihood of the entire pedigree structure, and thus does not provide specific p-values for each of the pair assignments, and allows the simultaneous inference of parentage and sibship. To obtain high confidence in these assignments, we also investigated the discriminative power of the microsatellite data by conducting an allele frequency analysis in cervus [70]. We calculated the number of alleles per locus, and the polymorphic information content (PIC), as well as combined non-exclusion probabilities for parent pairs, and combined non-exclusion probabilities for an individual identity for each mesocosm separately using only parental genotypes.
(c). Statistical analyses
Fecundity of females (egg size, clutch size) and reproductive success were analysed as generalized linear mixed effect models using the MCMCglmm package [71] in the R statistical environment [72]. For all models, we ran Markov chains of 106 iterations. After burn-in removal of 105 iterations, we kept every 1000th estimate after thinning. We fitted proper but uninformative priors covering half the variance of the trait for each random and fixed effect (i.e. V = 0.5, nu = 0.002) when fitting Poisson or Gaussian response variables, but fixed the variance to 1 when fitting binomial response variables. We checked the resulting Markov chains for autocorrelation and stationary distribution, and only kept chains with an effective sampling size of greater than 500 for each estimated parameter. All models contained mesocosm as a random effect.
Egg size was analysed as a Gaussian response variable using the mean of 10 measured eggs per clutch, and we fitted female size, clutch size, female developmental temperature and oviposition (mesocosm) temperature plus the interactions of female developmental temperature with clutch size and mesocosm temperature. Clutch size was modelled as a Poisson distributed response variable as a function of female size, mean egg size, female developmental temperature and mesocosm temperature plus the interactions of female developmental temperature with mean egg size and mesocosm temperature. Reproductive success was analysed in two ways: first, we investigated the role of within-generation plasticity (developmental temperature) on mate choice and reproductive success and checked whether potential combinations of F1 males and females within one mesocosm had any offspring (mating success) in the two reproductive environment temperatures. Mating success was estimated as the proportion of clutches sired, determined as the number of realized matings divided by the number of all potential matings a given male could have achieved. We analysed this as a binomial response variable using male and female size as covariates, male and female developmental temperature and mesocosm temperature plus all their interactions as fixed effects. Second, we investigated the role of TGP on F1 male mating success (using only those males whose F0 parents were acclimated to either 17°C or 21°C; n = 45) by analysing the proportion of clutches sired (number of realized matings divided by all potential matings) as a binomial response with F1 male size as a covariate, and F0 acclimation temperature, F1 male developmental temperature and mesocosm temperature plus all interactions as fixed effects. Note: we could not fit meaningful models for a potential four-way interaction because F0 temperatures differed between males and females in many cases. Therefore, we decided to fit separate models for each sex, but could not fit a full model for females owing to one missing three-way treatment combination.
3. Results
(a). Offspring assignments
Except for one individual, all 144 parental fish were recovered at the end of the experiment, and all parental genotypes could be reliably determined. In total, 108 egg clutches were collected from the 12 mesocosms over the course of the 18 day experiment, and we randomly chose six clutches per mesocosm for parentage analysis, resulting in 72 clutches genotyped. Of the 16 larvae per clutch genotyped (total 1152 larvae), 51 larval genotypes (4.4%) could not be determined owing to PCR failure. The microsatellite makers were highly informative, with a mean number of alleles per locus across mesocosms of 11.22 (s.d. = 0.71), an average PIC of 0.85 (s.d. = 0.02), an average combined non-exclusion probability per parent pair of 1.1 × 10−5, and a combined non-exclusion probability of identity of 3.05 × 10−8. Consequently, the power of our markers to reliably infer parentage within each mesocosm was very high.
(b). Egg size, clutch size and hatching success
Of the 72 clutches genotyped, 39 contained eggs assigned to a single female. Only these 39 clutches were used for egg size and clutch size analyses. Mean egg size was significantly influenced by mesocosm temperature and the interaction between female developmental temperature and mesocosm temperature (table 1). Overall, eggs laid in 21°C mesocosms were smaller than eggs laid in 17°C mesocosms, and the reduction in egg size in 21°C mesocosms was driven by females that developed at 17°C (figure 2a). Clutch size, on the other hand, was not significantly influenced by female size, egg size, female developmental temperature or mesocosm temperature (table 1). In general, however, females that developed at 21°C tended to have smaller mean clutch sizes (119.44 ± 28.58) than females that developed at 17°C (154.1 ± 50.89; ANOVA F1,37 = 3.77, p = 0.06), but showed a trend of increased clutch size in 21°C mesocosms (figure 2b). Hatching success was nearly 100% for all clutches (mean hatching success 96.7 ± 6%), and did not differ between mesocosm temperatures (ANOVA F1,37 = 1.81, p = 0.19). Fertilization success (embryo clearly visible) ranged from 52.4 to 100%, with a mean fertilization success of 89.6 ± 11%.
Table 1.
Mean egg size and clutch size of stickleback (Gasterosteus aculeatus) females depending on developmental temperature (female temperature; F°C) and oviposition temperature (Mesocosm temperature; Mes°C). Female size, clutch size and mean egg size were included as covariates, and mesocosm was modelled as a random effect. Mean egg size was modelled as a Gaussian distributed response and clutch size was modelled as a Poisson distributed response. Fixed term estimates with 95% confidence intervals are shown, and significant terms are highlighted in bold.
| (A) mean egg size (Gaussian) DIC = −124.720 | |||
|---|---|---|---|
| fixed effects | estimate | 95% CI | p-value |
| female size | 0.036 | −0.026 to 0.092 | 0.264 |
| clutch size | −0.001 | −0.001 to 0.002 | 0.811 |
| female temperature (F°C) | 0.025 | −0.140 to 0.169 | 0.716 |
| mesocosm temperature (Mes°C) | −0.059 | −0.097 to −0.025 | <0.001 |
| clutch size × F°C | −0.001 | −0.002 to 0.001 | 0.251 |
| F°C × Mes°C | 0.080 | 0.002–0.156 | 0.038 |
| random effects | variance component | 95% CI | |
|---|---|---|---|
| mesocosm | 0.000 | 0.000–0.001 | |
| units | 0.002 | 0.001–0.003 |
| (B) clutch size (Poisson) DIC = 340.649 | |||
|---|---|---|---|
| fixed effects | estimate | 95% CI | p-value |
| female size | 0.260 | −0.258 to 0.731 | 0.271 |
| mean egg size | −0.450 | −3.545 to 2.750 | 0.773 |
| female temperature (F°C) | 5.505 | −7.534 to 19.893 | 0.409 |
| mesocosm temperature (Mes°C) | −0.131 | −0.477 to 0.269 | 0.442 |
| mean egg size × F°C | −3.788 | −13.212 to 4.620 | 0.389 |
| F°C × Mes°C | 0.325 | −0.312 to 0.953 | 0.304 |
| random effects | variance component | 95% CI | |
|---|---|---|---|
| mesocosm | 0.003 | 0.000–0.016 | |
| units | 0.142 | 0.073–0.226 |
Figure 2.
Fecundity traits ((a) mean egg size ± s.e. and (b) mean clutch size ± s.e.) of stickleback (Gasterosteus aculeatus) females that developed at either 17°C or 21°C under different oviposition (mesocosm) temperatures (17°C or 21°C). Mean egg size for each clutch was estimated from the diameter (mm) of 10 eggs, and clutch size was estimated as the total number of eggs in a clutch.
(c). Mating success of males and females
In total, 41 out of 72 males in the experiment (i.e. both mesocosm temperatures) managed to sire some offspring, but mating success was heavily skewed toward 17°C males (28 of the 41 males developed at 17°C; p = 0.001; figure 3). Fifty-six of the 72 clutches (77.8%) were sired by a single father (nest owner), 12 clutches contained eggs sired by sneaker males (e.g. father of some larvae within a nest but not the nest owner), of which 10 sneaking events occurred in 17°C mesocosms and two in 21°C mesocosms, and three clutches contained stolen eggs (two between-male thefts at 21°C). Parentage of one clutch was equally split between two males and a nest owner could not be assigned. Of the 56 single-male clutches, 24 were found in 17°C mesocosms and 32 in 21°C mesocosms. Sneaking and egg theft events were equally distributed between male developmental temperatures. The overall distribution of mating success was similar for females (figure 3). Here, 46 out of 72 females managed to reproduce, of which 30 females developed at 17°C and 16 females developed at 21°C ( p = 0.001). Females shared nests more frequently than males, because only 39 out of 72 clutches (54.2%) contained eggs from a single female ( p = 0.004). These were, however, biased towards 17°C females which laid eggs alone in 30 nests, while 21°C females had nine single-female nests.
Figure 3.
Within-generation plasticity effects on mating success of stickleback (Gasterosteus aculeatus) males and females depending on developmental temperature combinations in either 17°C or 21°C reproductive environments (mesocosm temperature). Mating success was estimated as the proportion of clutches sired (number of realized matings divided by the number of all potential matings). Bars depict the proportion for each combination of male–female developmental temperature and mesocosm temperature.
There was a positive relationship between male size and mating success in general (table 2); however, male size also varied with the status of the males in relation to their developmental temperature (electronic supplementary material, figure S1). Specifically, while nest-owning 17°C males were comparatively larger than non-reproducing males, nest owners were significantly smaller when they developed at 21°C (interaction: status (nest owner) × male developmental temperature (17°C) versus status (non-reproducer) × male developmental temperature (21°C); estimate = −0.108, p = 0.043, electronic supplementary material, table S1). Sneaker males, on the other hand, were significantly smaller than nest owners but tended to be larger when they developed at 21°C (estimate = −0.152, p = 0.004, see electronic supplemental material).
Table 2.
Mating success of stickleback (Gasterosteus aculeatus) depending on within-generation plasticity of male and female developmental temperature (male temperature, M°C and female temperature, F°C) and reproductive environment temperature (mesocosm temperature, Mes°C). Male size and female size (standard length, mm) were included as covariates, and mesocosm was modelled as a random effect. Mating success (proportion of clutches sired) was modelled as a binomial response. Fixed term estimates with 95% confidence intervals are shown, and significant terms are highlighted in bold.
| mating success (within-generation plasticity) DIC = 975.237 | |||
|---|---|---|---|
| fixed effects | estimate | 95% CI | p-value |
| male size | 0.895 | 0.089–1.762 | 0.033 |
| female size | −0.019 | −0.734–0.694 | 0.949 |
| male temperature (M°C) | −1.646 | −2.460 – −0.815 | <0.001 |
| female temperature (F°C) | −1.286 | −1.998–0.066 | <0.001 |
| mesocosm temperature (Mes°C) | −0.320 | −0.956–0.318 | 0.300 |
| M°C × F°C | 0.637 | −0.669–2.158 | 0.353 |
| M°C × Mes°C | 1.205 | 0.097–2.162 | 0.011 |
| F°C × Mes°C | 0.250 | −0.899–1.140 | 0.584 |
| M°C × F°C × Mes°C | −1.388 | −3.297–0.563 | 0.180 |
| random effects | variance component | 95% CI | |
|---|---|---|---|
| mesocosm | 0.074 | 0.001–0.269 |
(d). Within-generation plasticity and TGP effects on F1 mating success
Both within-generation plasticity and TGP had significant effects on mating success. In terms of within-generation plasticity, mating success was significantly influenced by male developmental temperature, female developmental temperature and the interaction between male developmental temperature and mesocosm temperature (table 2). Males and females that developed at 17°C had more mating success than those that developed at 21°C (figure 3). The difference was more pronounced for females, as males could partly compensate for their loss in mating success in 21°C mesocosms if they developed at 21°C (male °C × mesocosm °C interaction; table 2 and figure 3). This was also reflected in single-male clutches, where 21°C males sired 18% more clutches in 21°C mesocosms than in 17°C mesocosms (12/32 clutches at 21°C versus 4/20 clutches at 17°C), while mesocosm temperature had no influence on single-female clutches (19 clutches at 17°C and 20 clutches at 21°C).
Male mating success was also influenced by TGP, with significant effects of F0 acclimation temperature, F1 male developmental temperature and their interaction (i.e. parent environment × offspring environment interaction; table 3). Overall, males with a 17°C thermal history across two generations had the highest mating success, males with a 17 × 21 F0 × F1 thermal history had the lowest, and males with F0 parents acclimated to 21°C were intermediate (figure 4). In other words, the mismatch between parent and offspring environments resulted in lower mating success, but only for males with F0 parents acclimated to 17°C, likely driving the F0 × F1 temperature interaction. That both combinations of 21°C F0 × F1 male developmental temperature showed the same response pattern (i.e. only TGP (21 × 17) or both developmental and TGP (21 × 21); figure 4) suggests that the relatively higher mating success for these males was due to effects carried over from F0 parents acclimated to 21°C. Although no terms that included mesocosm temperature were significant in the model (likely owing to low statistical power), there was a trend of higher mating success in 21°C mesocosms for males with F0 parents acclimated to 21°C ( p = 0.087), while no trend across mesocosm temperatures was apparent for males with 17°C F0 parents ( p = 0.200; figure 4).
Table 3.
Mating success of male stickleback (Gasterosteus aculeatus) depending on transgenerational plasticity (TGP) of F0 parents acclimation temperature (F0°C), F1 male developmental temperature (F1 male temperature; M°C) and reproductive environment temperature (Mesocosm temperature; Mes°C). Male size was included as a covariate, and mesocosm was modelled as a random effect. Mating success was modelled as a binomial response for each possible clutch yielding the proportion of clutches sired. Fixed term estimates with 95% confidence intervals are shown, and significant terms are highlighted in bold.
| mating success (TGP) DIC = 569.658 | |||
|---|---|---|---|
| fixed effects | estimate | 95% CI | p-value |
| male size | 0.897 | −1.087 to 2.542 | 0.333 |
| F0°C | −2.183 | −3.690 to −0.401 | 0.004 |
| F1 male temperature (M°C) | −2.231 | −3.924 to −0.593 | 0.007 |
| mesocosm temperature (Mes °C) | 0.018 | −1.803–2.112 | 0.984 |
| F0°C × M°C | 2.825 | 0.803–4.869 | 0.004 |
| F0°C × Mes°C | 0.216 | −1.680–2.254 | 0.824 |
| M°C × Mes°C | −0.047 | −2.080–2.230 | 0.962 |
| F0°C × M°C × Mes°C | 0.582 | −2.631–3.196 | 0.711 |
| random effects | variance component | 95% CI | |
|---|---|---|---|
| mesocosm | 1.552 | 0.063–3.935 |
Figure 4.
Transgenerational plasticity (TGP) effects on mating success of F1 male stickleback (Gasterosteus aculeatus) depending on F0 parent acclimation temperature (F0 °C) and F1 male developmental temperature in either 17°C or 21°C reproductive environments (mesocosm temperature). Mating success was estimated as the proportion of clutches sired (number of realized matings divided by the number of all potential matings). Bars depict the proportion for each combination of F0 temperature–F1 male developmental temperature and mesocosm temperature.
4. Discussion
The most striking finding of our study is that mate choice and reproductive success of stickleback may be influenced by not only within-generation plasticity but also TGP. Most studies of TGP conducted to date have investigated isolated proxies of fitness (e.g. growth, body size, fecundity), but the integration of these components into direct measures of fitness such as reproductive success has not yet been shown. Here, we found that maternal developmental temperature and oviposition environment affected egg size plasticity. We also showed within-generation plasticity of mate choice, in that mating success was influenced by the interaction between paternal developmental temperature and reproductive environment. Additionally, F1 male mating success was also influenced by F0 parent acclimation temperature (parent environment by offspring environment interaction). Our study thus demonstrates the potential role of both within-generation plasticity and TGP in shaping sexual traits involved in mate choice and resulting reproductive success, which may further facilitate rapid responses of species to environmental change.
(a). Thermal plasticity and fecundity under climate change
Stickleback females allocated resources to eggs differently depending on their developmental temperature and immediate oviposition (mesocosm) temperature within 18 days of exposure to changed environmental conditions. Females that developed at 17°C laid smaller eggs in 21°C mesocosms, whereas females that developed at 21°C laid similar sized eggs in both ambient and elevated temperature mesocosms. The reduction in egg size for 17°C females at elevated temperature was not accompanied by an increase in clutch size at 21°C, so likely does not reflect a classic trade-off between egg size and clutch size or adaptive plasticity [3,73,74], but rather some kind of physiological cost associated with higher oviposition temperature. Likewise, females that developed at 21°C did not show a temperature-dependent trade-off between egg size and clutch size, but tended to produce larger clutches at elevated temperature, indicative of a possible physiological benefit (or at least no cost) at higher oviposition temperature.
Previous studies of this population found that adaptive egg size plasticity occurred when mothers were acclimated to different temperatures for as few as six weeks during reproductive conditioning [59]; however, the effects of changed oviposition temperature were not tested. Here, despite life-long development at a specific temperature, females adjusted the allocation of resources to eggs within 18 days of exposure to changed conditions. This demonstrates that mothers can dynamically modify offspring size in the very last stages of egg development (just prior to laying), despite any base-line egg size allocation due to thermal history [58,75]. Nevertheless, changes to egg size due to oviposition temperature probably do not reflect adaptive TGP in this case (e.g. smaller eggs at higher temperature owing to lower egg oxygen demands [56,58,59,76,77]), as females that developed at 21°C did not lay (even) smaller eggs in 21°C mesocosms. Rather, females that developed at 17°C may have spent more energy on metabolism in 21°C mesocosms, resulting in less energy available for egg provisioning, whereas 21°C females should have optimized metabolism at higher temperature [56,60]. Alternatively, 21°C females could not take advantage of 17°C oviposition conditions owing to long-term effects of higher temperature over multiple generations, i.e. smaller body size and smaller egg and clutch sizes [57]. Although female size was not a significant factor in our egg trait analyses, 21°C females were on average 3.1 mm smaller than 17°C females within the single-female clutches analysed (ANOVA: F1,29 = 7.213, p = 0.012).
Hatching success was much higher here under semi-natural conditions (nearly 100%) than in previous laboratory experiments using artificial fertilization [56–58]. While a large difference in hatching success between experiment types is likely due to methods issues (e.g. strip-spawning, high sperm solution concentrations leading to polyspermy (multiple sperms entering an oocyte) resulting in failed fertilizations (see also [78]), and/or calculation bias due to inclusion of unfertilized eggs), the high fertilization and hatching success of the current study speaks to the importance of conducting experiments under semi-natural conditions or in wild populations. For instance, arguments for selective mortality at early developmental stages based on low hatching success in laboratory experiments has led to uncertainty about the importance of TGP as a response mechanism to rapid environmental change [79]. However, while selection may indeed be occurring, it can also be that some of these arguments might be confounded by methodological issues as described above. Furthermore, by allowing breeding adults to express the behavioural and physical components involved in mate choice in direct competition with conspecifics under controlled conditions, we remove any effect of selective breeding and gain an unbiased view of the role of within-generation plasticity and TGP under climate change for phenotypic characters potentially signalling mate quality. Since choice is expected to maximize reproductive success, we reduce the risk of over- or underestimating the role of TGP in offspring viability.
(b). Sneaking males, male size and reproductive environment temperature
Males showed differential expression of alternative reproductive tactics (sneaking and egg theft) depending on the environmental temperature in which mating occurred. That 22.2% of clutches contained either stolen eggs or eggs fertilized by sneaking males demonstrates that these ‘parasitic’ male reproductive tactics are a common strategy in stickleback (see also [44,80]), with potentially large impacts on mating and reproductive success. The majority of sneaking events occurred in 17°C mesocosms under ambient, presumably good, environmental conditions, whereas the two instances of egg theft at 21°C occurred under more stressful temperature conditions. For both tactics, male developmental temperature did not seem to play a role, as males from either thermal history were equally likely to engage in alternative reproductive strategies. Instead, reproductive environment temperature had a strong influence on these behaviours, especially on sneaking, with 83.3% of sneaking events occurring in ambient (17°C) environments. This is in line with another study of stickleback mating which showed a reduction in sneaking in disturbed, eutrophied habitats with dense vegetation [81]. In that study, increased habitat structure reduced the opportunity for sexual selection by relaxing the competition among males for nesting sites [81]. Here, conditions in elevated temperature mesocosms may have been too energetically costly for males to engage in additional reproductive tactics. For example, a study of sailfin mollies (Poecilia latipinna) showed that males exhibited variation in plasticity for mating behaviour (some genotypes switched between courting and sneaking, whereas other genotypes were fixed), and that sneaking was associated with upregulation of genes involved in learning and memory, suggesting that sneaking is more cognitively demanding and energetically costly than courtship [82]. These costs will be exacerbated at high temperature, and male stickleback have been shown to build fewer nests and incubate them less actively at elevated temperature [83]. Therefore, accounting for within-generation plasticity and possibly TGP in mating tactics under climate change is key to understanding the evolutionary potential of these alternative strategies, as TGP could lead to compensation of sneaking costs at high temperature, facilitating an adaptive response over time.
Overall, we found a positive relationship between male size and mating success, but males of smaller size tended to have higher mating success in 21°C mesocosms. Body size is probably the most important visual mate choice cue in stickleback, and numerous studies have shown that females choose either large males [49–51] or males of similar size to themselves [29,30,32,36,52,53]. Here, females chose smaller males under elevated temperature conditions, regardless of their own size, suggesting that small size may be advantageous at high temperature, potentially owing to lower metabolic demands for males and eggs [49]. Furthermore, the chosen males were all nest owners, so the pattern of response was not due to sneaking males. Consequently, our results for mating success do not fit with the hypothesis of phenotype matching based on size (at least for small males), but more likely with female choice based on environment-dependent male condition [21,84]. Such context- or condition-dependent validity of sexual cues can lead to environmentally dependent mate choice preferences with consequences for the strength and direction of selection [21,85].
(c). Within-generation plasticity and TGP effects on mating success
Our results suggest that mate choice and resulting reproductive success of stickleback may be both condition-dependent and context-dependent according to thermal history within and across generations, and reproductive environment temperature. In terms of within-generation plasticity, mating success was highest for 17°C males mated with 17°C females at both mesocosm temperatures, whereas 21°C males had higher reproductive success in 21°C mesocosms when mated with 17°C females. For this population, 17°C is the average summer water temperature [58], and we have previously shown 17°C to be a low- (or no)-stress environment with no negative effects on development, growth and parasite resistance in comparison with 21°C [54–56,58,59]. We have further shown that a thermal history of 21°C across three generations led to smaller fish (males and females) and less fecund females [57]. Here, males that developed at 17°C were likely in better condition, and thus more attractive to 17°C females, who were themselves in better condition and had larger body and clutch sizes, resulting in the condition-dependent choice of 17°C males by 17°C females. Females that developed at 21°C, on the other hand, did not show a strong preference for males from a specific temperature (see also [86] for a similar pattern), and the lower fecundity of 21°C females will also lead to lower reproductive success for both 17°C and 21°C males mating with 21°C females. Females that developed at 21°C were also less choosy because they laid their eggs more often in shared nests. Most interesting, 17°C females chose 21°C males more in 21°C mesocosms, possibly reflecting context- (environment)-dependent choice of within-generation plasticity and/or TGP-optimized phenotypes under climate warming.
TGP may have played a role in F1 male mating success under simulated ocean warming. Previous studies indicate that under these conditions, males with a 21°C thermal history should have an optimized metabolism [56,60], and females may have chosen these males because they should have more energy available to spend on courting behaviour, nest construction, competition, etc. Specifically, while males with a matching 17°C parent–offspring environment thermal history had the highest mating success overall, the mismatch between F0 acclimation temperature and F1 developmental temperature led to the lowest mating success for males with a 17°C × 21°C thermal history. The same pattern was not observed for males with F0 parents acclimated to 21°C, which had on average higher mating success than males in the 17°C × 21°C treatment group. Indeed, the benefits of 21°C F0 parents on mating success were evident for males that developed at both temperatures, indicating that the increase in mating success for these males was due to acclimation effects transferred from 21°C F0 parents and not developmental temperature. In other words, the expression of sexual traits and mate quality cues (e.g. condition-dependent metabolism), and possibly also a mating preference for these traits, may have been altered by TGP [17,18,20]. Interestingly, there was also a pattern of higher mating success in 21°C mesocosms for males with 21°C F0 parents, but no such trend across mesocosm temperatures for males with F0 parents acclimated to 17°C, suggesting that any potential transgenerational compensation between F0 parent environment and F1 offspring reproductive environment was only apparent under simulated climate change.
Mate choice is an important evolutionary process that contributes to the selection for a vast array of traits, but how non-genetic inheritance mechanisms influence sexual selection and resulting mate choice and reproductive success has been little investigated. In their recent review, Head et al. [20] outline how a number of different mechanisms of non-genetic inheritance might contribute to sexual selection. These include maternal provisioning of eggs which can influence expression of condition-dependent traits in offspring which can then become targets of mate choice, maternal transfer of somatic factors like hormones which can influence the expression of sexual traits, and the transmission of epigenetic state (e.g. DNA methylation) which has the potential to supply a source of renewable phenotypic variation in condition, thus maintaining benefits of choice (the same is true for paternal transfer of ejaculate-borne substances; [20]). Here, mate choice was likely influenced by maternal provisioning of eggs in the previous generation [59] leading to differences in growth and condition of F1 offspring breeders depending on thermal history, and possibly to epigenetic state underlying TGP at elevated temperature [60]. Intriguingly, models for different modes of female preference for male condition found that female choice is most probable when male condition is environmentally induced and transmitted over one generation [17], and that environmentally mediated modification of the sperm epigenome via e.g. DNA methylation is one possible mechanism to transmit environmentally induced male condition to offspring [20]. In our study, males displaying high quality mating cues owing to TGP benefits under climate change may have been more attractive to females owing to a greater reproductive effort at elevated temperature, resulting in higher reproductive success, not only for themselves, but potentially also for their offspring owing to attractiveness and/or survival [87,88]. Whether the quality of these mating cues is based on epigenetic modifications to the stickleback sperm epigenome deserves further investigation, as studies of zebrafish sperm methylomes indicate an important role for paternal transgenerational epigenetic effects for offspring traits [89].
5. Conclusion
While we observed both within-generation plasticity and TGP, especially the modification of mate choice and reproductive success by TGP may facilitate faster responses of species to environmental change. Whether these effects are adaptive will depend on the match between parent and offspring environments, as well as the specific environmental conditions under which mating occurs. Transgenerational effects on offspring mate choice cues may be underlain by maternal provisioning of resources and somatic factors, but also parental (maternal and paternal) variation in epigenetic state. If parents transmit their condition to offspring via transgenerational epigenetic inheritance, then non-genetic inheritance of fitness provides an alternative to genetic variation in fitness as a potential mechanism for the maintenance of costly mate preference [17]. As such, transgenerational effects on reproductive phenotypes may have implications for individual fitness and population dynamics under climate change.
Supplementary Material
Acknowledgements
Many thanks to Petra Kadel for keeping the mesocosms running, Kaibil Escobar Wolf for taking care of the fish, Andreas Kornmann for help installing the flow-through system, Nancy Kühne for help with the genotyping, the Community and Evolutionary Ecology group for feeding fish on weekends, and two anonymous reviewers for helpful comments. This paper is dedicated to Martin Kalbe.
Ethics
The study was conducted in accordance with German animal welfare standards (Schleswig-Holstein Ministerium für Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung (Tierschutz), permit no. V244-17922/2018(38-4/18).
Data accessibility
Data deposited at PANGAEA: https://doi.pangaea.de/10.1594/PANGAEA.892840.
Authors' contributions
L.N.S.S. and K.M.W. conceived the experiment, L.F. and S.W. conducted the experiment, L.F., S.W. and H.A. collected the genotype and phenotypic data, L.F. and E.R. conducted the parentage analysis, L.N.S.S. and K.M.W. carried out the statistical analyses, L.N.S.S. wrote the first draft of the manuscript, and all authors contributed to the final version. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
The study was funded by the PACES II Research Programme of the Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung. E.R. was funded by the Austrian Science Fund (FWF) via a Hertha Firnberg Fellowship (FWF T699-B24).
References
- 1.IPCC. 2014. Climate change 2014: synthesis report. Contribution of Working Groups I, I and III to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri RK, Meyer LA). Geneva, Switzerland: IPCC. See https://www.ipcc.ch/report/ar5/syr/. [Google Scholar]
- 2.Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. ( 10.1111/ele.12185) [DOI] [PubMed] [Google Scholar]
- 3.Mousseau AT, Fox CW. 1998. The adaptive significance of maternal effects. TREE 13, 4037. [DOI] [PubMed] [Google Scholar]
- 4.Crozier LG, et al. 2008. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol. Appl. 1, 252–270. ( 10.1111/j.1752-4571.2008.00033.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engqvist L, Reinhold K, Davey M. 2016. Adaptive trans-generational phenotypic plasticity and the lack of an experimental control in reciprocal match/mismatch experiments. Methods Ecol. Evol. 7, 1482–1488. ( 10.1111/2041-210X.12618) [DOI] [Google Scholar]
- 6.Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonduriansky R, Crean AJ, Day T. 2012. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 5, 192–201. ( 10.1111/j.1752-4571.2011.00213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donelson JM, Salinas S, Munday PL, Shama LNS. 2018. Transgenerational plasticity and climate change experiments: where do we go from here? Glob. Change Biol. 24, 13–34. ( 10.1111/gcb.13903) [DOI] [PubMed] [Google Scholar]
- 9.Salinas S, Brown SC, Mangel M, Munch SB. 2013. Non-genetic inheritance and changing environments. Non-Genetic Inheritance 1, 38–50. [Google Scholar]
- 10.Donelson JM, Wong M, Booth DJ, Munday PL. 2016. Transgenerational plasticity of reproduction depends on rate of warming across generations. Evol. Appl. 9, 1072–1081. ( 10.1111/eva.12386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenfield MD, Rodriguez RL. 2004. Genotype–environment interaction and the reliability of mating signals. Anim. Behav. 68, 1461–1468. ( 10.1016/j.anbehav.2004.01.014) [DOI] [Google Scholar]
- 12.Clutton-Brock TH. 1988. Reproductive success. Studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 13.Bateson PPG. 1983. Mate choice. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Kokko H, Brooks R, Jennions MD, Morley J. 2003. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B 270, 653–664. ( 10.1098/rspb.2002.2235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edward DA. 2015. The description of mate choice. Behav. Ecol. 26, 301–310. ( 10.1093/beheco/aru142) [DOI] [Google Scholar]
- 16.Ingleby FC, Hunt J, Hosken DJ. 2010. The role of genotype-by-environment interactions in sexual selection. J. Evol. Biol. 23, 2031–2045. ( 10.1111/j.1420-9101.2010.02080.x) [DOI] [PubMed] [Google Scholar]
- 17.Bonduriansky R, Day T. 2013. Nongenetic inheritance and the evolution of costly female preference. J. Evol. Biol. 26, 76–87. ( 10.1111/jeb.12028) [DOI] [PubMed] [Google Scholar]
- 18.Qvarnström A, Price DT. 2001. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 16, 95–100. ( 10.1016/S0169-5347(00)02063-2) [DOI] [PubMed] [Google Scholar]
- 19.Pfennig DW, Servedio MR. 2013. The role of transgenerational epigenetic inheritance in diversification and speciation. Non-Genetic Inheritance 1, 17–26. ( 10.2478/ngi-2013-0002) [DOI] [Google Scholar]
- 20.Head ML, Jennions MD, Zajitschek SRK. 2016. Sexual selection: incorporating non-genetic inheritance. Curr. Opin. Behav. Sci. 12, 129–137. ( 10.1016/j.cobeha.2016.10.005) [DOI] [Google Scholar]
- 21.Heuschele J, Mannerla M, Gienapp P, Candolin U. 2009. Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 20, 1223–1227. ( 10.1093/beheco/arp123) [DOI] [Google Scholar]
- 22.Head ML, Fox RJ, Barber I. 2017. Environmental change mediates mate choice for an extended phenotype, but not for mate quality. Evolution 71, 135–144. ( 10.1111/evo.13091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Candolin U, Salesto T, Evers M. 2007. Changed environmental conditions weaken sexual selection in sticklebacks. J. Evol. Biol. 20, 233–239. ( 10.1111/j.1420-9101.2006.01207.x) [DOI] [PubMed] [Google Scholar]
- 24.Sundin J, Berglund A, Rosenqvist G. 2010. Turbidity hampers mate choice in a pipefish. Ethology 116, 713–721. [Google Scholar]
- 25.Michelangeli M, Wong BBM, Chapple DG. 2015. It's a trap: sampling bias due to animal personality is not always inevitable. Behav. Ecol. 27, 62–67. ( 10.1093/beheco/arv123) [DOI] [Google Scholar]
- 26.Lacy RCS. 1983. Kin recognition by phenotype matching. Am. Nat. 121, 489–512. ( 10.1086/284078) [DOI] [Google Scholar]
- 27.Jiang Y, Bolnick DI, Kirkpatrick M. 2013. Assortative mating in animals. Am. Nat. 181, E125–E138. ( 10.1086/670160) [DOI] [PubMed] [Google Scholar]
- 28.Shine R, O'Connor D, Lemaster MP, Mason RT. 2001. Pick on someone your own size: ontogenetic shifts in mate choice by male garter snakes result in size-assortative mating. Anim. Behav. 61, 1133–1141. ( 10.1006/anbe.2001.1712) [DOI] [Google Scholar]
- 29.McKinnon JS, Rundle HD. 2002. Speciation in nature: the threespine stickleback model systems. Trends Ecol. Evol. 17, 480–488. ( 10.1016/S0169-5347(02)02579-X) [DOI] [Google Scholar]
- 30.McKinnon JS, Mori S, Blackman BK, Lior D, Kingsley DM, Jamieson L, Chou J, Schluter D. 2004. Evidence for ecology's role in speciation. Nature 429, 294–298. ( 10.1038/nature02556) [DOI] [PubMed] [Google Scholar]
- 31.Baldauf SAK, Schroth SH, Thünken T, Bakker TCM. 2009. You can't always get what you want: size assortative mating by mutual mate choice as a resolution of sexual conflict. BMC Evol. Biol. 9, 129 ( 10.1186/1471-2148-9-129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conte GL, Schluter D. 2013. Experimental confirmation that body size determines mate preference via phenotype matching in a stickleback species pair. Evolution 67, 1477–1484. ( 10.1111/evo.12041) [DOI] [PubMed] [Google Scholar]
- 33.Bay RA, et al. 2017. Genetic coupling of female mate choice with polygenic ecological divergence facilitates stickleback speciation. Curr. Biol. 27, 3344–3349. ( 10.1016/j.cub.2017.09.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzi DK, Bakker TCM. 2003. Female preference for symmetry in computer-animated three-spined stcklebacks, Gasterosteus aculeatus. Behav. Ecol. Sociobiol. 54, 156–161. [Google Scholar]
- 35.Jiggins CDN, Coe RL, Mallet J. 2001. Reproductive isolation caused by colour pattern mimicry. Nature 411, 302–305. ( 10.1038/35077075) [DOI] [PubMed] [Google Scholar]
- 36.Kitano J, Mori S, Peichel CL. 2007. Phenotypic divergence and reproductive isolation between sympatric forms of Japanese threespine sticklebacks. Biol. J. Linn. Soc. 91, 671–685. ( 10.1111/j.1095-8312.2007.00824.x) [DOI] [Google Scholar]
- 37.Milinski M. 2006. The major histocompatibility complex, sexual selection, and mate choice. Ann. Rev. Ecol. Evol. Syst. 37, 159–186. ( 10.1146/annurev.ecolsys.37.091305.110242) [DOI] [Google Scholar]
- 38.Roth O, Sundin J, Berglund A, Rosenqvist G, Wegner KM. 2014. Male mate choice relies on major histocompatibility complex class I in a sex-role-reversed pipefish. J. Evol. Biol. 27, 929–938. ( 10.1111/jeb.12365) [DOI] [PubMed] [Google Scholar]
- 39.Bell AM, Foster SA. 1994. The evolutionary biology of the threespine stickleback. Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Hendry AP, Boughman JW, Peichel CL, Matthews B, Nosil P. 2013. Stickleback research: the now and the next. Evol. Ecol. Res. 15, 111–141. [Google Scholar]
- 41.Tinbergen N. 1951. The study of instinct. Oxford, UK: Clarendon Press/University of Michigan. [Google Scholar]
- 42.Wooten RJ. 1984. A functional biology of sticklebacks. Berkely, CA: University of California Press. [Google Scholar]
- 43.Östlund-Nilsson SM, Huntingford FA. 2007. Biology of the three-spined stickleback. London, UK: Taylor & Francis. [Google Scholar]
- 44.Lagiadér RC, Fries V, Bakker TCM. 2001. Genetic analysis of sneaking and egg thievery in a natural population of the three-spined stickleback (Gasterosteus aculeatus L.). Heredity 86, 459–468. ( 10.1046/j.1365-2540.2001.00850.x) [DOI] [PubMed] [Google Scholar]
- 45.Candolin U, Tukiainen I, Bertell E. 2016. Environmental change disrupts communication and sexual selection in a stickleback population. Ecology 97, 969–979. ( 10.1890/15-1090.1) [DOI] [PubMed] [Google Scholar]
- 46.Milinsky M, Bakker TCM. 1990. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344, 330–333. ( 10.1038/344330a0) [DOI] [Google Scholar]
- 47.Eizaguirre C, Yeates SE, Lenz TL, Kalbe M, Milinski M. 2009. MHC-based mate choice combines good genes and maintenance of MHC polymorphism. Mol. Ecol. 18, 3316–3329. ( 10.1111/j.1365-294X.2009.04243.x) [DOI] [PubMed] [Google Scholar]
- 48.Kalbe M, et al. 2009. Lifetime reproductive success is maximized with optimal major histocompatibility complex diversity. Proc. R. Soc. B 276, 925–934. ( 10.1098/rspb.2008.1466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraak SBM, Bakker TCM, Mundwiler B. 1999. Sexual selection in sticklebacks in the field: correlates of reproductive, mating, and paternal success. Behav. Ecol. 10, 696–706. ( 10.1093/beheco/10.6.696) [DOI] [Google Scholar]
- 50.Jones FC, Brown C, Braithwaite VA. 2008. Lack of assortative mating between incipient species of stickleback from a hybrid zone. Behaviour 145, 463–484. ( 10.1163/156853908783929179) [DOI] [Google Scholar]
- 51.Sparkes TC, Rush V, Kopp DA, Foster SA. 2013. Reproductive success in a natural population of male three-spined stickleback Gasterosteus aculeatus: effects of nuptial colour, parasites and body size. J. Fish Biol. 82, 1720–1727. ( 10.1111/jfb.12083) [DOI] [PubMed] [Google Scholar]
- 52.Boughman JW, Rundle HD, Schluter D. 2005. Parallel evolution of sexual isolation in sticklebacks. Evolution 59, 361–373. ( 10.1111/j.0014-3820.2005.tb00995.x) [DOI] [PubMed] [Google Scholar]
- 53.Nagel L, Schluter D. 1998. Body size, natural selection and speciation in sticklebacks. Evolution 52, 209–218. ( 10.1111/j.1558-5646.1998.tb05154.x) [DOI] [PubMed] [Google Scholar]
- 54.Ramler D, Mitteroecker P, Shama LNS, Wegner KM, Ahnelt H. 2014. Nonlinear effects of temperature on body form and developmental canalization in the threespine stickleback. J. Evol. Biol. 27, 497–507. ( 10.1111/jeb.12311) [DOI] [PubMed] [Google Scholar]
- 55.Schade FM, Shama LNS, Wegner KM. 2014. Impact of thermal stress on evolutionary trajectories of pathogen resistance in three-spined stickleback (Gasterosteus aculeatus). BMC Evol. Biol. 14, 164 ( 10.1186/s12862-014-0164-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shama LNS, Strobel A, Mark FC, Wegner KM. 2014. Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct. Ecol. 28, 1482–1493. ( 10.1111/1365-2435.12280) [DOI] [Google Scholar]
- 57.Shama LNS, Wegner KM. 2014. Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J. Evol. Biol. 27, 2297–2307. ( 10.1111/jeb.12490) [DOI] [PubMed] [Google Scholar]
- 58.Shama LNS. 2015. Bet hedging in a warming ocean: predictability of maternal environment shapes offspring size variation in marine sticklebacks. Glob. Change Biol. 21, 4387–4400. ( 10.1111/gcb.13041) [DOI] [PubMed] [Google Scholar]
- 59.Shama LNS. 2017. The mean and variance of climate change in the oceans: hidden evolutionary potential under stochastic environmental variability in marine sticklebacks. Sci. Rep. 7, 8889 ( 10.1038/s41598-017-07140-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shama LN, Mark FC, Strobel A, Lokmer A, John U, Wegner KM. 2016. Transgenerational effects persist down the maternal line in marine sticklebacks: gene expression matches physiology in a warming ocean. Evol. Appl. 9, 1096–1111. ( 10.1111/eva.12370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryu T, Veilleux HD, Donelson JM, Munday PL, Ravasi T. 2018. The epigenetic landscape of transgenerational acclimation to ocean warming. Nat. Clim. Change 8, 504–509. ( 10.1038/s41558-018-0159-0) [DOI] [Google Scholar]
- 62.Daufresne M, Lengfeller K, Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793. ( 10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaplan RH. 1992. Greater maternal investment can decrease offspring survival in the frog Bombina orientalis. Ecology 73, 280–288. ( 10.2307/1938739) [DOI] [Google Scholar]
- 64.Morrongiello JR, Bond NR, Crook DA, Wong BB. 2012. Spatial variation in egg size and egg number reflects trade-offs and bet-hedging in a freshwater fish. J. Anim. Ecol. 81, 806–817. ( 10.1111/j.1365-2656.2012.01961.x) [DOI] [PubMed] [Google Scholar]
- 65.Wong BB, Jarvenpaa M, Lindstrom K. 2009. Risk-sensitive mating decisions in a visually compromised environment. Biol. Lett. 5, 600–602. ( 10.1098/rsbl.2009.0350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pansch A, Winde V, Asmus R, Asmus H. 2016. Tidal benthic mesocosms simulating future climate change scenarios in the field of marine ecology. Limnol. Oceanogr. Methods 14, 257–267. ( 10.1002/lom3.10086) [DOI] [Google Scholar]
- 67.Matschiner M, Salzburger W. 2009. TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25, 1982–1983. ( 10.1093/bioinformatics/btp303) [DOI] [PubMed] [Google Scholar]
- 68.Wang J, Santure AW. 2009. Parentage and sibship inference from multilocus genotype data under polygamy. Genetics, 181, 1579–1594. ( 10.1534/genetics.108.100214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rausch AM, Hödl W, Ringler E, Jehle R, Sztatecsny M. 2014. Male body size and parental relatedness but not nuptial colouration influence paternity success during scramble competition in Rana arvalis. Behaviour 151, 1869–1884. ( 10.1163/1568539X-00003220) [DOI] [Google Scholar]
- 70.Kalinowski ST, Taper ML, Marshall TC. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. ( 10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 71.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 72.R Core Team RC. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 73.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 74.Räsänen K, Kruuk LEB. 2007. Maternal effects and evolution at ecological time-scales. Funct. Ecol. 21, 408–421. ( 10.1111/j.1365-2435.2007.01246.x) [DOI] [Google Scholar]
- 75.Burton T, Metcalfe NB.. 2014. Can environmental conditions experienced in early life influence future generations? Proc. R. Soc. B 281, 20140311 ( 10.1098/rspb.2014.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolm N, Ahnnesjö I. 2005. Do egg size and parental care coevolve in fishes? J. Fish Biol. 66, 1499–1515. ( 10.1111/j.0022-1112.2005.00777.x) [DOI] [Google Scholar]
- 77.Bownds C, Wilson R, Marshall DJ. 2010. Why do colder mothers produce larger eggs? An optimality approach. J. Exp. Biol. 213, 3796–3801. ( 10.1242/jeb.043356) [DOI] [PubMed] [Google Scholar]
- 78.Mehlis M, Bakker TCM. 2014. The influence of ambient water temperature on sperm performance and fertilization success in three-spined sticklebacks (Gasterosteus aculeatus). Evol. Ecol. 28, 655–667. ( 10.1007/s10682-014-9707-x) [DOI] [Google Scholar]
- 79.Kielland ON, Bech C, Einum S. 2017. No evidence for thermal transgenerational plasticity in metabolism when minimizing the potential for confounding effects. Proc. R. Soc. B 284, 20162494 ( 10.1098/rspb.2016.2494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones AG, Ostlund-Nilsson S, Avise JC. 1998. A microsattelite assessment of sneaked fertilizations and egg thievery in the fifteenspine stickleback. Evolution 52, 848–858. ( 10.1111/j.1558-5646.1998.tb03709.x) [DOI] [PubMed] [Google Scholar]
- 81.Candolin UV. 2013. Estimating the dynamics of sexual selection in changing environments. Evol. Biol. 40, 589–600. ( 10.1007/s11692-013-9234-7) [DOI] [Google Scholar]
- 82.Fraser BA, Janowitz I, Thairu M, Travis J, Hughes KA. 2014. Phenotypic and genomic plasticity of alternative male reproductive tactics in sailfin mollies. Proc. R. Soc. B 281, 20132310 ( 10.1098/rspb.2013.2310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopkins K, Moss BR, Gill AB. 2011. Increased ambient temperature alters the parental care behaviour and reproductive success of the three-spined stickleback (Gasterosteus aculeatus). Environ. Biol. Fishes 90, 121–129. ( 10.1007/s10641-010-9724-8) [DOI] [Google Scholar]
- 84.Robinson MR, van Doorn GS, Gustafsson L, Qvarnstrom A.. 2012. Environment-dependent selection on mate choice in a natural population of birds. Ecol. Lett. 15, 611–618. ( 10.1111/j.1461-0248.2012.01780.x) [DOI] [PubMed] [Google Scholar]
- 85.McGhee KE, Feng S, Leasure S, Bell AM. 2015. A female's past experience with predators affects male courtship and the care her offspring will receive from their father. Proc. R. Soc. B 282, 20151840 ( 10.1098/rspb.2015.1840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bakker TCMK, Mazzi D. 1999. Condition related mate choice in sticklebacks. Nature 401, 234 ( 10.1038/45727) [DOI] [Google Scholar]
- 87.Reynolds JDG. 1992. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc. R. Soc. Lond. B 250, 57–62. ( 10.1098/rspb.1992.0130) [DOI] [Google Scholar]
- 88.Evans JP, Kelley JL, Bisazza A, Finazzo E, Pilastro A. 2004. Sire attractiveness influences offspring performance in guppies. Proc. R. Soc. Lond. B 271, 2035–2042. ( 10.1098/rspb.2004.2815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang L, et al. 2013. Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell 153, 773–784. ( 10.1016/j.cell.2013.04.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data deposited at PANGAEA: https://doi.pangaea.de/10.1594/PANGAEA.892840.