Abstract
Phenotypic plasticity is frequently assumed to be an adaptive mechanism by which organisms cope with rapid changes in their environment, such as shifts in temperature regimes owing to climate change. However, despite this adaptive assumption, the nature of selection on plasticity within populations is still poorly documented. Here, we performed a systematic review and meta-analysis of estimates of selection on thermal plasticity. Although there is a large literature on thermal plasticity, we found very few studies that estimated coefficients of selection on measures of plasticity. Those that did do not provide strong support for selection on plasticity, with the majority of estimates of directional selection on plasticity being weak and non-significant, and no evidence for selection on plasticity overall. Although further estimates are clearly needed before general conclusions can be drawn, at present there is not clear empirical support for any assumption that plasticity in response to temperature is under selection. We present a multivariate mixed model approach for robust estimation of selection on plasticity and demonstrate how it can be implemented. Finally, we highlight the need to consider the environments, traits and conditions under which plasticity is (or is not) likely to be under selection, if we are to understand phenotypic responses to rapid environmental change.
This article is part of the theme issue ‘The role of plasticity in phenotypic adaptation to rapid environmental change’.
Keywords: adaptive plasticity, climate change, global warming, reaction norm, selection gradient, selection on plasticity
1. Introduction
Rapid changes to the global climate in the Anthropocene are generating similarly rapid responses in phenotypic traits of many taxa [1], much of which may be driven by phenotypic plasticity: the change in the expression of phenotype by a given genotype as the environment it experiences changes [2]. Once simply regarded as random noise [3,4], phenotypic plasticity and its contribution to evolutionary dynamics are now the focus of a continually expanding research area [5–8] that aims to determine how both wild and domestic populations of plants and animals might respond to environmental change (e.g. [9–11]). However, the nature of selection on phenotypic plasticity in response to changing environmental conditions in general, and to changing climate in particular, is less well understood. Here, we use a systematic review of published studies on plasticity in response to temperature to assess the evidence to date for quantitative selection on thermal plasticity.
If phenotypic plasticity (hereafter ‘plasticity’) improves a genotype's fitness when environmental change occurs, it can be considered to be adaptive [2,12] (studies of phenotypic plasticity abound with diverse terminology, hence we set out the definitions we use here in table 1). This enticing concept has propagated a frequent ‘adaptationist’ assumption (see [2,13]) that any observed plasticity should be adaptive. However, as we discuss below, plasticity could obviously also be non-adaptive, in other words not related to fitness, or even maladaptive, whereby it reduces fitness [2,14,15]. Determining whether plasticity is likely to be adaptive or not requires an understanding of the patterns of selection acting on it—namely whether variation in plasticity is related to variation in fitness [2,16–20]. However, despite the long-standing realization that the adaptive nature of plasticity may be complex and the burgeoning interest in the role that plasticity plays in eco-evolutionary dynamics, understanding the nature of selection on plasticity across contexts is still a challenging and open research area [2,5,6,8].
Table 1.
Definitions of terms used in studies of plasticity and selection on plasticity.
| term | definition (as used here) |
|---|---|
| phenotype | the observed realization of a single or multiple traits (characteristics) of an individual organism, resulting from both genetic and environmental influences |
| genotype | the realization of genetic differences among individuals of a species, which could be variation in a particular gene, nucleotide, or genome region (this could constitute an individual, clone, family, or line) |
| fitness | the contribution of an individual or genotype to the gene pool of subsequent generations. In practical terms, one of the best measures of an individual's fitness may be the number of surviving offspring produced through an organism's entire life (‘lifetime reproductive success’, LRS), but fitness is often assessed by components of LRS such as survival or fecundity rates |
| global fitness | the fitness of a genotype averaged or summed across the range of environments experienced |
| (phenotypic) plasticity | the ability of a single genotype to express different phenotypes under different environmental conditions. Often measured as the slope of a regression of the phenotypic trait across environments. Property of a reaction norm when the reaction norm slope is non-zero |
| reaction norm | describes the shape or form of the phenotypic response to environment: the value of the trait as a function of the environmental variable |
| acclimation | plasticity that encompasses short term physiological changes in response to a changed environment |
| canalization | weak or no phenotypic response to environmental variation (i.e. lack of plasticity in a trait) |
| genotype × environment interactions (G×E) | interactions between genotypes and the environment determine the phenotype such that reaction norm slopes vary among genotypes |
| adaptive plasticity | the scenario when a plastic response to the environment results in higher fitness than if a genotype maintains a constant phenotype across environments |
| non-adaptive plasticity | the scenario when a genotype shows a plastic response to the environment but this does not affect fitness |
| maladaptive plasticity | the scenario when a genotype shows a plastic response to the environment and this results in a decrease in fitness |
| selection | the primary mechanism leading to adaptive evolution. Measured as the covariance between a phenotypic trait (or plasticity) and fitness |
| directional selection | selection that changes the mean trait value, typically measured as the covariance between trait and fitness (selection differential, S), or the linear slope of the regression of fitness on the trait (selection gradient, β) |
| stabilizing selection | selection that favours intermediate values of a trait, typically measured as the quadratic selection gradient (γ) |
| selection on plasticity | an association between phenotypic plasticity (slope of the reaction norm) and fitness, i.e. selection on the response rather than the value of the phenotypic trait itself |
Here, we examine the strength of the evidence for selection on phenotypic plasticity, with a particular focus on phenotypic responses to varying temperatures. While anthropogenic environmental change involves many abiotic factors (e.g. water availability, CO2, extreme or aseasonal weather events) that might affect phenotypic traits, temperature is arguably the most prominent variable to be shifting under the changing climate [21–23], and certainly one of the most important environmental parameters determining fundamental life-history rates or reproduction and survival, as well as distributions and dispersal of biota [24]. Shifts in the mean, variability, and extremes of temperatures around the globe are affecting the phenotypic responses of diverse species and threatening the stability and persistence of terrestrial and aquatic ecosystems from the tropics to the poles [25–31]. Experimental studies on plasticity and acclimation often therefore assess changes in organism phenotype and performance across temperatures to determine whether ‘at risk’ populations are likely to be able to respond to predicted rates of climate change within their lifetimes [32–34]. The obvious need to understand species' responses to global temperature changes and the accelerating research interest in phenotypic plasticity generates a clear need to understand whether plasticity in response to temperature change is under selection.
Our aims with this review are therefore threefold. We consider: (i) the assumptions and assessment of whether plasticity is under selection; (ii) a review of published literature of empirical estimates of the nature of selection on phenotypic plasticity to temperature; and (iii) methods and statistical approaches to guide future estimation of selection on plasticity.
2. The assessment of selection on plasticity
(a). The spectrum of the adaptive nature of plasticity
There is good evidence for adaptive benefits of plasticity in some traits. Classic examples include the development of defensive structures in the presence of predators, which increases survival probability (e.g. protuberance of dorsal spines in water fleas Daphnia pulex [35] and increased shell thickness in the freshwater snail Physa acuta [36]), or plasticity in the growth of plants to avoid conspecific shading (e.g. in the orange jewelweed Impatiens capensis, in which more elongated plants have higher fitness at high density, whereas shorter plants have higher fitness at low density [37]). An example of selection for increased plasticity in response to temperature is found in an Australian herb, the waxy bluebell Wahlenbergia ceracea, in which low-elevation populations (which experience warmer and more variable temperatures than those at high elevations) have greater plasticity in height in response to growth temperature, which results in a greater number of seed capsules [38]. In this system, increased plasticity in height allows the plants to optimize growth habit and light interception based on the relative suitability of the conditions they experience. The term ‘acclimation’ is often used to refer to plasticity that encompasses short term physiological changes in response to a changed environment and there is a large body of work on acclimation testing whether these responses are likely to be adaptive [26,39–42]. For example, in the snow gum Eucalyptus pauciflora, alteration of pigment complexes in response to cold improves recovery of photosynthetic performance in spring [43], and hence is most likely adaptive. Similarly, reversible alteration of gut morphology in response to seasonal variation in food availability in laboratory mice Mus musculus and white rats Rattus norvegicus leads to better energy balance over the course of the year [44].
However, there are other situations where a change in phenotype may not represent an adaptive response, or may even be maladaptive, for example when competition (e.g. in cases of density-dependent population regulation) or resource limitation hinder development and reproduction [6,15,45,46]. As an extreme generic example, reduced food availability will probably result in loss of individual condition, and hence lower rates of both survival and fecundity. Such a change in phenotype is in line with the standard definition of plasticity (table 1), but is unlikely to be adaptive: what may be considered as plasticity to an evolutionary biologist could be seen as density-dependence by a population ecologist. The likelihood of plastic responses being maladaptive may also increase when the environment to which an individual is exposed differs markedly from that in which its ancestors evolved [47], as could occur when the environment changes rapidly. For example, exposing high elevation genotypes of the alpine herbs W. ceracea (see above) and Campanula thyrsoides to warm conditions typical of lower elevation sites elicits phenotypic and phenological shifts, but is accompanied by significant fitness reductions [38,48].
Further, both costs and limits of plasticity may constrain its dynamics [6,15,49–52]. Even adaptive plastic responses may come with costs. Costs may be owing to maintaining the ‘machinery’ that confers the ability to be plastic or the costs of producing a plastic response (so that the plastic genotype has lower global fitness over multiple environments). Plasticity may also incur costs if it results in the ‘wrong’ phenotype being produced in a new environment [49,50]. Limits refer to developmental, physiological, temporal, and ecological constraints on the expression of plasticity beyond limits to the expression of the phenotype itself, which includes trade-offs between linked traits. But while costs and limits are frequently invoked in theoretical models of plasticity (e.g. [9,53]) and when anticipating species' responses to climate change [54], detecting the highly variable constraints on plasticity remains a significant challenge [6,15,55].
As the above examples illustrate, the fitness implications of plasticity may range from maladaptive through to adaptive, but conclusions as to where along this spectrum a given scenario falls requires more than just subjective inference of likely benefits. If there is variation in plasticity among individuals within a population, then a quantitative analysis of selection on plasticity in a population at a given time can provide valuable insights into its adaptive nature. For plasticity to be under selection requires variation in plasticity (e.g. in reaction norm slopes; see table 1) to be related to variation in fitness [2,16–20]. Natural selection can only directly ‘see’ phenotypic trait values that are expressed in a given environment (individual points on a reaction norm), rather than the plasticity itself (reaction norm slope)—but selection on plasticity will summarize the net effect of selection on the change in trait values expressed, which will reflect combined benefits and costs of plasticity [6]. A genotype's average trait value (reaction norm intercept) and trait plasticity (reaction norm slope) can indeed be strongly correlated, and when this is the case, plasticity may be under indirect selection when the trait value is under selection [18,49,50]. As we outline below, these processes can be investigated within the statistical framework of a Lande-Arnold [56] selection analysis. Thus, overall selection on plasticity will be determined by selection on the expressed trait values themselves combined across the continuum of environments [9,56–59]. While any such analysis of current selection obviously cannot provide a full picture of the pressures that have shaped plasticity in the past, it can indicate the current nature of selection: evidence that selection favours increased plasticity might indicate adaptive benefits to plasticity, whereas evidence against plasticity would indicate the opposite.
(b). Perspectives on plasticity from previous meta-analyses
To date, several meta-analyses have aimed to evaluate the spectrum of the adaptive nature of plasticity, and have largely indicated that plasticity cannot always (and indeed not necessarily often) affect fitness or be considered as an adaptive response [55,60–62]. These meta-analyses each took different approaches. Acasuso-Rivero et al. [60] compared estimates of coefficient of variation of trait expression across environments of life-history (close to fitness) versus non-life-history (further from fitness) traits, and concluded that both categories of traits are similarly plastic. By contrast, Davidson et al. [61] assessed the relationship between plasticity and fitness proxies in invasive versus non-invasive plant species, and concluded that although invasive species are generally more plastic, the plasticity itself did not confer a fitness benefit. Palacio-López et al.'s [62] meta-analysis focused on reciprocal transplant experiments and found that about one-third of all trait responses appeared to be adaptive, where plants could alter their phenotype to match the non-resident environment.
To our knowledge, the only assessment to date of estimates of selection on plasticity has been van Buskirk & Steiner's [55] review of selection gradient coefficients for the effect of plasticity on fitness. They found 27 studies that contained suitable data from which they were able to estimate selection gradients on plasticity. Their analysis showed, remarkably, exactly equal frequency of positive and negative selection gradient coefficients (262 positive, 262 negative and 12 zero-slope) of fitness against trait plasticity across environments. This is obviously precisely the expected outcome of regressing a random variable against fitness. However, of the 27 studies in [55], only three related to temperature. As it is now 10 years since this review, and given the increased interest in the effects of warming temperatures on biological populations, we aimed here to determine whether additional empirical studies had been conducted or could be identified. Hence, we performed a systematic review and meta-analysis that explicitly targeted selection on plasticity in the context of rapid climate change, to address the following question: is there evidence for selection on plasticity in response to temperature?
(c). Quantifying selection on plasticity
Analysis of selection on plasticity requires estimation of the association between a genotype's plasticity and its fitness. There are many different methods to quantify and model plasticity [63–65]. The simplest conceptual method is to regress the phenotypic trait value against the environments in which it was measured to visualize a reaction norm, the slope of which provides a measure of plasticity for each genotype or individual. A selection analysis then typically tests for associations between these measures of plasticity and a genotype's overall (‘global’) fitness, measured across the different environments experienced. Measuring fitness across individuals' entire lifetimes is challenging but not impossible: for example, lifetime reproductive success (LRS) has been measured for several wild animal populations [66]. In cases where fitness is not readily measured directly (e.g. in long-lived trees), then components of fitness such as fecundity in animals, or number of seeds produced or survival of seedlings in one season in plants, may be suitable substitutes as proximal fitness estimates [67].
Selection on plasticity can then be estimated from the regression of global fitness (either the average or summed fitness across all environments) on the respective plasticity values (e.g. [15,68,69]). This approach is effectively a selection gradient analysis on reaction norms [56,70,71], from which the direction and strength of selection on plasticity can be assessed by the relationship between global fitness and plasticity of the different genotypes. However, it has the disadvantage of requiring a two-step approach (first extracting estimates of plasticity, and second associating them with fitness, but typically without accounting for the error inherent in the first step [72]). In §4 below, we consider alternative approaches that circumvent this problem. Given the potential for correlations between a genotype's average trait value (the elevation or intercept of their reaction norm) and their plasticity (the slope of the reaction norm [49,73]), it is also important to separate direct selection on slopes from selection acting indirectly through associations with trait value. This is most efficiently dealt with by estimating selection gradients from an analysis that also considers genotypes' intercepts [49,55].
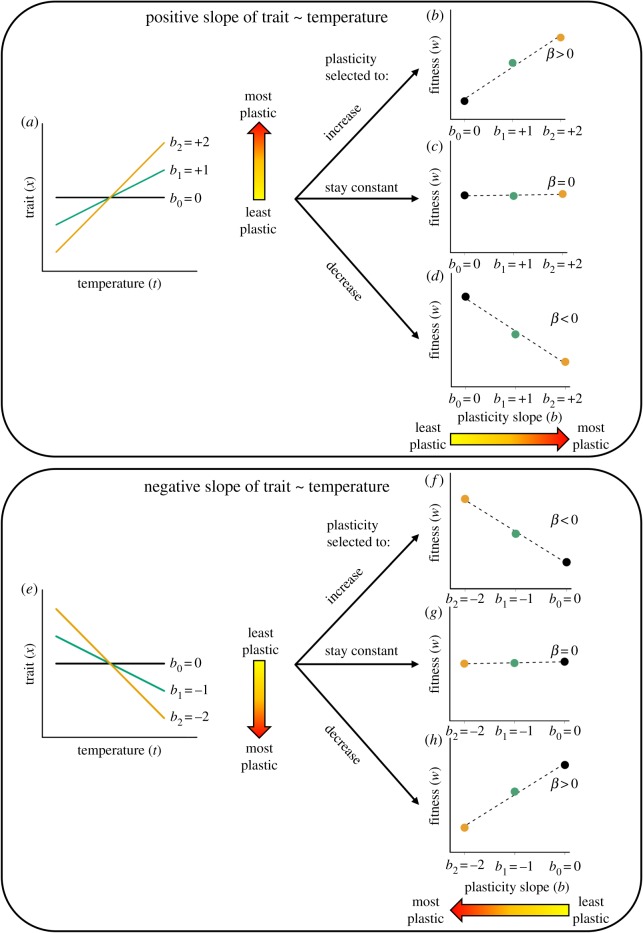
Care also needs to be taken in interpretation of the resulting selection gradients given their dependence on the average direction of plasticity. We set out the alternative, potentially confusing, scenarios in figure 1. Importantly, when the average reaction norm slope (e.g. of the trait against temperature regression) is positive (figure 1a), then if plasticity is under positive selection, the most plastic genotypes will have higher fitness and the selection gradient will be positive (figure 1b). Likewise, a selection gradient around zero is a lack of selection on plasticity (figure 1c), and a negative selection gradient is negative selection against plasticity (figure 1d). However, when the reaction norm slope (e.g. of the trait against temperature regression) is on average negative (figure 1e), then the converse is true: a negative selection gradient on reaction norm slopes indicates plasticity is selected to increase (figure 1f), selection gradient around zero is again lack of selection on plasticity, and finally a positive selection gradient indicates plasticity is selected to decrease (figure 1g). These issues are pertinent to the meta-analysis we present below.
Figure 1.
Interpretation of selection on plasticity will be dependent on the direction of average plasticity. The two halves of the figure represent the contrasting scenarios of positive (top half, panels (a–d)) or negative (bottom half, panels (e–h)) plasticity in a trait in response to temperature, and the corresponding selection analysis when plasticity is selected to increase, stay constant, or decrease. The scenarios on the right side of the figure (b–d; f–h) illustrate how fitness changes with plasticity. Note that for simplicity we do not include variation between genotypes in average trait values here. b0, b1 and b2 are the slopes of the three genotypes' reaction norms, and β is the selection gradient from the regression of fitness (w) on slope values. (a) The scenario where the average slope of the plastic response is positive (e.g. the trait increases with increasing temperature), illustrated by three genotypes that vary in reaction norm slope (bn) of phenotypic trait (x) across a continuous gradient of temperatures (t). The large arrow with yellow-red gradient indicates least to most plasticity, here and elsewhere. (b) Where plasticity is positive and selected to increase, the genotype with the greatest plasticity across temperatures (yellow; b2 = +2) has the highest fitness (w). Here, the linear selection gradient coefficient for the fitness ∼ plasticity relationship is positive (β > 0). (c) Where plasticity is selected to stay constant, plasticity does not affect fitness (all genotypes have equal fitness) and the selection gradient is zero (β = 0). (d) Where plasticity is positive but selected to decrease, the genotype with the least trait plasticity across temperatures (black; b0 = 0) has the highest fitness and the selection gradient is negative (β < 0). (e) The converse scenario to that described in (a); here, the reaction slope of plasticity is negative (e.g. the trait value declines with increasing temperature). (f) Where plasticity is negative and selected to increase, the genotype with the greatest plasticity across temperatures (yellow; b2 = −2) has the highest fitness and the selection gradient is negative (β < 0). (g) The same outcome as the scenario described in (c) above (β = 0). (h) Where plasticity is negative but selected to decrease, the genotype with the least plasticity across temperature (black; b0 = 0) has the highest fitness and the selection gradient is positive (β > 0).
As an alternative to the reaction norm approach of describing the shape of the phenotypic response across multiple environments, plasticity can also be modelled with a ‘character-state’ approach, which considers the phenotypic values expressed in each discrete environment as different traits [70]. In the same way that changes in variance across environments in a character-state model are equivalent to variance in the slope of reaction norms [74,75], selection on reaction norm slopes (via a covariance between slope and fitness) will generate changes in selection across environments (i.e. changes in the covariance between trait and fitness in each environment [76,77]). As such, the abundance of evidence of selection on phenotypic traits changing with time and environments [78,79] can arguably be taken as indirect selection on plasticity, and one which evolutionary theory predicts will shape the evolution of adaptive plasticity [9]. However, such patterns could also be driven by changes in the variance in fitness between environments and so could occur without the variance in reaction norm slopes that is required for selection on plasticity. The character-state inference also provides no indication of the nature of selection on reaction norms.
3. Review of the evidence for selection on thermal plasticity
(a). Motivation and literature search
We conducted a systematic review of the literature, with the aim of identifying empirical studies that have quantitatively assessed the nature of selection on plasticity across environmental gradients that have a temperature basis. To this end, we employed the PRISMA framework [80] by searching the Web of Science with the following search terms: topic: (selection near/3 plasticity or selection near/3 reaction norm or selection near/3 genotype near/1 environment or selection near/3 G × E or selection near/3 ‘G×E’) and topic: (temperature or thermal or ‘climate change’ or ‘climate-change’ or ‘global warming’ or ‘warming world’ or heat or hot or cold) in July 2018. The Boolean operator ‘near/n’ allows n words to appear between the topic words (e.g. ‘selection near/3 plasticity’ will capture phrase variants such as selection on/for/of thermal/phenotypic plasticity). Our search was thus explicitly targeted at those studies that investigated selection on plasticity.
Our initial search resulted in 139 articles. We then screened the titles and abstracts of these articles to determine which met all of the following five criteria: (i) analysed empirical data; (ii) included a measure of trait plasticity; (iii) included a measure or proxy of thermal environment; (iv) reported a measure of fitness or some component or close proxy of fitness (reproduction or survival), and finally; (v) assessed the relationship between the trait plasticity across thermal environment and the fitness component. Screening at this level reduced the number of articles that matched these criteria to 47. We found five papers that presented data that were eligible for qualitative assessment in that they contained estimates of selection coefficients on one or more measures of plasticity, and three additional papers from previously known sources. From each study, we extracted the following details: class and species of study organism, type of selection (e.g. directional, stabilizing), type of data collection (e.g. laboratory or field, wild population or transplant), type of environmental gradient (e.g. temperature, year as temperature proxy), plastic phenotypic trait, sample size, the type of analysis, selection gradient coefficient and associated standard error, whether the selection gradient was standardized (only standardized gradients with errors could be compared in the meta-analysis) and whether it was reported as significant (p < 0.05 in the original study). Where the average slope of the reaction norm between the trait and environment was negative (figure 1e), we reversed the sign of the selection coefficient so that a positive β indicated selection for steeper reaction norms (more plastic genotypes) and a negative β slope indicated selection for less steep reaction norms (less plastic genotypes). We also recorded relevant details on the context of the study and the authors' interpretation of their findings.
(b). Qualitative systematic review summary
The eight studies that explicitly tested for selection on plasticity [38,68,81–86] are summarized in table 2. These contained a total of 42 estimates of selection coefficients: 39 examples of tests for directional selection and two of stabilizing selection across two major taxa across four species of plants and three species of birds. All the studies on birds were field studies and the thermal environments of these studies were all indirect substitutions for temperature (e.g. a climate index, or year). The phenotypic traits that were quantified for plasticity were either size (e.g. plant height) or growth in the plant studies, or phenology (e.g. laying date) in the bird studies. All of the size-based traits in plants had a positive correlation with environment (e.g. plant height increased as temperature increased), whereas all phenological traits in birds had a negative correlation with thermal environment (i.e. laying date occurred earlier in the year as temperature increased). Fitness measures were all close proxies for reproduction and three studies used measures of LRS (‘total’ fitness).
Table 2.
Qualitative summary of studies that investigated selection on plasticity in thermal environments identified by our systematic review. (NAO = North Atlantic Oscillation; cond. = measurement conditions; the average slope of plasticity on environment is the sign of the regression of plasticity against temperature; the average slope of fitness on plasticity is such that positive slopes indicate directional selection for more plasticity and negative slopes indicate directional selection for less plasticity; the original author's interpretation is given as non-significant when the associated p-value was reported as non-significant in the author's analysis, otherwise if significant the coefficient direction and their interpretation of selection on plasticity is given in bold.)
| species | n | thermal environment, laboratory or field study | plasticity measure | fitness measure | average slope of plasticity on environment | average slope of fitness on plasticity | original author's interpretation | ref. |
|---|---|---|---|---|---|---|---|---|
| directional selection | ||||||||
| plants | linear coefficient | |||||||
| Arabidopsis thaliana var. MAGIC (thale cress) | 320 | current and elevated temperatures, field | flowering time | fruit number | negative | positive | selection favours more plasticity | [82] |
| rosette diameter | fruit number | positive | negative | non-significant | ||||
| Arabidopsis thaliana (thale cress) | 200 | spring and winter treatments, laboratory | flowering time (bred in spring) | fruit count (spring cond., three replicate populations) | positive | negative (2), positive (1) | non-significant (3) | [81] |
| fruit count (winter cond., three replicate populations) | positive | positive (2), negative (1) | non-significant (3) | |||||
| flowering time (bred in winter) | fruit count (spring cond., three replicate populations) | positive | negative (2), positive (1) | non-significant (3) | ||||
| fruit count (winter cond., three replicate populations) | positive | positive (2), zero (1) | non-significant (3) | |||||
| Arabidopsis thaliana (thale cress) | 630 | natural temperature gradient, laboratory | flowering time | fruit set | positive | negative | selection favours less plasticity | [68] |
| plant height | fruit set | positive | negative | non-significant | ||||
| leaf number | fruit set | positive | positive | non-significant | ||||
| Arabidopsis thalianaa (thale cress mutant) | 360 | natural temperature gradient, laboratory | flowering time | fruit set | positive | positive | non-significant | [68] |
| plant height | fruit set | positive | negative | non-significant | ||||
| leaf number | fruit set | positive | negative | non-significant | ||||
| Ranunculus bulbosus (bulbous buttercup) | 88 | current and elevated temperatures, laboratory | growth rate | number of inflorescences | positive | positive | non-significant | [86] |
| specific leaf area | number of inflorescences | positive | negative | non-significant | ||||
| biomass | number of inflorescences | positive | negative | non-significant | ||||
| budding start | number of inflorescences | positive | positive | non-significant | ||||
| flowering start | number of inflorescences | positive | positive | non-significant | ||||
| Trifolium montanum (mountain clover) | 174 | current and elevated temperatures, laboratory | growth rate | number of inflorescences | positive | positive | non-significant | [86] |
| specific leaf area | number of inflorescences | positive | positive | non-significant | ||||
| biomass | number of inflorescences | positive | negative | non-significant | ||||
| budding start | number of inflorescences | positive | positive | non-significant | ||||
| flowering start | number of inflorescences | positive | positive | non-significant | ||||
| Wahlenbergia ceracea (waxy bluebell) | 124 | current and elevated temperatures, laboratory | height (8 weeks) | seed capsule number | positive | positive | selection favours more plasticity | [38] |
| juvenile height growth | seed capsule number | positive | negative | non-significant | ||||
| rosette diameter | seed capsule number | positive | positive | selection favours more plasticity | ||||
| leaf number (11 weeks) | seed capsule number | positive | positive | selection favours more plasticity | ||||
| leaf number (14 weeks) | seed capsule number | positive | positive | non-significant | ||||
| days to flowering | seed capsule number | positive | negative | selection favours less plasticity | ||||
| birds | ||||||||
| Ficedula albicollis (collared flycatcher) | 694 | average temperature in May + June, field | laying date | lifetime reproductive success | negative | positive | selection favours more plasticity | [83] |
| laying date | lifetime fledgling production | negative | positive | selection favours more plasticity | ||||
| Parus major (great tit) | 833 | year substitutionb, field | laying date | lifetime reproductive success | negative | positive | selection favours more plasticity | [84] |
| Uria aalge (common guillemot) | 245 | winter NAO index substitution, field | laying date | breeding success | negative | positive | non-significant | [85] |
| stabilizing selection | ||||||||
| birds | quadratic coefficient | |||||||
| Ficedula albicollis (collared flycatcher) | 694 | average temperature in May and June, field | laying date | lifetime reproductive success | negative | negative | non-significant | [83] |
| Uria aalge (common guillemot) | 245 | winter NAO index substitution, field | laying date | breeding success | negative | negative | stabilizing selection around intermediate plasticity | [85] |
Across the eight studies, there were 19 negative, one zero and 20 positive linear selection coefficients (table 2). Of these, there were two significant negative coefficients (indicating selection for less steep reaction norms and less plasticity, in flowering time of Arabidopsis [68] and Wahlenbergia [38]), and seven significant positive coefficients (indicating selection for steeper reaction norms and more plasticity, again in Arabidopsis flowering time [82], and also in Wahlenbergia height, rosette diameter, and leaf number [38], and breeding time in collared flycatchers [83] and great tits [84]). There were two nonlinear selection coefficients, of which one was significant and indicated stabilizing selection on plasticity (favouring intermediate plasticity, in breeding time of common guillemots [85]). We therefore have evidence for three different types of selection on plasticity from a relatively small sample of significant coefficients, and fourfold more examples finding no evidence of any selection on plasticity (i.e. non-significant selection gradients). The two estimates in opposing directions on Arabidopsis flowering time highlight just how inconsistent the pattern of selection on plasticity can be, although considerable spatial, temporal and genetic differences between the sources of lines used in the two studies could obviously also be contributing to this difference [68,82]. We also note that the two collared flycatcher estimates [83] involve measures of fitness that are highly correlated, so do not represent independent points.
Our findings are therefore qualitatively congruent with previous reviews (especially van Buskirk & Steiner [55]) that plasticity is apparently inconsequential for fitness more often than not. To quantitatively test this assertion, we then conducted a meta-analysis on that subset of these studies with suitable coefficients.
(c). Meta-analysis of directional selection on plasticity in response to temperature
The dataset used for the qualitative systematic review was subset to those studies that reported estimates of standardized linear directional selection gradients and their associated standard errors, so that the selection gradient coefficient β could be used as the measure of effect size (following [87]). This reduced dataset contained 22 standardized selection gradients across four species from five studies: two on plants [68,81] and three on birds [83–85]. All five studies also had included trait means (or intercepts) in their models of fitness ∼ plasticity, to account for correlation between the trait mean and plasticity [49]. We conducted a multi-level meta-analysis using the metafor package [88] in R v. 3.5.1 [89]. Random effects of study and observation within study were included in the analysis to control for potential non-independence of data and to estimate residual variance [90]. All estimates are means with 95% confidence intervals (CI) and measurement error variance was the squared selection coefficient standard error as in [87]. We further quantified heterogeneity between selection gradients by calculating modified I2 statistics for multi-level models (the ratio of true heterogeneity to the total variance including sampling error [90,91]): represents variation between studies and represents variation within studies.
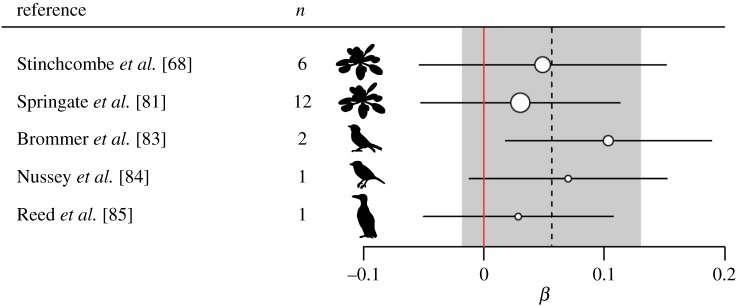
The mean standardized selection gradient coefficients (β) of all included studies were weakly positive (figure 2), however there was no evidence for selection on plasticity (βmean = 0.06 (−0.02 to 0.13 95% CI), p = 0.136). Heterogeneity between selection gradients was low by conventional standards [91] both between and within studies . The overall weak positive selection gradient from these 22 estimates of plasticity comprising both size and phenology related traits across thermal environments supports the null model: plasticity is not under significant directional selection. It is also worth noting that—as predicted by theory [49]—there was evidence for stronger directional selection on the mean trait value (intercept) rather than plasticity (slope) in several of the studies in our review [83–85]. In Brommer et al. [83], the expected evolutionary response of the population of collared flycatchers to increased mean annual temperature would be earlier laying dates (stronger selection on the intercept), but no substantial change in laying date plasticity (weaker selection on reaction norm slopes).
Figure 2.
Forest plot showing among-study heterogeneity in mean standardized linear selection gradient coefficients (β) of fitness in relation to trait thermal plasticity. Number of selection gradients reported for each study is shown by n and by the relative size of the data points, species used in the study are represented by silhouettes, and mean estimates are shown ±95% confidence intervals (CIs). The 95% CIs are calculated from the metafor model and do not match with standard error estimates from each individual study. The overall meta-analysis βmean ± 95% CIs is represented by the dashed black vertical line and grey shaded area, and the solid red vertical line is centred at β = 0 (no selection on plasticity). These studies collectively indicate no evidence for significant selection on thermal plasticity. (Online version in colour.)
However, we recognize that this is a very small sample from which to interpret anything (other than the distinct need for additional data); therefore, we do so with caution. The unambiguous outcome of our meta-analysis is that we require more direct tests for selection on plasticity in response to climate change from more taxonomic groups to evaluate these patterns if we are to posit any sort of informed conclusion about selection on thermal plasticity. Quantifying selection on plasticity is clearly challenging, but in the hope of encouraging more studies, we conclude by setting out recent developments in relevant statistical methods.
4. A multivariate mixed model approach to analysing selection on plasticity
We outlined above common approaches taken to assess selection on plasticity, but these are not without their problems. Several issues with the analysis of plasticity have been raised in recent years, including, but not limited to, the problems of multi-step analyses, misleading conclusions when other covariates such as mean trait values are not included [49,72], the oversimplification of reaction norms across just two environments [64], and consideration of only single traits rather than multivariate phenotypes [92]. In this final section, we outline a mixed model approach to analysing selection on plasticity that avoids these potential drawbacks.
The inference of selection on plasticity requires measures of individual plasticity and individual fitness. As outlined above, in the case of a linear reaction norm, a straightforward approach to estimate selection on a plastic response is to regress a genotype's global fitness against the slope of plasticity (e.g. figure 1b) using selection gradient analyses [38,56,68]. The simplest implementation of this approach is to use linear regressions for each individual of trait ∼ environment to provide estimates of the linear slope of plasticity (reaction norm slope; figure 1a), which can then be standardized and used as predictor variables for modelling individual fitness in a separate model: fitness ∼ slope of plasticity (e.g. figure 1b). Random regression mixed models can also provide estimates of plasticity slopes from best linear unbiased predictors, and fitness can then be regressed on these to estimate the selection gradient on plasticity. With both approaches, reaction norm intercepts (elevations) also need to be fitted to account for correlated selection [49]. However, both methods require two steps of models and thereby an undesirable reliance on ‘statistics-on-statistics’ [72,93]. Deriving estimates of selection on plasticity in this way neglects the uncertainty associated with estimates of plasticity, which could generate misleading levels of statistical confidence [93].
These potential pitfalls can be avoided by using multivariate random regression mixed models of trait and fitness, such that selection is assessed directly from estimates of the covariance of fitness with reaction norm slopes within a single model [73,93]. First, consider a random regression mixed model to model the variation between individuals in their change of the trait x across environments, with xi,j being the measurement of each individual i at time j in environment tj:
| 4.1 |
where μx is the mean of trait x, t is the mean-centred environmental covariate such as temperature, parentheses indicate random effects, indx,i is the random intercept and tj : indx,i is the random slope for individual i, and ɛx,ij is a residual term. This model has the associated individual-level variance–covariance matrix:
where estimates the between-individual variance in x, estimates the between-individual variance in the slopes of individuals' reaction norms of x against t, and is the intercept–slope covariance. Note that this model assumes a linear relationship between x and t, but can be extended to higher-order nonlinear models (such as quadratic functions) by the addition of further terms into equation (4.1) and the corresponding variance–covariance matrix. Scaling x to unit standard deviation will generate standardized estimates of the selection coefficients outlined below, and will also facilitate convergence.
Selection on both trait values and plasticity can then be assessed from the covariance of individuals' intercepts and slopes with their fitness, by extending to a bivariate model that also includes fitness:
| 4.2 |
where μw is the mean of the fitness measure w. Here, because fitness is measured only once per individual, the individual-level random effect indw,i is the deviation of individual i's relative fitness from the mean: this is effectively a residual term in the model, but is also equivalent to an individual-level effect for fitness. It is this individual-level fitness term whose covariance with the trait's reaction norm we want to assess. Doing so involves fitting equations (4.1) and (4.2) together in a bivariate model and considering the resulting individual-level variance–covariance matrix:
| 4.3 |
where is the variance in relative fitness w, σx,w gives the covariance between the reaction norm intercept and w, and is the covariance between the reaction norm slope and w. The two covariances σx,w and can thus be treated as a vector S of two selection differentials. Multivariate mixed models of this form can be implemented in software packages such as ASReml [94] or MCMCglmm [95] in R [89]. The approach has been used in analyses of selection on tolerance to parasite infection in a feral sheep Ovis aries population [96], and of selection on growth rates in swordtail fish Xiphophorus birchmanni [97], and maternal ageing in red deer Cervus elaphus [98]. It has also been used to assess selection on plasticity of clutch size in Ural owls, Strix uralensis [93], but, to our knowledge, has not yet been used more widely for analyses of selection on plasticity. However, with recent emphasis on the benefits of using random regression mixed models for the analysis of plasticity [64,72], we hope that this may change.
The selection differentials in S represent the total selection on reaction norm intercepts and slopes, incorporating both direct and indirect selection. These can then be transformed to give a vector of selection gradients β on intercepts and slope, via , where P2 is the 2 × 2 variance–covariance matrix for intercept and slopes of x (i.e. a subset of Pind). The selection gradients in β are then the direct selection on intercept and slope respectively, correcting for the covariance between them [56]. Where relatedness information is available for individuals, such analyses can also be extended to consider the additive genetic components of the relevant variances and covariances [99]. To our knowledge, adding additive genetic components of (co)variance to a multivariate model random regression model with fitness has not yet been attempted. It offers promising potential, but the demands on the data in doing so will be substantial.
On a technical note, analyses of fitness are rarely straightforward. Selection differentials or gradients should be calculated using relative fitness (absolute fitness divided by the population mean [56]), and models are typically fitted assuming Gaussian errors; see [93,96] for examples for selection on reaction norms. However, where the fitness measure follows a non-Gaussian distribution, as is typically the case with skewed distributions of fitness, a generalized linear mixed model (GLMM) of absolute fitness will be preferable [95,100]. The resulting covariances returned by the model will then be between the trait on the data scale and fitness on a ‘latent’ (link-function) scale. These estimates need to be transformed if data-scale estimates of selection are required [101]. However, in the case of a GLMM with a log-link function (e.g. Poisson, over-dispersed Poisson, or negative binomial distribution), it is possible to exploit the fact that the latent-scale covariance with absolute fitness is equivalent to the data-scale covariance of relative fitness [102]: consequently, and conveniently, the covariance components of Pind on the latent scale can simply be treated as selection differentials S. By extension, estimates of β as indicated above will also provide data-scale selection gradients.
The mixed model framework also offers a powerful means of extension beyond equations (4.1) and (4.2). In an ideal experiment or analysis, the range of environments would have at least three levels that go beyond historical averages, so that the assumption of linear reaction norms can be directly tested, and if required, nonlinear, higher-order reaction norm components can be estimated [64]. The models outlined above can also be extended to include reaction norms in response to more than one environmental variable (say t1 and t2), such that equation (4.1) would contain random regression terms of (t1,j : indx,i) and (t2,j : indx,i), and Pind would be a 4 × 4 matrix with additional terms of and . For example, the models used by Hayward et al. [96] contain random regressions of sheep weight on both parasite load and age. It is also possible to consider an additional response variable y, so that the model becomes an analysis of the random regressions of both x and y and their relationship with fitness—although, again, data demands will be high. In the electronic supplementary material for this paper, we set out the implementation of a bivariate model in MCMCglmm [95] with R code and an example dataset, with the aim of encouraging use of this approach.
5. Conclusion and future directions
A search of the Web of Science on topic: (temperature or thermal) and topic: (plasticity or acclimation) refined to biologically-relevant categories returns more than 4400 articles in the last 5 years alone. How is it that the literature abounds with studies reporting responses to temperature, with adaptive interpretation for a wide array of traits across diverse organisms, and yet there are so few quantitative tests of whether thermal plasticity is under selection? Quantifying selection on plasticity in heterogeneous environments is clearly challenging, but its contribution to our understanding of the role of plasticity in response to rapid environmental change will be substantial.
We have outlined here the reasons why phenotypic plasticity may not always be adaptive, and why analysis of the selection on plasticity can inform our understanding of the adaptive nature of plasticity. We have also shown that there appear to be very few published estimates of selection on plasticity across thermal environments. Those few selection estimates for plasticity in response to temperature support the equivocal evidence for the adaptive nature of, or for selection on, plasticity found by other generalized meta-analyses that considered a wide range of environmental types. However, given we found only a handful of studies that examine this question, we consider it premature to conclude that there is no selection on plasticity in response to temperature. We are also very aware that our systematic review may have failed to identify all published estimates of selection coefficients. Despite the various challenges inherent in estimating these parameters, they will be invaluable for basic and applied fields as rapid environmental change continues to drive mean annual temperatures upwards and increase the frequency of extreme temperature and weather events.
Global patterns of advancement in spring events (earlier onset of reproductive-related traits or behaviours) have been documented for decades across diverse species and geographical regions [103,104]. Shifts in phenology owing to plasticity may be beneficial for individuals to respond to variation in their present environment across time, but this plasticity does not seem to alter fitness in a substantial way that selection could act on this variation. The lack of substantial evidence for selection on plasticity in response to thermal environments does not necessarily mean that plasticity plays no role in evolutionary responses to environmental change.
The evolutionary dynamics of wild populations in response to current environmental changes will reflect the interplay between genetic and environmental variation and phenotypic plasticity, among other factors. Although selection may have previously favoured plasticity, it may not be sufficient to match environmental conditions that have large inter-annual variation because optimal reaction norms (and selection on them) are inconsistent across time and space [105]. For example, in the bush brown butterfly Bicyclus anynana, when previously consistent signals for wet–dry seasonal transitions are disrupted by climate stochasticity, plasticity that was once adaptive for optimizing growth and behaviour to match resource availability may no longer confer fitness benefits because of a mismatch between phenotype and the altered environment [106]. Environmental conditions that natural populations are exposed to are obviously not static, and crucially, extreme climatic events (e.g. heatwaves, frosts, droughts) are now occurring more frequently and with greater intensity or duration [107]. Although incorporating thermal variation and extreme events as treatments in experimental designs can be challenging [108], these dimensions of environmental stochasticity can have a disproportionate impact on selection and the evolution of plasticity relative to shifts in mean environmental conditions [109–111]. Thus, empirical studies that estimate selection on plasticity in response to climate variability and extreme events will be especially valuable. These data are required if we are to discern the conditions under which plasticity is adaptive or not, and for which taxa, phenotypic traits, environments, and contexts selection operates on plasticity. A key objective now is to apply appropriate statistical models to obtain robust estimates of selection on plasticity, as these will be fundamental to understand and predict phenotypic responses to rapid environmental change. For now, thousands of articles on thermal plasticity notwithstanding, the trail of selection on thermal plasticity remains fairly cold.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the organizers of this special issue for the invitation to submit a paper, Timothée Bonnet, Jarrod Hadfield, Michael Jennions, and Alastair Wilson for useful discussions and two anonymous reviewers for constructive comments that improved the manuscript.
Data accessibility
The dataset supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
P.A.A. conducted the systematic review and meta-analysis, and wrote the first draft of the manuscript. L.E.B.K. and A.B.N. conceived the ideas for the review and contributed extensive revisions to the manuscript.
Competing Interests
We declare we have no competing interests.
Funding
Australian Research Council DP170101681.
References
- 1.Palkovacs EP, Kinnison MT, Correa C, Dalton CM, Hendry AP. 2012. Fates beyond traits: ecological consequences of human-induced trait change. Evol. Appl. 5, 183–191. ( 10.1111/j.1752-4571.2011.00212.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: The John Hopkins University Press. [Google Scholar]
- 3.Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155. ( 10.1016/S0065-2660(08)60048-6) [DOI] [Google Scholar]
- 4.Bradshaw AD. 2006. Unravelling phenotypic plasticity—why should we bother? New Phytol. 170, 644–648. ( 10.1111/j.1469-8137.2006.01761.x) [DOI] [PubMed] [Google Scholar]
- 5.Forsman A. 2015. Rethinking phenotypic plasticity and its consequences for individuals, populations and species. Heredity 115, 276–284. ( 10.1038/hdy.2014.92) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendry AP. 2016. Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J. Hered. 107, 25–41. ( 10.1093/jhered/esv060) [DOI] [PubMed] [Google Scholar]
- 7.Huey RB, Buckley LB, Du W. 2018. Biological buffers and the impacts of climate change. Integr. Zool. 13, 349–354. ( 10.1111/1749-4877.12321) [DOI] [PubMed] [Google Scholar]
- 8.Grenier S, Barre P, Litrico I. 2016. Phenotypic plasticity and selection: nonexclusive mechanisms of adaptation. Scientifica 2016, 1–9. ( 10.1155/2016/7021701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 10.Valladares F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351–1364. ( 10.1111/ele.12348) [DOI] [PubMed] [Google Scholar]
- 11.Nicotra AB, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 15, 684–692. ( 10.1016/j.tplants.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 12.Sultan SE. 1995. Phenotypic plasticity and plant adaptation. Acta Bot. Neerl. 44, 363–383. ( 10.1111/j.1438-8677.1995.tb00793.x) [DOI] [Google Scholar]
- 13.Gould SJ, Lewontin RC. 1979. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc. R. Soc. Lond. B 205, 581–598. ( 10.1098/rspb.1979.0086) [DOI] [PubMed] [Google Scholar]
- 14.Newman RA. 1992. Adaptive plasticity in amphibian metamorphosis. Bioscience 42, 671–678. ( 10.2307/1312173) [DOI] [Google Scholar]
- 15.van Kleunen M, Fischer M. 2005. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 166, 49–60. ( 10.1111/j.1469-8137.2004.01296.x) [DOI] [PubMed] [Google Scholar]
- 16.Pigliucci M, Schlichting CD. 1996. Reaction norms of Arabidopsis. IV. Relationships between plasticity and fitness. Heredity 76, 427–436. ( 10.1038/hdy.1996.65) [DOI] [PubMed] [Google Scholar]
- 17.Weis AE, Gorman WL. 1990. Measuring selection on reaction norms: an exploration of the Eurosta-Solidago system. Evolution 44, 820–831. ( 10.1111/j.1558-5646.1990.tb03807.x) [DOI] [PubMed] [Google Scholar]
- 18.Via S. 1993. Adaptive phenotypic plasticity: target or by-product of selection in a variable environment? Am. Nat. 142, 352–365. ( 10.1086/285542) [DOI] [PubMed] [Google Scholar]
- 19.Stearns SC. 1989. The evolutionary significance of phenotypic plasticity. Bioscience 39, 436–445. ( 10.2307/1311135) [DOI] [Google Scholar]
- 20.Schlichting CD, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 21.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 22.Moles AT, et al. 2014. Which is a better predictor of plant traits: temperature or precipitation? J. Veg. Sci. 25, 1167–1180. ( 10.1111/jvs.12190) [DOI] [Google Scholar]
- 23.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 24.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. New York, NY: Oxford University Press. [Google Scholar]
- 25.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 26.Colesie C, Büdel B, Hurry V, Green TGA. 2017. Can Antarctic lichens acclimatize to changes in temperature? Glob. Change Biol. 24, 1123–1135. ( 10.1111/gcb.13984) [DOI] [PubMed] [Google Scholar]
- 27.Lee JR, Raymond B, Bracegirdle TJ, Chadès I, Fuller RA, Shaw JD, Terauds A. 2017. Climate change drives expansion of Antarctic ice-free habitat. Nature 547, 49–54. ( 10.1038/nature22996) [DOI] [PubMed] [Google Scholar]
- 28.Kraemer BM, et al. 2017. Global patterns in lake ecosystem responses to warming based on the temperature dependence of metabolism. Glob. Change Biol. 23, 1881–1890. ( 10.1111/gcb.13459) [DOI] [PubMed] [Google Scholar]
- 29.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacifici M, Visconti P, Butchart SHM, Watson JEM, Cassola FM, Rondinini C. 2017. Species’ traits influenced their response to recent climate change. Nat. Clim. Change 7, 205–208. ( 10.1038/nclimate3223) [DOI] [Google Scholar]
- 31.Munday PL, Warner RR, Monro K, Pandolfi JM, Marshall DJ. 2013. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 16, 1488–1500. ( 10.1111/ele.12185) [DOI] [PubMed] [Google Scholar]
- 32.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Heerwaarden B, Kellermann V, Sgrò CM. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 34.Sgrò CM, Terblanche JS, Hoffmann AA. 2016. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 20, 433–451. ( 10.1146/annurev-ento-010715-023859) [DOI] [PubMed] [Google Scholar]
- 35.Parejko K, Dodson SI. 1991. The evolutionary ecology of an antipredator reaction norm: Daphnia pulex and Chaoborus americanus. Evolution 45, 1665–1674. ( 10.1111/j.1558-5646.1991.tb02671.x) [DOI] [PubMed] [Google Scholar]
- 36.Auld JR, Relyea RA. 2011. Adaptive plasticity in predator-induced defenses in a common freshwater snail: altered selection and mode of predation due to prey phenotype. Evol. Ecol. 25, 189–202. ( 10.1007/s10682-010-9394-1) [DOI] [Google Scholar]
- 37.Dudley SA, Schmitt J. 1996. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. Am. Nat. 147, 445–465. ( 10.1086/285860) [DOI] [Google Scholar]
- 38.Nicotra AB, Segal DL, Hoyle GL, Schrey AW, Verhoeven KJF, Richards CL. 2015. Adaptive plasticity and epigenetic variation in response to warming in an alpine plant. Ecol. Evol. 5, 634–647. ( 10.1002/ece3.1329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaman JE, White CR, Seebacher F. 2016. Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 31, 237–249. ( 10.1016/j.tree.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 40.Wilson RS, Franklin CE. 2002. Testing the beneficial acclimation hypothesis. Trends Ecol. Evol. 17, 66–70. ( 10.1016/S0169-5347(01)02384-9) [DOI] [Google Scholar]
- 41.Yamori W, Hikosaka K, Way DA. 2014. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosyn. Res. 119, 101–117. ( 10.1007/s11120-013-9874-6) [DOI] [PubMed] [Google Scholar]
- 42.Morley SA, Nguyen KD, Peck LS, Lai CH, Tan KS. 2017. Can acclimation of thermal tolerance, in adults and across generations, act as a buffer against climate change in tropical marine ectotherms? J. Therm. Biol. 68, 195–199. ( 10.1016/j.jtherbio.2016.09.007) [DOI] [PubMed] [Google Scholar]
- 43.Gilmore AM, Ball MC. 2000. Protection and storage of chlorophyll in overwintering evergreens. Proc. Natl Acad. Sci. USA 97, 11 098–11 101. ( 10.1073/pnas.150237697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naya DE, Karasov WH, Bozinovic F. 2007. Phenotypic plasticity in laboratory mice and rats: a meta-analysis of current ideas on gut size flexibility. Evol. Ecol. Res. 9, 1363–1374. [Google Scholar]
- 45.Weiner J. 2004. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. Evol. Syst. 6, 207–215. ( 10.1078/1433-8319-00083) [DOI] [Google Scholar]
- 46.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 47.Price TD, Qvarnström A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440. ( 10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheepens JF, Stöcklin J. 2013. Flowering phenology and reproductive fitness along a mountain slope: maladaptive responses to transplantation to a warmer climate in Campanula thyrsoides. Oecologia 171, 679–691. ( 10.1007/s00442-012-2582-7) [DOI] [PubMed] [Google Scholar]
- 49.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 51.Murren CJ, et al. 2015. Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity 115, 293–301. ( 10.1038/hdy.2015.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytol. 176, 749–763. ( 10.1111/j.1469-8137.2007.02275.x) [DOI] [PubMed] [Google Scholar]
- 53.Chevin L-M, Lande R. 2015. Evolution of environmental cues for phenotypic plasticity. Evolution 69, 2767–2775. ( 10.1111/evo.12755) [DOI] [PubMed] [Google Scholar]
- 54.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Buskirk J, Steiner UK. 2009. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860. ( 10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- 56.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.1111/j.1558-5646.1983.tb00236.x) [DOI] [PubMed] [Google Scholar]
- 57.de Jong G. 2005. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phytol. 166, 101–118. ( 10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- 58.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522. ( 10.1111/j.1558-5646.1985.tb00391.x) [DOI] [PubMed] [Google Scholar]
- 59.de Jong G. 1999. Unpredictable selection in a structured population leads to local genetic differentiation in evolved reaction norms. J. Evol. Biol. 12, 839–851. ( 10.1046/j.1420-9101.1999.00118.x) [DOI] [Google Scholar]
- 60.Acasuso-Rivero C, Murren CJ, Schlichting CD, Steiner UK. 2018. Adaptive phenotypic plasticity for life history and less fitness-related traits. bioRxiv. ( 10.1101/367284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 14, 419–431. ( 10.1111/j.1461-0248.2011.01596.x) [DOI] [PubMed] [Google Scholar]
- 62.Palacio-López K, Beckage B, Scheiner S, Molofsky J. 2015. The ubiquity of phenotypic plasticity in plants: a synthesis. Ecol. Evol. 5, 3389–3400. ( 10.1002/ece3.1603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valladares F, Sanchez-Gomez D, Zavala MA. 2006. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 94, 1103–1116. ( 10.1111/j.1365-2745.2006.01176.x) [DOI] [Google Scholar]
- 64.Arnold PA, Kruuk LEB, Nicotra AB. In press How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. ( 10.1111/nph.15656) [DOI] [PubMed] [Google Scholar]
- 65.Murren CJ, et al. 2014. Evolutionary change in continuous reaction norms. Am. Nat. 183, 453–467. ( 10.1086/675302) [DOI] [PubMed] [Google Scholar]
- 66.Clutton-Brock T, Sheldon BC. 2010. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 25, 562–573. ( 10.1016/j.tree.2010.08.002) [DOI] [PubMed] [Google Scholar]
- 67.Primack RB, Kang H. 1989. Measuring fitness and natural selection in wild plant populations. Annu. Rev. Ecol. Syst. 20, 367–396. ( 10.1146/annurev.es.20.110189.002055) [DOI] [Google Scholar]
- 68.Stinchcombe JR, Dorn LA, Schmitt J. 2004. Flowering time plasticity in Arabidopsis thaliana: a reanalysis of Westerman & Lawrence (1970). J. Evol. Biol. 17, 197–207. ( 10.1046/j.1420-9101.2003.00641.x) [DOI] [PubMed] [Google Scholar]
- 69.van Kleunen M, Fischer M. 2001. Adaptive evolution of plastic foraging responses in a clonal plant. Ecology 82, 3309–3319. ( 10.2307/2680154) [DOI] [Google Scholar]
- 70.Via S, Gomulkiewicz R, de Jong G, Scheiner SM, Schlichting CD, van Tienderen PH. 1995. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217. ( 10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- 71.Weinig C, Johnston J, German ZM, Demink LM. 2006. Local and global costs of adaptive plasticity to density in Arabidopsis thaliana. Am. Nat. 167, 826–836. ( 10.1086/503530) [DOI] [PubMed] [Google Scholar]
- 72.Morrissey MB, Liefting M. 2016. Variation in reaction norms: statistical considerations and biological interpretation. Evolution 70, 1944–1959. ( 10.1111/evo.13003) [DOI] [PubMed] [Google Scholar]
- 73.Nussey DH, Wilson AJ, Brommer JE. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844. ( 10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 74.Roff DA. 1997. Evolutionary quantitative genetics. New York, NY: Chapman & Hall. [Google Scholar]
- 75.Roff DA, Wilson AJ. 2014. Quantifying genotype-by-environment interactions in laboratory systems. In Genotype-by-environment interactions and sexual selection (eds Hunt J, Hosken D), pp. 101–134. Chichester, UK: Wiley Blackwell. [Google Scholar]
- 76.de Jong G. 1995. Phenotypic plasticity as a product of selection in a variable environment. Am. Nat. 145, 493–512. ( 10.1086/285752) [DOI] [PubMed] [Google Scholar]
- 77.Wade MJ, Kalisz S. 1990. The causes of natural selection. Evolution 44, 1947–1955. ( 10.1111/j.1558-5646.1990.tb04301.x) [DOI] [PubMed] [Google Scholar]
- 78.Siepielski AM, et al. 2017. Precipitation drives global variation in natural selection. Science 355, 959–962. ( 10.1126/science.aag2773) [DOI] [PubMed] [Google Scholar]
- 79.Siepielski AM, Gotanda KM, Morrissey MB, Diamond SE, DiBattista JD, Carlson SM. 2013. The spatial patterns of directional phenotypic selection. Ecol. Lett. 16, 1382–1392. ( 10.1111/ele.12174) [DOI] [PubMed] [Google Scholar]
- 80.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Springate DA, Scarcelli N, Rowntree J, Kover PX. 2011. Correlated response in plasticity to selection for early flowering in Arabidopsis thaliana. J. Evol. Biol. 24, 2280–2288. ( 10.1111/j.1420-9101.2011.02360.x) [DOI] [PubMed] [Google Scholar]
- 82.Springate DA, Kover PX. 2014. Plant responses to elevated temperatures: a field study on phenological sensitivity and fitness responses to simulated climate warming. Glob. Change Biol. 20, 456–465. ( 10.1111/gcb.12430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brommer JE, Merilä J, Sheldon BC, Gustafsson L. 2005. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution 59, 1362–1371. ( 10.1111/j.0014-3820.2005.tb01785.x) [DOI] [PubMed] [Google Scholar]
- 84.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. ( 10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 85.Reed TE, Wanless S, Harris MP, Frederiksen M, Kruuk LEB, Cunningham EJA. 2006. Responding to environmental change: plastic responses vary little in a synchronous breeder. Proc. R. Soc. B 273, 2713–2719. ( 10.1098/rspb.2006.3631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frei ER, Ghazoul J, Pluess AR. 2014. Plastic responses to elevated temperature in low and high elevation populations of three grassland species. PLoS ONE 9, e98677 ( 10.1371/journal.pone.0098677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kingsolver JG, Diamond SE, Siepielski AM, Carlson SM. 2012. Synthetic analyses of phenotypic selection in natural populations: lessons, limitations and future directions. Evol. Ecol. 26, 1101–1118. ( 10.1007/s10682-012-9563-5) [DOI] [Google Scholar]
- 88.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 89.R Development Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 90.Nakagawa S, Santos ESA. 2012. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274. ( 10.1007/s10682-012-9555-5) [DOI] [Google Scholar]
- 91.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. 2003. Measuring inconsistency in meta-analyses. Brit. Med. J. 327, 557–560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonser SP, Ladd B, Monro K, Hall MD, Forster MA. 2010. The adaptive value of functional and life-history traits across fertility treatments in an annual plant. Ann. Bot. 106, 979–988. ( 10.1093/aob/mcq195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brommer JE, Kontiainen P, Pietiäinen H. 2012. Selection on plasticity of seasonal life-history traits using random regression mixed model analysis. Ecol. Evol. 2, 695–704. ( 10.1002/ece3.60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gilmour AR, Gogel BJ, Cullis BR, Thompson R. 2009. ASReml User Guide release 3.0. Hemel Hempstead, UK: VSN International Ltd. [Google Scholar]
- 95.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 96.Hayward AD, Nussey DH, Wilson AJ, Berenos C, Pilkington JG, Watt KA, Pemberton JM, Graham AL. 2014. Natural selection on individual variation in tolerance of gastrointestinal nematode infection. PLoS Biol. 12, e1001917 ( 10.1371/journal.pbio.1001917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boulton K, Walling CA, Grimmer AJ, Rosenthal GG, Wilson AJ. 2018. Phenotypic and genetic integration of personality and growth under competition in the sheepshead swordtail, Xiphophorus birchmanni. Evolution 72, 187–201. ( 10.1111/evo.13398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nussey DH, Wilson AJ, Morris A, Pemberton J, Clutton-Brock T, Kruuk LEB. 2008. Testing for genetic trade-offs between early- and late-life reproduction in a wild red deer population. Proc. R. Soc. B 275, 745–750. ( 10.1098/rspb.2007.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stinchcombe JR, Simonsen AK, Blows MW. 2014. Estimating uncertainty in multivariate responses to selection. Evolution 68, 1188–1196. ( 10.1111/evo.12321) [DOI] [PubMed] [Google Scholar]
- 100.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 101.de Villemereuil P, Schielzeth H, Nakagawa S, Morrissey M. 2016. General methods for evolutionary quantitative genetic inference from generalised mixed models. Genetics 204, 1281–1294. ( 10.1534/genetics.115.186536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morrissey MB, Bonnet T. In press. Analogues of the fundamental and secondary theorems of selection, assuming a log-normal distribution of expected fitness. Heredity. [DOI] [PubMed] [Google Scholar]
- 103.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 104.Thackeray SJ, et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob. Change Biol. 16, 3304–3313. ( 10.1111/j.1365-2486.2010.02165.x) [DOI] [Google Scholar]
- 105.Kingsolver JG, Buckley LB. 2018. How do phenology, plasticity, and evolution determine the fitness consequences of climate change for montane butterflies? Evol. Appl. 11, 1231–1244. ( 10.1111/eva.12618) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oostra V, Saastamoinen M, Zwaan BJ, Wheat CW. 2018. Strong phenotypic plasticity limits potential for evolutionary responses to climate change. Nat. Commun. 9, 1005 ( 10.1038/s41467-018-03384-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ummenhofer CC, Meehl GA. 2017. Extreme weather and climate events with ecological relevance: a review. Phil. Trans. R. Soc. B 372, 20160135 ( 10.1098/rstb.2016.0135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dowd WW, King FA, Denny MW. 2015. Thermal variation, thermal extremes and the physiological performance of individuals. J. Exp. Biol. 218, 1956–1967. ( 10.1242/jeb.114926) [DOI] [PubMed] [Google Scholar]
- 109.Chevin L-M, Hoffmann AA. 2017. Evolution of phenotypic plasticity in extreme environments. Phil. Trans. R. Soc. B 372, 20160138 ( 10.1098/rstb.2016.0138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kingsolver JG, Buckley LB. 2017. Quantifying thermal extremes and biological variation to predict evolutionary responses to changing climate. Phil. Trans. R. Soc. B 372, 20160147 ( 10.1098/rstb.2016.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buckley LB, Huey RB. 2016. How extreme temperatures impact organisms and the evolution of their thermal tolerance. Integr. Comp. Biol. 56, 98–109. ( 10.1093/icb/icw004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article have been uploaded as part of the electronic supplementary material.