Abstract
Climate change is leading to shifts in species geographical distributions, but populations are also probably adapting to environmental change at different rates across their range. Owing to a lack of natural and empirical data on the influence of phenotypic adaptation on range shifts of marine species, we provide a general conceptual model for understanding population responses to climate change that incorporates plasticity and adaptation to environmental change in marine ecosystems. We use this conceptual model to help inform where within the geographical range each mechanism will probably operate most strongly and explore the supporting evidence in species. We then expand the discussion from a single-species perspective to community-level responses and use the conceptual model to visualize and guide research into the important yet poorly understood processes of plasticity and adaptation.
This article is part of the theme issue ‘The role of plasticity in phenotypic adaptation to rapid environmental change’.
Keywords: climate change, marine ecology, phenotypic plasticity, range shift, species distribution, species range limits
1. Introduction
Global anthropogenic climate change is a significant threat to the persistence of species and the biodiversity of ecosystems [1]. Consequently, predicting the response of organisms to projected environmental change is critical to conservation and management planning [2]. Environmental warming—one manifestation of climate change—is inducing a variety of pervasive and observable changes to species distributions, abundance and phenology [3–6]. Species can respond to climate warming in several ways; they can move to new areas that become available within their thermal niche, they can acclimate and/or adapt their physiology or behaviour to extend their in situ thermal niche, and they can experience range contractions where a modified climate exceeds their thermal niche and thus prevents persistence. Although these responses are regularly characterized as ‘move, acclimate/adapt or die’ [7], they are rarely studied together to understand climate change-driven biological responses. This compartmentalization of responses is an oversimplification and unlikely to reflect reality. The influences of plasticity, adaptation and distributional changes are not mutually exclusive, and will exhibit different levels of importance within and across the species range. Moreover, the interplay between processes is likely to vary across a species range in complex but conceptually predictable ways.
Here, our aim is to synthesize understanding of the ecological and evolutionary processes that determine the pace and magnitude of range shifts in the sea. For this, we define three main areas of a species’ range in the context of climate change, where mature individuals are present: (i) the trailing edge—the warmest area of a species range, (ii) the core—the middle of a species’ range that may also have the greatest abundance, and (iii) the leading edge—the coolest area of a species’ range [8]. Previous reviews and meta-analyses have discussed global trends of range shifts [4,6], life-history traits that influence range-shift success [9–11], and proposed research directions to address taxonomic deficiencies and improvements in the classification of geographical patterns [12]: these predominantly involve data from terrestrial species. In addition, range-shift research has largely focused on observing their occurrence, primarily at the leading edge of the species distribution [13,14], although some studies have also observed changes in abundance within the core of a species range [12,15]. This is probably an artefact of the ease in observing the appearance of a species, and difficulty in observing changes in performance and abundance within a population that are additional to natural demographic fluctuations through time [16,17]. Documented species movements are often compared against the known rate of warming for the region. Conclusions are then drawn about the rate of movement compared to the expected rate given environmental change (i.e. environmental tracking [4,18,19]); or comparisons are made to describe the relationship between the environmental warming and species distribution (‘crash’, ‘lean’, ‘expand’, ‘extinct’, ‘retract’ and ‘march’; [12]). However, to explain and ultimately predict the speed and extent of movement, as well as the ecological consequences, a concerted effort is needed to advance our understanding of the ecological and evolutionary processes occurring in the core, leading and trailing areas of a species range. For example, similar observed responses in species' range shifts—such as a lag between warming and range contractions—may result from very different underlying mechanisms—e.g. phenotypic plasticity, selection, competition, predation and facilitation. Predicting future outcomes and managing conservation actions (including interventions) within this system, and predicting similar responses in regions that lack observational range-shift data, require understanding the ecological and evolutionary mechanisms that underlie range-shift responses.
Here, we conceptualize the interplay between ecological and evolutionary processes as species respond to climate change, and explore how these processes might play out differently throughout a species’ range, particularly in the context of marine ecosystems. Our intent is to advance the understanding of the role that evolutionary and ecological processes play in range shifts and their consequences, and to identify key gaps in our understanding and highlight future research priorities. The importance of understanding ecological and evolutionary processes in response to climate change and range shifts has been highlighted before (e.g. [20]). Some previous studies have also attempted to include aspects of local adaptation [21–24], plasticity [25] and species interactions [26–28] into species distribution modelling; however, the majority of these studies are based on terrestrial ecosystems (see for exceptions [24,26,29]). One of the likely reasons for the limited investigation of plasticity, adaptation and range shifts in unison is owing to the disparate timescales over which they each occur. Moreover, it is likely that the processes and the relative influence of processes at play may differ significantly between terrestrial species and marine ectotherms owing to a number of key differences (see box 1). For example, because most marine species possess a pelagic larval dispersal phase, it means that dispersal is occurring at the most environmentally sensitive period, and also potentially a highly plastic window owing to developmental plasticity [32]. Finding a way to connect plasticity, adaptation and changes in distribution will improve predictions about the spatial distribution of climate-sensitive traits and therefore the dynamics of climate-driven range shifts. Especially the predictability of how climate change will affect population demography and genetic structure across the species' range, both within the contemporary range and in newly colonized regions. Although it represents an enormous challenge, understanding multiple species’ responses and informing community-level predictions are also key to projecting future biological outcomes (e.g. [33,34]).
Box 1. Key differences between marine and terrestrial species that influence the process of plasticity, adaptation, changes in distribution and ultimately range-shift dynamics.
-
—
Marine species typically fill the geographical range of their fundamental thermal niche more fully than terrestrial species [30].
-
—
Marine species tend to have larger geographical ranges than terrestrial species because of relatively fewer geographical barriers [31].
-
—
The majority of marine ectotherms have a bipartite life history, with a pelagic larval stage that leads to high levels of gene flow and high dispersal potential. Pelagic early life-history stages also tend to have ecological requirements, including thermal niches, that differ vastly from adults.
-
—
Climate-driven changes in the distribution of marine species are occurring much faster than for terrestrial species, 72 km per decade on average compared to 17 km per decade in terrestrial species [4]. Thus, the greater rate of change in marine systems may ultimately facilitate our description and understanding of range-shift processes more generally.
-
—
Species in the ocean are adapted to less thermal variability at a given latitude, so small change in the ocean makes a big difference, i.e. marine life is adapted to less variability.
-
—
Time lags are reduced on average in the ocean compared to on land owing to few dispersal barriers (i.e. range edges better keep pace with temperature change).
Here, we describe a conceptual model of the interrelationship between plasticity, adaptive processes and movement for a population (box 2). We expect that this model could be applied more broadly, but focus here on relationships between plasticity, adaptation and migration throughout the species’ range for marine ectotherms in which water temperature is the focal environmental factor. The focus on marine systems is because the relative importance of processes will probably be different in marine systems, compared to terrestrial, because of unique characteristics, such as the predominance of highly dispersive larval stages. We then use this model, and its components, to explore the likely importance of various processes in each area of the range (trailing edge, core and leading edge) on expected demographic outcomes. We outline the general expectations for these processes across species ranges, as well as the empirical evidence available in marine systems. Subsequently, we discuss the current understanding of community-level responses to environmental change, species in light of both known and novel interactions from range shifts. We show how the conceptual model is useful in visualizing multiple species’ responses and their interactions (box 3). Finally, we summarize and suggest key research directions critical to understanding the interplay between plastic and adaptive processes in determining range shifts.
Box 2. Interplay between plasticity, adaptation and movement for a single species.
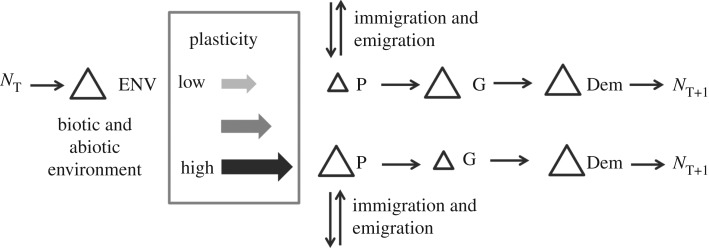
While it may be intuitive that plasticity, adaptation and geographical movement will all interact to produce the net changes in phenotypes that dictate a population's demographic response to climate change, there is yet to be a conceptualization of exactly how this might occur (figure 1). Environmental factors, including warming owing to climate change, act on populations over time to select suitable phenotypes. A change in the environment, whether biotic or abiotic, can lead to a change in the distribution of phenotypes (phenotypic variation) through either natural selection or plasticity in phenotypes. Selection on heritable phenotypic variation produces a change in genetic variation, which can promote adaptation to environmental change (figure 1: top-line example, figure 2: star example). Alternatively, phenotypic plasticity may enable individuals to buffer the effects of environmental change without genetic selection (figure 1: bottom-line example, figure 2: hexagon). When phenotypic plasticity is high, the population may be buffered against the effects of environmental change without any change in genetic variation. Ultimately, however, the tolerant phenotypes produced by plasticity could become fixed in the population through genetic assimilation. Intermediate levels of plasticity may provide some buffering against the effects of environmental change, and also reduce the intensity of selection by shifting the mean phenotype of the population, consequently limiting the pace of adaptation. Immigration and emigration of individuals through the system also lead to changes in phenotypic variation in the population, which again may promote or limit adaptation. Heritable or not, phenotypic changes can lead to demographic changes that affect population size in the next time-step. Changes in population size and the distribution of phenotypes in the population will then interact with the changing populations of other species in the community. This model also could describe stochastic changes to demography and populations that occur naturally without human-induced shifts to the environmental conditions.
Figure 1.
Model of evolution, plasticity and migration as response to changes in the environment. NT, population size at time T; P, phenotype; G, genotype; Dem, demography; NT + 1, population size at time T + 1. Arrows represent state changes within a time-step.
Box 3. Using the model to predict multispecies responses.
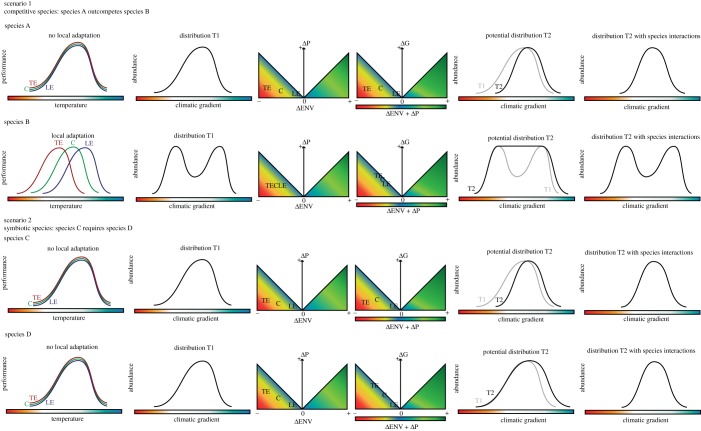
The model proposed in box 2 can be used to conceptualize the potential outcomes for various species’ interaction scenarios, across a species range (figure 3). Knowledge on the thermal performance, effect of environmental change and the capacity for plasticity and adaptation could be used to project likely demographic effects for a given species that would also allow comparisons between species in a community. Species’ interactions could include any situation where the presence of one species either directly or indirectly influences another, e.g. competition, predation, facilitation, mutualism or symbiosis. Here, we present two example scenarios to highlight the potential usefulness of the model. In scenario (1), there are two competitive species where species A outcompetes species B, unless species B has enhanced performance. Species B is locally adapted to the thermal environment experienced in the range sections, and both species have the capacity for dispersal. Species B has a greater capacity for plastic and adaptive change resulting in it both maintaining and extending its range, but it is still impacted when species A has equal or greater performance. Scenario (2) displays two symbiotic species, where species D requires species C for persistence. Both species possess similar thermal performance across their current range, have the capacity for dispersal, but have differing capacity for phenotypic and adaptive change. While in isolation, species D would be expected to maintain and extend their range with future warming, including the symbiotic requirements would result in a reduced distributional range.
Figure 3.
Multispecies scenarios for example model use. In this figure, each row represents a species. The far left panels depict the current-day thermal performance of a species across the range sections (TE, trailing edge; C, core; LE, leading edge), followed by the current-day distribution at time 1. The middle two sections are the model visualization from figure 2 (colour scale blue is a neutral, green positive and red negative response). The right two panels depict the expected distribution at time 2. The first shows time 2 distribution without species interactions in black and the time 1 distribution in grey for comparison (second panel from the right). Finally, the far right panel shows time 2 distribution with species' interactions.
2. Plasticity, adaptation, movement and range shifts
In relation to global warming, essential components of an organism's performance are the fundamental and realized (observed in light of other processes) thermal niches (survival limits sensu [35]). The shape of a thermal performance curve is expected to relate to the variety and variability of environmental conditions experienced [36]. The climatic variability hypothesis proposes that as seasonal climatic fluctuations increase, individuals which experience higher fluctuations should possess broader thermal tolerance ranges and/or greater physiological flexibility [37]. For a broad range of taxa, thermal niches consistent with the climatic variability hypothesis are observed. Specifically, species from temperate regions, which experience higher seasonal thermal variation, generally possess wider thermal performance niches and are living further below warm critical thermal limits, than tropical species [38–41]. Differences in thermal performance can also be observed across the range of a species, when locations within the range experience differing thermal variation (e.g. [39,40]).
For many species, an individual's thermal niche can shift depending on the history of thermal conditions experienced [42–44]. Phenotypic plasticity is traditionally defined as the capacity of a given genotype to render alternative phenotypes under different environmental conditions [32], but in relation to climate change is often more broadly considered as environmentally induced phenotypic variation [45]. Phenotypic plasticity can include changes to individual morphology, physiology and behaviour [42], which may enhance performance under altered environmental conditions (often called acclimation). In relation to climate change, plasticity is usually considered in terms of improving or optimizing performance to altered conditions, although plasticity is not always beneficial [42,46]. Behavioural plasticity, such as changes in microhabitat use or depth movements, could also allow individuals to avoid unfavourable environmental conditions at their geographical location, but maintain the same thermal performance niche. Theoretically, adaptive phenotypic plasticity should be greater when populations or species experience greater temporal environmental heterogeneity [47,48]. This has been observed in latitudinal trends for some species [49,50], but is far from ubiquitous [43,51–53], and in some cases plasticity may relate to localized environmental variation [54,55]. Plasticity is often thought of as the first response to environmental change because it can occur over relatively rapid timescales [56,57]. However, the costs of and limits to phenotypic change can mean that there is a time lag in the response to environmental change or that full phenotypic compensation does not occur [42].
Adaptation in a population occurs through the selection of favourable phenotypes that are heritable from one generation to another, leading to a shift in the frequency of alleles that confer greater fitness under the altered conditions. Selection can occur on pre-existing genetic variation as well as selection on new mutations (reviewed in [48]). Adaptation requires time for natural selection to take place, so it is often considered to have limitations as a rapid response to climate change, especially in longer-lived organisms [3,58,59]. Limitations can be owing to low levels of heritable variation, a lack of genetic variation owing to small population size or slow rates of beneficial mutation, as well as mismatches between the direction of selection and the standing genetic (co)variance within the population [60,61]. Nevertheless, examples are emerging where adaptation can occur over timescales relevant to the rate of human-induced climate change [62–64]. There is also speculation that phenotypic plasticity may facilitate adaptation if plastic traits are selected for and become fixed in the population (i.e. genetic assimilation; [65,66]). In the case of genetic assimilation, the originally environmentally plastic trait becomes robust to environmental change. Plasticity could also maintain effective population sizes, thereby reducing the costs of selection and risk of extirpation [67]. Alternatively, phenotypic plasticity (e.g. behavioural thermoregulation) may retard the pace of adaptation by shifting the mean phenotype of the population, and thus reducing selection pressure, without any change in allele frequencies [68]. The relationship between plasticity and adaptation has been discussed previously in the context of climate change responses (see reviews [55,57,67,69]); however, there is yet to be a conceptual model proposed that can be used to help understand the role of plasticity and adaptation specifically in relation to geographical range shifts (box 2).
In addition to plasticity and adaptation, geographical movement represents a further process by which distributions of phenotypes can be altered by environmental change, ultimately leading to species’ range shifts. Range shifts involve the extension, contraction and/or movement of individuals within and outside of the species' historical geographical boundaries [13], including colonization of new populations as well as immigration and emigration between populations (i.e. gene flow). Within the literature, we are making the implicit assumption that our contemporary documentation of a species range represents a range that is at equilibrium; however, this may not be the case for many species [70]. The ecological implications of dispersal and connectivity depend on the frequency and nature of dispersal events (i.e. from rare events to continuous flow [71]), as well as the relative phenotypic and genetic differences between locations. When dispersal or movement is limited to the pelagic larval stages (i.e. adult life stages are sedentary), this early life stage is expected to have narrower thermal tolerance [72], which will significantly influence the impact of dispersal upon range shifts. Indeed, species that brood their young on average have range extensions that are equally fast as species with long-lived pelagic larval stages [9]. Predictions of future migration will also need to take into account changes to ocean currents, for example, the general expectation of strengthening boundary currents globally [73–75]. It is also important to consider how warming may influence larval development directly, with elevated (non-lethal) temperatures predicted to limit mean dispersal distance owing to reduced pelagic larval duration [76].
Gene flow has the potential to enhance local adaptation by importing beneficial genotypes [77]. However, gene flow can also hinder the potential for adaptation by providing maladaptive phenotypes/genotypes that decrease the mean fitness, and when large amounts of migration occur, this can lead to gene swamping [77–79]. Populations that experience warmer conditions on average, or which have a wider thermal range, can possess warmer-suited phenotypes/genotypes [62,80,81]. However, there is also evidence in some species, for homogeneous thermal performance among populations that experience different maximum and average temperatures [40,82], possibly owing to high levels of gene flow between populations. In these cases, there would be limited phenotypic and genetic implications of emigration, because it would not be providing thermally adapted phenotypes. Alternatively, while strong genetic isolation could favour local adaptation, it will probably restrict the spread of favourable alleles under warming when dispersal connections remain the same [83,84]. For most marine species, the phenotypic and genetic differences that exist among populations, as well as the connectivity between them, are poorly understood, so our ability to predict the influence of movement or dispersal on outcomes of selection is limited (exception see [85]). Some marine species have a high capacity for both self-recruitment and long-distance dispersal, which can allow for both local adaptation and high levels of population connectivity [71,74,86–90]. Active transplantation of warmer performance phenotypes/genotypes has been proposed as a conservation technique to enhance demographic outcomes at particular locations within the range [91]. Although this practice could potentially allow for the short-term persistence of species in locations where they would otherwise not survive, its efficacy in establishing viable heat-tolerant populations is contested and may be limited to situations where selective advantage is high. In particular, the small number of heat-tolerant individuals that could be introduced compared to the massive effective population size of most marine species means that favourable alleles will be swamped by existing genetic diversity, which would impede their ability to spread rapidly through the population.
3. Expected processes operating in the trailing edge, core and leading edge of the range
Across a species’ range, the importance of particular ecological and evolutionary processes in response to environmental warming is expected to vary. Although we can predict the processes (e.g. plasticity, selection, movement) likely to shape the thermal performance of populations across a species range, we have limited empirical data to test these predictions for the majority of marine species. Below we outline, the general expectations for processes operating in three areas of a warming range, as well as the supporting or opposing evidence from marine systems.
(a). Trailing edge
Close to the trailing edge, the existing habitat is generally becoming warmer than the species' present realized niche. Here, populations will need to exhibit the maximum amount of plasticity and/or adaptation to warming in order to persist. There is potential for behavioural buffering of environmental change [92], especially in mobile species or habitats with heterogeneous microclimates [93,94]. Selection will favour the tolerance of hotter climates [95] and/or the tolerance of new biotic interactions as other species encroach as a result of their own range shifts. Population declines are expected at the trailing edge of a species range when the maximum plastic and/or adaptation potential is reached [80], and environmental conditions surpass the fundamental thermal niche [13,36]. Although trailing-edge populations are expected to be thermally adapted, in some species there is evidence that these populations show similar or even less resilience than core populations [96–99]. Additionally, trailing-edge populations are believed to have relatively low capacity for plasticity; or have reached the limit of their plasticity. However, there is evidence that plasticity may be higher compared to core populations or it can increase in peripheral populations (i.e. moving away from the core) [43,80,100,101]. In the trailing-edge region, there is likely to be limited opportunity for genetic rescue as low to no immigration from thermally tolerant populations would occur. In the case of environmental variability, rather than directional climate change, there could be instances where trailing-edge locations are rescued by migration from the core (e.g. sources and sinks [102,103]). However, large amounts of migration to small peripheral populations risk negative effects of gene swamping [104]. Strong selection for warm-tolerant phenotypes in the trailing edge could reduce genetic diversity and constrain the potential for adaptation to other stressors, unless there are positive genetic correlations between traits [56,105]. Evidence for warming-induced effects to trailing-edge populations has been observed in a marine macroalga (Fucus vesiculosus) in the northeastern Atlantic with extinction and loss of genetic diversity in the trailing edge [105].
As population size and genetic diversity decline, populations in the trailing edge will become susceptible to inbreeding and allele effects that can further compromise population performance [60]. It is generally expected that population size and genetic diversity are positively correlated, thus as population sizes decline so does genetic diversity. However in nature, the relationship between population decline and genetic diversity can be complex, because genetic diversity can still be substantial at low population densities or when spatial fragmentation and demographic bottlenecks occur [106]. Numerical population declines may involve a time lag, especially for longer-lived species, as the adult population can persist for some time even when reproductive and recruitment failure is occurring [107]. This suggests that monitoring genetic diversity, in addition to abundance, could be a useful tool for detecting population changes. The presence of other species that possess warmer thermal distributions (e.g. leading edge) or enhanced thermal performance (i.e. more thermally tolerant) will also change the dynamics of ecological processes, such as competition and predation, which further impinge on the ability of the population to persist in the trailing edge. If we simplistically assume all other factors are equal, species that possess superior warm tolerance and/or plasticity are likely to be favoured, at the expense of species with poorer thermal tolerance and/or limited plasticity [108,109].
(b). Core
In the core of a species range, phenotypic plasticity, movement and selection will all be influential in determining demographic effects. Genetic variation is likely to be greatest in the core of the range where population size is assumed to be greater [83], reducing the demographic costs of selection compared with the leading and trailing edge, and owing to immigration of climate-related alleles from warmer parts of the range. Migration of climate-related alleles will be especially important in species that exhibit local climate adaptation [99]. As individuals move or disperse from warmer-adapted populations, they can transfer alleles that will be favoured in response to warming in the core of the species range, although whether this occurs will depend on the interplay between local adaptation and gene flow [79,84,85,110]. In large populations, the potential for positive effects of warm-adapted migration is likely to be controlled by the relative influx of individuals with warm-adapted genotypes compared to the retention of local genotypes, and the extent of selection for those warm-adapted individuals. Limitations could occur if thermally unsuitable phenotypes are retained locally [62] and thermally superior individuals fail to immigrate. To the best of our knowledge, no empirical studies in marine systems have established migration rates and local thermal adaptation in unison. Nevertheless, theoretical models suggest that the reef-building coral, Acropora millepora, on the Great Barrier Reef has sufficient genetic variation and connectivity to allow adaptation of populations to future projected ocean warming [110].
The influence of phenotypic plasticity in the core of the range will be determined by the rate at which it can occur relative to environmental change. As environmental conditions within the core region are likely to be well within the bounds of the species’ fundamental thermal niche, there is expected to be high capacity for plasticity within the core (figure 2 hexagon example). However, there are potential circumstances where physiological plasticity could be limited (figure 2 star example), such as when behavioural thermoregulation occurs [51,111]. While expected to be less common in marine than terrestrial species, populations using spatial thermal heterogeneity to maintain body temperature will decline if warming shifts the thermal environment beyond the capacity for behaviour to effectively buffer body temperature [112]. Generally, core populations are expected to be at lower risk than populations at the trailing edge as there is more time for adaptive processes to occur before thermal limits are exceeded. However, the median phenotype will still lag behind the fitness optimum in a rapidly changing environment [42,67]. Tolerance to new biotic interactions may also be important in this region if new species are expanding into the core area of the current species; however, this is likely to be less influential than in the trailing edge because core populations are large and have not yet exhausted all their plastic and adaptive potential to maintain performance at higher temperatures.
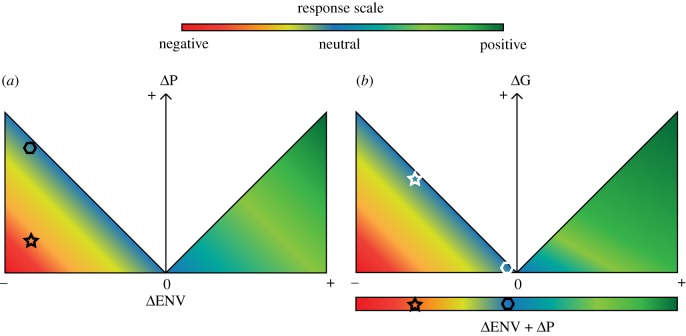
Figure 2.
Visualization of conceptual model (from figure 1) showing how environmental change (ΔENV), phenotypic change (ΔP: plasticity, immigration and emigration) and genotypic change (ΔG: selection) shift demography within a time-step. The first part of the equation including ΔENV and ΔP is displayed in (a). This result (black shape) becomes the x-axis of (b) which displays the subsequent relationship with ΔG. The result is the expected demographic outcome in the next time-step (i.e. white shapes). Example 1 (star) shows a neutral demographic response (blue) with low plasticity (a) and high response to selection (b). Example 2 (hexagon) shows a neutral demographic response with high plasticity (a) and low response to selection (b).
There are a number of factors that could alter the expectations outlined above for the processes occurring in the core of the species range in response to warming. For example, while it is often assumed that populations which experience warmer conditions on average will have warm-adapted phenotypes, this is not always the case [40,53,62,82]. In these instances, immigration of climate-related alleles from warmer parts of the range is unlikely to result in a shift in thermal tolerance of the receiving population. High gene flow among populations, as occurs in many marine species, may restrict local adaptation and thus the potential for genetic rescue from warm-adapted populations. Shifts in oceanographic currents owing to climate change [73,75,113] could provide instances where migration can deliver warm-adapted genotypes to previously isolated populations [114]. Restriction in the migration of warm-adapted phenotypes from the trailing edge could also occur when warming negatively impacts on reproduction or larval survival in trailing-edge individuals [115]. The prediction that the core region contains the largest populations may not hold true in all instances [16,116–118]. For example, intertidal invertebrates were found to have diverse abundance patterns across their range [119], highlighting a need to understand what sets present range boundaries and distribution patterns for species so that projections into the future are possible. Furthermore, even when there are differences in genetic diversity between core and edge populations, and this is not always the case [120], the amount of diversity may not be large [121], thus reducing the impact of immigration and emigration.
(c). Leading edge
At the leading edge, where new regions are becoming climatically similar to the species' core range, selection is expected to be relaxed on thermal physiology, but may be intensified on a species’ ability to cope with novel combinations of physical habitat, climate variables and biotic community. For example, vagrant tropical fish in temperate southeast Australia were found to have better growth and survival if they formed social groups with local temperate species rather than their tropical conspecifics [122]. A number of other abiotic factors in the novel environment are likely to influence the population, including temperature variability (interannual, seasonal or daily [123]), photoperiod, and physical abiotic habitat; while biotic interactions, such as competition, diminished biotic habitat or food source, may further limit population distribution and/or abundance. This means that species with the broadest fundamental niche are often favoured in range expansion, as it allows success in diverse ecological conditions [9,124]. It is intuitive that a generalist genotype (thermal or otherwise) would be beneficial in range expansion (e.g. invasive species generally; [125]), because the species is better able to make use of novel resources as it moves into new areas compared with an ecological specialist. However, when a required resource is readily available, ecological specialization could confer superior performance and competitive ability for the given resource [126,127].
Predicting range expansion success may not be a simple case of whether a species possesses a broad ecological niche, as success may be determined by the strength of competition within the receiving community [128,129]. The cooler limits of current-day realized thermal niches (i.e. contemporary geographical ranges) can correlate to the magnitude of seasonal thermal variation experienced across a species range [36], and as such may also influence future range-shift potential. New populations are generally composed of a relatively small number of individuals, so there is potential for genetic bottlenecks and reduced allele diversity, runaway selection, as well as Allee effects [130] to all contribute to the challenges in establishing a population at the leading edge. However, if species' geographical distributions are able to perfectly track their thermal niche, it is possible that populations in the leading edge of the range could be maintained without major effects on genetic structure [74,120]. For example, genetic bottlenecks and changes in population size were detected throughout the range expansion axis in the range-shifting gloomy octopus [131]. However, persistent gene flow from throughout the historical zone and moderate genetic diversity may buffer the genetic bottlenecks and favour the range expansion. A number of biological attributes and conditions could also exist that limit the ability of species to expand their range, one of which is poor migration capacity or larval ‘lottery’, which limits the ability to track thermal conditions and extend the leading edge past contemporary boundaries [132,133].
4. Importance of a biological community perspective
Single-species predictions are the starting point, but a multispecies perspective, while challenging, is required to fully comprehend and predict the effects of climate change to communities, as well as the occurrence and consequences of range shifts [134]. Environmental change can alter ecological processes such as predation dynamics, competition and mutualisms [3,70,135], thus also influencing the realized niche of multiple species. The presence of one species can influence another through effects on growth rates, displacement rates and carrying capacity [136]. In regards to environmental change and range shifts, understanding how species differences in thermal sensitivity, plasticity and adaptive capacity will interact to result in demographic changes in the community is of interest. For example, loss of a dominant competitor, owing to difference in thermal sensitivity, could lead to competitive release favouring subordinate competitors [135]. Effects on cellular and organismal physiology induced by temperature change are expected to influence energetic requirements, foraging rates and assimilation rates, and consumptive and non-consumptive effects on lower tropic levels [137,138]. Owing to physiological and ecological differences between trophic levels, changes in environmental temperature can disproportionally affect levels causing resource limitation [139,140] or shifts in predator–prey relationships [141,142]. Species in the community may indirectly affect each other through mechanisms such as behaviourally mediated indirect interactions or when the presence of a species changes the environmental conditions experienced [143,144]. In addition to the effect of temperature, plasticity and adaptation have the potential to affect ecological processes, both directly and indirectly [145]. For example, differences in plasticity could shift the balance of interspecific competition (box 3 scenario 1) or allow maintenance of the usual competitive balance that would not occur by species' thermal sensitivity [146]. Predicting species’ interactions within areas of known range overlap could be more easily completed with historic compared to novel interactions, because there is potential for some prior knowledge of the species relationships [33]. However, multi-species investigations have highlighted that the effects of environmental change can shift substantially compared to single-species responses [147–149].
As the environment warms, species’ movement is allowing colonization of new locations that is leading to novel species’ interactions. As previously discussed, having a broad ecological niche [9,124] or a unique ecological niche [128] can be of benefit to incoming (i.e. leading-edge) species because it probably reduces resource limitation and competition. Arriving species can have negative impacts on species within the local community with whom they compete directly for resources [150] or whom they consume [73,151]. The addition of novel species can induce habitat modification with critical indirect flow-on effects to the resident community [125,152]. By contrast, the addition of a new species can reduce predation on an existing species, by attracting predation from a shared consumer [151]. Human activities can also influence the effect of species’ range shifts, such as fishing which can reduce competition or lead to predatory release of the invading species (this could also be true within a species range but would be difficult to detect). For example in the historically kelp-dominant regions of eastern Tasmania, the fishing of spiny lobster (predator) has facilitated range expansion of the long-spined sea urchin (prey) [153]. Adding to the complexity of predicting species range shifts is the impact of historical relationships between range-shifting species. Specifically, whether species that have a history arrive together or mismatched in time, this can affect range-shift dynamics by slowing or facilitating expansion [154]. Facilitation may be critical in many cases, for example in coral reef fishes with dependence on coral habitat or specific coral species [155,156] (box 3 scenario 2). Facilitation can also occur through the presence of a key species (e.g. biotic modifier, ecosystem engineer, niche constructor) that modifies the abiotic environment, allowing habitable conditions for another range-shifting species [157].
When predicting community-level responses to environmental change it is naive to consider each species and its range independently given the diversity of species interaction types occurring in a community. Within a given community, species may be at different locations within their range, at different mean temperatures along their thermal performance niche, and have different plastic and adaptive potentials; thus different processes will be predicted to influence demography of each species if considered in isolation. A framework to conceptualize the comparison of species responses on the basis of the impact of environmental change, as well as plasticity and adaptive capacity is outlined in box 3. This framework is useful in consideration of the complexity of comparisons among species because different interplay between environmental change and adaptation can produce divergent results, or divergent processes can produce similar demographic outcomes. For example, it would be useful to understand the relative importance of plasticity to adaptation/selection in shifting the population phenotype, if high levels of selection mean high mortality and population size reduction. A large reduction in abundance of a given species could have flow-on effects to predators, competitors or those in mutualistic relationships. We propose that knowledge of the effect of environmental change compared with the relative effects of plastic and adaptive potential in response could be used to estimate the likely demographic outcomes. By combining this assessment with knowledge of species’ interactions, and future climate scenarios, a strong basis for predicting future outcomes is possible. However, in most natural systems, including marine, more information on the long-term effect of environmental change to the performance, plasticity, adaptation and interactions between species holistically is required to make and validate predictions.
5. Conclusion
Predicting range shifts in the ocean is clearly a substantial challenge, especially with the range of environmental stressors affecting populations (e.g. temperature, pH, oxygen, salinity, sea-level change, nutrients). Our focus on the ubiquitous stressor, ocean warming, illustrates the challenges that exist for projecting species responses and persistence. As marine species tend to more fully inhabit their fundamental niche than terrestrial species [30], species distributions and thermal conditions across the distribution could be used to estimate thermal sensitivity. However, to project species responses into the future, data regarding the capacity for plastic and adaptive responses across the species' range are needed.
We have identified additional knowledge gaps that are important to fill, including connectivity, gene flow, genetic variation (both within and between populations), and local thermal adaptation across the species range. We also need to understand what aspects of the environment populations are adapted to (e.g. the average, extremes or variation in conditions) and phenotypic plasticity (table 1). Technological advances provide new tools to understand populations in terms of genetic connectivity (e.g. microsatellite and parentage analysis), population genetic variation (e.g. low-cost genomes and environmental DNA), and phenotypic plasticity (e.g. transcriptomics and metabolomics). Some global regions offer useful natural experiments to understand the interaction between plastic, adaptive and migration processes occurring, such as in fast-warming areas [8] and regions where vagrant species are yet to establish breeding populations (e.g. vagrant tropical fishes in southeastern Australia [124]). The starting point to testing our conceptual model would begin with a better understanding of the interplay between plasticity, adaptation and migration in a number of representative species. As more information becomes available, the generalized expectation of mechanisms that operate in each area of the range outlined here can be more formally tested and empirically modelled.
Table 1.
Outstanding questions for future research and approaches to address the knowledge gaps in marine systems.
| questions | approaches |
|---|---|
| how does plasticity differ between populations across a species range? | experimental approaches for testing plasticity across life stages (e.g. developmental, reversible, transgenerational plasticity) |
| what is the connectivity and gene flow between populations? | field sampling through time in combination with genetic approaches such as microsatellite and/or parentage analysis |
| what is the genetic variation within and between populations across a species range? | quantitative genetic approaches |
| what is the heritability and GxE interactions of key traits for persistence? | experimental quantitative genetic approaches, including structured breeding designs |
| how do plasticity and selection outcomes differ in multi-stressor situations? | experimental approaches inducing potential multi-stressor scenarios. Make use of natural settings that vary in both temperature and other environmental parameters |
| how do species interactions shift along a species range? | greater field-based investigation across species' ranges (i.e. diverse environments). Ideally investigating areas with and without species overlap including in the leading edge where species are encountering novel communities |
| how do plasticity and selection interact in various areas of a species range? | experimental evolution approaches with short-lived species. Use areas and regions where vagrant or new species are yet to establish breeding populations to measure phenotypic and genetic difference of arriving versus persisting individuals |
While beyond the scope of this paper, there is also a clear need for multi-stressor investigations and discussion in relation to range shifts [158]. Throughout a species' range, changes to the environment other than temperature could lead to performance and survival differences that reflect other biotic or abiotic factors rather than direct and indirect effects of warming [159]. The direction of change in environmental variables can also differ such that opposing selection can occur, further complicating outcomes. Any environmental change can be visualized in the model at the ΔENV term (box 2). It is expected that populations which experience changes in additional environmental conditions (e.g. pH, salinity, resource availability) would either experience greater change in demographic rates (e.g. growth and survival) or require greater phenotypic plasticity and/or adaptive capacity to maintain performance, regardless of where in the range these environmental changes occur. As discussed above in relation to temperature change, trailing-edge populations are potentially at greater risk of population declines because the capacity for plastic and evolutionary responses is likely to be already pushed to the maximum. Populations at the trailing edge will experience the strongest selection for tolerance to higher temperatures, which may reduce genetic variation and adaptive potential to other environmental changes. Additionally, adaptation would be impeded if there are negative genetic correlations between performance in warmer conditions and changes in other environmental parameters [56,160]. However, predicting multi-stressor responses is difficult as a range of potential phenotypic and genotypic responses may exist depending on the combination of stressors involved (e.g. [161]).
Gaining this knowledge will take an approach that combines broad-scale quantitative information on the abundances of species across space and time with both laboratory and field-based experimentation. Ultimately the goal is to predict and validate species and community-level range-shift responses, which allows for more effective conservation and management in a changing climate. While there are substantial challenges in gaining information on processes for numerous species and areas of the range for marine species, the knowledge acquired is essential for robust biological predictions. Our conceptual model provides framework for prioritizing research on species’ responses to environmental change.
Acknowledgements
The authors thank their relevant funding sources and the Species on the Move Conference Organisers (2016) Hobart Tasmania for hosting the workshop. These include the ARC Centre of Excellence for Coral Reef Studies, the University of Technology Sydney, McGill University, University of Sydney, CSIRO Oceans and Atmosphere, The University of Hong Kong, Institute for Marine and Antarctic Studies (University of Tasmania), Australian Museum Research Institute, Monash University and James Cook University. The authors thank the three reviewers whose helpful comments substantially improved the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
This paper is the result of a workshop led by J.M.D., P.L.M., D.J.B. and G.G.R. This manuscript was written from ideas discussed at the workshop. J.M.D. wrote the manuscript with input from co-authors. All authors contributed to writing and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
G.P. was supported by an Australian Research Council Future Fellowship.
References
- 1.Scheffers BR, et al. 2016. The broad footprint of climate change from genes to biomes to people. Science 354, eaaf7671 ( 10.1126/science.aaf7671) [DOI] [PubMed] [Google Scholar]
- 2.Bonebrake TC, et al. 2018. Managing consequences of climate-driven species redistribution requires integration of ecology, conservation and social science. Biol. Rev. 93, 284–305. ( 10.1111/brv.12344) [DOI] [PubMed] [Google Scholar]
- 3.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 4.Poloczanska ES, et al. 2013. Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925. ( 10.1038/nclimate1958) [DOI] [Google Scholar]
- 5.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 6.Pecl GT, et al. 2017. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214 ( 10.1126/science.aai9214) [DOI] [PubMed] [Google Scholar]
- 7.Maggini R, Lehmann A, Kéry M, Schmid H, Beniston M, Jenni L, Zbinden N. 2011. Are Swiss birds tracking climate change? Detecting elevational shifts using response curve shapes. Ecol. Modell. 222, 21–32. ( 10.1016/j.ecolmodel.2010.09.010) [DOI] [Google Scholar]
- 8.Hampe A, Petit RJ. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecol. Lett. 8, 461–467. ( 10.1111/j.1461-0248.2005.00739.x) [DOI] [PubMed] [Google Scholar]
- 9.Sunday JM, et al. 2015. Species traits and climate velocity explain geographic range shifts in an ocean-warming hotspot. Ecol. Lett. 18, 944–953. ( 10.1111/ele.12474) [DOI] [PubMed] [Google Scholar]
- 10.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species' traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689. ( 10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 11.MacLean SA, Beissinger SR. 2017. Species’ traits as predictors of range shifts under contemporary climate change: a review and meta-analysis. Glob. Change Biol. 23, 4094–4105. ( 10.1111/gcb.13736) [DOI] [PubMed] [Google Scholar]
- 12.Lenoir J, Svenning JC. 2015. Climate-related range shifts: a global multidimensional synthesis and new research directions. Ecography (Cop.) 38, 15–28. ( 10.1111/ecog.00967) [DOI] [Google Scholar]
- 13.Bates AE, et al. 2014. Defining and observing stages of climate-mediated range shifts in marine systems. Glob. Environ. Change 26, 27–38. ( 10.1016/j.gloenvcha.2014.03.009) [DOI] [Google Scholar]
- 14.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 12, 450–455. ( 10.1111/j.1365-2486.2006.01116.x) [DOI] [Google Scholar]
- 15.Kiessling W, Simpson C, Beck B, Mewis H, Pandolfi JM. 2012. Equatorial decline of reef corals during the last Pleistocene interglacial. Proc. Natl Acad. Sci. USA 109, 21 378–21 383. ( 10.1073/pnas.1214037110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40, 415–436. ( 10.1146/annurev.ecolsys.110308.120317) [DOI] [Google Scholar]
- 17.Robinson LM, Gledhill DC, Moltschaniwskyj NA, Hobday AJ, Frusher S, Barrett N, Stuart-Smith J, Pecl GT. 2015. Rapid assessment of an ocean warming hotspot reveals ‘high’ confidence in potential species' range extensions. Glob. Environ. Change 31, 28–37. ( 10.1016/j.gloenvcha.2014.12.003) [DOI] [Google Scholar]
- 18.Przeslawski R, Falkner I, Ashcroft MB, Hutchings P. 2012. Using rigorous selection criteria to investigate marine range shifts. Estuar. Coast. Shelf Sci. 113, 205–212. ( 10.1016/j.ecss.2012.08.005) [DOI] [Google Scholar]
- 19.Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA. 2013. Marine taxa track local climate velocities. Science 341, 1239–1242. ( 10.1126/science.1239352) [DOI] [PubMed] [Google Scholar]
- 20.Lavergne S, Mouquet N, Thuiller W, Ronce O. 2010. Biodiversity and climate change: integrating evolutionary and ecological responses of species and communities. Annu. Rev. Ecol. Evol. Syst. 41, 321–350. ( 10.1146/annurev-ecolsys-102209-144628) [DOI] [Google Scholar]
- 21.Wang T, O'Neill GA, Aitken SN. 2010. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 20, 153–163. ( 10.1890/08-2257.1) [DOI] [PubMed] [Google Scholar]
- 22.Atkins KE, Travis JMJ. 2010. Local adaptation and the evolution of species' ranges under climate change. J. Theor. Biol. 266, 449–457. ( 10.1016/j.jtbi.2010.07.014) [DOI] [PubMed] [Google Scholar]
- 23.O'Neill GA, Hamann A, Wang T. 2008. Accounting for population variation improves estimates of the impact of climate change on species' growth and distribution. J. Appl. Ecol. 45, 1040–1049. ( 10.1111/j.1365-2664.2008.01472.x) [DOI] [Google Scholar]
- 24.Cacciapaglia C, van Woesik R.. 2018. Marine species distribution modelling and the effects of genetic isolation under climate change. J. Biogeogr. 45, 154–163. ( 10.1111/jbi.13115) [DOI] [Google Scholar]
- 25.Valladares F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351–1364. ( 10.1111/ele.12348) [DOI] [PubMed] [Google Scholar]
- 26.Torres LG, Read AJ, Halpin P. 2008. Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity? Ecol. Appl. 18, 1702–1717. ( 10.1890/07-1455.1 ) [DOI] [PubMed] [Google Scholar]
- 27.Wisz MS, et al. 2013. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. 88, 15–30. ( 10.1111/j.1469-185X.2012.00235.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araújo MB, Luoto M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753. ( 10.1111/j.1466-8238.2007.00359.x) [DOI] [Google Scholar]
- 29.Jonsson PR, Kotta J, Andersson HC, Herkül K, Virtanen E, Sandman AN, Johannesson K. 2018. High climate velocity and population fragmentation may constrain climate-driven range shift of the key habitat former Fucus vesiculosus. Divers. Distrib. 24, 892–905. ( 10.1111/ddi.12733) [DOI] [Google Scholar]
- 30.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 31.Rapoport EH. 1994. Remarks on marine and continental biogeography: an areographical viewpoint. Phil. Trans. R. Soc. Lond. B 343, 71–78. ( 10.1098/rstb.1994.0009) [DOI] [Google Scholar]
- 32.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Marzloff MP, Melbourne-thomas J, Hamon KG, Hoshino E. 2016. Modelling marine community responses to climate-driven species redistribution to guide monitoring and adaptive ecosystem-based management. Glob. Change Biol. 22, 2462–2474. ( 10.1111/gcb.13285) [DOI] [PubMed] [Google Scholar]
- 34.Fulton E, et al. 2018. Decadal scale projection of changes in Australian fisheries stocks under climate change. See http://www.frdc.com.au/project?id=3000.
- 35.Connell JH. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42, 710–723. ( 10.2307/1933500) [DOI] [Google Scholar]
- 36.Stuart-Smith RD, Edgar GJ, Bates AE. 2017. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 1, 1846–1852. ( 10.1038/s41559-017-0353-x) [DOI] [PubMed] [Google Scholar]
- 37.Janzen DH. 1967. Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249. ( 10.1086/282487) [DOI] [Google Scholar]
- 38.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296 ( 10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 39.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rummer JL, Couturier CS, Stecyk JAW, Gardiner NM, Kinch JP, Nilsson GE, Munday PL. 2014. Life on the edge: thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob. Change Biol. 20, 1055–1066. ( 10.1111/gcb.12455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naya DE, Spangenberg L, Naya H, Bozinovic F. 2012. Latitudinal patterns in rodent metabolic flexibility. Am. Nat. 179, E172–E179. ( 10.1086/665646) [DOI] [PubMed] [Google Scholar]
- 42.Angilletta MJJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 43.Seebacher F, White CR, Franklin CE. 2015. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5, 61–66. ( 10.1038/nclimate2457) [DOI] [Google Scholar]
- 44.Schulte PM, Healy TM, Fangue NA. 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702. ( 10.1093/icb/icr097) [DOI] [PubMed] [Google Scholar]
- 45.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: John Hopkins Press. [Google Scholar]
- 46.Welch MJ, Munday PL. 2017. Heritability of behavioural tolerance to high CO2 in a coral reef fish is masked by nonadaptive phenotypic plasticity. Evol. Appl. 10, 682–693. ( 10.1111/eva.12483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balaguer L, Martínez-Ferri E, Valladares F, Pérez-Corona ME, Baquedano FJ, Castillo FJ, Manrique E. 2001. Population divergence in the plasticity of the response of Quercus coccifera to the light environment. Funct. Ecol. 15, 124–135. ( 10.1046/j.1365-2435.2001.00505.x) [DOI] [Google Scholar]
- 48.Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763. ( 10.1111/j.1469-8137.2007.02275.x) [DOI] [PubMed] [Google Scholar]
- 49.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65 ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 50.Faulkner KT, Clusella-Trullas S, Peck LS, Chown SL. 2014. Lack of coherence in the warming responses of marine crustaceans. Funct. Ecol. 28, 895–903. ( 10.1111/1365-2435.12219) [DOI] [Google Scholar]
- 51.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calosi P, Bilton DT, Spicer JI. 2008. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 4, 99–102. ( 10.1098/rsbl.2007.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donelson JM, Munday PL. 2012. Thermal sensitivity does not determine acclimation capacity for a tropical reef fish. J. Anim. Ecol. 81, 1126–1131. ( 10.1111/j.1365-2656.2012.01982.x) [DOI] [PubMed] [Google Scholar]
- 54.Barria AM, Bacigalupe LD, Lagos NA, Lardies MA. 2018. Thermal physiological traits and plasticity of metabolism are sensitive to biogeographic breaks in a rock-pool marine shrimp. J. Exp. Biol. 221, jeb.181008 ( 10.1242/jeb.181008) [DOI] [PubMed] [Google Scholar]
- 55.Veilleux HD, Ryu T, Donelson JM, Ravasi T, Munday PL. 2018. Molecular response to extreme summer temperatures differs between two genetically differentiated populations of a coral reef fish. Front. Mar. Sci. 5, 349 ( 10.3389/fmars.2018.00349) [DOI] [Google Scholar]
- 56.Munday P, Warner RR, Monro K, Pandolfi JM, Marshall DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. ( 10.1111/ele.12185) [DOI] [PubMed] [Google Scholar]
- 57.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 58.Reznick DN, Ghalambor CK. 2001. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112, 183–198. ( 10.1023/A:1013352109042) [DOI] [PubMed] [Google Scholar]
- 59.Colautti RI, Barrett SCH. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342, 364–366. ( 10.1126/science.1242121) [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann AA, Sgró CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 61.Pease CM, Lande R, Bull JJ. 1989. A model of population growth, dispersal and evolution in a changing environment. Ecology 70, 1657–1664. ( 10.2307/1938100) [DOI] [Google Scholar]
- 62.Sanford E, Kelly MW. 2011. Local adaptation in marine invertebrates. Annu. Rev. Mar. Sci. 3, 509–535. ( 10.1146/annurev-marine-120709-142756) [DOI] [PubMed] [Google Scholar]
- 63.Hendry AP, et al. 2011. Evolutionary principles and their practical application. Evol. Appl. 4, 159–183. ( 10.1111/j.1752-4571.2010.00165.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopp M, Matuszewski S. 2014. Rapid evolution of quantitative traits: theoretical perspectives. Evol. Appl. 7, 169–191. ( 10.1111/eva.12127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ehrenreich IM, Pfennig DW. 2016. Genetic assimilation: a review of its potential proximate causes and evolutionary consequences. Ann. Bot. 117, 769–779. ( 10.1093/aob/mcv130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 67.Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huey RB, Hertz PE, Sinervo B. 2003. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 161, 357–366. ( 10.1086/346135) [DOI] [PubMed] [Google Scholar]
- 69.Barrett RDH, Schluter D. 2008. Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44. ( 10.1016/j.tree.2007.09.008) [DOI] [PubMed] [Google Scholar]
- 70.Case TJ, Taper ML. 2000. Interspecific competition, environmental gradients, gene flow, and the coevolution of species’ borders. Am. Nat. 155, 583–605. ( 10.1086/303351) [DOI] [PubMed] [Google Scholar]
- 71.Banks SC, Piggott MP, Williamson JE, Bové U, Holbrook NJ, Beheregaray LB. 2007. Oceanic variability and coastal topography shape genetic structure in a long-dispersing sea urchin. Ecology 88, 3055–3064. ( 10.1890/07-0091.1) [DOI] [PubMed] [Google Scholar]
- 72.Pörtner HO, Peck MA. 2010. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779. ( 10.1111/j.1095-8649.2010.02783.x) [DOI] [PubMed] [Google Scholar]
- 73.Vergés A, et al. 2014. The tropicalization of temperate marine ecosystems : climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 281, 1–10. ( 10.1098/rspb.2014.0846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Banks SC, Ling SD, Johnson CR, Piggott MP, Williamson JE, Beheregaray LB. 2010. Genetic structure of a recent climate change-driven range extension. Mol. Ecol. 19, 2011–2024. ( 10.1111/j.1365-294X.2010.04627.x) [DOI] [PubMed] [Google Scholar]
- 75.van Gennip SJ, Popova EE, Yool A, Pecl GT, Hobday AJ, Sorte CJB. 2017. Going with the flow: the role of ocean circulation in global marine ecosystems under a changing climate. Glob. Change Biol. 23, 2602–2617. ( 10.1111/gcb.13586) [DOI] [PubMed] [Google Scholar]
- 76.O'Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM. 2007. Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc. Natl Acad. Sci. USA 104, 1266–1271. ( 10.1073/pnas.0603422104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bridle JR, Vines TH. 2007. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 22, 140–147. ( 10.1016/j.tree.2006.11.002) [DOI] [PubMed] [Google Scholar]
- 78.Lenormand T. 2002. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189. ( 10.1016/S0169-5347(02)02497-7) [DOI] [Google Scholar]
- 79.Kirkpatrick M, Barton NH. 1997. Evolution of a species' range. Am. Nat. 150, 1–23. ( 10.1086/286054) [DOI] [PubMed] [Google Scholar]
- 80.Kelly MW, Sanford E, Grosberg RK. 2012. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. R. Soc. B 279, 349–356. ( 10.1098/rspb.2011.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howells EJ, Berkelmans R, Van Oppen MJH, Willis BL, Bay LK.. 2013. Historical thermal regimes define limits to coral acclimatization. Ecology 94, 1078–1088. ( 10.1890/12-1257.1) [DOI] [PubMed] [Google Scholar]
- 82.Johansen JL, Messmer V, Coker DJ, Hoey AS, Pratchett MS. 2014. Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob. Change Biol. 20, 1067–1074. ( 10.1111/gcb.12452) [DOI] [PubMed] [Google Scholar]
- 83.Frankham R. 1996. Relationship of genetic variation to population size in wildlife. Conserv. Biol. 10, 1500–1508. ( 10.1046/j.1523-1739.1996.10061500.x) [DOI] [Google Scholar]
- 84.Nosil P, Vines TH, Funk DJ. 2005. Reproductive isolation by natural selection against immigrants from divergent habitats. Evolution 59, 705–719. ( 10.1111/j.0014-3820.2005.tb01747.x) [DOI] [PubMed] [Google Scholar]
- 85.Marshall DJ, Monro K, Bode M, Keough MJ, Swearer S. 2010. Phenotype-environment mismatches reduce connectivity in the sea. Ecol. Lett. 13, 128–140. ( 10.1111/j.1461-0248.2009.01408.x) [DOI] [PubMed] [Google Scholar]
- 86.Cowen RK, Paris CB, Srinivasan A. 2006. Scaling of connectivity in marine populations. Science 311, 522 ( 10.1126/science.1122039) [DOI] [PubMed] [Google Scholar]
- 87.Palumbi SR. 2014. Population genetics, demographic connectivity, and the design of marine reserves. Ecol. Appl. 13, 146–158. ( 10.1890/1051-0761(2003)013%5B0146:PGDCAT%5D2.0.CO;2) [DOI] [Google Scholar]
- 88.Jones GP, Milicich MJ, Emslie MJ, Lunow C. 1999. Self-recruitment in a coral reef fish population. Nature 402, 802–804. ( 10.1038/45538) [DOI] [Google Scholar]
- 89.Palmer MA, Allan JD, Butman CA. 1996. Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends Ecol. Evol. 11, 322–326. ( 10.1016/0169-5347(96)10038-0) [DOI] [PubMed] [Google Scholar]
- 90.Williamson DH, et al. 2016. Large-scale, multidirectional larval connectivity among coral reef fish populations in the Great Barrier Reef Marine Park. Mol. Ecol. 25, 6039–6054. ( 10.1111/mec.13908) [DOI] [PubMed] [Google Scholar]
- 91.Van Oppen M, Oliver HJ, James K, Putnam HM, Gates RD.. 2015. Building coral reef resilience through assisted evolution. Proc. Natl Acad. Sci. USA 112, 2307–2313. ( 10.1073/pnas.1422301112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kearney M, Shine R, Porter WP. 2009. The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc. Natl Acad. Sci. USA 106, 3835–3840. ( 10.1073/pnas.0808913106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holland KN, Brill RW, Chang RKC, Sibert JR, Fournier DA. 1992. Physiological and behavioural thermoregulation in bigeye tuna (Thunnus obesus). Nature 358, 410–412. ( 10.1038/358410a0) [DOI] [PubMed] [Google Scholar]
- 94.Wiggins P, Frappell P. 2002. Behavioural thermoregulation in Daphnia carinata from different depths of a natural water body: influence of environmental oxygen levels and temperature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 133, 771–780. ( 10.1016/S1095-6433(02)00238-6) [DOI] [PubMed] [Google Scholar]
- 95.Baker AC. 2004. Corals' adaptive response to climate change. Nature 430, 2004 ( 10.1016/j.beem.2008.09.010) [DOI] [PubMed] [Google Scholar]
- 96.Sorte CJB, Hofmann GE. 2004. Changes in latitudes, changes in aptitudes: Nucella canaliculata (Mollusca: Gastropoda) is more stressed at its range edge. Mar. Ecol. Prog. Ser. 274, 263–268. ( 10.3354/meps274263) [DOI] [Google Scholar]
- 97.Pearson GA, Lago-Leston A, Mota C. 2009. Frayed at the edges: selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J. Ecol. 97, 450–462. ( 10.1111/j.1365-2745.2009.01481.x) [DOI] [Google Scholar]
- 98.Rodgers GG, Donelson JM, McCormick MI, Munday PL. 2018. In hot water: sustained ocean warming reduces survival of a low-latitude coral reef fish. Mar. Biol. 165, 1–10. ( 10.1007/s00227-018-3333-z) [DOI] [Google Scholar]
- 99.Bennett S, Wernberg T, Arackal Joy B, de Bettignies T, Campbell AH.. 2015. Central and rear-edge populations can be equally vulnerable to warming. Nat. Commun. 6, 10280 ( 10.1038/ncomms10280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pereira RJ, Sasaki MC, Burton RS. 2017. Adaptation to a latitudinal thermal gradient within a widespread copepod species: the contributions of genetic divergence and phenotypic plasticity. Proc. R. Soc. B 284, 20170236 ( 10.1098/rspb.2017.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sorte CJB, Jones SJ, Miller LP. 2011. Geographic variation in temperature tolerance as an indicator of potential population responses to climate change. J. Exp. Mar. Biol. Ecol. 400, 209–217. ( 10.1016/j.jembe.2011.02.009) [DOI] [Google Scholar]
- 102.Munguia P. 2015. Role of sources and temporal sinks in a marine amphipod. Biol. Lett. 11, 20140864 ( 10.1098/rsbl.2014.0864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dias P. 1996. Sources and sinks in population biology. Trends Ecol. Evol. 11, 326–330. ( 10.1016/0169-5347(96)10037-9) [DOI] [PubMed] [Google Scholar]
- 104.Alleaume-Benharira M, Pen IR, Ronce O. 2006. Geographical patterns of adaptation within a species’ range: interactions between drift and gene flow. J. Evol. Biol. 19, 203–215. ( 10.1111/j.1420-9101.2005.00976.x) [DOI] [PubMed] [Google Scholar]
- 105.Nicastro KR, Zardi GI, Teixeira S, Neiva J, Serrão EA, Pearson GA. 2013. Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biol. 11, 6 ( 10.1186/1741-7007-11-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Assis J, Castilho Coelho N, Alberto F, Valero M, Raimondi P, Reed D, Alvares Serrão E. 2013. High and distinct range-edge genetic diversity despite local bottlenecks. PLoS ONE 8, 1–11. ( 10.1371/journal.pone.0068646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Warner RR, Chesson PL. 1985. Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am. Nat. 125, 769–787. ( 10.1086/284379) [DOI] [Google Scholar]
- 108.Wethey DS. 1983. Geographic limits and local zonation: the barnacles Semibalanus (Balanus) and Chthamalus in New England. Biol. Bull. 165, 330–341. ( 10.2307/1541373) [DOI] [Google Scholar]
- 109.Southward AJ, et al. 2004. Long-term oceanographic and ecological research in the western English Channel. Adv. Mar. Biol. 47, 1–105. ( 10.1016/S0065-2881(04)47001-1) [DOI] [PubMed] [Google Scholar]
- 110.Matz MV, Treml EA, Aglyamova GV, Bay LK. 2018. Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLoS Genet. 14, 1–19. ( 10.1371/journal.pgen.1007220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buckley LB, Ehrenberger JC, Angilletta MJ. 2015. Thermoregulatory behaviour limits local adaptation of thermal niches and confers sensitivity to climate change. Funct. Ecol. 29, 1038–1047. ( 10.1111/1365-2435.12406) [DOI] [Google Scholar]
- 112.Willis J, Hobday AJ. 2007. Influence of upwelling on movement of southern bluefin tuna (Thunnus maccoyii) in the Great Australian Bight. Mar. Freshw. Res. 58, 699 ( 10.1071/MF07001) [DOI] [Google Scholar]
- 113.Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. 2006. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241. ( 10.1111/j.1461-0248.2005.00871.x) [DOI] [PubMed] [Google Scholar]
- 114.Roughan M, Macdonald HS, Baird ME, Glasby TM. 2011. Modelling coastal connectivity in a Western Boundary Current: seasonal and inter-annual variability. Deep. Res. Part II Top. Stud. Oceanogr. 58, 628–644. ( 10.1016/j.dsr2.2010.06.004) [DOI] [Google Scholar]
- 115.Pankhurst NW, Munday PL. 2011. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62, 1015–1026. ( 10.1071/MF10269) [DOI] [Google Scholar]
- 116.Du WG, Yan SJ, Ji X.. 2000. Selected body temperature, thermal tolerance and thermal dependence of food assimilation and locomotor performance in adult blue-tailed skinks, Eumeces elegans. J. Therm. Biol. 25, 197–202. ( 10.1016/S0306-4565(99)00022-4) [DOI] [Google Scholar]
- 117.Sagarin RD, Gaines SD, Gaylord B. 2006. Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol. Evol. 21, 524–530. ( 10.1016/j.tree.2006.06.008) [DOI] [PubMed] [Google Scholar]
- 118.Sagarin RD, Gaines SD. 2002. The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecol. Lett. 5, 137–147. ( 10.1046/j.1461-0248.2002.00297.x) [DOI] [Google Scholar]
- 119.Sagarin RD, Gaines SD. 2002. Geographical abundance distributions of coastal invertebrates: using one-dimensional ranges to test biogeographic hypotheses. J. Biogeogr. 29, 985–997. ( 10.1046/j.1365-2699.2002.00705.x) [DOI] [Google Scholar]
- 120.Zardi GI, Nicastro KR, Serrao EA, Jacinto R, Monteiro CA, Pearson GA. 2015. Closer to the rear edge: ecology and genetic diversity down the core-edge gradient of a marine macroalga. Ecosphere 6, art23 ( 10.1890/ES14-00460.1) [DOI] [Google Scholar]
- 121.Eckert CG, Samis KE, Lougheed SC. 2008. Genetic variation across species' geographical ranges: the central-marginal hypothesis and beyond. Mol. Ecol. 17, 1170–1188. ( 10.1111/j.1365-294X.2007.03659.x) [DOI] [PubMed] [Google Scholar]
- 122.Smith SM, Fox RJ, Booth DJ, Donelson JM. 2018. ‘Stick with your own kind, or hang with the locals?’ Implications of shoaling strategy for tropical reef fish on a range-expansion frontline. Glob. Change Biol. 24, 1663–1672. ( 10.1111/gcb.14016) [DOI] [PubMed] [Google Scholar]
- 123.Figueira WF, Booth DJ. 2010. Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Glob. Change Biol. 16, 506–516. ( 10.1111/j.1365-2486.2009.01934.x) [DOI] [Google Scholar]
- 124.Feary DA, Pratchett MS, Emslie MJ, Fowler AM, Figueira WF, Luiz OJ, Nakamura Y, Booth DJ. 2013. Latitudinal shifts in coral reef fishes: why some species do and others do not shift. Fish Fish. 15, 593–615. ( 10.1111/faf.12036) [DOI] [Google Scholar]
- 125.Whitney KD, Gabler CA. 2008. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Divers. Distrib. 14, 569–580. ( 10.1111/j.1472-4642.2008.00473.x) [DOI] [Google Scholar]
- 126.Schoener TW. 1971. Resource partitioning in ecological communities. Science 185, 27–39. ( 10.1126/science.185.4145.27) [DOI] [PubMed] [Google Scholar]
- 127.Sorensen JS, Turnbull CA, Dearing MD. 2004. A specialist herbivore (Neotoma stephensi) absorbs fewer plant toxins than does a generalist (Neotoma albigula). Physiol. Biochem. Zool. Ecol. Evol. Approach. 77, 139–148. ( 10.1086/378923) [DOI] [PubMed] [Google Scholar]
- 128.Azzurro E, Tuset VM, Lombarte A, Maynou F, Simberloff D, Rodríguez-Pérez A, Solé RV. 2014. External morphology explains the success of biological invasions. Ecol. Lett. 17, 1455–1463. ( 10.1111/ele.12351) [DOI] [PubMed] [Google Scholar]
- 129.Bates AE, Barrett NS, Stuart-Smith RD, Holbrook NJ, Thompson PA, Edgar GJ. 2014. Resilience and signatures of tropicalization in protected reef fish communities. Nat. Clim. Change 4, 62–67. ( 10.1038/nclimate2062) [DOI] [Google Scholar]
- 130.Allee WC. 1938. The social life of animals. New York, NY: W. W. Norton & Company Inc. [Google Scholar]
- 131.Ramos JE, Pecl GT, Moltschaniwskyj NA, Semmens JM, Souza CA, Strugnell JM. 2018. Population genetic signatures of a climate change driven marine range extension. Sci. Rep. 8, 1–12. ( 10.1038/s41598-018-27351-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keith SA, Herbert RJH, Norton PA, Hawkins SJ, Newton AC. 2011. Individualistic species limitations of climate-induced range expansions generated by meso-scale dispersal barriers. Divers. Distrib. 17, 275–286. ( 10.1111/j.1472-4642.2010.00734.x) [DOI] [Google Scholar]
- 133.Knutsen H, Jorde PE, Gonzalez EB, Robalo J, Albretsen J, Almada V. 2013. Climate change and genetic structure of leading edge and rear end populations in a northwards shifting marine fish species, the corkwing wrasse (Symphodus melops). PLoS ONE 8, e67492 ( 10.1371/journal.pone.0067492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
- 135.Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT. 2008. Modeling the response of populations of competing species to climate change. Ecology 89, 3138–3149. ( 10.1890/07-1169.1) [DOI] [PubMed] [Google Scholar]
- 136.Svenning JC, et al. 2014. The influence of interspecific interactions on species range expansion rates. Ecography (Cop.) 37, 1198–1209. ( 10.1111/j.1600-0587.2013.00574.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Del Toro-Silva FM, Miller JM, Taylor JC, Ellis TA. 2008. Influence of oxygen and temperature on growth and metabolic performance of Paralichthys lethostigma (Pleuronectiformes: Paralichthyidae). J. Exp. Mar. Biol. Ecol. 358, 113–123. ( 10.1016/j.jembe.2008.01.019) [DOI] [Google Scholar]
- 138.Somero GN. 2002. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr. Comp. Biol. 42, 780–789. ( 10.1093/icb/42.4.780) [DOI] [PubMed] [Google Scholar]
- 139.Richardson A. 2008. In hot water: zooplankton and climate change. ICES J. Mar. Sci. 65, 279–295. ( 10.1093/icesjms/fsn028) [DOI] [Google Scholar]
- 140.Pratchett MS, Thompson CA, Hoey AS, Cowman PF, Wilson SK. 2018. Effects of coral bleaching and coral loss on the structure and function of reef fish assemblages. In Coral bleaching: patterns, processes, causes and consequences. Ecological studies, 233 (eds MJH van Oppen, JM Lough), pp. 265–293. Cham, Switzerland: Springer. [Google Scholar]
- 141.Allan BJM, Domenici P, Munday PL, Mccormick MI. 2015. Feeling the heat: the effect of acute temperature changes on predator – prey interactions in coral reef fish. Conserv. Physiol. 3, 1–8. ( 10.1093/conphys/cov011.Introduction) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kordas RL, Harley CDG, O'Connor MI. 2011. Community ecology in a warming world: the influence of temperature on interspecific interactions in marine systems. J. Exp. Mar. Biol. Ecol. 400, 218–226. ( 10.1016/j.jembe.2011.02.029) [DOI] [Google Scholar]
- 143.Dill LM, Heithaus MR, Walters CJ. 2003. Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecol. Modell. 84, 1151–1157. [Google Scholar]
- 144.Ling SD, Barrett NS, Edgar GJ. 2018. Facilitation of Australia's southernmost reef-building coral by sea urchin herbivory. Coral Reefs 37, 1053–1073. ( 10.1007/s00338-018-1728-4) [DOI] [Google Scholar]
- 145.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 146.Seebacher F, Wilson RS. 2006. Fighting fit: thermal plasticity of metabolic function and fighting success in the crayfish Cherax destructor. Funct. Ecol. 20, 1045–1053. ( 10.1111/j.1365-2435.2006.01194.x) [DOI] [Google Scholar]
- 147.Warren DT, Donelson JM, McCormick MI, Ferrari MCO, Munday PL. 2016. Duration of exposure to elevated temperature affects competitive interactions in juvenile reef fishes. PLoS ONE, 11, e0164505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goldenberg SU, Nagelkerken I, Marangon E, Bonnet A, Ferreira CM, Connell SD. 2018. Ecological complexity buffers the impacts of future climate on marine consumers. Nat. Clim. Change 8, 229–233. ( 10.1038/s41558-018-0086-0) [DOI] [Google Scholar]
- 149.Nagelkerken I, Goldenberg SU, Ferreira CM, Russell BD, Connell SD. 2017. Species interactions drive fish biodiversity loss in a high-CO2 world. Curr. Biol. 27, 2177– 2184.e4 ( 10.1016/j.cub.2017.06.023) [DOI] [PubMed] [Google Scholar]
- 150.Zeidberg L, Robison B. 2007. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc. Natl Acad. Sci. USA 104, 12 948–12 950. ( 10.1073/pnas.0702043104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hollebone AL, Hay ME. 2008. An invasive crab alters interaction webs in a marine community. Biol. Invasions 10, 347–358. ( 10.1007/s10530-007-9134-9) [DOI] [Google Scholar]
- 152.Ling SD. 2008. Range expansion of a habitat-modifying species leads to loss of taxonomic diversity: a new and impoverished reef state. Oecologia 156, 883–894. ( 10.1007/s00442-008-1043-9) [DOI] [PubMed] [Google Scholar]
- 153.Ling SD, Johnson CR, Frusher SD, Ridgway KR. 2009. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl Acad. Sci. USA 106, 22 341–22 345. ( 10.1073/pnas.0907529106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carrasco D, Desurmont GA, Laplanche D, Proffit M, Gols R, Becher PG, Larsson MC, Turlings TCJ, Anderson P. 2018. With or without you: effects of the concurrent range expansion of an herbivore and its natural enemy on native species interactions. Glob. Change Biol. 24, 631–643. ( 10.1111/gcb.13836) [DOI] [PubMed] [Google Scholar]
- 155.Nakamura Y, Feary DA, Kanda M, Yamaoka K. 2013. Tropical fishes dominate temperate reef fish communities within western Japan. PLoS ONE 8, 1–8. ( 10.1371/journal.pone.0081107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Booth DJ, Sear J. 2018. Coral expansion in Sydney and associated coral-reef fishes. Coral Reefs 37, 995 ( 10.1007/s00338-018-1727-5) [DOI] [Google Scholar]
- 157.Linder PH, et al. 2012. Biotic modifiers, environmental modulation and species distribution models. J. Biogeogr. 39, 2179–2190. ( 10.1111/j.1365-2699.2012.02705.x) [DOI] [Google Scholar]
- 158.Boyd PW, et al. 2018. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—a review. Glob. Change Biol. 24, 2239–2261. ( 10.1111/gcb.14102) [DOI] [PubMed] [Google Scholar]
- 159.Britton D, Cornwall CE, Revill AT, Hurd CL, Johnson CR. 2016. Ocean acidification reverses the positive effects of seawater pH fluctuations on growth and photosynthesis of the habitat-forming kelp, Ecklonia radiata. Sci. Rep. 6, 1–10. ( 10.1038/srep26036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pistevos JCA, Nagelkerken I, Rossi T, Connell SD. 2017. Antagonistic effects of ocean acidification and warming on hunting sharks. Oikos 126, 241–247. ( 10.1111/oik.03182) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.