Abstract
Little is known about the life-history trade-offs and limitations, and the physiological mechanisms that are associated with phenotypic adaptation to future ocean conditions. To address this knowledge gap, we investigated the within- and trans-generation life-history responses and aerobic capacity of a marine polychaete, Ophryotrocha labronica, to elevated temperature and elevated temperature combined with elevated salinity for its entire lifespan. In addition, transplants between treatments were carried out at both the egg mass and juvenile stage to identify the potential influence of developmental effects. Within-generation, life-history trade-offs caused by the timing of transplant were only detected under elevated temperature combined with elevated salinity conditions. Polychaetes transplanted at the egg mass stage grew slower and had lower activities of energy metabolism enzymes but reached a larger maximum body size and lived longer when compared with those transplanted as juveniles. Trans-generation exposure to both elevated temperature and elevated temperature and salinity conditions restored 20 and 21% of lifespan fecundity, respectively. Trans-generation exposure to elevated temperature conditions also resulted in a trade-off between juvenile growth rates and lifespan fecundity, with slower growers showing greater fecundity. Overall, our results suggest that future ocean conditions may select for slower growers. Furthermore, our results indicate that life-history trade-offs and limitations will be more prevalent with the shift of multiple global change drivers, and thus there will be greater constraints on adaptive potential.
This article is part of the theme issue ‘The role of plasticity in phenotypic adaptation to rapid environmental change’.
Keywords: adaptive phenotypic plasticity, natural selection, costs, aerobic capacity, longevity, fecundity
1. Introduction
Adaptive phenotypic plasticity and genetic adaptation are two processes which can enable phenotypic adaptation, and thus population persistence, in the face of rapid environmental change [1–3]. Adaptive phenotypic plasticity refers to the ability of a genotype to produce multiple phenotypes that have higher fitness across multiple environments [1] and can function both within (i.e. developmental plasticity [4]) and across generations (i.e. trans-generation plasticity [5]). By contrast, genetic adaptation is the process by which a population evolves towards a phenotype that best suits the present environmental conditions over multiple generations and is driven by natural selection [6]. These two adaptive processes are not mutually exclusive as phenotypic plasticity can act to facilitate genetic adaptation [7–10]. Importantly, both phenotypic plasticity and genetic adaptation are subject to various costs and limitations that can constrain the evolution of fitness-related life-history traits [11–13]. Understanding the potential for adaptive processes, as well as assessing the fitness consequences associated with them, is paramount to accurately forecast how populations will be affected by a rapid environmental change, such as ongoing global climate change [14–16].
It has been recognized for some time that genetic adaptation can occur over climate change relevant time-scales [17–20]. However, only in recent years has interest in the potential for adaptive processes to facilitate marine metazoan population persistence in the face of rapid climate change grown [6,14,15]. Numerous studies have now demonstrated that both adaptive phenotypic plasticity and natural selection can buffer the negative effects on life-history traits that are often associated with various ocean change stressors, including ocean warming [21–23], ocean acidification [24–26] and changes in salinity [27,28]. The ability of an organism to maintain life-history trait performance under future ocean conditions will ultimately depend on its ability to adjust both its energetic demands and aerobic capacity to match new environmental conditions [29]. To this extent, mitochondria are likely to be a key player in defining the environmental conditions that an organism can tolerate and in which it can thrive, owing to the predominant role they play in aerobic metabolism and bioenergetics [30,31]. Mitochondrial efficiency and respiration rates can be impaired under acute environmental stress owing to the failure of metabolic enzymes and mitochondrial complexes that govern substrate oxidation, electron transport and oxidative phosphorylation [32,33]. Given enough time, however, alterations in mitochondrial properties (e.g. mitochondrial volume density and enzymatic function) can allow aerobic functions to be accommodated to new conditions [23,30].
Although most findings from experiments investigating adaptive processes to-date are encouraging, many studies share similar limitations. Firstly, most focus on a single global change scenario, and thus less is known about how effective plastic responses will be, and whether adaptation may occur, when multiple global change drivers interact (but see [21,34,35]). Secondly, most studies have focused solely on the performances of early life-history stages (i.e. larvae and juveniles) [16,36], thus neglecting adult life stages (but see [21,34,37]). Longevity in particular has been ignored when investigating the impacts of future ocean change on marine organisms, despite its fundamental importance in life-history evolution [11], most probably owing to the technical issues involved in performing studies that span the entire life of an organism. Consequently, our current understanding of the life-history trade-offs and limitations associated with phenotypic plasticity and natural selection is limited [16,36], particularly for marine organisms, which reduces the accuracy of models that predict how populations and ecosystems will respond to future climate change [15].
Adaptive processes, such as adjusting aerobic capacity, entail energetically demanding physiological costs [12]. Consequently, they can result in trade-offs between traits owing to resource limitations. For example, through developmental plasticity, the coral reef fish Acanthochromis polyacanthus was able to restore aerobic scope following exposure to elevated temperature, but at the cost of reduced body size [38]. In turn, trade-offs represent the fitness costs paid when a positive change in one trait, physiological or life-history, causes a negative change in another [11,13]. The enhanced performances of organisms observed in early life stages in experiments conducted to-date [23,24,39] could therefore have negative consequences later in life. Indeed, trade-offs between early life-stage traits and adult traits are common [40–42]. In addition to trade-offs, adaptive processes also have limitations that become evident when a trait cannot be recovered to its optimum and can be imposed by physiological costs [43]. While some studies have shown that adaptive processes can fully restore a life-history trait under future ocean conditions [21,25,44], others have observed only partial compensation [23,26]. By contrast, the behavioural abnormalities observed in coral reef fishes subjected to ocean acidification conditions were not rectified through trans-generation exposure [45,46]. Our understanding of how adaptive processes affect key fitness-associated life-history traits such as total reproductive output, maximum body size and longevity is limited. Determining which phenotypic traits respond to adaptive processes, and which do not, in addition to identifying relationships between traits (in particular trade-offs) has been highlighted as a research priority [16,36].
Here, we investigate the life-history and metabolic responses of an emerging marine model organism, the marine benthic polychaete Ophryotrocha labronica (La Greca & Bacci, 1962), following within- and trans-generation exposure to future ocean conditions. The aims of the present study were two-fold. Firstly, to determine the within- and trans-generation responses to future ocean conditions. Secondly, to determine the presence of trade-offs between early life and adult life-history traits after within-generation exposure to future ocean conditions and how these are modified by trans-generation exposure. The population of O. labronica used in this study originated from the Mediterranean Sea, an area that has been identified as one of the most prominent ‘hot-spots’ in future climate change projections [47]. Additionally, precipitation is expected to decrease significantly, causing the Mediterranean Sea to become more saline [48]. Consequently, we chose to use both elevated temperature and salinity as our climate change drivers. The parental generation (F1) was reared under control (27°C and salinity 35 parts per thousand (ppt)), elevated temperature (30°C and salinity 35 ppt) and elevated temperature combined with elevated salinity (30°C and salinity 39 ppt) conditions. Juveniles from control parents were reared in all conditions (within-generation exposure), whereas juveniles from the two experimental treatments were only reared in the same conditions as their parents (trans-generation exposure). Comparisons between treatments allowed us to determine the within-generation effects of experimental conditions and how these were modified by parental exposure. Ophryotrocha labronica undergoes direct development. Therefore, developmental responses can be identified by transplanting egg masses and juveniles between treatments. Throughout the experiment we measured a range of early life-stage (juvenile survival and growth) and adult (maximum body size, lifespan fecundity and longevity) life-history traits. Furthermore, one enzyme (citrate synthase (CS)) and one enzymatic complex (electron transport system (ETS)) were measured in adults as proxies for energy metabolism and aerobic capacity to uncover the underlying physiological mechanisms behind any observed responses.
2. Material and methods
(a). Study organism, collection and maintenance
Ophryotrocha labronica is a gonochoric, iteroparous species with semi-continuous reproduction: females reproduce many times over an extended breeding period (average number of eggs produced per spawning event = 120 [49]). Eggs are externally fertilized by males and parental care is required until they hatch [26]. Ophryotrocha labronica individuals are easy to culture in large numbers and have a relatively short generation time (approx. three to four weeks at 24°C to produce the first progeny) and lifespan (between five and six months) [50]. These traits make it an ideal study species for trans- and multi-generation studies [21,25,34]
The population of O. labronica used in this study originally came from approximately 100 individuals collected in January 2014 in the harbour of Porto Empedocle (Sicily, Italy; 37°17′4″ N, 13°31′3″ E). Polychaetes were kept at the Marine Biology and Ecology Laboratory of the University of Modena and Reggio Emilia (Modena, Italy) in culture for approximately six generations at relatively constant salinity (mean ± s.e.: 35 ppt ± 1) (obtained by dissolving an artificial sea salt—Reef Crystals, Instant Ocean—in distilled water) and photoperiod (12 L : 12 D), but at variable temperature (min/max = 15–27°C), to mimic natural seasonal variation. Polychaetes were fed once a week on minced spinach [50]. In December 2014, 80 individuals were transported to the Marine Evolutionary Physiology Laboratory at the Université du Québec à Rimouski (Canada) and reared for two generations at 27°C (current day maximum summer temperature and control temperature for the experiment) under the same salinity and photoperiod conditions to allow time for acclimation to the new stable thermal control conditions.
(b). Experimental design
(i). Experimental conditions
Our elevated temperature level (30°C) represents + 3°C relative to the current maximum summer temperatures recorded in the collection site, consistent with warming projections [51]. Our elevated salinity level (39 ppt) represents conditions that may be experienced by O. labronica in the field in some locations of the Mediterranean and which this species will be exposed to much more frequently in the future owing to the significant rise in the frequency of extreme heat events that are predicted to occur over the next century leading to increased evaporation [52]. The effect of elevated salinity in isolation was not investigated as it is less ecologically relevant under future ocean scenarios.
(ii). Parental conditioning
Twelve pairs (F0) were formed and bred. Three days after hatching, 20 offspring (F1) from each pair were assigned to either control (C: 27°C, salinity 35 ppt), elevated temperature (T: 30°C, salinity 35 ppt) or elevated temperature and salinity conditions (TS: 30°C, salinity 39 ppt) (four six-well culture plates per treatment; three broods per plate). This ensured that there was similar genetic diversity between all treatments at the start of the experiment and aided comprehension of genetic and non-genetic offspring responses [14]. Stable temperatures were achieved by placing the culture plates in incubators (Sanyo MLR-351 HI, Moriguchi City, Japan) and a 12 L : 12 D regime was implemented. Plates from the same treatment were kept on separate shelves in the incubator. To reduce evaporation and maintain a stable salinity, plates were covered with a breathable seal (Aeraseal, Alpha Laboratories Ltd, Eastleigh, UK). Throughout the experiment, polychaetes were fed ad libitum on minced spinach every day [50] and water changes were performed using sea water at the appropriate temperature and salinity to maintain good water quality.
(iii). Treatment transplants
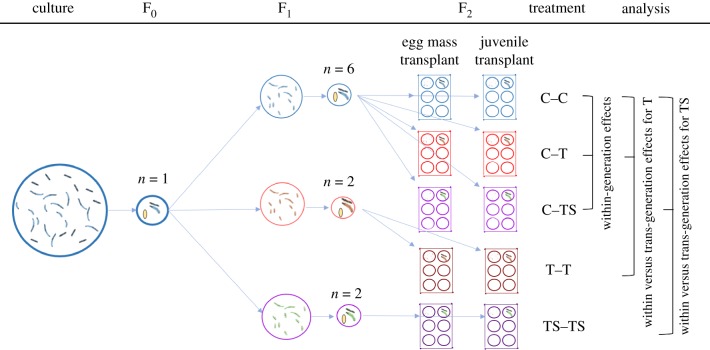
When F1 individuals in the C treatment reached sexual maturity, six pairs per brood were created by crossing males and females from different wells. Half of the pairs were used to produce broods for the egg mass transplants and half to produce broods for the juvenile transplants. This enabled us to perform transplants to C (C–C), T (C–T) and TS conditions (C–TS) with individuals originating from the first egg mass that was produced by a pair, thus removing the potentially confounding issue of inter-clutch variation. In the T and TS treatments, only two F1 pairs per brood were formed (one for the egg mass and one for the juvenile transplants), because offspring were only transplanted to the same conditions (T–T and TS–TS). Again, these originated from the first egg mass. Several pairs were unable to produce viable egg masses under T and TS conditions. When this occurred, additional pairs were formed to ensure a sufficient level of replication was achieved [21]. Comparisons between treatments allowed us to determine the within-generation effects of exposure to T and TS conditions (C–C versus C–T versus C–TS) and how these were modified by trans-generation exposure (C–C versus C–T versus T–T and C–C versus C–TS versus T–TS). Please refer to figure 1 for a summary diagram of the experimental design and analysis.
Figure 1.
Summary diagram of experimental design and analysis. For simplicity, only one family line of the 12 formed is shown (n = the number of adult pairs formed). Offspring from each F0 pair were assigned to either control (C: 27°C, salinity 35 ppt), elevated temperature (T: 30°C, salinity 35 ppt) or elevated temperature and salinity conditions (TS: 30°C, salinity 39 ppt). When F1 individuals in the C treatment reached sexual maturity, six F1 pairs per family were created. Offspring from control parents were reared in all conditions. In the T and TS treatments, only two F1 pairs per family were formed, because offspring were only transplanted to the same conditions as their parents. In all treatments, half of the pairs were used to produce broods for the egg mass transplants and half to produce broods for the juvenile transplants.
Egg mass transplants were performed by moving the plate containing the egg mass and the parents to the new treatment, to allow the provision of parental care. Pairs that had not spawned yet within the same plate were transferred to a new plate and kept in the same treatment until spawning occurred. Three days post-hatch, up to 20 juveniles were transferred to a new well within the same plate. Any spare polychaetes were left in the original well and the parents removed. Juvenile transplants were made by moving all offspring in a brood the day of hatching into a new plate. Then, where possible, 20 juveniles were transferred to a new well within the same plate 3 days post-hatch. Any spare individuals from a brood were kept in their well. Spare polychaetes were used to determine CS and ETS activity.
(c). Determination of traits
(i). Indicators of selection
To determine if any selection had occurred in generation F1 we calculated survival to sexual maturity in the F1 offspring. We also quantified reproductive success based on the number of initial pairs formed that produced a viable egg mass. Finally, as it was not always possible to transfer 20 F2 offspring, hatching event success was recorded as the percentage of F1 pairs that were able to produce at least 20 F2 offspring.
(ii). Life-history traits (generation F2)
Juvenile survival and growth rates were determined at 7 days post-hatch. Juvenile survival per brood was expressed as the percentage of individuals that survived relative to the total number of individuals transferred at 3 days post-hatch. Juvenile size was determined by counting the number of chaetigers (i.e. metameric segments bearing bristles) of five randomly selected individuals per well, using a digital camera (3.2 MP, Omax, Bucheon, South Korea) attached to a dissecting microscope (MS5, Leica, St Gallen, Switzerland) at ×2.5 magnification. Juvenile growth rates were then calculated as the number of chaetigers added per day (number of chaetigers d−1).
Upon the individuals of a brood reaching maturity, a pair was formed by crossing a male and female from different wells. All remaining polychaetes were transferred to the brood's spare well. Adult life-history traits were only measured in females because their contribution to life history is more relevant than males [11,50]. Furthermore, it allowed fecundity, size and lifespan to be measured in the same individual. Females' reproductive performances were evaluated by determining the total number of eggs that were produced in an individual's lifespan: here defined as ‘lifespan fecundity’. Lifespan fecundity was standardized to the number of chaetigers (lifespan fecundity cheatiger−1). Egg masses were unfolded under a dissecting microscope and then photographed so that egg counts could be completed later. Body size (defined as the number of chaetigers for each individual) was determined at each reproductive event and subsequently used to identify the maximum body size an individual reached during its lifespan. Egg volume, used as a proxy for egg quality [53], was determined using the second egg mass of each pair. The longest and shortest axes of 10 eggs were measured using ImageJ (http://rsb.info.nih.gov/ij/) and egg volume (expressed as ×10−3 mm3) calculated using the formula:
where A is the short radius and B the long radius. Longevity was defined as the number of days between the death of a female occurring and the day it hatched from its egg mass.
(iii). Activity of energy metabolism markers (generation F2)
CS activity is a proxy for mitochondrial density commonly used to detect changes in the aerobic capacity of marine organisms [54–56]. The ETS is an enzyme complex located on the inner mitochondrial membrane, which is widely accepted as a marker for maximum mitochondrial oxygen consumption [57]. When a pair had produced its second egg mass, the females in the spare well were frozen and stored at −80°C for later analysis of CS activity (two polychaetes per brood) and ETS activity (three–five polychaetes per brood). CS and ETS activity were determined via enzyme assays, conducted in triplicate at 27°C as described by Chakravarti et al. [20] and were expressed as units per mg protein. Detailed protocols on how CS and ETS activity were determined are referred to in the electronic supplementary material.
(d). Statistical analysis
(i). Indicators of selection
We determined the effects of T and TS conditions on juvenile survival and reproductive success of the F1 generation in addition to hatching event success, as indicators of the strength of the selection pressure imposed by the treatments. F1 juvenile survival was tested using a generalized linear model with a binomial link function and was weighted to the number of polychaetes initially placed in a well. Differences in reproductive success between treatments were determined using a chi-squared test (χ2), where we expected all the initial pairs created to be able to produce a viable egg mass under all exposure conditions. Similarly, χ2 tests were used to determine the effects that T and TS had on the number of offspring that could be transferred, where we expected to be able to move 20 offspring from each brood.
(ii). Offspring life-history and physiological performance
Within-generation (C–C versus C–T versus C–TS), within versus trans-generation for elevated temperature (C–C versus C–T versus T–T) and within versus trans-generation for elevated temperature combined with elevated salinity (C–C versus C–TS versus TS–TS) responses were analysed separately (figure 1). In all cases, the effects of ‘treatment’, ‘transplant’ and their interaction were tested with ‘incubator shelf’ as a random factor. To analyse the effects on survival, generalized linear mixed-effect models with binomial link functions were used. Additionally, models were weighted to the number of polychaetes initially placed in a well. For all other traits, linear mixed-effects models were used. Normality and homogeneity were assessed using residual plots. All pairwise comparisons were conducted using Tukey's tests. Please note that the purpose of the juvenile transplants in the T–T and TS–TS treatments was to test whether extra handling the polychaetes early in life affected their responses (this was not the case). They are not comparable to the juvenile transplants that were made within-generation and thus when a significant ‘treatment’ × ‘transplant’ interaction was present comparisons between within- and trans-generation juvenile transplant groups were not made. All analyses were conducted in R v. 3.4.0 [58] using the nlme [59], lme4 [60] and multcomp [61] packages. To see the models used and their outputs please refer to the electronic supplementary information.
(iii). Relationships between traits
The relationships between juvenile growth rates per brood and adult traits (lifespan fecundity, maximum body size and longevity) for the within-generation treatments (C–C, C–T and C–TS) were analysed separately. For the C–C and C–T treatments MANOVA tests were used. However, the assumptions of MANOVA were not met for the C–TS treatment and thus three sperate ANOVA were performed (a Bonferroni correction was applied to give a new alpha of 0.017).
To determine how trans-generation exposure affected the relationships between juvenile growth rates per brood and adult traits observed after within-generation exposure, we compared within and trans-generation treatments using egg mass transplant data only with ‘treatment’ as a fixed factor. Again, experimental treatments were analysed separately. MANOVA was used to analyse C–T versus T–T and three separate ANOVA to analyse C–TS versus TS–TS (a Bonferroni correction was applied to give a new alpha of 0.017).
We observed increased lifespan fecundity after trans-generation exposure in both experimental treatments. To determine if these improvements were being driven by changes in longevity and/or maximum body size, we compared relationships between these traits. Analysis was performed as described above (MANOVA was used to analyse C–T versus T–T and ANOVA to analyse C–TS versus TS–TS (a Bonferroni correction was applied to give a new alpha of 0.025)) with ‘treatment’ as a fixed factor.
Finally, if trade-offs between juvenile growth rates and adult life-history trait were observed, we tested the relationship between the adult trait and CS/ETS activity, using ANOVA with either ‘treatment’ or ‘transplant’ as fixed factors, to determine the underlying physiological mechanism. This analysis had to be carried out separately because there was not a CS/ETS activity data point for each family line.
In all models, if ‘treatment’ or ‘transplant’ had no significant effect on the relationship between traits it was removed from the model. Analyses were conducted in R and Prism 7. To see the models used and their outputs, please refer to the electronic supplementary material.
3. Results
(a). Indicators of selection
Juvenile survival in the F1 generation was significantly affected by ‘treatment’ (figure 2a; χ2 = 10.81, p = 0.004). Juvenile survival was 8% lower under elevated TS conditions compared with C conditions (p = 0.004). T conditions had no significant effect on F1 juvenile survival (p = 0.440),
Figure 2.
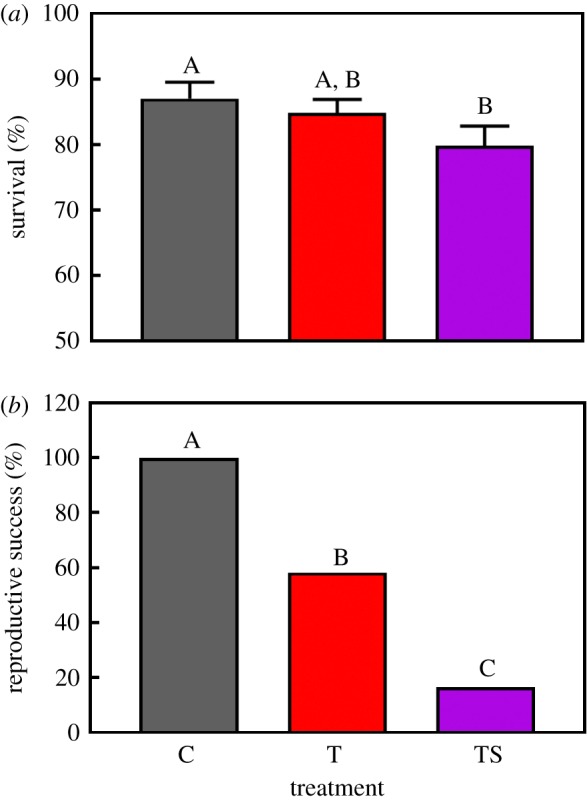
Effects of elevated temperature (T) and elevated temperature combined with elevated salinity conditions (TS) on (a) juvenile survival (n = 12 per treatment) and (b) reproductive success (n = 12 per treatment) of the parental generation (F1) of O. labronica. C = control conditions. Bar chart represents mean values ± s.e. (when applicable). Capital letters represent significant differences between treatments.
Reproductive success was 100% under C conditions but was significantly reduced in the T (58.2%) and TS (16.7%) treatments (figure 2b; min. χ2 = 12.63, p < 0.001). Reproductive success was significantly greater in the T treatment compared with the TS treatment (χ2 = 8.89, p = 0.003). In the T and TS treatments an average of 1.75 and 2.26 pairs per brood, respectively, were required to obtain an egg mass that resulted in offspring hatching.
Hatching event success of egg masses that were kept in C conditions was 100%. This value was significantly greater than egg masses transplanted from C–T and C–TS (figure 3; min. χ2 = 4.80, p = 0.028), which averaged 50 and 8.3%, respectively. There was no significant difference for this trait between either the C–T and T–T treatments or the C–TS and TS–TS treatments (max. χ2 = 1.20, p = 0.273). In contrast to the egg mass transplants, there were no significant differences in hatching event success between the C–C, C–T, C–TS and T–T juvenile transplant treatments (max. χ2 = 2.27, p = 0.132). However, all were significantly greater than the TS–TS treatment (χ2 = 4.20, p = 0.041). Finally, there was a significant difference in hatching event success between egg mass and juvenile C–T and C–TS transplants, being greater for juvenile transplants (min. χ2 = 5.04, p = 0.025).
Figure 3.
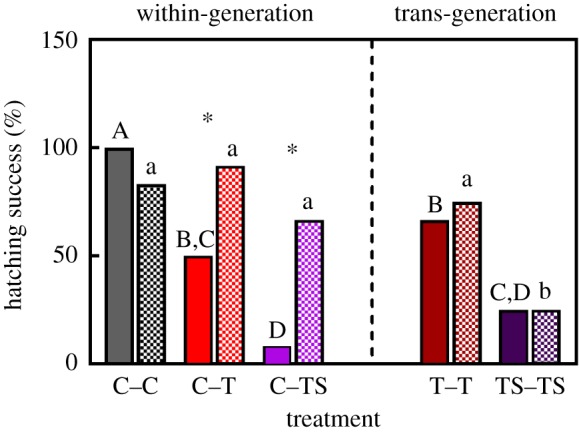
Effects of within-generation and trans-generation exposure to elevated temperature (T) and elevated temperature combined with elevated salinity (TS) between egg mass (solid bars) and juvenile transplants (hatched bars) on hatching event success (i.e. the percentage of F1 broods from which 20 F2 offspring could be moved) of O. labronica (n = 10–12 per treatment). C = control conditions. Capital and lower-case letters represent significant differences between egg mass or juvenile transplants between treatments, respectively. Asterisk (*) represents a significant difference between egg mass and juvenile transplants within the same treatment.
(b). Offspring life-history performance
Juvenile survival was significantly reduced by within-generation exposure to TS conditions (figure 4a; electronic supplementary material, tables S1 and S3). Trans-generation exposure to TS had no significant effect on survival with levels still comparable to those observed after within-generation exposure (figure 4a; electronic supplementary material, table S3). Juvenile survival was not significantly affected by either within- or trans-generation exposure to T conditions (figure 4a; electronic supplementary material, tables S1 and S2).
Figure 4.
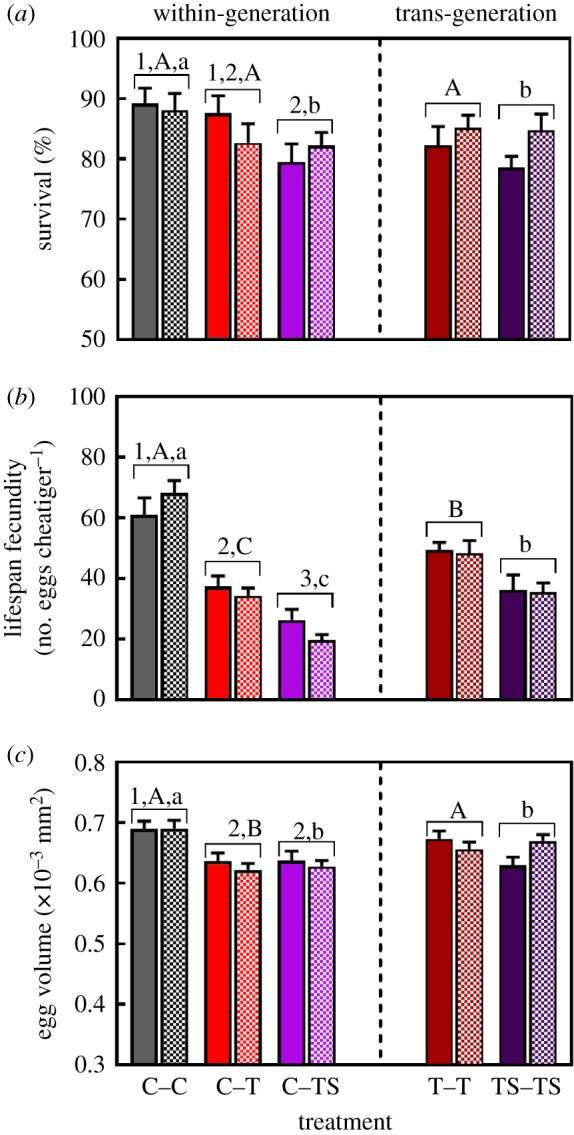
Effects of within-generation and trans-generation exposure to elevated temperature (T) and elevated temperature combined with elevated salinity (TS) conditions on traits which were only affected by ‘treatment’. (a) Juvenile survival (n = 10–12 per treatment), (b) lifespan fecundity (n = 10–12 per treatment) and (c) egg volume (n = 100–120 per treatment) of O. labronica. C = control conditions. Solid and hatched bars represent egg mass and juvenile transplants, respectively. Bar chart represents mean values ± s.e. Numbers represent significant differences between within-generation treatments. Capital and lower-case letters represent significant differences between within- versus trans-generation treatments for T and TS condition, respectively.
Juvenile growth rates were significantly increased by within-generation exposure to T conditions (figure 5a, electronic supplementary material, tables S1 and S2). By contrast, within-generation exposure to TS conditions significantly reduced growth rates. However, individuals that were transplanted at the egg mass stage responded differently to individuals transplanted as juveniles, as shown by the significant interaction between ‘treatment’ and ‘transplant’ (figure 5a; electronic supplementary material, tables S1 and S3). Growth rates of individuals from the C–TS egg mass and the juvenile transplants were both significantly lower than the control (p < 0.05), but individuals from the egg mass transplants grew slower than those transplanted as juveniles (p < 0.05). Juvenile growth rates were also significantly greater after trans-generational exposure to T conditions but were comparable to levels observed in the within-generation treatment (figure 5a; electronic supplementary material, table S2). There was no significant difference in juvenile growth rates between individuals exposed within- and trans-generationally to TS conditions (figure 5a; electronic supplementary material, table S3).
Figure 5.
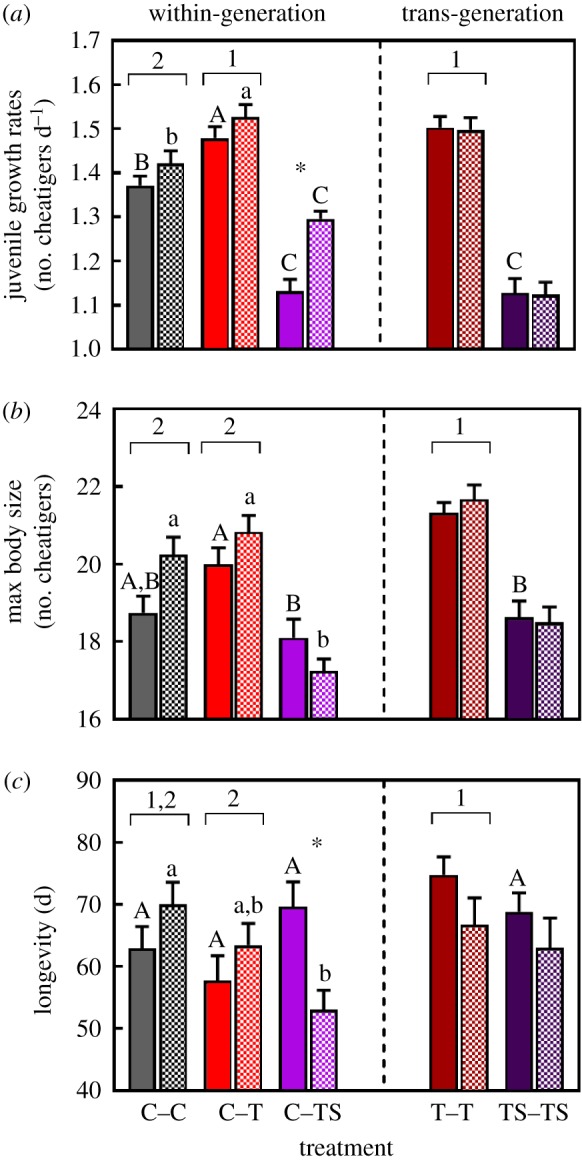
Effects of within-generation and trans-generation exposure to elevated temperature (T) and elevated temperature combined with elevated salinity (TS) conditions on traits for which there was an interactive effect of ‘treatment’ and ‘transplant’. (a) Juvenile growth rates (n = 50–60 per treatment), (b) maximum body size (n = 10–12 per treatment) and (c) longevity (n = 10–12 per treatment) of O. labronica. Solid and hatched bars represent egg mass and juvenile transplants respectively. C = control conditions. Solid and hatched bars represent egg mass and juvenile transplants respectively. Bar chart represents mean values ± s.e. Capital and lower-case letters represent significant differences between egg mass and juvenile transplants, respectively, for within-generation treatments and for within- versus trans-generation TS comparisons. Numbers represent significant differences between within- versus trans-generation T treatments. Asterisk (*) represents a significant difference between egg mass and juvenile transplants within the same treatment.
Lifespan fecundity was significantly reduced by within-generation exposure to both T and TS, with exposure to TS conditions having a significantly greater negative effect (figure 4b; electronic supplementary material, tables S1–S3). Trans-generation exposure to T and TS conditions increased lifespan fecundity to levels that were intermediate, and significantly different, compared to those reported for individuals kept in control conditions or exposed to T and TS conditions within-generation (figure 4b; electronic supplementary material, tables S1–S3).
Within-generation exposure to T and TS conditions caused a significant reduction in egg volume (figure 4c; electronic supplementary material, tables S1–S3). Trans-generation exposure to T conditions increased egg volume to levels comparable to control individuals and significantly greater than those following within-generation exposure to T conditions (figure 4c; electronic supplementary material, table S2). Trans-generation exposure to TS conditions had no significant effect on mean egg volume (figure 4c; electronic supplementary material, table S3).
Within-generation exposure to TS conditions had a ‘transplant’ dependent effect on maximum body size, as highlighted by the significant interaction between ‘treatment’ and ‘transplant’ (figure 5b; electronic supplementary material, tables S1 and S3). Maximum body size was significantly lower in the C–TS egg mass and juvenile transplants compared with their C–T counterparts. Maximum body size in the C–TS juvenile transplants was also significantly lower than the C–C juvenile transplants. There was, however, no significant difference in maximum body size between the C–C and C–TS egg mass transplants groups. Trans-generation exposure to T conditions had a positive effect on maximum body size, with individuals from the T–T treatment reaching a larger size than control individuals (figure 5b; electronic supplementary material, table S2). Maximum body size in the T–T treatment was significantly greater than the C–T treatment (electronic supplementary material, table S2). Finally, trans-generation exposure to TS conditions had no significant effect on maximum body size (figure 5b; electronic supplementary material, table S3).
Within-generation exposure to TS conditions had a ‘transplant’ dependent effect on longevity, evidenced by the significant interaction between ‘treatment’ and ‘transplant’ (figure 5c; electronic supplementary material, tables S1 and S3). There was no significant difference in longevity between the C–C and C–TS egg mass transplant groups. By contrast, longevity in the C–C juvenile transplant group was significantly greater than their C–TS counterparts. Furthermore, within the C–TS treatment, individuals that were transplanted as juveniles had significantly lower longevity than those that were transplanted as an egg mass (figure 5c; tables S1 and S3). Trans-generation exposure to TS conditions had no significant effect on longevity (figure 5b; electronic supplementary material, table S3). Similarly, longevity was not affected by within-generation exposure to T conditions (figure 5c; electronic supplementary material, tables S1 and S2). Finally, trans-generation exposure to T conditions had a positive effect on longevity but only compared with the within-generation exposure treatment (figure 5c; electronic supplementary material, table S2).
(c). Offspring energy metabolism markers
Within-generation exposure to treatment conditions only had a significant effect on CS activity in the juvenile transplant groups as is shown by the significant interaction between ‘treatment’ and ‘transplant’ (electronic supplementary material, table S1). CS activity in the C–TS juvenile transplant group was significantly higher than the C–T juvenile transplant group (p < 0.05), but both groups were comparable to all other treatments (p > 0.05). Trans-generation exposure to T and TS conditions had no effect on CS activity (electronic supplementary material, tables S2 and S3). Finally, ETS activity was not affected by either within- or trans-generation exposure to T and TS conditions (electronic supplementary material, tables S1 and S2).
(d). Relationships between traits
(i). Within-generation (egg mass versus juvenile transplants)
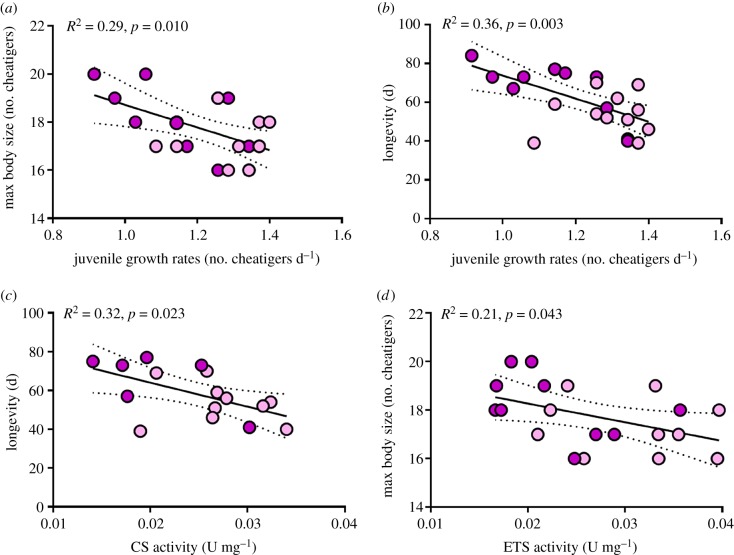
Maximum body size and longevity were both negatively correlated with juvenile growth rates in the C–TS treatment (table 1 and figure 6a,b; min. R2 = 0.29, p = 0.010). There was no significant relationship between juvenile growth rates and lifespan fecundity under C–TS conditions (table 1 and electronic supplementary material, figure S1; R2 = 0.002, p = 0.863). Finally, no significant relationship between juvenile growth rates and adult life-history traits were observed in the C–C and C–T treatments (table 1; electronic supplementary material, figure S1; max. R2 = 0.05, p = 0.299).
Table 1.
Within-generation relationships between juvenile growth rates and adult life-history traits (max body size, longevity and lifespan fecundity) of O. labronica exposed to control (C–C), elevated temperature (C–T) and elevated temperature combined with elevated salinity (C–TS) treatments. (Relationships between longevity and size versus citrate synthase (CS) and electron transport system (ETS) activity are also shown for C–TS. R2, p-value (p) and degrees of freedom (in brackets) are provided. Significant relationships are given in bold and the arrows highlight the direction of the relationship. Asterisk represents where a Bonferroni correction was applied to give a new alpha of 0.017.)
| juvenile growth rates | CS activity | ETS activity | |||||
|---|---|---|---|---|---|---|---|
| C–C | max body size |
R2 = 0.01, p = 0.638 (1,22) |
|||||
| longevity |
R2 = 0.01, p = 0.684 (1,22) |
||||||
| lifespan fecundity |
R2 = 0.02, p = 0.464 (1,22) |
||||||
| C–T | max body size |
R2 = 0.01, p = 0.581 (1,22) |
|||||
| longevity |
R2 = 0.05, p = 0.299 (1,22) |
||||||
| lifespan fecundity |
R2 = 0.04, p = 0.378 (1,22) |
||||||
| C–TS | max body size |
R2 = 0.29,
p = 0.010* (1,20) |
↓ |
R2 = 0.09, p = 0.268 (1,14) |
R2 = 0.21,
p = 0.043 (1,18) |
↓ | |
| longevity |
R2 = 0.36,
p = 0.003* (1,20) |
↓ |
R2 = 0.32, p = 0.023 (1,14) |
↓ |
R2 = 0.16, p = 0.078 (1,18) |
||
| lifespan fecundity |
R2 = 0.003, p = 0.799* (1,20) |
Figure 6.
Significant within-generation relationships between traits of O. labronica exposed to the control-elevated temperature combined with elevated salinity (C–TS) treatment. (a) Juvenile growth rates versus max body size, (b) juvenile growth rates versus longevity, (c) citrate synthase activity versus longevity and (d) electron transport system activity versus max body size. Dark and light colours represent egg mass and juvenile transplants, respectively. R2 and p-values are provided.
Significant negative relationships between juvenile growth rates and maximum body size/longevity were detected. Consequently, we tested the relationship between maximum body size/longevity and CS/ETS activity. There was a significant negative relationship between CS activity and longevity under TS conditions (table 1 and figure 6c; R2 = 0.32, p = 0.023), but not between CS activity and maximum body size (table 1; electronic supplementary material, figure S2; R2 = 0.09, p = 0.268). There was a negative relationship between ETS activity and longevity, although this was non-significant (table 1; electronic supplementary material, figure S2; R2 = 0.16, p = 0.078). Finally, there was a significant negative relationship between ETS activity and maximum body size (table 1 and figure 6d; R2 = 0.21, p = 0.043).
(ii). Within- versus trans-generation (egg mass transplants only)
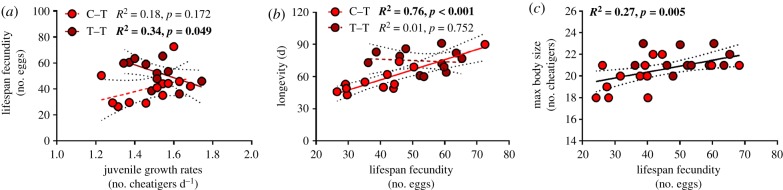
Under T conditions, the relationship between juvenile growth rates and lifespan fecundity was dependent on trans- or within-generation exposure as is highlighted by a significant interactive effect (p = 0.023). More specifically, there was no significant relationship following within-generation exposure to T conditions (table 2 and figure 7a; R2 = 0.21, p = 0.138), but trans-generation exposure resulted in a significant negative relationship (table 2 and figure 7a; R2 = 0.34, p = 0.049). There were no significant relationships between juvenile growth rates and maximum body size/longevity under T conditions (table 2 and electronic supplementary material, figure S3; max. R2 = 0.07, p = 0.139). Under TS conditions, there were no significant relationships between juvenile growth rates and any of the adult traits (table 2 and electronic supplementary material, figure S3; max. R2 = 0.26, p = 0.018 (α = 0.017)). No significant relationships were observed between CS/ETS activity and lifespan fecundity under T conditions (electronic supplementary material, figure S4; max. R2 = 0.16, p = 0.070).
Table 2.
Within-generation versus trans-generation relationships (egg mass transplants only) between juvenile growth rates and adult life-history traits of O. labronica (max body size, longevity and lifespan fecundity) for the C–T and C–TS treatments. (Relationships between lifespan fecundity and CS/ETS activity are also shown for T conditions. R2, p-value (p) and degrees of freedom (in brackets) are provided. Significant relationships are given in bold and the arrows highlight the direction of the relationship. Asterisk (*) represents where a Bonferroni correction was applied to give a new alpha of 0.017. Double asterisk (**) represents where a Bonferroni correction was applied to give a new alpha of 0.025.)
| juvenile growth rates | lifespan fecundity | CS activity | ETS activity | ||||
|---|---|---|---|---|---|---|---|
| C–T versus T–T | max body size |
R2 = 0.001, p = 0.866 (1,20) |
R2 = 0.27,
p = 0.005 (1,20) |
↑ | |||
| longevity |
R2 = 0.07, p = 0.139 (1,20) |
C–T
R2 = 0.76, p < 0.001 (1,10) T–T R2 = 0.01, p = 0.752 (1,10) |
↑ | ||||
| lifespan fecundity | C–T R2 = 0.18, p = 0.172 (1,10) T–T R2 = 0.34, p = 0.049 (1,10) |
R2 = 0.16, p = 0.070 (1,20) |
R2 = 0.14, p = 0.079 (1,21) |
||||
| C–TS versus TS–TS | max body size |
R2 = 0.01, p = 0.677* (1,19) |
↓ |
R2 = 0.06, p = 0.277** (1,19) |
|||
| longevity |
R2 = 0.26, p = 0.018* (1,19) |
R2 = 0.07, p = 0.239** (1,19) |
|||||
| lifespan fecundity |
R2 = 0.06, p = 0.274* (1,19) |
Figure 7.
Significant within- versus trans-generation effects on relationships between traits of O. labronica under elevated temperature conditions. (a) Juvenile growth rates versus lifespan fecundity, (b) lifespan fecundity versus longevity (c) and lifespan fecundity versus max body size. Light and dark colours represent egg mass transplants from the within- and trans-generation treatments, respectively. R2 and p-values are provided with significant relationships highlighted in bold.
Under T conditions, the relationship between lifespan fecundity and longevity was dependent on trans- or within-generation exposure as is highlighted by a significant interactive effect (p = 0.007). More specifically, there was significant positive relationship following within-generation exposure to T conditions (table 2 and figure 7b; R2 = 0.76, p < 0.001), but no relationship after trans-generation exposure (table 2 and figure 7b; R2 = 0.01, p = 0.752). There was also a significant positive relationship between lifespan fecundity and maximum body size (table 2 and figure 7c; R2 = 0.27, p = 0.006). Under TS conditions, there were no significant relationships between lifespan fecundity and maximum body size or longevity (table 2 and electronic supplementary material, figure S5; max. R2 = 0.07, p = 0.239 (α = 0.025)).
4. Discussion
Adaptive phenotypic plasticity and natural selection can mediate the negative life-history effects of future ocean conditions. However, most studies to-date have focused on early life stages and thus our understanding of the trade-offs and limitations that are associated with adaptive processes is limited, particularly in the presence of multiple stressors. Here, we reared a marine polychaete for its entire lifespan under two global change scenarios: one mimicking warming and one both warming and the associated change (increase) in salinity caused by a reduction in rainfall predicted to occur in coastal areas of the Mediterranean [48]. We found that adaptive processes cause trade-offs between juvenile growth rates and adult life-history traits both within and across generations. Furthermore, we identified the presence of limitations that are associated with trans-generation exposure. Overall, our results demonstrate that the early life-stage exposure influences responses later in life and that trade-offs and limitations will be more prevalent in a multi-stressor environment.
(a). Within-generation responses
Temperature and salinity are among the most important environmental factors that govern the physiology of marine organisms and ultimately determine their geographical distribution [62,63]. Here, we observed that juvenile polychaetes, O. labronica, exhibit increased growth rates under T, which is a common response to warming in marine ectotherms [64]. This effect was not observed when salinity is also elevated (TS), with polychaetes displaying reduced juvenile growth rates and survival. Owing to the experimental design used in this study, it is not possible to ultimately discriminate whether the negative effects observed under TS conditions were driven by the interaction between elevated temperature and elevated salinity or purely by elevated salinity. However, we believe the former is more likely. We base this conclusion on the responses to high salinity exposure of the closely related species O. diadema, which showed that tolerance to high salinity is lowered by exposure to elevated temperature, with both reduced survivorship and development observed [65].
Adult polychaetes were negatively affected by both T and TS conditions. Reduced lifespan fecundity was observed in both treatments, however, TS conditions had a more negative impact. This could be caused by the additive or (most likely) synergistic effect of elevated TS. A similar interaction has been reported in the life-history response of O. diadema, where reproductive rates at low temperatures were greater under higher salinities, while at higher temperatures, this relationship was reversed [65]. Maximum body size and aerobic capacity, measured here as CS and ETS activity, were largely unaffected by within-generation exposure to T and TS. Thus, our results suggest that energy investment into maintenance of aerobic capacity and growth, measured as body size, is favoured as a short-term fitness advantage. This trade-off is consistent with life-history theory for invertebrates with indeterminate growth (i.e. growth that continues after maturation), such as O. labronica, where investment into somatic growth in later life stages increases organisms' reproductive performance [66]. Indeed, such life-history trade-offs and associated fitness costs are a common consequence of alterations in the allocation of energy caused by adaptive processes [67,68].
Hatching event success in O. labronica was measured as an indicator of selection. Under T conditions, hatching event success in the egg mass transplant group (50%) was significantly lower than the juvenile transplant group (91.6%), demonstrating that selection pressure was greater. Despite this, we report that there were no significant differences between egg mass and juvenile transplants in any other trait measured, which suggests that phenotypic plastic responses were not expressed or that they showed ‘perfect compensation’ [69]. This was not the case under TS conditions, where ‘timing of transplant’ significantly impacted the response of several traits. Polychaetes transplanted at the egg mass stage grew significantly slower than polychaetes transplanted as juveniles. However, polychaetes from the egg mass transplant group also maintained their maximum body size and longevity at levels comparable to the control, whereas polychaetes from the juvenile transplant group did not, with observed reductions in both traits. In addition, polychaetes from the egg mass transplant group had a significantly greater lifespan than their juvenile transplant counterparts under TS conditions. Likewise, adult polychaetes, Janua sp, derived from larger larvae had shorter lifespans [70]. The differences observed in the mean responses between transplant groups appear to be driven by trade-offs that exist between juvenile growth rate and maximum body size/longevity under TS conditions. Furthermore, the differences between egg mass and juvenile transplants under TS conditions appear to be related to hatching success. Hatching event success in the C–TS egg mass transplant group was significantly lower than both the control (−91.7%) and juvenile transplant (−58.3%) groups. Thus, it appears that selection during embryonic development and/or the first 3 days after hatching is an important factor in determining growth rate, maximum body size and longevity. Trade-offs between early growth rate and longevity/body size have also been observed in the brown trout, Salmo trutta, and the three-spined stickleback, Gasterosteus aculeatus [40,41], as well as other taxa [71]. Finally, mean lifespan fecundity levels were 24% higher in polychaetes from egg mass transplants, although this was not significantly different compared to individuals transplanted as juveniles, which suggests that these trade-offs may have consequences for polychaetes reproductive performance.
The physiological mechanism behind the observed trade-offs under TS conditions appears to be related to a shift in metabolic capacity, potentially leading to the alteration of energy allocation. CS and ETS activity were 23.8% and 26.2% greater in the juvenile transplant group, although these differences were not significant (min. p = 0.053). This suggests that polychaetes in the juvenile transplant group may have had a greater aerobic scope, which would explain their higher growth rates. Faster growth rates are generally linked to greater levels of oxidative stress within the cell and can lead to reduced investment into protein maintenance, which ultimately accelerates senescence and reduces longevity [71]. Indeed, the production of reactive oxygen species (ROS) is thought to be a central mediator of trade-offs between life-history traits [72,73]. In line with this, we report significant negative relationships between CS activity and longevity, and ETS activity and maximum body size. Considered together, our results indicate that lower aerobic capacity is selected for during early development under TS conditions. Reduced metabolic capacity is also the adaptive mechanism that enables some marine polychaete species to inhabit CO2 vents [74]. We hypothesize that reduced aerobic capacity is selected for during early (developmental) exposure to TS conditions, ultimately leading to slower growth and less energy spent on combating the negative effects of ROS. This could potentially make more energy available to support core functions, such as the maintenance of homeostasis and other life-history traits: i.e. maximum body size and longevity.
(b). Trans-generation responses
Trans-generation exposure to T and TS conditions partially alleviated the negative within-generation effects on lifespan fecundity, although the levels observed under TS were still lower compared with control conditions. Egg volume, however, was fully restored by trans-generation exposure to T. Under T conditions, the observed trans-generation improvement in lifespan fecundity was driven by larger maximum body size and longer lifespan, highlighted by the significant positive relationship between maximum body size/longevity and lifespan fecundity. Female polychaetes exposed to T conditions across a generation reached a larger maximum body size than control or within-generation polychaetes and lived longer than their within-generation counterparts (i.e. larger maximum body size was driven by a longer lifespan). The observed increase in maximum body size is a surprising result, as a reduction in body size is expected to be the usual adaptive outcome in response to elevated temperature [38,75–77], as well as for other global change drivers such as ocean acidification [74,78]. Body size is a primary determinant of many biological and ecological processes, including competitive and predator–prey interactions [79,80]. Thus, the changes in body size that are associated with adaptive processes could have major consequences at the ecosystem level. In previous studies, trans-generation improvements in life-history traits have been linked to increased aerobic performance [23,24,39]. However, we did not detect changes in CS and ETS activity following trans-generation exposure to T and TS conditions. This suggests that cellular homeostasis can be maintained without adjustment of mitochondrial content, which could be supported by higher mobilization or efficiency of mitochondria [81].
It was not an aim of this study to determine the exact mechanism (i.e. selection or adaptive phenotypic plasticity) responsible for the trans-generation response. However, juvenile survival and reproductive success in the F1 generation provide some clues as to how much selection occurred prior to the F2 generation. Juvenile survival in the parental generation (F1) was significantly reduced by 8% under TS conditions, which suggests that a small level of selection had occurred before the polychaetes reached sexual maturity. By contrast, reproductive success was significantly lower under both T and TS conditions; only 58.2 and 16.7% of the initial F1 pairs were able to produce viable egg masses, demonstrating that strong selection pressures were present at the fertilization and/or the embryonic development stage [82–84]. Selection has been shown to be important in trans-generation studies. For example, the calanoid copepod Pseudocalanus acuspes showed increased fecundity after trans-generation exposure to elevated CO2 levels [26]. In this case, selection resulted in genetic changes in translation mechanisms such as ribosome formation and mitochondrial function, in addition to altering the expression of genes related to transcription/replication, ultimately modifying energy allocation [85].
Trans-generation phenotypic plasticity (TGP) is another mechanism that improves offspring performance under stressful conditions [21–24]. Within the context of TGP, the environment experienced by parents alters the phenotype of their offspring via non-genetic inheritance mechanisms [5]. Trans-generation plasticity can occur through the transfer of nutritional material (e.g. maternal provisioning) and/or via epigenetic processes (i.e. changes in gene expression; Bonduriansky et al. [10]). Egg size is often used as an indicator for maternal provisioning. Here, trans-generation improvements were observed despite egg volume being significantly reduced by within-generation exposure to both T and TS conditions. It is possible that while eggs were smaller their nutritional components were altered in a way that increased energetic content, indeed, egg size does not always correlate with egg quality [86]. Similar findings have been observed in cinnamon clownfish, Amphiprion melanopus, after trans-generation exposure to elevated CO2; the clownfish produced hatchlings that had a smaller yolk sac area, but at no apparent cost to the fitness of the offspring [24]. Thus, we cannot rule out the occurrence of maternal provisioning. However, as all traits affected by trans-generation exposure were adult traits, an alternative and more plausible explanation for our observed trans-generation response is that changes in metabolic gene expression caused a shift in energy production towards the maintenance of aerobic performance, as observed in the spiny damselfish A. polyacanthus, and the marine stickleback G. aculeatus [87,88].
The partial restoration of fecundity observed across a generation suggests that the efficacy of undergoing high levels of selection and possibly phenotypic plasticity are constrained by the physiological limits imposed by extreme environmental conditions [43]. Under TS conditions, lifespan fecundity was the only trait positively affected by trans-generation exposure, whereas under T conditions, four traits (lifespan fecundity, egg volume, maximum body size and longevity) were positively affected. This suggests that multi-stressor environments impose greater limits on trans-generation responses, at least in terms of the cost of maintaining aerobic performance. Additionally, the trade-offs that arose between juvenile growth rates and lifespan fecundity following trans-generation exposure to T were not present after within-generation exposure to T. This discrepancy suggests that the energetic costs associated with the changes in metabolic performance are modified by trans-generation exposure. No such trade-offs were observed after trans-generation exposure to TS.
5. Summary
Here, we identify several life-history trade-offs and limitations that are associated with adaptive processes under future ocean conditions in an emerging marine model species, O. labronica. We only observed trade-offs within-generationally after exposure to elevated temperature combined with elevated salinity conditions. Juvenile growth rates were negatively related to both maximum body size and longevity, which appears to have been driven by selection during early development. Trans-generation exposure to T and TS conditions only partially restored lifespan fecundity, despite high levels of selection. Trans-generation exposure to elevated temperature also resulted in a trade-off between juvenile growth rates and lifespan fecundity: slower growers showing a greater reproductive output. Overall, our results suggest that future ocean conditions may select for the slow grower and that life-history trade-offs and limitations will be more prevalent in a multi-stressor environment, resulting in greater constraints on adaptive potential.
The Mediterranean Sea has been identified as an area likely to suffer high levels of local extinction under future climate change because it is semi-enclosed, which prevents organisms from shifting their distribution [89]. Consequently, the species/populations that inhabit this area will be disproportionately reliant on adaptive processes to ensure their survival. Our results indicate that adaptive processes under future conditions in the Mediterranean Sea will produce slower growing individuals with reduced reproductive output. Furthermore, our results suggest that, under future ocean warming, periods of elevated salinity have the potential to cause severe population crashes. Benthic polychaete populations play an important role in the marine nitrogen cycle through bioturbation, driving the exchange of nutrients between sediments and overlying waters [90–92]. The trade-offs and limitations associated with adaptive processes could thus have repercussions for ecosystem structure and function at a regional scale. More studies that rear organisms for an extended period of time are needed to characterize trade-offs and limitations associated with adaptive processes. Only with such studies will we be able to accurately predict future climate change impacts on populations and ecosystems [93] and thus any associated socio-economic implications [94–96].
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Vincent Turpin for assisting with data collection and Jennifer Donelson for providing feedback on an earlier draft.
Data accessibility
Data are available as the electronic supplementary material.
Authors' contributions
M.D.J., L.J.C., G.M.-N. and P.C. designed the experiment. M.D.J., L.J.C. and E.M.G. carried out the experiment. P.C. and P.U.B. provided funding. F.C. carried out the enzyme assays. M.D.J. carried out statistical analyses with input from P.C. M.D.J. drafted the manuscript with input from all authors. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This work was financed by a Natural Sciences and Engineering Research Council of Canada Discovery Program grant (RGPIN-2015-06500), the Programme Établissement de Nouveaux Chercheurs Universitaires of the Fonds de Recherche du Québec—Nature et Technologies No.199173, and the Fonds de recherche de l'Université du Québec á Rimouski, all awarded to P.C. Physiological analyses were financed by a Natural Sciences and Engineering Research Council of Canada Discovery Program grant awarded to P.U.B. and P.C. (RGPIN 155926 and RGPIN-2015-06500, respectively), and by funding from the Research Group BORÉAS de l'Université du Québec á Rimouski to P.C. G.M.-N. was supported by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement, no. 659359.
References
- 1.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 2.Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 3.Salinas S, Brown SC, Mangel M, Munch SB. 2013. Non-genetic inheritance and changing environments. Non-Genetic Inherit. 1, 38–50. ( 10.2478/ngi-2013-0005) [DOI] [Google Scholar]
- 4.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 5.Mousseau T, Fox C. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 6.Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. ( 10.1111/ele.12185) [DOI] [PubMed] [Google Scholar]
- 7.Chevin L-M, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scoville AG, Pfrender ME. 2010. Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl Acad. Sci. USA 107, 4260–4263. ( 10.1073/pnas.0912748107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latta LC, Bakelar JW, Knapp RA, Pfrender ME. 2007. Rapid evolution in response to introduced predators II: the contribution of adaptive plasticity. BMC Evol. Biol. 7, 21 ( 10.1186/1471-2148-7-21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonduriansky R, Crean AJ, Day T. 2012. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 5, 192–201. ( 10.1111/j.1752-4571.2011.00213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stearns S. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 13.Patridge L, Sibly RM. 1991. Constraints in the evolution of life histories. Phil. Trans. R. Soc. Lond. B 332, 3–13. ( 10.1098/rstb.1991.0027) [DOI] [Google Scholar]
- 14.Donelson JM, Salinas S, Munday PL, Shama LNS. 2018. Transgenerational plasticity and climate change experiments: where do we go from here? Global Change Biol. 24, 13–34. ( 10.1111/gcb.13903) [DOI] [PubMed] [Google Scholar]
- 15.Calosi P, De Wit P, Thor P, Dupont S. 2016. Will life find a way? Evolution of marine species under global change. Evol. Appl. 9, 1035–1042. ( 10.1111/eva.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross PM, Parker L, Byrne M. 2016. Transgenerational responses of molluscs and echinoderms to changing ocean conditions. ICES J. Mar. Sci. 73, 537–549. ( 10.1093/icesjms/fsv254) [DOI] [Google Scholar]
- 17.Reznick ND, Ghalambor KC. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113, 183–198. ( 10.1023/A:1013352109042) [DOI] [PubMed] [Google Scholar]
- 18.Stockwell CA, Hendry AP, Kinnison MT. 2003. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94–101. ( 10.1016/S0169-5347(02)00044-7) [DOI] [Google Scholar]
- 19.Carroll SP, Hendry AP, Reznick DN, Fox CW. 2007. Evolution on ecological time-scales. Funct. Ecol. 21, 387–393. ( 10.1111/j.1365-2435.2007.01289.x) [DOI] [Google Scholar]
- 20.Thompson JN. 1998. Rapid evolution as an ecological process. Trends Ecol. Evol. 5347, 329–332. ( 10.1016/S0169-5347(98)01378-0) [DOI] [PubMed] [Google Scholar]
- 21.Chakravarti LJ, Jarrold MD, Gibbin EM, Christen F, Massamba-N'Siala G, Blier PU, Calosi P. 2016. Can trans-generational experiments be used to enhance species resilience to ocean warming and acidification? Evol. Appl. 9, 1133–1146. ( 10.1111/eva.12391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donelson JM, Wong M, Booth DJ, Munday PL. 2016. Transgenerational plasticity of reproduction depends on rate of warming across generations. Evol. Appl. 9, 1072–1081. ( 10.1111/eva.12386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shama LNS, Strobel A, Mark FC, Wegner KM. 2014. Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct. Ecol. 28, 1482–1493. ( 10.1111/1365-2435.12280) [DOI] [Google Scholar]
- 24.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. 2012. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861. ( 10.1038/nclimate1599) [DOI] [Google Scholar]
- 25.Rodríguez-Romero A, Jarrold MD, Massamba-N'Siala G, Spicer JI, Calosi P. 2016. Multi-generational responses of a marine polychaete to a rapid change in seawater pCO2. Evol. Appl. 9, 1082–1095. ( 10.1111/eva.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thor P, Dupont S. 2015. Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Global Change Biol. 21, 2261–2271. ( 10.1111/gcb.12815) [DOI] [PubMed] [Google Scholar]
- 27.Renborg E, Johannesson K, Havenhand J. 2014. Variable salinity tolerance in ascidian larvae is primarily a plastic response to the parental environment. Evol. Ecol. 28, 561–572. ( 10.1007/s10682-013-9687-2) [DOI] [Google Scholar]
- 28.Jensen N, Allen RM, Marshall DJ. 2014. Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Funct. Ecol. 28, 724–733. ( 10.1111/1365-2435.12195) [DOI] [Google Scholar]
- 29.Pörtner H-O, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–98. ( 10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 30.Blier PU, Lemieux H, Pichaud N. 2014. Holding our breath in our modern world: will mitochondria keep the pace with climate changes? Can. J. Zool. 92, 591–601. ( 10.1139/cjz-2013-0183) [DOI] [Google Scholar]
- 31.Schulte PM. 2015. The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J. Exp. Biol. 218, 1856–1866. ( 10.1242/jeb.118851) [DOI] [PubMed] [Google Scholar]
- 32.Ekström A, Sandblom E, Blier PU, Dupont Cyr B-A, Brijs J, Pichaud N. 2017. Thermal sensitivity and phenotypic plasticity of cardiac mitochondrial metabolism in European perch, Perca fluviatilis. J. Exp. Biol. 220, 386–396. ( 10.1242/jeb.150698) [DOI] [PubMed] [Google Scholar]
- 33.Iftikar FI, MacDonald JR, Baker DW, Renshaw GMC, Hickey AJR. 2014. Could thermal sensitivity of mitochondria determine species distribution in a changing climate? J. Exp. Biol. 217, 2348–2357. ( 10.1242/jeb.098798) [DOI] [PubMed] [Google Scholar]
- 34.Gibbin E, Chakravarti LJ, Jarrold MD, Christen F, Turpin V, Massamba N'Siala G. 2017. Can multi-generational exposure to ocean warming and acidification lead to the adaptation of life history and physiology in a marine metazoan? J. Exp. Biol. 220, 551–563. ( 10.1242/jeb.149989) [DOI] [PubMed] [Google Scholar]
- 35.Putnam HM, Gates RD. 2015. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J. Exp. Biol. 218, 2365–2372. ( 10.1242/jeb.123018) [DOI] [PubMed] [Google Scholar]
- 36.Munday PL. 2014. Transgenerational acclimation of fishes to climate change and ocean acidification. F1000Prime Rep. 6, 99 ( 10.12703/P6-99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen SA, Håkedal OJ, Salaberria I, Tagliati A, Gustavson LM, Jenssen BM, Olsen AJ, Altin D. 2014. Multigenerational exposure to ocean acidification during food limitation reveals consequences for copepod scope for growth and vital rates. Environ. Sci. Technol. 48, 12 275–12 284. ( 10.1021/es501581j) [DOI] [PubMed] [Google Scholar]
- 38.Donelson JM, Munday PL, Mccormick MI, Nilsson GE. 2011. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Global Change Biol. 17, 1712–1719. ( 10.1111/j.1365-2486.2010.02339.x) [DOI] [Google Scholar]
- 39.Parker LM, Ross PM, O'Connor WA, Borysko L, Raftos DA, Pörtner H-O. 2012. Adult exposure influences offspring response to ocean acidification in oysters. Global Change Biol. 18, 82–92. ( 10.1111/j.1365-2486.2011.02520.x) [DOI] [Google Scholar]
- 40.Jonsson B, L'Abée-Lund JH, Heggberget TG, Jensen AJ, Johnsen BO, Næsje TF, Sættem LM. 1991. Longevity, body size, and growth in anadromous brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 48, 1838–1845. ( 10.1139/f91-217) [DOI] [Google Scholar]
- 41.Lee W-S, Monaghan P, Metcalfe NB. 2013. Experimental demonstration of the growth rate-lifespan trade-off. Proc. R. Soc. B 280, 20122370 ( 10.1098/rspb.2012.2370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travers LM, Garcia-Gonzalez F, Simmons LW. 2015. Live fast die young life history in females: evolutionary trade-off between early life mating and lifespan in female Drosophila melanogaster. Sci. Rep. 5, 15469 ( 10.1038/srep15469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann AA. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880. ( 10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 44.Allan BJM, Miller GM, McCormick MI, Domenici P, Munday PL. 2014. Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc. R. Soc. B 281, 20132179 ( 10.1098/rspb.2013.2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch MJ, Watson S-A, Welsh JQ, McCormick MI, Munday PL. 2014. Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat. Clim. Change 4, 1086–1089. ( 10.1038/nclimate2400) [DOI] [Google Scholar]
- 46.McMahon SJ, Donelson JM, Munday PL. 2018. Food ration does not influence the effect of elevated CO2 on antipredator behaviour of a reef fish. Mar. Ecol. Prog. Ser. 586, 155–165. ( 10.3354/meps12397) [DOI] [Google Scholar]
- 47.Giorgi F, Lionello P. 2008. Climate change projections for the Mediterranean region. Global Planet Change 63, 90–104. ( 10.1016/j.gloplacha.2007.09.005) [DOI] [Google Scholar]
- 48.Borghini M, Bryden H, Schroeder K, Sparnocchia S, Vetrano A. 2014. The Mediterranean is becoming saltier. Ocean Sci. 10, 693–700. ( 10.5194/os-10-693-2014) [DOI] [Google Scholar]
- 49.Paxton H, Åkesson B. 2007. Redescription of Ophryotrocha puerilis and O. labronica (Annelida, Dorvilleidae). Mar. Biol. Res. 3, 3–19. ( 10.1080/17451000601024373) [DOI] [Google Scholar]
- 50.Massamba-N'Siala G, Simonini R, Cossu P, Maltagliati F, Castelli A, Prevedelli D. 2011. Life-history and demographic spatial variation in Mediterranean populations of the opportunistic polychaete Ophryotrocha labronica (Polychaeta, Dorvilleidae). Mar. Biol. 158, 1523–1535. ( 10.1007/s00227-011-1668-9) [DOI] [Google Scholar]
- 51.Collins M, et al. 2013. Long-term climate change: projections, commitments and irreversibility. In Climate change 2013: the physical science basis. IPCC Working Group I contribution to AR5 (eds IPCC). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 52.Giorgi F. 2006. Climate change hot-spots. Geophys. Res. Lett. 33, L08707 ( 10.1029/2006GL025734) [DOI] [Google Scholar]
- 53.Allen RM, Marshall D. 2014. Egg size effects across multiple life-history stages in the marine annelid Hydroides diramphus. PLoS ONE 9, e102253 ( 10.1371/journal.pone.0102253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morley SA, Lurman GJ, Skepper JN, Pörtner HO, Peck LS. 2009. Thermal plasticity of mitochondria: a latitudinal comparison between Southern Ocean molluscs. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 152, 423–430. ( 10.1016/j.cbpa.2008.11.015) [DOI] [PubMed] [Google Scholar]
- 55.Strobel A, Graeve M, Poertner HO, Mark FC. 2013. Mitochondrial acclimation capacities to ocean warming and acidification are limited in the antarctic Nototheniid fish, Notothenia rossii and Lepidonotothen squamifrons. PLoS ONE 8, e68865 ( 10.1371/journal.pone.0068865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivest EB, Hofmann GE. 2014. Responses of the metabolism of the larvae of Pocillopora damicornis to ocean acidification and warming. PLoS ONE 9, e96172 ( 10.1371/journal.pone.0096172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidlin L, von Fumetti S, Nagel P. 2015. Temperature effects on the feeding and electron transport system (ETS) activity of Gammarus fossarum. Aquat. Ecol. 49, 71–80. ( 10.1007/s10452-015-9505-8) [DOI] [Google Scholar]
- 58.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 59.Pinheiro J, Bates D, DebRoy DS, Team RC. 2017. nlme: linear and nonlinear mixed effects models. R package version 3.1-131. See https://CRAN.R-project.org/package=nlme.
- 60.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. [Google Scholar]
- 61.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometric J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 62.Kinne O. 1971. Salinity-invertebrates. In Marine ecology I part 2 (ed. Kinne O.), pp. 821–874. London, UK: Wiley-Interscience. [Google Scholar]
- 63.Fernandez C, Pasqualini V, Boudouresque CF, Johnson M, Ferrat L, Caltagirone A, Mouillot D. 2006. Effect of an exceptional rainfall event on the sea urchin (Paracentrotus lividus) stock and seagrass distribution in a Mediterranean coastal lagoon. Estuar. Coast Shelf Sci. 68, 259–270. ( 10.1016/j.ecss.2006.02.020) [DOI] [Google Scholar]
- 64.Puzzle L, Angilletta MJ, Steury TD, Sears MW. 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Comp. Biol. 44, 498–509. ( 10.1093/icb/44.6.498) [DOI] [PubMed] [Google Scholar]
- 65.Åkesson B, Costlow JD. 1978. Effects of temperature and salinity on the life cycle of Ophryotrocha diadema (Polychaeta, dorvilleidae). Ophelia 17, 215–229. ( 10.1080/00785326.1978.10425485) [DOI] [Google Scholar]
- 66.Reznick DN. 1983. The structure of guppy life histories: the tradeoff between growth and reproduction. Ecology 64, 862–873. ( 10.2307/1937209) [DOI] [Google Scholar]
- 67.Angilletta MJ, Wilson RS, Navas CA, James RS. 2003. Tradeoffs and the evolution of thermal reaction norms. Trends Ecol. Evol. 18, 234–240. ( 10.1016/S0169-5347(03)00087-9) [DOI] [Google Scholar]
- 68.Hoffmann AA. 1995. Acclimation: increasing survival at a cost. Trends Ecol. Evol. 10, 1–2. ( 10.1016/S0169-5347(00)88949-1) [DOI] [Google Scholar]
- 69.Precht H. 1958. Marine invertebrate mitochondria and oxidative stress. In Concepts of temperature adaptation of unchanging reaction system of cold-blooded animal in physiological adaptation (ed. Prossser CL.), pp. 50–78. Washington, DC: American Physiological Society. [Google Scholar]
- 70.Kesselring H, Wheatley R, Marshall DJ. 2012. Initial offspring size mediates trade-off between fecundity and longevity in the field. Mar. Ecol. Prog. Ser. 465, 129–136. ( 10.3354/meps09865) [DOI] [Google Scholar]
- 71.Metcalfe NB, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 72.Dowling DK, Simmons LW. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 20081791 ( 10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monaghan P, Metcalfe NB, Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92. ( 10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 74.Calosi P, et al. 2013. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Phil. Trans. R. Soc. B 368, 20120444 ( 10.1098/rstb.2012.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daufresne M, Lengfellner K, Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793. ( 10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291. ( 10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 77.Kingsolver JG, Huey RB. 2008. Size, temperature, and fitness: three rules. Evol. Ecol. Res. 10, 251–268. [Google Scholar]
- 78.Garilli V, et al. 2015. Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat. Clim. Change 5, 678–682. ( 10.1038/nclimate2616) [DOI] [Google Scholar]
- 79.Arendt J. 2007. Ecological correlates of body size in relation to cell size and cell number: patterns in flies, fish, fruits and foliage. Biol. Rev. 82, 241–256. ( 10.1111/j.1469-185X.2007.00013.x) [DOI] [PubMed] [Google Scholar]
- 80.Peters R. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 81.Cortassa S, O'Rourke B, Winslow RL, Aon MA. 2009. Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys. J. 96, 2466–2478. ( 10.1016/j.bpj.2008.12.3893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parker LM, Ross PM, O'Connor WA. 2009. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Global Change Biol. 15, 2123–2136. ( 10.1111/j.1365-2486.2009.01895.x) [DOI] [Google Scholar]
- 83.Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, Davis AR. 2009. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. R. Soc. B 276, 1883–1888. ( 10.1098/rspb.2008.1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griffin FJ, Pillai MC, Vines CA, Kaaria J, Hibbard-Robbins T, Yanagimachi R, Cherr GN. 1998. Effects of salinity on sperm motility, and development in the Pacific herring, Clupea pallasi. Biol. Bull. 194, 25–35. ( 10.2307/1542510) [DOI] [PubMed] [Google Scholar]
- 85.De Wit P, Dupont S, Thor P. 2016. Selection on oxidative phosphorylation and ribosomal structure as a multigenerational response to ocean acidification in the common copepod Pseudocalanus acuspes. Evol. Appl. 9, 1112–1123. ( 10.1111/eva.12335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moran AMYL, Alister JSMC. 2009. Egg size as a life history character of marine invertebrates: is it all it's cracked up to be? Biol. Bull. 216, 226–242. ( 10.1086/BBLv216n3p226) [DOI] [PubMed] [Google Scholar]
- 87.Shama LNS, Mark FC, Strobel A, Lokmer A, John U, Mathias Wegner K. 2016. Transgenerational effects persist down the maternal line in marine sticklebacks: gene expression matches physiology in a warming ocean. Evol. Appl. 9, 1096–1111. ( 10.1111/eva.12370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Veilleux HD, et al. 2015. Molecular processes of transgenerational acclimation to a warming ocean. Nat. Clim. Change 5, 1074–1078. ( 10.1038/nclimate2724) [DOI] [Google Scholar]
- 89.Cheung WWL, Lam VWY, Sarmiento JL, Kearney K, Watson R, Pauly D. 2009. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 10, 235–251. ( 10.1111/j.1467-2979.2008.00315.x) [DOI] [Google Scholar]
- 90.Hansen K, Kristensen E. 1997. Impact of macrofaunal recolonization on benthic metabolism and nutrient fluxes in a shallow marine sediment overgrown with macroalgal mats. Estuar. Coast Shelf Sci. 45, 613–628. ( 10.1006/ecss.1996.0229) [DOI] [Google Scholar]
- 91.Nizzoli D, Bartoli M, Cooper M, Welsh DT, Underwood GJC, Viaroli P. 2007. Implications for oxygen, nutrient fluxes and denitrification rates during the early stage of sediment colonisation by the polychaete Nereis spp. in four estuaries. Estuar. Coast Shelf Sci. 75, 125–134. ( 10.1016/j.ecss.2007.03.035) [DOI] [Google Scholar]
- 92.Laverock B, Gilbert JA, Tait K, Osborn AM, Widdicombe S. 2011. Bioturbation: impact on the marine nitrogen cycle. Biochem. Soc. Trans. 39, 315–320. ( 10.1042/BST0390315) [DOI] [PubMed] [Google Scholar]
- 93.Doney S, et al. 2012. Climate Change Impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 94.Cheung WWL, et al. 2010. Large-scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biol. 16, 24–35. ( 10.1111/j.1365-2486.2009.01995.x) [DOI] [Google Scholar]
- 95.Sumaila UR, Cheung WWL, Lam VWY, Pauly D, Herrick S. 2011. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Change 1, 449–456. ( 10.1038/nclimate1301) [DOI] [Google Scholar]
- 96.Hilmi N, et al. 2013. Towards improved socio-economic assessments of ocean acidification's impacts. Mar. Biol. 160, 1773–1787. ( 10.1007/s00227-012-2031-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as the electronic supplementary material.