Abstract
Plant and animal parents may respond to environmental conditions such as resource stress by altering traits of their offspring via heritable non-genetic effects. While such transgenerational plasticity can result in progeny phenotypes that are functionally pre-adapted to the inducing environment, it is unclear whether such parental effects measurably enhance the adult competitive success and lifetime reproductive output of progeny, and whether they may also adversely affect fitness if offspring encounter contrasting conditions. In glasshouse experiments with inbred genotypes of the annual plant Polygonum persicaria, we tested the effects of parental shade versus sun on (a) competitive performance of progeny in shade, and (b) lifetime reproductive fitness of progeny in three contrasting treatments. Shaded parents produced offspring with increased fitness in shade despite competition, as well as greater competitive impact on plant neighbours. Inherited effects of parental light conditions also significantly altered lifetime fitness: parental shade increased reproductive output for progeny in neighbour and understorey shade, but decreased fitness for progeny in sunny, dry conditions. Along with these substantial adaptive and maladaptive transgenerational effects, results show complex interactions between genotypes, parent environment and progeny conditions that underscore the role of environmental variability and change in shaping future adaptive potential.
This article is part of the theme issue ‘The role of plasticity in phenotypic adaptation to rapid environmental change’.
Keywords: adaptive plasticity, shade plasticity, non-genetic inheritance, parental effects, plant competition, polygonum
1. Introduction
It is increasingly recognized that even the relatively rapid process of contemporary selective evolution [1] may be too slow to permit organisms to adaptively keep pace with rapidly changing environments [2–6], and that individual plasticity may provide a critical source of adaptive adjustment over very short timescales (e.g. [7,8]; reviewed in [9–12]). However, the adaptive effectiveness of plastic response may be limited by the time required for the developing individual to perceive its environment and initiate appropriate phenotypic adjustments [13–16]. This time lag is eliminated (in all but the inducing generation) in plant and animal taxa that express adaptive transgenerational plasticity, whereby individuals respond to specific environmental states by modifying traits of their progeny in ways that preadapt them to those same conditions ([17–28] in plants and [29–33] in animals). Because this mode of phenotypic change can be induced after just one generation in a new environment, and may be expressed in many offspring at once in that environment, transgenerational effects may enhance a population's persistence in the face of variable or rapidly changing conditions [34–38]. Note that these inherited changes to progeny phenotypes are not simply ‘silver spoon’ effects [39], in which maternal plants and animals in favourable conditions produce higher quality, more well-provisioned progeny that have universally enhanced growth, competitive success and fecundity (reviewed in [40–46]). These positive effects of favourable maternal conditions are unlikely to provide adaptation to anthropogenically changed environments, which generally entail abiotic or biotic stresses. Rather, plastic transgenerational effects may provide such ‘adaptive rescue’, because they consist of changes to offspring made by parents in response to particular—often stressful—environments that confer the specific traits necessary for maximizing fitness in those environments.
Not surprisingly, this remarkable aspect of plasticity has excited a great deal of interest as a potential source of rapid adaptive change in natural populations facing new challenges. Yet two key questions remain to be answered in order to evaluate its potential impact in natural systems [47,48]. First, do transgenerational effects of parental environment significantly alter the realized success of offspring? Among published studies that show beneficial transgenerational effects of parental stresses on progeny development in similar conditions (see references above), very few have directly tested effects on either key ecological interactions such as competition [49] or lifetime reproductive fitness [50]. Apart from a small number of cases that document positive effects on juvenile survival [23,26,31,51] or reproductive output (to date, in arthropods only: [52–54]), the vast majority of such studies in both animals and plants focus on progeny size traits such as rosette diameter [55], larval size [56], or biomass [24,27,57], or on the size or number of defensive structures [29,58]. While such growth traits may influence reproductive output in various circumstances, direct measures of fitness impact are essential to assess the adaptive significance of transgenerational effects.
Second, does the direction of such fitness effects (positive or negative) vary depending on the environment? The ecological and fitness consequences of inherited plastic modifications (unlike ‘silver spoon’ effects) will likely be context-dependent: if parent individuals respond to an environmental challenge by producing progeny able to withstand that particular challenge; this phenotype may comprise an adaptive mismatch in contrasting conditions with different phenotypic optima [50,59–61]. In other words, inherited effects of parental environments on development may be maladaptive rather than adaptive, if progeny individuals encounter dissimilar rather than similar environmental conditions. If this is the case, the fitness consequences of transgenerational effects will depend crucially on the interplay of spatial and temporal environmental variability with both dispersal and seed (or egg) longevity. Testing for context-dependent fitness impacts requires transgenerational studies designed to include ecologically realistic alternative offspring environments that can reveal potentially maladaptive effects (e.g. [23,24,54,62]).
Rigorous tests for adaptive consequences of transgenerational effects require a two-step experimental design that isolates progeny variation due to parental environment from variation due to parental genotype [38,50]: (i) replicate parents of each experimental genotype must be raised in two (or more) treatments to generate progeny differing only in parental environment, and (ii) these sets of progeny must be tested factorially in two (or more) offspring treatments; these treatments need not be identical to the parent environments, but they must have different adaptive optima. Clearly, such tests will be most meaningful if they are carried out with naturally evolved systems, and in ecologically relevant alternative environmental states; in addition, an accurate measurement of lifetime fitness is essential. Here, we present a study using naturally evolved (field-based) genotypes of Polygonum persicaria, a widespread herbaceous plant of diverse temperate habitats. This species offers three key experimental advantages: first, it has a mixed breeding system (i.e. populations undergo both outcrossing and self-fertilization; [63]), so genotypes are diverse, as in most systems, yet can be intensively inbred to produce isogenic replicate parents [64]. Second, P. persicaria is an obligate annual, so total reproductive output (i.e. fitness) can be directly measured. Finally, the range and variability of major environmental factors have been characterized for natural source populations [65], providing a robust context for the design of experimental treatments [15].
We investigated transgenerational effects of parental environment on progeny competitive performance and lifetime fitness, in response to a key environmental variable for plants: light. Light conditions vary in all natural plant habitats [66], as incident solar radiation is mediated in both quantity and spectral quality by canopy and neighbour shade [67]. Because different phenotypes are required for maximizing growth and competitive success in shaded versus full-sun conditions ([66,68,69], and references therein), any transgenerational effects of parental light environment could potentially influence progeny fitness in alternative conditions. Within- and among-site patterns of light variation are expected to change in future climatic and atmospheric conditions, reflecting denser canopies in some systems [66] and sunnier, drier conditions in others [70–73]. Moreover, increased variability in temperature and precipitation [74–76] may lead to greater year-to-year variation for patterns of neighbour shade in herbaceous communities.
We carried out two related experiments to test the transgenerational fitness effects of full sun versus simulated understorey shade as parental environments. The design allowed us to separately evaluate the effects of parental environment and genotype, and to test for genotypic differences in transgenerational effects. For a multi-population sample of five genotypes, we grew replicate parent plants in contrasting glasshouse light treatments and then examined the effects of parental sun versus shade on (a) progeny competitive performance; and (b) total lifetime fitness in three alternative offspring environments: sunny dry conditions, severe understorey shade and neighbour shade. To gain insight to the causes of fitness variation, we also measured three growth traits: height extension, which plays a key role in competitive interactions [77]; timing of reproductive onset, which can strongly affect lifetime reproductive output in plants [63,78], and total vegetative biomass, which contributes to reproductive potential [64]. These data provide evidence that transgenerational plasticity in response to parental shade may have a surprisingly strong positive effect on the ecological interactions and reproductive fitness of progeny growing in shade, but an even stronger negative effect on fitness if progeny instead encounter sunny, dry conditions.
2. Methods
(a). Study system
Polygonum persicaria is a common Eurasian annual plant naturalized in North America [79,80]. Previous studies have documented inherited effects of both parental moisture and parental light conditions on seedling development in this species [24,81,82]. In order to sample from the species' genotypic diversity, genotypes were drawn from three typical northeastern US populations: a moist pasture in full sun (MHF population; Northfield, MA), a moist, moderately shaded field (TP population; Dover, MA) and an organic farm (full sun with neighbour shade; NAT population, Natick, MA; see [65] for site details). Note that this multi-population sample is intended to provide a robust basis for (i) evaluating transgenerational effects in this species and (ii) testing for potential genotypic variation in these effects, and not to resolve the distribution of such variation within versus among populations; see [24,26] for related studies using this same sample design. Field-collected achenes (one-seeded propagules) were inbred under uniform glasshouse conditions for four generations to produce highly inbred (selfed full-sib) genetic lines (hereafter ‘genotypes’).
(b). Parental generation
Replicate parent plants of each inbred genotype were grown in both sun and shade glasshouse treatments to produce genetically uniform offspring that differed only in parental light environment (see [26,81,83]).
Fifth-generation inbred achenes of 5 genotypes (2 MHF, 2 TP and 1 NAT; see above) were stratified in distilled water at 4°C for seven weeks, sown into flats of moist vermiculite, and randomly positioned on a glasshouse bench (1 June 2012). At the first true leaf stage (4–6 days after emergence), seedlings of each genotype were individually transplanted into 1 l clay pots filled with a 1 : 1 : 1 mix of sterilized topsoil : horticultural sand : fritted clay (Turface™, Profile Products, Buffalo Grove, IL, USA) pre-moistened with 250 ml water. Five days after transplant, replicate seedlings of each genotype were randomly assigned to each of two parental glasshouse treatments. In the parental sun treatment, plants received 100% of incident light (ca 1300–1800 µmol m–2 s–1 midday photosynthetically active radiation (PAR)) with a red : far red (R : FR) spectral ratio of ca 1.0 (measured with an SKR R : FR meter; Skye Instruments, Llandrindod Wells, UK). The parental shade treatment consisted of a metal frame covered by 80% neutral-density shade cloth (PAK Unlimited, GA, USA) overlaid with strips of green plastic filter (no. 138; Lee Filters, Burbank, CA, USA), providing plants with ca 260 µmol m–2 s–1 midday PAR and an R : FR ratio ≈ 0.7, which agrees with measured R : FR ratios in shaded natural Polygonum habitats [84]. Equidistant 3.5 cm diameter holes cut in the shade cloth provided parental shade plants with a daily 15 min sunfleck, simulating understorey conditions [85]. Parental plants in both treatments were kept at field capacity moisture and grown for nine weeks, with bench positions re-randomized weekly. Self-fertilized, full-sib achenes produced by the 10 experimental parent units (5 genotypes × 2 parental treatments) were harvested, air-dried and stored at 4°C.
(c). Competition experiment
For each genotype, 250 achenes from a parent plant grown in parental sun and 250 achenes of that genotype grown in parental shade were germinated in 100 mm Petri plates lined with moist filter paper and positioned randomly on a glasshouse bench (7 June 2017). Plates were monitored twice daily for germination. As soon as the radicle began to emerge, new germinants were immediately transplanted into 1 l clay pots (filled as described above but with a protective 1 cm top layer of moist vermiculite) in pentagonal competitive arrays that each consisted of a central target plant and five surrounding, equidistant neighbour (competitive background) plants. These spatial arrays were set up to test competitive interactions in all four possible combinations of parental sun or shade target plants, and parental sun or shade competitive backgrounds (i.e. parental sun target/parental sun background, parental sun target/parental shade background; parental shade target/parental sun background; parental shade target/parental shade background, figure 1a). For each genotype, 10 replicate arrays were set up for each of the four parental treatment combinations. The overall experimental design was: 5 genotypes × 2 parental treatments of target plant (target PT) × 2 parental treatments of competitive background plants (background PT) × 1 replicate array per block × 10 blocks = 200 competitive arrays.
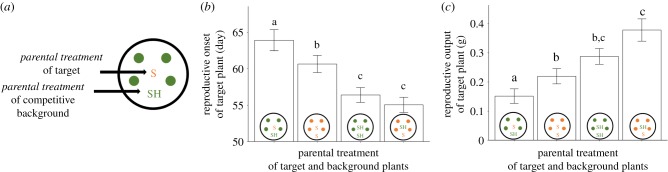
Figure 1.
Effects of parental light treatment on the performance of central target plants in competitive arrays. (a) Design and labelling of competitive arrays (one of four factorial arrays is shown as an example): one parental sun (S) target plant is surrounded by five parental shade (SH) background plants of the same genotype. Means ± s.e. for each type of array are shown (pooled across 5 P. persicaria genotypes) for (b) number of days to reproductive onset and (c) lifetime reproductive output. Letters indicate significant differences based on post hoc Tukey's HSD tests (details in Methods). (Online version in colour.)
Competitive arrays were set in a randomized complete block design (with separate blocks set across multiple glasshouse benches) under moderate shade tents (as described above in §2b) at ca 235 ± 32 µmol m–2 s–1 midday PAR (R : FR ≈ 0.7) and grown at 100% field capacity moisture for 13–14 weeks, a period of time corresponding to the full length of a natural growth season for the source Polygonum populations. The distance between individual plants in the competitive arrays (equivalent to 490 individuals per m2) corresponds to high-density conditions observed in natural Polygonum field populations [86,87].
(d). Contrasting offspring treatments
Eight replicate offspring from each (genotype and parental treatment) experimental unit (1–3 replicate parent individuals per unit) were stratified (see §2a), germinated as described below, and grown in a randomized split-plot design in each of three glasshouse growth treatments: neighbour shade, severe shade and sunny dry. Plants were harvested after 13–14 weeks in treatment. In each treatment, midday light measurements were taken daily for 11 consecutive days (midway through the experiment) to calculate mean midday PAR, and six soil moisture measurements were taken (SM 150 soil moisture kit, Delta-T Devices, Cambridge, UK) to determine mean soil moisture. R : FR light wavelength ratios are reported based on prior studies using the same glasshouse treatments [84,88]. The experimental design was: 5 genotypes × 2 parental treatments × 3 offspring treatments × 8 blocked replicates per offspring treatment (total N = 240 plants).
Severe shade and sunny dry offspring treatments: for each genotype, 48 achenes produced by a parent individual grown in parental sun, and 48 achenes from a parent individual of the same genotype grown in parental shade, were sown as described in §2b (31 May 2017) and individually transplanted at the first true leaf stage into 1 l clay pots (see §2b; 15 June 2017). In the severe shade treatment, plants were grown at 100% field capacity moisture under the shade tents described in §2b but with an additional layer of 30% neutral-density shade cloth (PAK Unlimited, GA, USA), resulting in midday PAR levels of ca 126 ± 20 µmol m−2 s−1 and R : FR ≈ 0.7. In the sunny dry treatment, plants received 100% of full glasshouse sun (ca 1569 ± 252 µmol m−2 s−1 midday PAR, R : FR ≈ 1) and were manually given 10–15 ml water 1–4 times per day as needed to maintain uniform moisture stress in all pots (ca 23% of soil field capacity by weight), such that every plant wilted for 2–3 h at midday.
Neighbour shade offspring treatment: the neighbour shade treatment was set up as described in §2c for competitive arrays, except that all plants in a single array were from the same genotype and parental treatment (8 pots per genotype×parental treatment combination).
(e). Data collection
Flowering (defined as the first day on which the open perianth of at least one flower on the plant was visible) was monitored daily to determine the number of days to reproductive onset. Plant height (cm from base to apex) was measured weekly in juvenile plants (weeks 3–6 in severe shade, sunny dry and neighbour shade treatments; weeks 1–6 in the competition experiment). Starting at week 9 in treatment, mature achenes were collected weekly (to prevent the loss of ripe achenes), air-dried and weighed. At final harvest, vegetative and reproductive tissues (including mature and immature achenes, flowers and peduncles; mature achenes typically compose ≥ 95% of reproductive tissue mass, S. E. Sultan 2001, unpublished data) were separately harvested. The air-dried masses of reproductive tissues collected at harvest were summed with previously collected achenes to determine lifetime reproductive output (g). Vegetative tissues were collected, dried at 100°C for ≥1 h and then at 65°C for ≥48 h, and weighed, to determine vegetative biomass (g). For the neighbour shade treatment and the competition experiment, traits were measured only for the target plant in each array. Owing to insufficient germination, nine drought-stressed plants that never reached maturity, and the exclusion of one to six outliers per trait (data points that >1.5 times the interquartile range below the first quartile or above the third quartile), the final samples sizes for each trait were N = 223 (days to reproductive onset), N = 225 (lifetime reproductive output), N = 238 (plant height) and N = 201 (vegetative biomass, owing to oven malfunction) in the contrasting offspring environments, and N = 189 (days to reproductive onset), N = 191 (lifetime reproductive output), N = 129 (vegetative biomass, owing to oven malfunction) and N = 193 (plant height) in the competition experiment.
(f). Data analysis
Statistical analyses were performed with JMP Pro 13 (SAS Institute, Cary, NC, USA) and graphing was done with R v. 3.3.3 (R Core Team 2017; https://www.r-project.org/). Type I error was controlled using false discovery rate (FDR)-adjusted p-values following the Benjamini & Hochberg method, with an FDR of 5% [89].
(i). Competition experiment
Analysis of variance (ANOVA) with type III sums of squares was used to analyse the (fixed) effects on target plant traits of target parental treatment (PT), background PT, genotype, all two-way and three-way interactions and block. These main and interaction effects on plant height over time were tested by multivariate repeated-measures ANOVA [90]; following a significant sphericity χ2 test, multivariate Wilks' lambda was used to assess effect significance [91]. To examine the extent to which variation in lifetime reproductive output was explained by transgenerational effects on reproductive timing, we carried out analysis of covariance (ANCOVA), testing the main and interaction effects of target and background plant parental treatments, genotype and block, and including days to reproductive onset as a covariate. Genotype was treated as a fixed effect because the sample was drawn from specific populations representing the species' ecological breadth and used in previous studies [26]. Lifetime reproductive output and vegetative biomass were Box–Cox transformed to meet the ANOVA assumption of homoscedasticity. Effect sizes were calculated as partial eta-squared [92], a metric that is robust for comparing effect sizes across traits within a single dataset [92,93].
To evaluate the magnitude of the main effects of target PT (averaged across both genotypes and background plant PT), we calculated the mean per cent change of all target plants due to their parents' light treatment, using the equation: 100% × (trait meanPARENTAL SHADE − trait meanPARENTAL SUN)/trait meanPARENTAL SUN. We similarly calculated the mean per cent change of target plants due to the parental treatment of the background plants. To precisely resolve significant target PT × background PT interaction effects, post hoc Tukey's honest significant difference (HSD) tests were carried out to test for differences between target plant trait means in the four types of competitive array. To examine possible genotype-specific effects of parental sun versus shade, we followed up significant genotype × target PT and genotype × background PT interaction terms with simple effects tests [94].
(ii). Contrasting offspring treatments
ANOVA with type III sums of squares was used to analyse the (fixed) effects on offspring traits of parental treatment (PT, parental shade versus parental sun), offspring treatment (OT, severe shade, neighbour shade or sunny dry), genotype, all two-way and three-way interactions and block (nested within offspring treatment) (see [26] for a similar analysis). We used ANCOVA to test these main and interaction effects on lifetime reproductive output while including day of reproductive onset as a covariate. As described above, multivariate repeated-measures ANOVA was used to analyse changes in plant height over time. Effect sizes were calculated as partial eta-squared . All traits were Box–Cox transformed to meet the assumptions of ANOVA.
Significant (and marginally non-significant) parental treatment × offspring treatment interaction effects were followed with simple main effects tests of differences due to parental treatment within each offspring treatment. To further examine the offspring treatment-specific effects of parental treatment on each trait, the mean per cent change (pooled across genotypes) due to parental shade versus parental sun was calculated in each offspring treatment using the equation: 100% × (trait meanPARENTAL SHADE − trait meanPARENTAL SUN)/trait meanPARENTAL SUN. To examine genotype-specific effects, the significant genotype × parental treatment×offspring treatment three-way interaction effect was followed up with simple effects tests to separately assess for each genotype the effect of parental treatment within each offspring treatment.
3. Results
(a). Competition experiment
(i). Progeny of shaded parents showed enhanced performance for both competitive response to neighbours and competitive impact on them
Target plants that were progeny of shaded parents (averaged across the 5 genotypes and 2 background conditions) maintained high growth and fitness despite competition (competitive response), flowering 6.6 days earlier than parental sun target plants, growing 25% taller by week 6, and producing 47% greater vegetative biomass and 92% greater lifetime reproductive output (table 1, effect of target PT on all traits p < 0.0001***; figures 1 and 2). When competing against each type of competitive background (either sun progeny or shade progeny), parental shade target plants maintained higher fitness than parental sun targets (cf. Tukey's tests, figure 1b,c).
Table 1.
Results of ANOVA for parental effects on competitive performance. Effects of parental treatment of target plant (target PT; parental shade versus parental sun), parental treatment of competitive background (background PT; parental shade versus parental sun) and genotype (G) on target plant fitness traits from three-way ANOVA. Significant p-values (adjusted for false discovery rate) and partial eta-squared values for each term are shown in italics (†p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001, non-significant p ≥ 0.10). Details in Methods.
| target plant height at 6 weeks N = 193; ; d.f. = 28, 164 |
target plant vegetative biomass N = 129; ; d.f. = 25, 103 |
target plant no. days to reproductive onset N = 189; ; d.f. = 28, 160 |
target plant lifetime reproductive output N = 191; ; d.f. = 28, 162 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| source of variation | d.f. | p-value | p-value | p-value | p-value | ||||
| parental treatment of target | 1 | <0.0001*** | 0.262 | <0.0001*** | 0.184 | <0.0001*** | 0.351 | <0.0001*** | 0.284 |
| parental treatment of competitive background | 1 | <0.0001*** | 0.089 | 0.0006*** | 0.123 | 0.0072** | 0.052 | <0.0001*** | 0.116 |
| genotype (G) | 4 | <0.0001*** | 0.293 | 0.0001*** | 0.220 | <0.0001*** | 0.591 | <0.0001*** | 0.289 |
| target PT×background PT | 1 | 0.0002*** | 0.316 | 0.2131 | 0.077 | 0.1903 | 0.100 | 0.0771† | 0.183 |
| G × target PT | 4 | 0.0297* | 0.019 | 0.0160* | 0.018 | <0.0001*** | 0.012 | <0.0001*** | 0.020 |
| G × background PT | 4 | 0.0778† | 0.117 | 0.0689† | 0.124 | 0.0723† | 0.311 | 0.0001*** | 0.188 |
| G × target PT × background PT | 4 | 0.0164* | 0.065 | 0.2131 | 0.090 | 0.3541 | 0.056 | 0.3115 | 0.134 |
| block | 9a | 0.0007*** | 0.075 | 0.2131 | 0.057 | 0.0723† | 0.027 | 0.0001*** | 0.029 |
aOwing to oven malfunction, block d.f. = 6 for target plant vegetative biomass.
Figure 2.
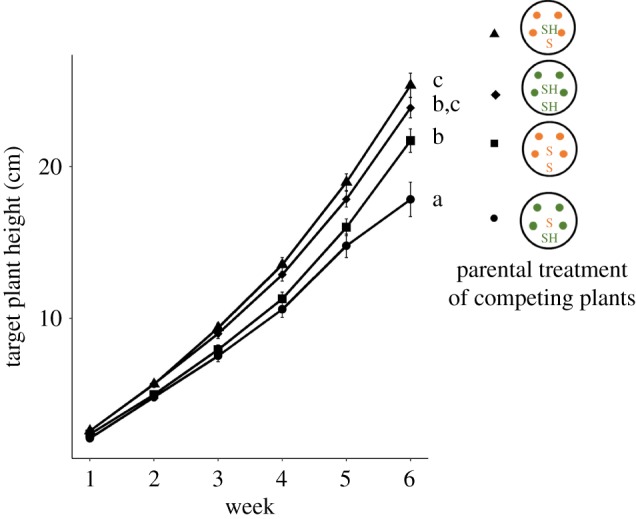
Effects of parental light treatment on target plant height extension over time in competitive arrays. Means ± s.e for each type of array are shown (pooled across 5 genotypes); parental treatment of target and background plants (parental sun, S; parental shade, SH) labelled as in figure 1. Letters indicate significant differences based on post hoc Tukey's HSD tests (details in Methods). (Online version in colour.)
The offspring of shaded parents were also better at competitively suppressing the growth and fitness of neighbours (competitive effect) than the offspring of full-sun parents. When grown with parental shade competitive backgrounds, target plants (averaged across both target parental treatments) flowered 2.3 days later than target plants competing with parental sun competitive backgrounds, grew 11% shorter, and produced 26% less vegetative biomass and 30% lower lifetime reproductive output (table 1: effect of background PT on all traits p < 0.0072**). Together, the positive effects of parental shade on both response as target plants and impact as background plants resulted in consistent rank ordering of target plant growth and fitness in the four combinatorial arrays: the tallest, earlier-reproducing, highest biomass and highest fitness target plants under competition were shade progeny competing against a competitive background of sun progeny, and the target plants with the lowest fitness were sun progeny competing against a competitive background of shade progeny (cf. Tukey's tests; figures 1b,c and 2). Based on weekly height measurements, these effects did not diminish over developmental time, and indeed target progeny of sun parents increasingly reduced height extension (significant interaction effects of target PT×time, background PT×time; table 2), especially when competing with a shade-progeny background (significant effect of target PT×background PT on height at week 6, table 1; Tukey's tests, figure 2). Based on ANCOVA, timing of reproductive onset was a significant covariate for lifetime reproductive output (p < 0.0001***), but the main effects of target PT and background PT on target plant fitness remained significant (p < 0.0235* and p < 0.0008***, respectively; electronic supplementary material, table S1).
Table 2.
Results of repeated-measures ANOVA for parental effects on height extension over time. Effects of parental treatment of target plant (target PT; parental shade versus parental sun), parental treatment of competitive background (background PT; parental shade versus parental sun), genotype (G) and time on target plant height measured weekly over six weeks from a multivariate repeated-measures ANOVA. Significant p-values (adjusted for false discovery rate) are shown in italics (†p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001, non-significant (n.s.) p ≥ 0.10). Details in Methods.
| source of variation | d.f. | p-value |
|---|---|---|
| time | 5, 160 | <0.0001*** |
| target PT × time | 5, 160 | <0.0001*** |
| background PT × time | 5, 160 | 0.0034** |
| genotype (G) × time | 20, 531.6 | <0.0001*** |
| target PT × background PT × time | 5, 160 | 0.229 n.s. |
| G × target PT × time | 20, 531.6 | 0.0012** |
| G × background PT × time | 20, 531.6 | 0.0138* |
| G × target PT × background PT × time | 20, 531.6 | 0.061† |
| block × time | 45, 718.8 | <0.0001*** |
(ii). Effects of parental shade versus sun on competitive performance varied among genotypes
Polygonum genotypes varied in the impact of parental shade versus sun on target plant performance (significant genotype×target PT interaction effects for all traits; table 1). Genotype by parent treatment interaction effects on the competitive impact of background plants was also highly significant for lifetime reproductive output, but marginally non-significant for growth traits (genotype × background PT effects; table 1; see electronic supplementary material, figure S1 for effects of target PT and background PT on individual genotypes). Genotypic differences for the effects of both target and competitive background parent treatment significantly affected height over time (significant effects of genotype × target PT × time and genotype × background PT × time; table 2).
For every target-plant trait (except number of days to reproductive onset), the target PT and background PT together explained more variation than genotype (cf. values, table 1: target PT η2 ≈ 0.18–0.28; background PT ≈ 0.09–0.12; and genotype ≈ 0.22–0.29 for those three traits). For lifetime reproductive output, the combined effects of target PT and background PT explained more variation than did genotype, and the parental environment of the target plant alone had virtually equivalent impact on fitness to its genotype (table 1: , 0.116 and 0.289, respectively). However, genotype explained substantially more of the variation for number of days to reproductive onset (table 1: : target PT = 0.351; background PT = 0.052; and genotype = 0.591).
(b). Contrasting offspring treatments
(i). Parental shade increased growth and fitness of progeny in both severe and neighbour shade, but reduced growth and fitness in sunny, dry conditions
Parental treatment resulted in substantial, lifetime effects on progeny growth and fitness; these effects varied significantly depending on offspring treatment (table 3, PT × OT interaction effects on all traits p < 0.0001***; figure 3). Because the effects of parental shade versus sun were positive in the two progeny shade treatments but negative in the progeny sun treatment, the main effect of parental treatment was generally non-significant (table 3). In both severe and neighbour shade, progeny of shaded parents grew taller and larger, and had earlier reproductive onset and greater lifetime reproductive output, than progeny of full-sun parents. However, shade-produced progeny were shorter, smaller in biomass, slower to reproduce and less fecund than progeny of full-sun parents in the sunny dry offspring treatment (figure 3a–d).
Table 3.
Results of ANOVA for parental effects on growth and fitness in contrasting environments. Effects of parental treatment (PT; parental shade versus parental sun), offspring treatment (OT; severe shade versus neighbour shade versus sunny dry), genotype (G), all two- and three-way interactions and block (nested within offspring treatment) on growth and fitness traits, based on significance tests from a three-way ANOVA. Significant p-values (adjusted for false discovery rate) and partial eta-squared (η2p) values for each term are shown in italics (†p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001, non-significant p ≥ 0.10). Details in Methods.
| plant height N = 238; ; d.f. = 50, 187 |
vegetative biomass N = 201; d.f. = 47, 153 |
no. days to reproductive onset = 223; d.f. = 50, 172 |
lifetime reproductive output N = 225; d.f. = 50, 174 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| source of variation | d.f. | p-value | p-value | p-value | p-value | ||||
| parental treatment (PT) | 1 | 0.0411* | 0.022 | 0.2462 | 0.012 | 0.4662 | 0.003 | 0.0857† | 0.018 |
| offspring treatment (OT) | 2 | <0.0001*** | 0.937 | <0.0001*** | 0.678 | <0.0001*** | 0.806 | <0.0001*** | 0.612 |
| genotype (G) | 4 | 0.0501† | 0.049 | 0.0925† | 0.061 | <0.0001*** | 0.263 | 0.0002*** | 0.130 |
| PT×OT | 2 | 0.0017** | 0.066 | <0.0001*** | 0.174 | <0.0001*** | 0.169 | <0.0001*** | 0.277 |
| G×PT | 4 | 0.5371 | 0.016 | 0.2462 | 0.039 | 0.0045** | 0.087 | 0.0290* | 0.063 |
| G×OT | 8 | 0.0191* | 0.092 | 0.7872 | 0.035 | <0.0001*** | 0.271 | 0.0008*** | 0.148 |
| G×PT×OT | 8 | 0.0985† | 0.068 | 0.7979 | 0.029 | 0.0221* | 0.099 | 0.3892 | 0.047 |
| block (offspring treatment) | 21a | <0.0001*** | 0.274 | 0.0308* | 0.192 | 0.0045** | 0.213 | 0.0084** | 0.202 |
aOwing to oven malfunction, block d.f. = 18 for vegetative biomass.
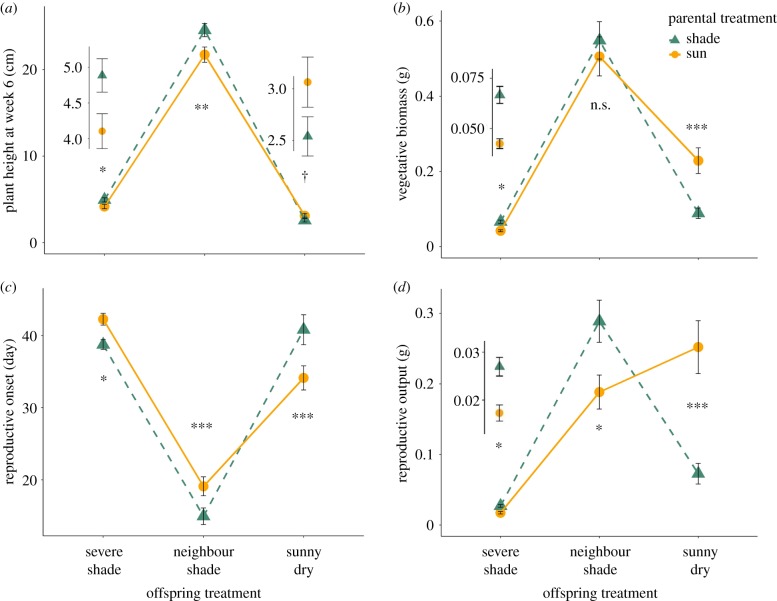
Figure 3.
Effects of parental sun versus parental shade on fitness traits of offspring grown in contrasting treatments. Means ± s.e are shown (pooled across 5 genotypes) for (a) plant height at week 6, (b) total vegetative biomass, (c) number of days to reproductive onset and (d) lifetime reproductive output. For each trait, significance tests for the effect of parental shade versus parental sun within each offspring treatment are shown (simple effects tests; †p < 0.10, *p < 0.05, **p < 0.01, ***p < 0.001, non-significant (n.s.) p ≥ 0.10; details in Methods). Insets show enlarged scale for significant or marginally n.s. results within stressful, low-growth treatments. (Online version in colour.)
In the severe shade and neighbour shade treatments, juvenile progeny of shaded parents grew significantly taller than progeny of full-sun parents (by 19 and 13%, respectively; p = 0.028* and 0.003** based on simple effects test of parental treatment within each offspring treatment; figure 3a). This height increment was consistent over time (electronic supplementary material, figure S2a,b; parental treatment×time interaction effects 0.269 > p > 0.074). In the sunny dry treatment, by contrast, progeny of shaded parents initially expressed this same height advantage, but starting in week 4 they became shorter than sun-parent progeny, a height gap that became more pronounced over time as the shade progeny increasingly slowed shoot extension (electronic supplementary material, figure S2c; parental treatment × time interaction p = 0.039*). By harvest, the offspring of shaded parents had produced significantly more vegetative biomass than the offspring of full-sun parents in severe offspring shade (+57%; p < 0.0188), and slightly (non-significantly) more in neighbour shade (+8%; p = 0.702; figure 3b). However, for offspring grown in sunny dry conditions, parental shade resulted in dramatically decreased vegetative biomass compared with parental sun (−61%, p < 0.0001***; figure 3b).
Offspring of shaded parents transitioned to reproduction earlier than offspring of full-sun parents in both severe shade and neighbour shade (8 and 22% earlier, respectively; p ≤ 0.023*; figure 3c). Parental-environment effects on fitness were surprisingly dramatic: parental shade resulted in 55% greater lifetime reproductive output compared with parental sun for progeny in severe shade (p ≤ 0.0228*), and 53% higher reproductive output in neighbour shade (p = 0.0117*) (figure 3d). Conversely, in sunny dry conditions, the offspring of shaded parents had a 20% later reproductive onset (p < 0.0001***; figure 3c) and 71% lower lifetime reproductive output (p < 0.0001***; figure 3d) than offspring of full-sun parents. The impact of parental treatment on lifetime fitness in contrasting environments was not entirely explained by effects on reproductive timing: although reproductive onset was a significant covariate for total reproductive output (p < 0.0001***), both the main effect of parental treatment and the PT × OT interaction remained significant after acccounting for this effect (p < 0.0355* and p < 0.0002***, respectively, in ANCOVA; electronic supplementary material, table S1).
(ii). Effects of parental and offspring treatment varied among genotypes
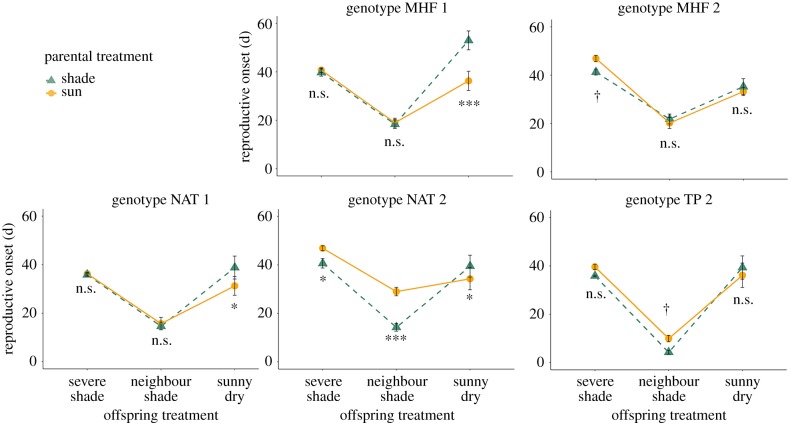
For most traits, the effects of parental as well as progeny treatment varied among genotypes (significant genotype × PT effects on reproductive onset and total fitness; significant genotype × OT effects on these traits as well as on plant height; and three-way genotype × PT × OT effects on reproductive onset and (marginally non-significantly) plant height; table 3). Such three-way interactions reflect the particular impact of parental environment on each genotype's pattern of trait expression in the three alternative progeny growth environments (figure 4). For instance, parental shade led to substantially faster reproductive onset for plants of genotype NAT 2 growing in neighbour shade, and a less pronounced but similar effect in severe shade, while plants of genotype MHF 1 showed a pronounced (negative) effect of parental shade on reproductive onset in the sunny dry progeny treatment, but no effect on life-history timing in the shade treatments (figure 4)
Figure 4.
Effects of parental sun versus parental shade on each genotype's time of reproductive onset in three contrasting offspring treatments. Means ± s.e. are shown. For each genotype, significance tests for the effect of parental shade versus parental sun within each offspring treatment are shown (simple effects tests; †p < 0.10, *p < 0.05, ***p < 0.001, non-significant (n.s.) p ≥ 0.10; details in Methods). (Online version in colour.)
The main effect of genotype was significant or marginally non-significant for all traits (table 3). However, with one exception (reproductive onset timing), differences due to offspring treatment-specific effects of parental treatment were greater than those due to genotype (η2p values, table 3; e.g. for total reproductive output, for PT × OT interaction effect and for genotype). Note that, because experimental genotypes were drawn from three distinct populations and thus were not closely related, our sample likely includes large genotypic differences (e.g. relative to genotypic differences within a single natural population). Accordingly, this was a conservative way to test the relative magnitude of inherited environmental versus genotype effects.
4. Discussion
(a). Parental shade significantly enhanced the competitive ability of offspring in shade
Because plants do not grow in isolation, competitive ability is a key fitness factor in natural populations [95,96]. This ability arises from two distinct aspects of plant performance: competitive effect, the ability to suppress the growth and reproduction of neighbour individuals, and competitive response, the ability to maintain growth and fitness despite the presence of neighbours [97,98]. Success relative to neighbours may result from either aspect of competitive ability [96]; the two are often positively correlated (e.g. [99–103]), but in some systems, individuals show just one type of competitive superiority [97,98,104,105]. We tested the effect of parental shade versus sun on each aspect of competitive ability by factorially varying the parental treatment of competing focal (target) and background plants. Both competitive response and competitive effect were substantially greater in progeny of shaded parents than progeny of full-sun parents: as target plants, they more successfully maintained high growth rates, early reproductive onset, and total reproductive output against a background of competing individuals, and as background plants, they more effectively suppressed the growth and fitness of target plants.
Such among-individual variation in competitive ability is generally assumed to result from genetic differences, and indeed many studies have confirmed that genotypes may differ in one or both aspects of competitive ability (e.g. [104,106–113]), including in a closely related Polygonum species [114]. By contrast, the possible influence of parental environment on competitive interactions has seldom been rigorously tested (i.e. by holding genotype constant; [49]). Here, we present the first evidence for a substantial and specifically adaptive effect of parental environment on competitive ability in a similar (shade) environment. Notably, although the growth and fitness of target plants in competitive arrays differed on average among Polygonum genotypes, more of the variation in height, biomass and lifetime reproductive output was explained by the parental treatments of the target and background plants than by their genotype, and the parental environment of just the target plant had as great an impact on its reproductive output as did its genotype. This finding raises the possibility that competitive outcomes in plant populations may be strongly shaped by environmentally induced transgenerational effects as well as by genotype.
Earlier studies have shown ‘silver-spoon’ environmental effects, in which progeny of resource-poor or environmentally stressed maternal individuals have lower growth and reproduction in competition than progeny of resource-rich mothers (e.g. [41,43]; reviewed in [40,115]). In other cases, resource-deprived maternal animals and plants (such as those grown at higher density) may express adaptive offspring size plasticity [116] by producing larger or higher-quality eggs or seeds [49,56,117] that are able to grow successfully under competitive conditions. Whether negative or positive, such overall provisioning effects are likely to influence growth and hence competitive performance in any offspring environment.
By contrast, the superior competitive effect and response of shade-produced Polygonum progeny likely reflect the specific developmental effects of parental shade on progeny height and shading power, two traits that allow plants to overtop and thereby suppress competitors while maximizing their own access to available photons ([77]; e.g. [118–122]). Along with the greater rate of height extension documented here—an effect that increased over time in contrast to expectations (see below, §4b last paragraph)—a previous study with the same P. persicaria genotypes and glasshouse treatments showed that seedling progeny of shaded parents produced more vegetative biomass, increased allocation to leaf tissue and produced larger, thinner leaves, resulting in greater whole-plant leaf area [82].
Unlike the ‘silver-spoon’ effects on competition discussed above, shaded P. persicaria parents altered these specific developmental traits of offspring without increasing overall provisioning [82]. Moreover, expression of these inherited environmental effects was context-dependent: trait changes due to parental shade were more pronounced when progeny were grown in glasshouse shade that mimicked the spectral signal of neighbour or canopy vegetation than in full sun [82]. Such specific changes to phenotypic expression of offspring may result from environmentally induced parental adjustments to cytoplasmic signalling constituents of egg or seed tissues, such as hormones, small or noncoding RNAs, and proteins, or to environment-specific epigenetic modifications of DNA [34,123–125]. Previous work with P. persicaria has confirmed that DNA methylation changes substantially mediate the transgenerational developmental effects of both shade and drought stress in this system [82,83]. Note that here we present data documenting the effects of parental shade on progeny competitive ability only in a shaded progeny treatment. Because the expression of specific transgenerational modifications (as well as possible fitness costs of those trait states) may vary depending on offspring conditions, the competitive consequences of parental shade effects could well differ in direction and/ or magnitude in alternative abiotic progeny conditions such as dry soil or intense insolation.
(b). Parental shade increased progeny growth and fitness in both severe and neighbour shade, but reduced growth and fitness in sunny, dry conditions
Contrasting parental light environments caused surprisingly large (and highly significant) fitness differences over the full life cycle of P. persicaria progeny. Offspring of shaded parents had faster reproductive onset and considerably higher lifetime reproductive output when grown in both severe simulated understorey shade and neighbour shade. These data provide one of very few documented examples of specifically adaptive transgenerational effects of parental conditions on the lifetime reproductive fitness of progeny in similar environments. To our knowledge, such fitness effects have previously been shown only in food-limited mosquitoes [53] and in planktonic marine crustaceans exposed to pathogens [52] or heavy metals [54]. Our data also revealed a substantial negative fitness effect of parental shade on progeny grown in dissimilar conditions: in a sunny, dry environment, the offspring of shaded parents had delayed reproductive onset and dramatically decreased lifetime reproductive output relative to progeny of parents that had grown in full sun. These findings indicate that, at least in certain taxa, environmental conditions experienced by parent individuals may lead to strongly adaptive or maladaptive effects on fitness, depending on progeny conditions. Note that the pronounced fitness effects of parental environment were not driven solely by changes in phenology, as these effects were highly significant even after accounting for flowering time as a covariate.
Most of the (relatively few) cases in which parental conditions have been shown to influence lifetime fitness of progeny reflect direct provisioning changes that consistently either reduce or enhance progeny growth (e.g. [45]; discussed in §4a above). By contrast, P. persicaria progeny showed context-dependent fitness effects that likely reflect specific transgenerational adjustments: as noted above, in a previous study with these same genotypes, progeny of shaded parents produced shade-appropriate phenotypes with greater leaf allocation and larger, thinner leaves [82]. Functionally, the resulting increase in photosynthetic surface area per unit plant mass would maximize growth in either canopy or neighbour shade [68,69,126–129]—as indicated by the higher total biomass of shade progeny in these conditions—but could also account for the maladaptive growth and fitness effects of parental shade on offspring in sunny, dry conditions, where larger, structurally thinner leaves would lose more water to transpiration [69]. In a different set of P. persicaria genotypes, offspring of low-light parents had equal biomass but significantly shorter roots by day 3 of development than offspring of isogenic full-sun parents [81], a developmental adjustment that would likewise be maladaptive in dry soil, where seedlings must quickly extend roots to gain access to available moisture [130–132].
The significantly greater lifetime fitness of shade-produced P. persicaria offspring that were themselves grown in shade treatments exemplifies adaptive transgenerational plasticity, in which parent individuals respond to environmental conditions by altering their progeny in ways that are specifically adaptive to those conditions (see [17–33]). Clearly, the fitness impact of these plastic adjustments will depend on whether progeny encounter similar or contrasting environmental challenges; the transgenerational effect of parental shade on fitness of progeny in sunny, dry conditions was even more strongly negative. When parent and offspring environments match, such specific transgenerational effects may help populations to persist in altered or stressful conditions, by allowing many individuals in the progeny generation to maintain fitness without the lag time (and serendipity) required for favourable allelic variants to selectively increase [34,37,47,133]. Yet when progeny encounter a different environmental state than that of the parent—for example, in the case of passive dispersal across a patchy landscape, or a temporal change in situ from one generation to the next—transgenerational developmental modifications can result in reduced fitness that may likewise be expressed in many individuals at once [50,60,134–136].
Although parental light environment clearly has a pronounced impact in P. persicaria, the extent to which such inherited effects may be important for realized fitness outcomes more generally, and in natural populations, is not yet known. Evidence for parental effects on lifetime competitive success and reproductive fitness may be lacking because studies have seldom tested for them: because any effects of parental environments on offspring phenotypes are generally expected to diminish during ontogeny ([50,137]; e.g. [29,33,138,139]), many studies that have identified putatively adaptive transgenerational effects have measured only developmental traits expressed early in the life cycle ([21], but see [23,26] for data on juvenile mortality). Similarly, studies of epigenetically mediated inherited effects (e.g. methylation changes in plants) have rarely examined fitness consequences directly [25,140,141], but have focused instead on differences in developmental and reproductive timing, allocation, and herbivore damage [140,142,143], or on gene expression changes [144]. In a careful meta-analysis of 58 transgenerational studies, Uller et al. [50] found that effects of parental environment on putatively fitness-related functional and developmental traits were generally ‘subtle’ compared with direct effects of the offspring's immediate environment. However, their analysis showed that the impact of parental environment on offspring traits varied enormously among studies, as well as among traits within studies (see also [38,137]). Like other aspects of plasticity, transgenerational effects will no doubt vary for different taxa, environmental states and progeny traits. A broader understanding of the possible impact of such effects in natural populations will require lifetime fitness data from appropriately designed experiments with diverse biological systems, in naturalistic alternative environments [50].
(c). Transgenerational effects of parental shade versus sun on competitive performance and fitness varied among genotypes
In addition to generally small but significant (or marginally non-significant) average differences, the five P. persicaria genotypes varied significantly in the effects of parental light environment on competitive and fitness traits of their progeny. Just as genotypes vary in their plastic responses to the immediate environment (references in [133,145,146]), genotypic variation for transgenerational plasticity is a common if not ubiquitous feature of these systems [61] that has been documented previously in other genotypes of P. persicaria [81,83] as well as many other plant and animal taxa (e.g. [49,147–151]). Such statistical genotype by parental environment effects reflect the influence of inherited, environmentally induced modulations of cytoplasmic and epigenetic signalling factors on the progeny individual's gene expression pathways (references in [133]). Hence, although heritable parent environment effects are often considered to be ‘decoupled’ from genetic variation [37], the two modes of inheritance interact, resulting in genotype-specific patterns of transgenerational plasticity ([83,151–154]). When such variation occurs within populations, it may provide a substrate for further adaptive evolution of parental effects [20,147,148,155]. Although our multi-population sample of genotypes was not designed to address this issue, the pronounced differences between the two pairs of genotypes drawn from the same populations (MHF 1 and 2, and NAT 1 and 2; figure 4) suggest that this type of variation is likely present in this system, but there is no indication in this limited sample of consistent population differences.
Because our design allowed us to test the effects of both parent and offspring treatment on individual genotypes, the results revealed an even more complex aspect of biological interaction. As discussed (see §4b), the fitness impact of parental shade versus sun was very different in alternative progeny environments, demonstrating how inherited and immediate environmental factors jointly shape individual phenotypic outcomes [24,34,82,137]. Genotypes also differed in their responses to both parental and immediate conditions, leading to genotype by environment by parent environment interactions that were statistically significant for reproductive onset (and nearly so for plant height, a key competitive trait). Plasticity studies use the term norm of reaction to describe an individual's pattern of phenotypic response to a given set of environments, such as the contrasting offspring treatments we studied ([145,156]; reviewed in [133]). This characteristic response pattern is usually considered to be genetically determined [35,157,158]. These results suggest that, instead, the norm of reaction entails response to a particular combination of parental and immediate environments [38,152]. For example, the effect of parental shade versus sun on reproductive onset in the P. persicaria genotypes was not to move their response norms similarly up or down, as would be predicted by a ‘silver spoon’ parental effect on overall offspring size or quality. Instead, the impact of parental environment on norms of reaction varied, depending on the particular genotype in question (cf. figure 4).
These data thus illustrate at the genotype level a view of transgenerational plasticity as ‘differences in offspring phenotype that occur due to the interaction between the current generation and the previous generation's environmental conditions' ([21], cited in [38]). Such highly complex effects on fitness-related traits can be expected to render natural selection based on genetic variants per se less efficient, altering selective trajectories on those variants, and potentially maintaining allelic variation in environmentally heterogeneous populations ([34,159–162]; further references in [133]). Conversely, if patterns of environmental variation are predictable within or across generations, and complex genotypic fitness differences are therefore consistently expressed, selection may shape the particular way a population integrates parental with immediate environmental factors to most effectively generate adaptive phenotypes [38,50,163–165]. This would lead to population-specific patterns of genotype by environment by parent environment interactions, rather than to simpler among-population differences in transgenerational effects per se. Testing for such potentially complex aspects of local adaptation poses a fascinating question but is beyond the scope of the present study: this requires comparing populations from sites that differ in quantified patterns of both environmental variation and temporal autocorrelation.
5. Conclusion
Both empiricists and theoreticians have emphasized the importance of a better understanding of plasticity—including transgenerational plasticity—to assess the prospects for adaptation to rapidly changing environments [35,38,50,166]. This consensus reflects the realization that it is not DNA sequence variation alone that will determine the potential for future adaptation, but rather the phenotypes that are actually expressed in future environments and their fitness consequences [2,38,167,168]. We identified strong adaptive and maladaptive effects of parental shade on both the competitive performance and the lifetime reproductive output of progeny, depending on whether the progeny were themselves growing in shaded or sunny, dry conditions. These data make clear that parental environment may substantially influence not only the early development but also the fitness of offspring, in ways that depend in turn on offspring environment. When adaptive transgenerational effects are context-dependent, as in this case, their potential contribution to adaptive rescue will depend on the precise distribution of environmental states, both spatially (with respect to dispersal) and temporally. Furthermore, when genotypes vary in these context-dependent effects, further adaptive evolution of transgenerational effects may be subject to complex selective dynamics, especially if environmental conditions become more variable in the future. Further studies testing genotypic responses to realistic combinations of parental and progeny environments may provide critical insights to the potential for future adaptation in diverse natural systems.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Tim Early and Ashley Morse for their capable assistance harvesting and processing experimental plants. We also thank Drs Frederick Cohan and Joseph Coolon for valuable discussion, and Dr Robert Kabacoff of the Wesleyan University Quantitative Analysis Center for statistical consultation.
Data accessibility
The datasets generated and analysed for this study are available as part of the electronic supplementary material.
Authors' contributions
B.H.B. and S.E.S. designed the experiments. B.H.B., M.L.-I. and R.W. conducted the experiments, B.H.B. carried out the statistical analyses, B.H.B. and S.E.S. interpreted results and co-wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Research funds were provided by a project grant from Wesleyan University to S.E.S. Support for B.H.B. was provided by a partial stipend from the Biology Department. M.L.-I. and R.W. were supported by summer research fellowships from Wesleyan's College of the Environment and College of Integrative Sciences, respectively, with additional support from the Biology Department.
References
- 1.Hendry AP, Gotanda KM, Svensson EI. 2017. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B372, 20160028. ( 10.1098/rstb.2016.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chevin LM, Collins S, Lefèvre F. 2013. Phenotypic plasticity and evolutionary demographic responses to climate change: taking theory out to the field. Funct. Ecol. 27, 967–979. ( 10.1111/j.1365-2435.2012.02043.x) [DOI] [Google Scholar]
- 3.Botero CA, Weissing FJ, Wright J, Rubenstein DR. 2015. Evolutionary tipping points in the capacity to adapt to environmental change. Proc. Natl Acad. Sci. USA 112, 184–189. ( 10.1073/pnas.1408589111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson SM, Cunningham CJ, Westley PA. 2014. Evolutionary rescue in a changing world. Trends Ecol. Evol. 29, 521–530. ( 10.1016/j.tree.2014.06.005) [DOI] [PubMed] [Google Scholar]
- 5.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. ( 10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479 ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 7.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LE, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 8.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, 482–485. ( 10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendry AP, Farrugia TJ, Kinnison MT. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29. ( 10.1111/j.1365-294X.2007.03428.x) [DOI] [PubMed] [Google Scholar]
- 10.Merilä J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matesanz S, Gianoli E, Valladares F. 2010. Global change and the evolution of phenotypic plasticity in plants. Ann. NY Acad. Sci. 1206, 35–55. ( 10.1111/j.1749-6632.2010.05704.x) [DOI] [PubMed] [Google Scholar]
- 12.Charmantier A, Gienapp P. 2014. Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evol. Appl. 7, 15–28. ( 10.1111/eva.12126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilla DK, Adolph SC. 1996. Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol. Ecol. 10, 105–117. ( 10.1007/BF01239351) [DOI] [Google Scholar]
- 14.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 15.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 16.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press. [Google Scholar]
- 18.Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 5, 537–542. ( 10.1016/S1360-1385(00)01797-0) [DOI] [PubMed] [Google Scholar]
- 19.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 20.Herman JJ, Sultan SE. 2011. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Front. Plant Sci. 2, 102 ( 10.3389/fpls.2011.00102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salinas S, Brown SC, Mangel M, Munch SB. 2013. Non-genetic inheritance and changing environments. Non-Genetic Inheritance 1, 38–50. ( 10.2478/ngi-2013-0005) [DOI] [Google Scholar]
- 22.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 23.Galloway LF, Etterson JR. 2007. Transgenerational plasticity is adaptive in the wild. Science 318, 1134–1136. ( 10.1126/science.1148766) [DOI] [PubMed] [Google Scholar]
- 24.Sultan SE, Barton K, Wilczek AM. 2009. Contrasting patterns of transgenerational plasticity in ecologically distinct congeners. Ecology 90, 1831–1839. ( 10.1890/08-1064.1) [DOI] [PubMed] [Google Scholar]
- 25.Scoville AG, Barnett LL, Bodbyl-Roels S, Kelly JK, Hileman LC. 2011. Differential regulation of a MYB transcription factor is correlated with transgenerational epigenetic inheritance of trichome density in Mimulus guttatus. New Phytol. 191, 251–263. ( 10.1111/j.1469-8137.2011.03656.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JJ, Sultan SE, Horgan-Kobelski T, Riggs C. 2012. Adaptive transgenerational plasticity in an annual plant: grandparental and parental drought stress enhance performance of seedlings in dry soil. Integr. Comp. Biol. 52, 77–88. ( 10.1093/icb/ics041) [DOI] [PubMed] [Google Scholar]
- 27.Latzel V, Janeček Š, Doležal J, Klimešová J, Bossdorf O. 2014. Adaptive transgenerational plasticity in the perennial Plantago lanceolata. Oikos 123, 41–46. ( 10.1111/j.1600-0706.2013.00537.x) [DOI] [Google Scholar]
- 28.Moriuchi KS, et al. 2016. Salinity adaptation and the contribution of parental environmental effects in Medicago truncatula. PLoS ONE 11, e0150350 ( 10.1371/journal.pone.0150350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401, 60–63. ( 10.1038/43425) [DOI] [Google Scholar]
- 30.Donelson J, Munday P, McCormick M, Pitcher C. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32. ( 10.1038/nclimate1323) [DOI] [Google Scholar]
- 31.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. 2012. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861. ( 10.1038/nclimate1599) [DOI] [Google Scholar]
- 32.Salinas S, Munch SB. 2012. Thermal legacies: transgenerational effects of temperature on growth in a vertebrate. Ecol. Lett. 15, 159–163. ( 10.1111/j.1461-0248.2011.01721.x) [DOI] [PubMed] [Google Scholar]
- 33.Shama LN, Strobel A, Mark FC, Wegner KM. 2014. Transgenerational plasticity in marine sticklebacks: maternal effects mediate impacts of a warming ocean. Funct. Ecol. 28, 1482–1493. ( 10.1111/1365-2435.12280) [DOI] [Google Scholar]
- 34.Bonduriansky R, Day T. 2009. Nongenetic inheritance and its evolutionary implications. Ann. Rev. Ecol. Evol. Syst. 40, 103–125. ( 10.1146/annurev.ecolsys.39.110707.173441) [DOI] [Google Scholar]
- 35.Chevin L-M, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357 ( 10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoyle RB, Ezard TH. 2012. The benefits of maternal effects in novel and in stable environments. J. R. Soc. Interface 9, 2403–2413. ( 10.1098/rsif.2012.0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klironomos FD, Berg J, Collins S. 2013. How epigenetic mutations can affect genetic evolution: model and mechanism. Bioessays 35, 571–578. ( 10.1002/bies.201200169) [DOI] [PubMed] [Google Scholar]
- 38.Donelson JM, Salinas S, Munday PL, Shama LN. 2018. Transgenerational plasticity and climate change experiments: where do we go from here? Glob. Change Biol. 24, 13–34. ( 10.1111/gcb.13903) [DOI] [PubMed] [Google Scholar]
- 39.Grafen A. 1988. On the uses of data on lifetime reproductive success. In Reproductive success: studies of individual variation in contrasting breeding systems (ed. T Clutton-Brock), ch. 28, pp. 454–471. Chicago, IL: University of Chicago Press.
- 40.Roach DA, Wulff RD. 1987. Maternal effects in plants. Annu. Rev. Ecol. Syst. 18, 209–235. ( 10.1146/annurev.es.18.110187.001233) [DOI] [Google Scholar]
- 41.Stratton D. 1989. Competition prolongs expression of maternal effects in seedlings of Erigeron annuus (Asteraceae). Am. J. Bot. 76, 1646–1653. ( 10.1002/j.1537-2197.1989.tb15149.x) [DOI] [Google Scholar]
- 42.Miao S, Bazzaz F, Primack R. 1991. Effects of maternal nutrient pulse on reproduction of two colonizing Plantago species. Ecology 72, 586–596. ( 10.2307/2937198) [DOI] [Google Scholar]
- 43.Miao SL, Bazzaz FA, Primack RB. 1991. Persistence of maternal nutrient effects in Plantago major: the third generation. Ecology 72, 1634–1642. ( 10.2307/1940963) [DOI] [Google Scholar]
- 44.Hafer N, Ebil S, Uller T, Pike N. 2011. Transgenerational effects of food availability on age at maturity and reproductive output in an asexual collembolan species. Biol. Lett. 7, 755–758. ( 10.1098/rsbl.2011.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blödner C, Goebel C, Feussner I, Gatz C, Polle A. 2007. Warm and cold parental reproductive environments affect seed properties, fitness, and cold responsiveness in Arabidopsis thaliana progenies. Plant Cell Environ. 30, 165–175. ( 10.1111/j.1365-3040.2006.01615.x) [DOI] [PubMed] [Google Scholar]
- 46.Whittle C, Otto S, Johnston MO, Krochko J. 2009. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 87, 650–657. ( 10.1139/B09-030) [DOI] [Google Scholar]
- 47.Uller T. 2012. The evolution of parental care. In Parental effects in development and evolution (eds NJ Royle, PT Smiseth, M Kölliker), ch.14, pp. 247–266. Oxford, UK: Oxford University Press. ( 10.1093/acprof:oso/9780199692576.003.0014) [DOI] [Google Scholar]
- 48.Grossniklaus U, Kelly WG, Ferguson-Smith AC, Pembrey M, Lindquist S. 2013. Transgenerational epigenetic inheritance: how important is it? Nat. Rev. Genet. 14, 228 ( 10.1038/nrg3435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bossdorf O, Shuja Z, Banta JA. 2009. Genotype and maternal environment affect belowground interactions between Arabidopsis thaliana and its competitors. Oikos 118, 1541–1551. ( 10.1111/j.1600-0706.2009.17559.x) [DOI] [Google Scholar]
- 50.Uller T, Nakagawa S, English S. 2013. Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170. ( 10.1111/jeb.12212) [DOI] [PubMed] [Google Scholar]
- 51.Storm JJ, Lima SL. 2010. Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am. Nat. 175, 382–390. ( 10.1086/650443) [DOI] [PubMed] [Google Scholar]
- 52.Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489–492. ( 10.1016/S0960-9822(03)00163-5) [DOI] [PubMed] [Google Scholar]
- 53.Grech K, Maung LA, Read AF. 2007. The effect of parental rearing conditions on offspring life history in Anopheles stephensi. Malar. J. 6, 130 ( 10.1186/1475-2875-6-130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwok KW, Grist EP, Leung KM. 2009. Acclimation effect and fitness cost of copper resistance in the marine copepod Tigriopus japonicus. Ecotoxicol. Environ. Saf. 72, 358–364. ( 10.1016/j.ecoenv.2008.03.014) [DOI] [PubMed] [Google Scholar]
- 55.Suter L, Widmer A. 2013. Environmental heat and salt stress induce transgenerational phenotypic changes in Arabidopsis thaliana. PLoS ONE 8, e60364 ( 10.1371/journal.pone.0060364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen RM, Buckley YM, Marshall DJ. 2007. Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am. Nat. 171, 225–237. ( 10.1086/524952) [DOI] [PubMed] [Google Scholar]
- 57.Lau JA, Peiffer J, Reich PB, Tiffin P. 2008. Transgenerational effects of global environmental change: long-term CO2 and nitrogen treatments influence offspring growth response to elevated CO2. Oecologia 158, 141 ( 10.1007/s00442-008-1127-6) [DOI] [PubMed] [Google Scholar]
- 58.Holeski L. 2007. Within and between generation phenotypic plasticity in trichome density of Mimulus guttatus. J. Evol. Biol. 20, 2092–2100. ( 10.1111/j.1420-9101.2007.01434.x) [DOI] [PubMed] [Google Scholar]
- 59.Bateson P, et al. 2004. Developmental plasticity and human health. Nature 430, 419–421. ( 10.1038/nature02725) [DOI] [PubMed] [Google Scholar]
- 60.Bateson P, Gluckman P, Hanson M. 2014. The biology of developmental plasticity and the predictive adaptive response hypothesis. J. Physiol. 592, 2357–2368. ( 10.1113/jphysiol.2014.271460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herman JJ, Spencer HG, Donohue K, Sultan SE. 2014. How stable ‘should' epigenetic modifications be? Insights from adaptive plasticity and bet hedging. Evolution 68, 632–643. ( 10.1111/evo.12324) [DOI] [PubMed] [Google Scholar]
- 62.Jensen N, Allen RM, Marshall DJ. 2014. Adaptive maternal and paternal effects: gamete plasticity in response to parental stress. Funct. Ecol. 28, 724–733. ( 10.1111/1365-2435.12195) [DOI] [Google Scholar]
- 63.Guilbaud CS, Dalchau N, Purves DW, Turnbull LA. 2015. Is ‘peak N' key to understanding the timing of flowering in annual plants? New Phytol. 205, 918–927. ( 10.1111/nph.13095) [DOI] [PubMed] [Google Scholar]
- 64.Bazzaz FA. 1996. Plants in changing environments: linking physiological, population, and community ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 65.Sultan S, Wilczek A, Hann S, Brosi B. 1998. Contrasting ecological breadth of co-occurring annual Polygonum species. J. Ecol. 86, 363–383. ( 10.1046/j.1365-2745.1998.00265.x) [DOI] [Google Scholar]
- 66.Valladares F, Niinemets Ü. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Ann. Rev. Ecol. Evol. Syst. 39, 237–257. ( 10.1146/annurev.ecolsys.39.110707.173506) [DOI] [Google Scholar]
- 67.Franklin KA. 2008. Shade avoidance. New Phytol. 179, 930–944. ( 10.1111/j.1469-8137.2008.02507.x) [DOI] [PubMed] [Google Scholar]
- 68.Marin M, Blandino C, Laverack G, Toorop P, Powell A. In press. Responses of Primula vulgaris to light quality in the maternal and germination environments. Plant Biol. ( 10.1111/plb.12849) [DOI] [PubMed] [Google Scholar]
- 69.Fitter AH, Hay RK. 2012. Environmental physiology of plants. Cambridge, MA: Academic Press. [Google Scholar]
- 70.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 71.Reusch TB, Wood TE. 2007. Molecular ecology of global change. Mol. Ecol. 16, 3973–3992. ( 10.1111/j.1365-294X.2007.03454.x) [DOI] [PubMed] [Google Scholar]
- 72.Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997. ( 10.1126/science.1098704) [DOI] [PubMed] [Google Scholar]
- 73.Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds.). 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 74.Rahmstorf S, Coumou D. 2011. Increase of extreme events in a warming world. Proc. Natl Acad. Sci. USA 108, 17 905–17 909. ( 10.1073/pnas.1101766108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katz RW, Brown BG. 1992. Extreme events in a changing climate: variability is more important than averages. Clim. Change 21, 289–302. ( 10.1007/BF00139728) [DOI] [Google Scholar]
- 76.Alexander L, et al. 2006. Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res. Atmos. 111 ( 10.1029/2005JD006290) [DOI] [Google Scholar]
- 77.Craine JM, Dybzinski R. 2013. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 27, 833–840. ( 10.1111/1365-2435.12081) [DOI] [Google Scholar]
- 78.Lockwood JL, Cassey P, Blackburn T. 2005. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 20, 223–228. ( 10.1016/j.tree.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 79.Mitchell RS, Dean JK. 1978. Polygonaceae (buckwheat family) of New York State. Bull. NY Mus. Sci. Serv, no. 431. [Google Scholar]
- 80.Staniforth R, Cavers PB. 1979. Distribution of habitats of four annual smartweeds in Ontario. Canad. Field-Nat. 93, 378–385. [Google Scholar]
- 81.Sultan SE. 1996. Phenotypic plasticity for offspring traits in Polygonum persicaria. Ecology 77, 1791–1807. ( 10.2307/2265784) [DOI] [Google Scholar]
- 82.Baker BH, Berg LJ, Sultan SE. 2018. Context-dependent developmental effects of parental shade versus sun are mediated by DNA methylation. Front. Plant Sci. 9, 1251 ( 10.3389/fpls.2018.01251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herman JJ, Sultan SE. 2016. DNA methylation mediates genetic variation for adaptive transgenerational plasticity. Proc. R. Soc. B 283, 20160988 ( 10.1098/rspb.2016.0988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griffith TM, Sultan SE. 2005. Shade tolerance plasticity in response to neutral vs green shade cues in Polygonum species of contrasting ecological breadth. New Phytol. 166, 141–147. ( 10.1111/j.1469-8137.2004.01277.x) [DOI] [PubMed] [Google Scholar]
- 85.Matesanz S, Horgan-Kobelski T, Sultan SE. 2014. Contrasting levels of evolutionary potential in populations of the invasive plant Polygonum cespitosum. Biol. Invasions 16, 455–468. ( 10.1007/s10530-013-0533-9) [DOI] [Google Scholar]
- 86.Horgan-Kobelski TP. 2010. Contemporary evolution, response to novel environments, and ecological breadth in the invasive annual Polygonum cespitosum. MSc thesis, Wesleyan University, Middletown, CT, USA. [Google Scholar]
- 87.Matesanz S, Horgan-Kobelski T, Sultan SE. 2015. Evidence for rapid ecological range expansion in a newly invasive plant. AoB Plants 7, plv038. ( 10.1093/aobpla/plv038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sultan SE, Horgan-Kobelski T, Nichols LM, Riggs CE, Waples RK. 2013. A resurrection study reveals rapid adaptive evolution within populations of an invasive plant. Evol. Appl. 6, 266–278. ( 10.1111/j.1752-4571.2012.00287.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 90.Scheiner SM, Gurevitch J. 2001. Design and analysis of ecological experiments, 2nd edn Oxford, NY: Oxford University Press. [Google Scholar]
- 91.Cole J, Grizzle JE. 1966. Applications of multivariate analysis of variance to repeated measurements experiments. Biometrics 22, 810–828. ( 10.2307/2528076) [DOI] [Google Scholar]
- 92.Olejnik S, Algina J. 2003. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol. Methods 8, 434 ( 10.1037/1082-989X.8.4.434) [DOI] [PubMed] [Google Scholar]
- 93.Lakens D. 2013. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 ( 10.3389/fpsyg.2013.00863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Winer BJ, Brown DR, Michels KM. 1991. Statistical principles in experimental design, 3rd edn New York: NY: McGraw-Hill. [Google Scholar]
- 95.Keddy PA. 2001. Competition, 2nd edn Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 96.Kunstler G, et al. 2016. Plant functional traits have globally consistent effects on competition. Nature 529, 204 ( 10.1038/nature16476) [DOI] [PubMed] [Google Scholar]
- 97.Goldberg DE, Landa K. 1991. Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J. Ecol. 79, 1013–1030. ( 10.2307/2261095) [DOI] [Google Scholar]
- 98.Wang P, Stieglitz T, Zhou DW, Cahill JF Jr. 2010. Are competitive effect and response two sides of the same coin, or fundamentally different? Funct. Ecol. 24, 196–207. ( 10.1111/j.1365-2435.2009.01612.x) [DOI] [Google Scholar]
- 99.Wilson SD, Keddy PA. 1986. Species competitive ability and position along a natural stress/disturbance gradient. Ecology 67, 1236–1242. ( 10.2307/1938679) [DOI] [Google Scholar]
- 100.Goldberg DE, Fleetwood L. 1987. Competitive effect and response in four annual plants. J. Ecol. 75, 1131–1143. ( 10.2307/2260318) [DOI] [Google Scholar]
- 101.Miller T, Werner P. 1987. Competitive effects and responses between plant species in a first-year old-field community. Ecology 68, 1201–1210. ( 10.2307/1939204) [DOI] [Google Scholar]
- 102.Gurevitch J, Wilson P, Stone JL, Teese P, Stoutenburgh RJ. 1990. Competition among old-field perennials at different levels of soil fertility and available space. J. Ecol. 78, 727–744. ( 10.2307/2260895) [DOI] [Google Scholar]
- 103.Thomsen MA, Corbin JD, D'Antonio CM. 2006. The effect of soil nitrogen on competition between native and exotic perennial grasses from northern coastal California. Plant Ecol. 186, 23–35. ( 10.1007/s11258-006-9109-4) [DOI] [Google Scholar]
- 104.Cahill JF, Kembel SW, Gustafson DJ. 2005. Differential genetic influences on competitive effect and response in Arabidopsis thaliana. J. Ecol. 93, 958–967. ( 10.1111/j.1365-2745.2005.01013.x) [DOI] [Google Scholar]
- 105.Fraser LH, Miletti TE. 2008. Effect of minor water depth treatments on competitive effect and response of eight wetland plants. Plant Ecol. 195, 33–43. ( 10.1007/s11258-007-9296-7) [DOI] [Google Scholar]
- 106.Turkington R, Harper JL. 1979. The growth, distribution and neighbour relationships of Trifolium repens in a permanent pasture: IV. Fine-scale biotic differentiation. J. Ecol. 67, 245–254. ( 10.2307/2259348) [DOI] [Google Scholar]
- 107.Kelley SE, Clay K. 1987. Interspecific competitive interactions and the maintenance of genotypic variation within two perennial grasses. Evol. 41, 92–103. ( 10.1111/j.1558-5646.1987.tb05773.x) [DOI] [PubMed] [Google Scholar]
- 108.Taylor DR, Aarssen LW. 1990. Complex competitive relationships among genotypes of three perennial grasses: implications for species coexistence. Am. Nat. 136, 305–327. ( 10.1086/285100) [DOI] [Google Scholar]
- 109.Bossdorf O, Prati D, Auge H, Schmid B. 2004. Reduced competitive ability in an invasive plant. Ecol. Lett. 7, 346–353. ( 10.1111/j.1461-0248.2004.00583.x) [DOI] [Google Scholar]
- 110.Fridley JD, Grime JP, Bilton M. 2007. Genetic identity of interspecific neighbours mediates plant responses to competition and environmental variation in a species-rich grassland. J. Ecol. 95, 908–915. ( 10.1111/j.1365-2745.2007.01256.x) [DOI] [Google Scholar]
- 111.Willis C, Brock M, Weinig C. 2010. Genetic variation in tolerance of competition and neighbour suppression in Arabidopsis thaliana. J. Evol. Biol. 23, 1412–1424. ( 10.1111/j.1420-9101.2010.02003.x) [DOI] [PubMed] [Google Scholar]
- 112.Wilson AJ, Gelin U, Perron M-C, Réale D. 2009. Indirect genetic effects and the evolution of aggression in a vertebrate system. Proc. R. Soc. B 276, 533–541. ( 10.1098/rspb.2008.1193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boulton K, Walling CA, Grimmer AJ, Rosenthal GG, Wilson AJ. 2018. Phenotypic and genetic integration of personality and growth under competition in the sheepshead swordtail, Xiphophorus birchmanni. Evolution 72, 187–201. ( 10.1111/evo.13398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Corliss CT, Sultan SE. 2016. Evolutionary potential for increased invasiveness: high-performance Polygonum cespitosum genotypes are competitively superior in full sun. Am. J. Bot. 103, 348–354. ( 10.3732/ajb.1500306) [DOI] [PubMed] [Google Scholar]
- 115.Fenner M, Thompson K. 2005. The ecology of seeds, pp. 1–31. Cambridge, UK; New York, NY, USA: Cambridge University Press. [Google Scholar]
- 116.Mousseau TA, Dingle H. 1991. Maternal effects in insect life histories. Annu. Rev. Entomol. 36, 511–534. ( 10.1146/annurev.en.36.010191.002455) [DOI] [Google Scholar]
- 117.Einum S, Fleming IA. 1999. Maternal effects of egg size in brown trout (Salmo trutta): norms of reaction to environmental quality. Proc. R. Soc. B 266, 2095–2100. ( 10.1098/rspb.1999.0893) [DOI] [Google Scholar]
- 118.Gaudet CL, Keddy PA. 1988. A comparative approach to predicting competitive ability from plant traits. Nature 334, 242 ( 10.1038/334242a0) [DOI] [Google Scholar]
- 119.Hikosaka K, Hirose T. 1997. Leaf angle as a strategy for light competition: optimal and evolutionarily stable light-extinction coefficient within a leaf canopy. Ecoscience 4, 501–507. ( 10.1080/11956860.1997.11682429) [DOI] [Google Scholar]
- 120.Schieving F, Poorter H. 1999. Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytol. 143, 201–211. ( 10.1046/j.1469-8137.1999.00431.x) [DOI] [Google Scholar]
- 121.Falster DS, Westoby M. 2003. Plant height and evolutionary games. Trends Ecol. Evol. 18, 337–343. ( 10.1016/S0169-5347(03)00061-2) [DOI] [Google Scholar]
- 122.Violle C, Garnier E, Lecoeur J, Roumet C, Podeur C, Blanchard A, Navas M-L. 2009. Competition, traits and resource depletion in plant communities. Oecologia 160, 747–755. ( 10.1007/s00442-009-1333-x) [DOI] [PubMed] [Google Scholar]
- 123.Jablonka E, Raz G. 2009. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176. ( 10.1086/598822) [DOI] [PubMed] [Google Scholar]
- 124.Feil R, Fraga MF. 2012. Epigenetics and the environment: emerging patterns and implications. Nat. Rev. Genet. 13, 97–109. ( 10.1038/nrg3142) [DOI] [PubMed] [Google Scholar]
- 125.Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475 ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 126.Evans J, Poorter H. 2001. Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 24, 755–767. ( 10.1046/j.1365-3040.2001.00724.x) [DOI] [Google Scholar]
- 127.Navas M-L, Garnier E. 2002. Plasticity of whole plant and leaf traits in Rubia peregrina in response to light, nutrient and water availability. Acta Oecologica 23, 375–383. ( 10.1016/S1146-609X(02)01168-2) [DOI] [Google Scholar]
- 128.Niinemets Ü, Valladares F, Ceulemans R. 2003. Leaf-level phenotypic variability and plasticity of invasive Rhododendron ponticum and non-invasive Ilex aquifolium co-occurring at two contrasting European sites. Plant Cell Environ. 26, 941–956. ( 10.1046/j.1365-3040.2003.01027.x) [DOI] [PubMed] [Google Scholar]
- 129.Herr-Turoff A, Zedler JB. 2007. Does morphological plasticity of the Phalaris arundinacea canopy increase invasiveness? Plant Ecol. 193, 265–277. ( 10.1007/s11258-007-9264-2) [DOI] [Google Scholar]
- 130.Mazer SJ. 1989. Ecological, taxonomic, and life history correlates of seed mass among Indiana dune angiosperms. Ecol. Monogr. 59, 153–175. ( 10.2307/2937284) [DOI] [Google Scholar]
- 131.Hofmann M, Isselstein J. 2004. Effects of drought and competition by a ryegrass sward on the seedling growth of a range of grassland species. J. Agron. Crop Sci. 190, 277–286. ( 10.1111/j.1439-037X.2004.00117.x) [DOI] [Google Scholar]
- 132.Moles AT, Westoby M. 2006. Seed size and plant strategy across the whole life cycle. Oikos 113, 91–105. ( 10.1111/j.0030-1299.2006.14194.x) [DOI] [Google Scholar]
- 133.Sultan SE. 2015. Organism and environment: ecological development, niche construction, and adaptation, New York: NY: Oxford University Press. [Google Scholar]
- 134.Beaman JE, White CR, Seebacher F. 2016. Evolution of plasticity: mechanistic link between development and reversible acclimation. Trends Ecol. Evol. 31, 237–249. ( 10.1016/j.tree.2016.01.004) [DOI] [PubMed] [Google Scholar]
- 135.Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. 2007. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr. Res. 61, 5R ( 10.1203/pdr.0b013e318045bedb) [DOI] [PubMed] [Google Scholar]
- 136.Gluckman PD, Hanson MA, Spencer HG. 2005. Predictive adaptive responses and human evolution. Trends Ecol. Evol. 20, 527–533. ( 10.1016/j.tree.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 137.Auge GA, Leverett LD, Edwards BR, Donohue K. 2017. Adjusting phenotypes via within- and across-generational plasticity. New Phytol. 216, 343–349. ( 10.1111/nph.14495) [DOI] [PubMed] [Google Scholar]
- 138.Rotem K, Agrawal AA, Kott L. 2003. Parental effects in Pieris rapae in response to variation in food quality: adaptive plasticity across generations? Ecol. Entomol. 28, 211–218. ( 10.1046/j.1365-2311.2003.00507.x) [DOI] [Google Scholar]
- 139.Lindholm AK, Hunt J, Brooks R. 2006. Where do all the maternal effects go? Variation in offspring body size through ontogeny in the live-bearing fish Poecilia parae. Biol. Lett. 2, 586–589. ( 10.1098/rsbl.2006.0546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kalisz S, Purugganan MD. 2004. Epialleles via DNA methylation: consequences for plant evolution. Trends Ecol. Evol. 19, 309–314. ( 10.1016/j.tree.2004.03.034) [DOI] [PubMed] [Google Scholar]
- 141.Richards CL, Verhoeven KJ, Bossdorf O. 2012. Evolutionary significance of epigenetic variation. In Plant genome diversity, vol. 1, pp. 257–274. Berlin, Germany: Springer. [Google Scholar]
- 142.Herrera CM, Bazaga P. 2011. Untangling individual variation in natural populations: ecological, genetic and epigenetic correlates of long-term inequality in herbivory. Mol. Ecol. 20, 1675–1688. ( 10.1111/j.1365-294X.2011.05026.x) [DOI] [PubMed] [Google Scholar]
- 143.Zhang YY, Fischer M, Colot V, Bossdorf O. 2013. Epigenetic variation creates potential for evolution of plant phenotypic plasticity. New Phytol. 197, 314–322. ( 10.1111/nph.12010) [DOI] [PubMed] [Google Scholar]
- 144.Colicchio JM, Miura F, Kelly JK, Ito T, Hileman LC. 2015. DNA methylation and gene expression in Mimulus guttatus. BMC Genomics 16, 507 ( 10.1186/s12864-015-1668-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scheiner SM, Gurevitch J. 1993. Design and analysis of ecological experiments. New York: NY: Chapman and Hall. [Google Scholar]
- 146.Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics (4th edn). Trends Genet. 12, 280 ( 10.1016/0168-9525(96)81458-2) [DOI] [Google Scholar]
- 147.Schmitt J, Niles J, Wulff RD. 1992. Norms of reaction of seed traits to maternal environments in Plantago lanceolata. Am. Nat. 139, 451–466. ( 10.1086/285338) [DOI] [Google Scholar]
- 148.Wulff R, Caceres A, Schmitt J. 1994. Seed and seedling responses to maternal and offspring environments in Plantago lanceolata. Funct. Ecol. 8, 763–769. ( 10.2307/2390236) [DOI] [Google Scholar]
- 149.Stjernman M, Little T. 2011. Genetic variation for maternal effects on parasite susceptibility. J. Evol. Biol. 24, 2357–2363. ( 10.1111/j.1420-9101.2011.02363.x) [DOI] [PubMed] [Google Scholar]
- 150.Plaistow SJ, Shirley C, Collin H, Cornell SJ, Harney ED. 2015. Offspring provisioning explains clone-specific maternal age effects on life history and life span in the water flea, Daphnia pulex. Am. Nat. 186, 376–389. ( 10.1086/682277) [DOI] [PubMed] [Google Scholar]
- 151.Vu WT, Chang PL, Moriuchi KS, Friesen ML. 2015. Genetic variation of transgenerational plasticity of offspring germination in response to salinity stress and the seed transcriptome of Medicago truncatula. BMC Evol. Biol. 15, 59 ( 10.1186/s12862-015-0322-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sultan SE. In press. Genotype-environment interaction and the unscripted reaction norm. In Cause and process in evolution (ed. Laland TUAK.). Cambridge, MA: MIT Press. [Google Scholar]
- 153.Bateson P, Gluckman P. 2011. Plasticity, robustness, development and evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 154.Herrera CM, Medrano M, Bazaga P. 2014. Variation in DNA methylation transmissibility, genetic heterogeneity and fecundity-related traits in natural populations of the perennial herb Helleborus foetidus. Mol. Ecol. 23, 1085–1095. ( 10.1111/mec.12679) [DOI] [PubMed] [Google Scholar]
- 155.Case AL, Lacey EP, Hopkins RG. 1996. Parental effects in Plantago lanceolata L. II. Manipulation of grandparental temperature and parental flowering time. Heredity 76, 287 ( 10.1038/hdy.1996.42) [DOI] [Google Scholar]
- 156.Stearns SC. 1989. The evolutionary significance of phenotypic plasticity. Bioscience 39, 436–445. ( 10.2307/1311135) [DOI] [Google Scholar]
- 157.Nager R, Keller L, Van Noordwijk A, Mousseau T, Sinervo B, Endler J. 2000. Understanding natural selection on traits that are influenced by environmental conditions. In Adaptive genetic variation in the wild (eds TA Mousseau, B Sinervo, JA Endler), pp. 95–115. Oxford, UK: Oxford University Press. [Google Scholar]
- 158.DeWitt T, Scheiner S. 2004. Phenotypic variation from single genotypes. In Phenotypic plasticity: functional and conceptual approaches (eds DeWitt TJ, Scheiner SM), pp. 1–9. Oxford, UK: Oxford University Press. [Google Scholar]
- 159.Kirkpatrick M, Lande R. 1989. The evolution of maternal characters. Evolution 43, 485–503. ( 10.1111/j.1558-5646.1989.tb04247.x) [DOI] [PubMed] [Google Scholar]
- 160.Gomulkiewicz R, Kirkpatrick M. 1992. Quantitative genetics and the evolution of reaction norms. Evolution 46, 390–411. ( 10.1111/j.1558-5646.1992.tb02047.x) [DOI] [PubMed] [Google Scholar]
- 161.Geoghegan JL, Spencer HG. 2012. Population-epigenetic models of selection. Theor. Popul. Biol. 81, 232–242. ( 10.1016/j.tpb.2011.08.001) [DOI] [PubMed] [Google Scholar]
- 162.Ledón-Rettig CC, Pfennig DW, Chunco AJ, Dworkin I. 2014. Cryptic genetic variation in natural populations: a predictive framework. Integr. Comp. Biol. 54, 783–793. ( 10.1093/icb/icu077) [DOI] [PubMed] [Google Scholar]
- 163.Ezard TH, Prizak R, Hoyle RB. 2014. The fitness costs of adaptation via phenotypic plasticity and maternal effects. Funct. Ecol. 28, 693–701. ( 10.1111/1365-2435.12207) [DOI] [Google Scholar]
- 164.Leimar O, McNamara JM. 2015. The evolution of transgenerational integration of information in heterogeneous environments. Am. Nat. 185, E55–E69. ( 10.1086/679575) [DOI] [PubMed] [Google Scholar]
- 165.Uller T, Pen I. 2011. A theoretical model of the evolution of maternal effects under parent–offspring conflict. Evolution 65, 2075–2084. ( 10.1111/j.1558-5646.2011.01282.x) [DOI] [PubMed] [Google Scholar]
- 166.Bonduriansky R, Crean AJ, Day T. 2012. The implications of nongenetic inheritance for evolution in changing environments. Evol. Appl. 5, 192–201. ( 10.1111/j.1752-4571.2011.00213.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sultan SE. 2007. Development in context: the timely emergence of eco-devo. Trends Ecol. Evol. 22, 575–582. ( 10.1016/j.tree.2007.06.014) [DOI] [PubMed] [Google Scholar]
- 168.Horgan-Kobelski T, Matesanz S, Sultan SE. 2015. Limits to future adaptation in the invasive plant Polygonum cespitosum: expression of functional and fitness traits at elevated CO2. J. Hered. 107, 42–50. ( 10.1093/jhered/esv070) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed for this study are available as part of the electronic supplementary material.