Abstract
This Feature Article focuses on recent advances in the bioconjugation of surface-bound zwitterionic polymers for biospecific antifouling surfaces. Various approaches for the functionalization of antifouling zwitterionic polymers are systematically investigated, such as chain-end and side-chain functionalization. Side-chain functionalization methods can be further classified as those that are achieved through homopolymerization of custom-synthesized zwitterionic monomers equipped with reactive groups, or those that are achieved via synthesis of random or block copolymers combining different monomers with antifouling functionality and others with reactive groups. Several of the pros and cons of these approaches are outlined and discussed. Finally, some perspective and future directions of research are presented toward long-term stable, generically repelling surfaces that strongly and specifically adhere to a single component in a complex mixture.
Introduction
Antifouling coatings play a crucial role in mitigating the response to artificial materials in biological environments. They reduce the nonspecific adsorption of biomaterials, such as proteins and cells, minimizing unwanted biofilm formation and suppressing foreign body reactions and inflammations. This enables the successful in vitro and in vivo application of, for example, biosensors,1 targeted drug delivery systems,2 and biomedical devices3 such as contact lenses, orthopedics, catheters, vascular stents, and grafts. Biosensors are devices that convert a biomolecular interaction into a measurable signal, ideally in a sensitive, specific, and quantifiable manner.4 The biomolecular interaction is often achieved by immobilizing bioactive molecules on the surface of the sensors. These surfaces should be antifouling to prevent nonspecific interactions from interfering species, requiring functionalized antifouling coatings.5 Achieving highly or even fully antifouling surfaces has gained importance, given the highly increased sensitivities of a wide range of biosensing techniques. Often it is, in principle, possible to measure biomarkers down to concentrations well below their bioavailability or physiologically relevant concentrations: techniques ranging from mass spectrometry6 via fluorescence to surface plasmon resonance (SPR)7 and surface-specific Raman analysis8 are repeatedly reported to achieve this. Yet, in marked contrast, this does not easily translate to a workable outcome in day-to-day biosensing: the issue is increasingly not the signal but the noise. This noise is due to instrument-specific issues and to interfering surface–analyte interactions that are prevalent in complex biological mixtures, ranging from urine and serum to milk and other foods. For example, in SPR without bioactive antifouling layers, the signal from such complex biological matrices would be dominated by nonspecific interactions. This Feature Article focuses on strategies to prevent these interfering surface–analyte interactions and aims to briefly describe the state-of-the-art in surfaces that we have labeled as romantic: long-term stable coatings, not wanting to induce any interactions with any species around apart from the “object of desire”, typically a biomarker present at low concentrations against a sea of other components. Pictorially this concept is indicated in Figure 1.
Figure 1.
Romantic surfaces are stable coatings that generally repel all components in a complex mixture but yet display a strong interaction to a specific compound. [Picture attributions: Top picture: Old couple crossing the street, CC-BY/2.0 - Copyright Ivan Mlinaric, flickr.com/photos/eye1/4517295809; Bottom right picture: Odd one out beside the A30, CC-BY-SA/2.0 - Copyright Maigheach-gheal - geograph.org.uk/p/889321.]
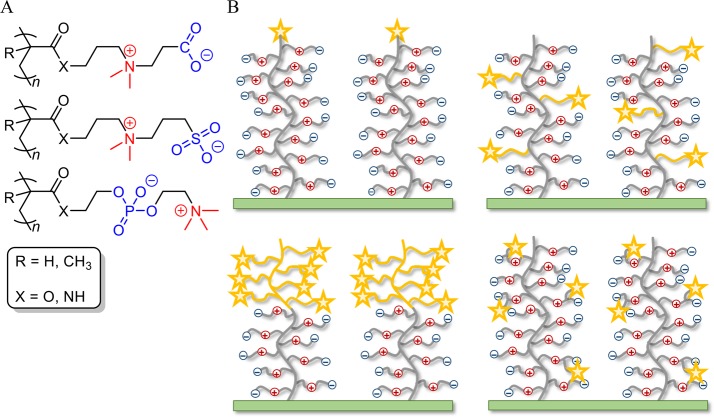
The most common strategy to reduce nonspecific adsorption is to immobilize hydrophilic, antifouling polymers onto the material’s surface. Different types of polymers have been used for this, including polysaccharides (dextran), polyacrylates, polyacrylamides, and poly(ethylene glycol) (PEG).9 PEG has been the most popular choice, and different coating methods have been studied, including self-assembled monolayers10−12 and polymer brushes.13 Concerns about the oxidative stability14,15 and the discovery of anti-PEG antibodies16 invoked more research on alternative materials. Whitesides and co-workers surveyed different classes of materials using self-assembled monolayers and found that a common feature shared by protein-resistant antifouling materials is their capability to strongly bind water.17,18 They postulated that antifouling materials in general should have the following properties: polar, hydrogen-bond acceptors, no hydrogen-bond donors, and electrically neutral. In addition, they observed that most of the known protein-resistant surfaces are based on displays of preferentially hydrated kosmotropes.19 Based on these observations, a new class of overall neutral, antifouling polymers based on (kosmotropic) zwitterionic moieties, containing permanent positive and negative charges, was discovered.20,21 Until then, only zwitterionic phosphoryl choline-containing polymers were known for their antifouling properties,22−24 inspired by their abundant presence in thrombo-resistant cell membranes.25 However, also polymer brushes of acrylates and acrylamides with sulfobetaine and carboxybetaine moieties (Figure 2A) have shown excellent antifouling behavior in complex media, such as undiluted blood and plasma.26−28 In addition, zwitterionic brushes are very stable in aqueous media: for example, sulfobetaine brushes grafted from silicon nitride showed no signs of degradation after continuous exposure in phosphate-buffered saline for 7 days.28 Also no degradation was observed for sulfobetaine methacrylamide polymers in solution, even after 1 year of exposure to acidic (1 M HCl) and basic (1 M NaOH) conditions at ambient temperature.29 The excellent hydrolytic stability can be attributed to the steric shielding of the ester and amide groups by the polymer backbone, since the monomers themselves showed hydrolysis upon prolonged exposure.29 The excellent stability and antifouling properties of zwitterionic polymer brushes make them good candidates for incorporation in bioactive layers of biosensors. The low degree of interaction and biocompatibility/low immunoactivity of such zwitterionic polymer brushes make them increasingly useful as coatings for, e.g., implants.30,31
Figure 2.
(A) Commonly used zwitterionic polymer brushes featuring as zwitterionic moiety a carboxybetaine (top), sulfobetaine (middle), or phosphoryl choline (bottom) group. (B) Schematic depiction of different strategies for introducing reactive sites: chain-end functionalization (top, left), random side-chain modification (top right), hierarchically structured copolymer brushes (bottom, left), and the development of zwitterionic monomers with intrinsic reactivity (bottom, right).
In this Feature Article, an overview is given of various approaches toward the biofunctionalization of zwitterionic brushes. Biofunctionalization requires the integration of reactive sites for the coupling of bioactive elements. We will discuss different strategies for introducing such reactive sites by exploiting specific characteristics of the brushes, such as side-chain or chain-end modification, random or hierarchically structured copolymer brushes, and the development of zwitterionic monomers with intrinsic reactivity (Figure 2B). Last, some perspective and future directions of this research field are presented.
Chain-End Biofunctionalization
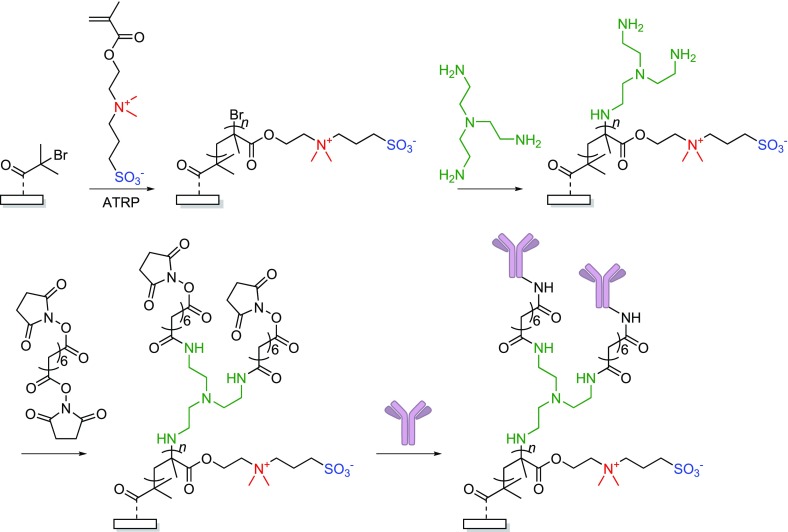
Most antifouling zwitterionic brushes are prepared using controlled polymerization techniques, like atom-transfer radical polymerization (ATRP), on surfaces functionalized with an appropriate polymerization initiator.32 After an ATRP-based surface-initiated (SI) polymerization, the active chain ends (typically a Br atom) are preserved, and this presents an opportunity for further functionalization.33 In the case of polymer brushes prepared with ATRP, the terminal alkyl halides can be post-polymerization functionalized using nucleophilic substitution reactions.34,35 In 2011, our group demonstrated the viability of this method for zwitterionic polymer brushes,36 using sulfobetaine methacrylate (SBMA) brushes grafted by SI-ATRP from α-bromoisobutyryl initiator-functionalized silicon nitride surfaces (Figure 3). The terminal alkyl bromides of the SBMA polymer chains were subsequently reacted with a trifunctional tris(2-aminoethyl)amine linker. This yielded amine-terminated brushes, which were further reacted with a bis-N-hydroxysuccinimide (NHS) linker to provide active ester-functionalized surfaces. To show the effectiveness of this approach, anti-Salmonella antibodies were subsequently immobilized onto the SBMA brushes through reaction of free amine groups present in the antibody with the NHS-activated esters. The resulting surfaces not only showed excellent protein repellence but also were highly efficient in capturing Salmonella bacteria.
Figure 3.
SI-ATRP of sulfobetaine methacrylate, followed by nucleophilic substitution of the brush’s terminal halide to create an amine-terminated zwitterionic layer. After reaction with a bifunctional NHS-activated ester, anti-Salmonella antibodies could be covalently bound.36
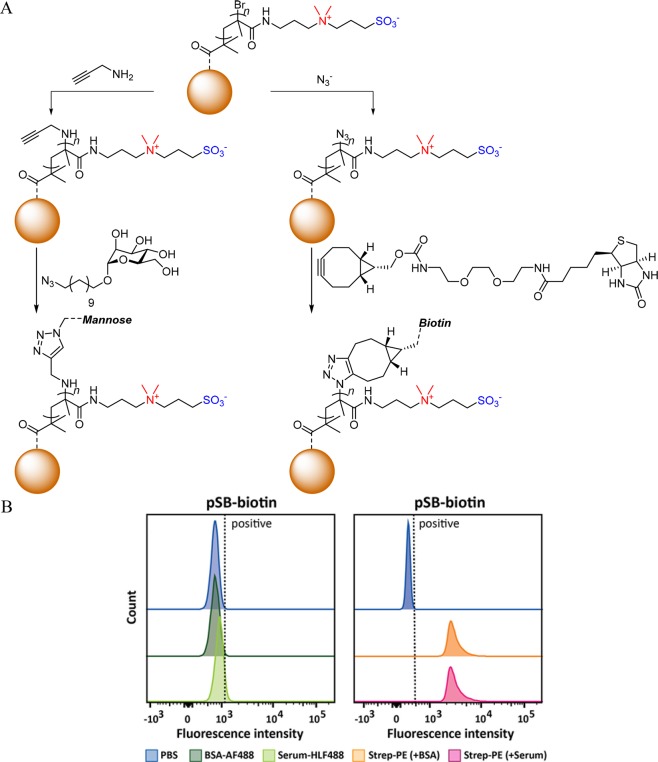
Chain-end functionalization of zwitterionic sulfobetaine methacrylamide (SBMAA) polymer brushes could also be achieved by nucleophilic substitution of the terminal halide with an azide.37 This enabled further reaction using a strain-promoted alkyne–azide cycloaddition (SPAAC) to attach a biotin ligand. More recently, the versatility of this method was further demonstrated by applying it on poly(2-methacryloyloxyethyl phosphorylcholine) (polyMPC)38,39 and polySBMAA40 brushes. As examples, azide, alkyne, and o-quinone moieties could be introduced at the chain ends of the brushes, allowing subsequent functionalization with terminal alkynes38,39 and cyclooctynes,40 with azides,40 or with cyclooctynes and cyclopropenes,41 respectively. This allowed, e.g., coupling with biotin and mannose to zwitterionic brush-coated beads for the specific binding of streptavidin or concanavalin A (ConA), respectively, from complex biological media (Figure 4A). These polySBMAA-coated beads showed highly selective binding of the protein of interest while maintaining excellent antifouling properties. In contrast to batch-based methods like SPR that analyze a relatively small number of sample surfaces, application of this approach to magnetic beads not only provides biospecific antifouling surfaces but also allows analysis of thousands of surfaces in a single experiment, thereby significantly improving the statistics of antifouling studies. For example, biotin-functionalized zwitterionic polymer brush-coated beads showed complete repellence to the nonspecific adhesion of BSA in a single-protein solution or of serum proteins (Figure 4B, left), while it strongly bound streptavidin (Figure 4B, right) under each of these conditions. The combination of these factors indicates that this bead-based platform shows great potential for the further development of a range of bioassays that require ultralow biofouling conditions.
Figure 4.
(A) Chain-end functionalization of bead-bound zwitterionic polymer brushes opens up novel modes of antifouling testing. (B) Biotin-functionalized zwitterionic polymer beads, obtained via the method outlined in (A), display a generic antifouling—i.e., no binding of fluorescently labeled BSA in a pure protein solution or upon contact with (fluorescently labeled) serum—while strongly and selectively binding to streptavidin in each of these conditions.40
Side-Chain Biofunctionalization: Hierarchically Structured Block Copolymers
In the case of a controlled surface-initiated polymerization (e.g., SI-ATRP), the presence of the active, or living, chain ends can also be exploited by grafting a second bioactive polymer segment on top of the antifouling brush. For example, Kitano et al. prepared SBMA polymer brushes and extended the polymer brush with a short second block of N-methacryloyloxy succinimide (MAOS), thus offering a reactive group for biomolecule immobilization (Figure 5A).42 Concanavalin A could subsequently be bound to the polymer brush through the NHS-activated acrylates. The concanavalin-functionalized copolymer brush showed specific interaction with colloidal gold particles decorated with mannose residues, as observed by the localized SPR. It was noted that there was not a significant difference in bound protein as a function of the length of the MAOS segment. A drawback of using MAOS is the instability of the NHS ester, which hydrolyzes in water within 3 h. For practical application, these surfaces would therefore need to be stored in a dry environment or used immediately after preparation.
Figure 5.
Two-step block copolymer brush synthesis, followed by covalent immobilization of protein concanavalin A (A)42 or covalent antibody binding mediated by SpA (B).43 (C) Different modes of antibody immobilization onto the block copolymer brush shown in (B).
One of the most used detection elements in biosensors are antibodies. For an optimal capture efficiency, it is important that the structure of the antibodies is preserved after immobilization on the biosensor surface and that the antigen binding sites are accessible,44 as up to 2 orders of magnitude increases in sensitivity can be obtained in this manner.45 The strong water-binding capacity of zwitterionic polymers contributes to stabilization of the antibodies on these surfaces by inhibiting denaturation.46−48 Ishihara and co-workers developed a method to orient antibodies on top of 2-methacryloyloxyethyl phosphorylcholine (MPC) polymers.43 To enable antibody immobilization on top of the MPC polymer brushes, they created a block-type polymer with a short (∼5 monomers) 2-aminoethyl methacrylate segment on top of the MPC polymer brushes (Figure 5B). They compared different modes of antibody immobilization: randomly oriented by physisorption, partially oriented by antibody binding to randomly physisorbed staphylococcal protein A (SpA), and antibody bound to surface-oriented SpA (Figure 5C). In the last case, the SpA protein was covalently coupled to the amino groups on the surface via enzymatic oxidation of the SpA tyrosine residues to o-quinones using tyrosinase. This resulted in oriented binding, because the tyrosine residues are predominantly located at the back side of the SpA protein’s Fc binding site. The oriented antibodies showed significantly higher binding efficiency (80% reacted with two binding sites) and 100-fold higher binding affinities compared to the random and partially oriented antibodies.
A drawback of using block copolymers is that the introduction of a reactive second block can reduce the antifouling performance. For this reason, the choice of the second block thickness is a trade-off between preserving antifouling properties and maximizing the binding capacity. This was also seen by Iwata et al., who grafted a block copolymer of MPC and glycidyl methacrylate (GMA).49 Reacting the epoxy groups in the GMA block with dithiothreitol (DTT) enabled subsequent oriented antibody immobilization by disulfide bridge formation with Fab′ fragments that could subsequently site-specifically bind a mouse IgG (the antigen). The block copolymers showed higher binding densities of Fab′ fragments and higher activity toward mouse IgG compared to a monolayer with epoxide moieties, due the presence of the MPC polymer segment. However, the antifouling properties were reduced compared to those of MPC homopolymer brushes.
Side-Chain Biofunctionalization: Random Copolymers
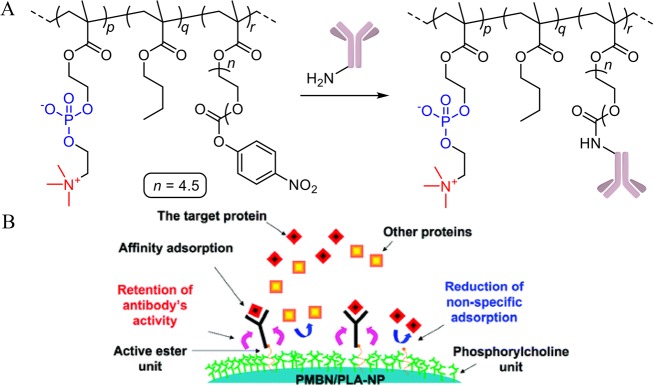
Another strategy for the biofunctionalization of zwitterionic polymers uses random copolymers that combine antifouling monomers with biofunctionalizable monomers. This approach allows a significant loading of an antibody. For example, the random copolymer poly[2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate-co-p-nitrophenyloxycarbonyl poly(ethylene glycol) methacrylate] (PMBN) was physisorbed on microwell surfaces (Figure 6). Subsequently, antibodies were attached by nucleophilic substitution of the reactive p-nitrophenol ester groups. The immobilized antibodies showed high binding capacity while maintaining the antibody activity over a significant period (>60 days).50 This polymer was also successfully tested for application on quartz crystal microbalance immunosensors51 and beads for bioaffinity separation (Figure 6B).52
Figure 6.
(A) Structure of the random copolymer and subsequent antibody functionalization reported by Nishizawa et al.50 (B) Schematic representation of the antibody functionalization of polymer nanoparticles using the physisorbed polymer depicted in (A), yielding nanoparticles that can be used for affinity-based separation methods.52
The presence of functional monomers in random copolymers can also reduce the antifouling properties. Therefore, optimization of the ratio between the zwitterionic monomer and functional monomer is needed to optimize the antibody loading and antifouling properties. Iwasaki and co-workers demonstrated this for a copolymer of MPC and methacrylic acid (MAA) synthesized by reversible addition–fragmentation chain-transfer (RAFT) polymerization.53 Subsequent aminolysis yielded a thiol-terminated copolymer, which was grafted to gold-coated SPR biosensor chips. Copolymers with 79% MPC and 21% MAA showed a minimal amount of fouling, near the low level observed for MPC homopolymers, while polymers with larger MAA content showed significantly more fouling. The methacrylic acid groups were activated with NHS/EDC chemistry to covalently couple biotin to the surface. The SPR chips were able to detect avidin in blood plasma with a limit of detection (LOD) of 1.5 nM (100 ng/mL), much lower than the LOD (150 nM) of biotinylated carboxylic acid monolayers. Later they improved the platform by using a clickable monomer, propargyl methacrylate (PMA), instead of MAA.54 In this case, the optimal composition was 55% MPC and 45% PMA. The smaller amount of MPC indicates that apparently the propargyl moieties have less of a disturbing effect on the antifouling properties than the carboxylic acids. This larger fraction of propargyl moieties enabled a large loading of biotin and a slightly lower LOD of 0.95 nM for capture of streptavidin.
The random copolymer approach has also been applied for oriented antibody immobilization. Iwasaki et al. reported an MPC-GMA random copolymer55 and reacted the epoxy groups with DTT for immobilization of anti-mouse IgG Fab′ fragments via a thiol–disulfide interchange reaction, similar to that described above for the MPC-GMA block copolymers. Song et al. also used GMA as a functional monomer but used SBMA as the antifouling zwitterionic monomer.56 The epoxy groups were further functionalized by attaching to them a boronic acid, which is reactive toward diols present in the carbohydrate moieties on the Fc chains of antibodies. This allowed site-specific immobilization of antibodies by coupling to the boronic acid residues in the polymer. These brushes showed a 6 times better signal-to-noise ratio compared to GMA homopolymer brushes. The boronic acid–diol binding is reversible at high and low pH values. The presence of the SBMA monomer led to an improved release of bound antibodies at higher pH.
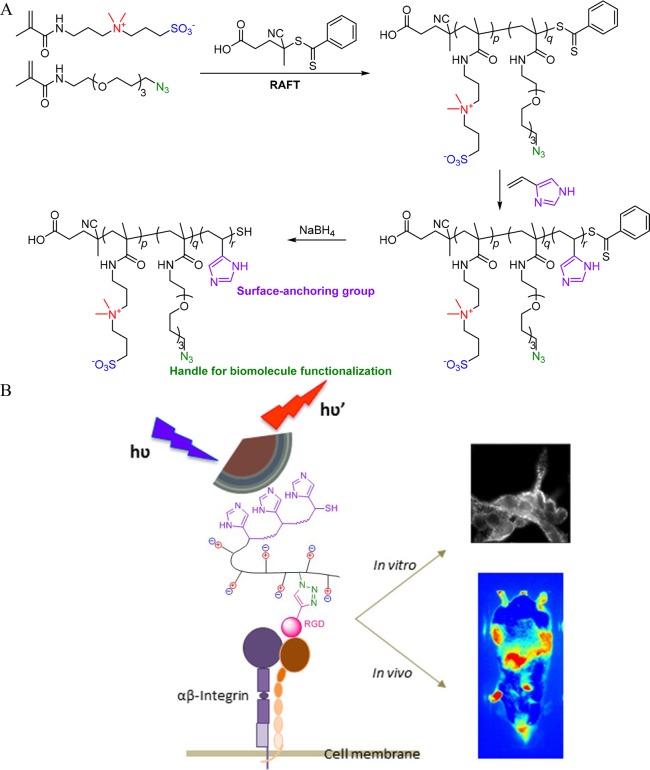
Most studies on “romantic surfaces” focus on in vitro biosensing. Recently, Trapiella-Alfonso et al. studied the specific targeting of tumor cells in vivo by zwitterionic fluorescent quantum dots (QDs) (Figure 7).57 They used RAFT polymerization to synthesize a copolymer of SBMAA and an oligo(ethylene glycol) acrylamide with an clickable azido function. A short second block of 4-vinylimidazole was subsequently added as an anchor for attaching the polymer to the QDs. Integrin-targeting arginylglycylaspartic acid (RGD) peptides were coupled via a SPAAC reaction to the azide groups. The RGD-functionalized QDs specifically adhered to integrin-presenting cell lines in vitro. However, the in vivo uptake of the zwitterionic RGD-functionalized QDs in mice bearing tumors did not show significant differences compared to that of nonfunctionalized QDs, but prolonged circulation times in blood were observed. Li et al. developed a similar approach based on zwitterionic nanogels, by copolymerizing ornithine methacrylamide with fluorescent cross-linkable carbon dots (CCDs).58,59 By functionalizing the nanogel carriers with folic acid using NHS/EDC chemistry, they showed in vitro selective uptake of these nanogels in cancer cells overexpressing folate receptors.
Figure 7.
(A) Synthesis of random copolymer of SBMAA and azido-functional oligo(ethylene glycol) acrylamide with terminal block of 4-vinylimidazole. (B) Tagging this polymer to fluorescent quantum dots and functionalizing it with integrin-targeting arginylglycylaspartic acid (RGD) peptides enables selective targeting of integrin-presenting cell lines in vitro and in vivo as observed via fluorescence imaging.57
Side-Chain Biofunctionalization: Carboxybetaine (Co)Polymers
The lack of reactive groups in the sulfobetaine and phosphorylcholine zwitterionic polymers fueled the development of the chain-end functionalization and copolymer strategies. However, carboxybetaines offer the opportunity of direct functionalization by NHS/EDC activation of carboxyl groups.60 The simplicity of this approach has made it a popular choice for developing romantic surfaces for various applications.27 For example, antibodies against activated leukocyte cell adhesion molecule (ALCAM, CD 166) were immobilized on carboxybetaine acrylamide (CBAA) brushes on gold-coated SPR chips (Figure 8).61,62 After functionalization and subsequent deactivation of the remaining NHS ester groups, the nonspecific protein adsorption remained ultralow (<3 ng cm–2 from undiluted blood plasma), and ALCAM was detected with a LOD of 10 ng/mL, which was 10 times lower than that observed with an antibody-functionalized PEG-based SAM. Jiang and co-workers had demonstrated the versatility of this platform for other applications such as glucose biosensors,63 silicon microring resonators for label-free biosensing,64 paper-based sensors,65 and 96-microwell plates.66
Figure 8.
Direct biofunctionalization of carboxybetaines by NHS/EDC activation of carboxyl groups.61
Not only is lowering the background signal by using antifouling coatings important for optimal detection with biosensors, but also maximizing the loading of the sensing element contributes to improved detection efficiency. Maximizing the loading is typically done by creating low-density hydrogel layers, enabling the sensing elements to bind not only on top of but also inside the polymer network.67 Jiang and co-workers developed hierarchical two-layer structures based on carboxybetaines (Figure 9).68,69 The first layer is a dense polymer brush with excellent antifouling properties. On top of this first layer, a second layer was grown with a lower density of polymer chains, enabling a high loading capacity for immobilization of antibodies inside the low-density polymer layer. Loading capacities of 800–1300 ng cm–2 were observed, which were 3–5 times higher compared to those found with a one-layer platform.
Figure 9.

Hierarchical two-layer structures based on carboxybetaines with a dense low-fouling base layer and a low-density polymer layer with high loading capacity for immobilization of antibodies using NHS/EDC chemistry, as shown in Figure 8.68
The simplicity and effectiveness of carboxybetaines for biofunctionalization also make it possible for their monomers to be used in copolymer-based approaches in combination with non-zwitterionic antifouling polymers. For example, surfaces with a hierarchical block copolymer were synthesized with a first block of oligo(ethylene glycol) methacrylate and on top a second block of CBAA.70 Lísalová et al. studied random copolymers of the antifouling monomers N-(2-hydroxypropyl) acrylamide (HPMA) with CBMAA as the biofunctionalizable monomer.71,72 They observed that a high loading of biorecognition elements leads to increased fouling from blood plasma. Therefore, a careful optimization of the loading capacity was performed to maintain the ultralow fouling properties (<5 ng cm–2). By attaching a DNA-ON probe, a successful detection of microRNA (miR-16) in 50% blood plasma could be achieved with a LOD of 6 nmol L–1.
Side-Chain Biofunctionalization: Zwitterionic Polymers with Intrinsic Reactivity
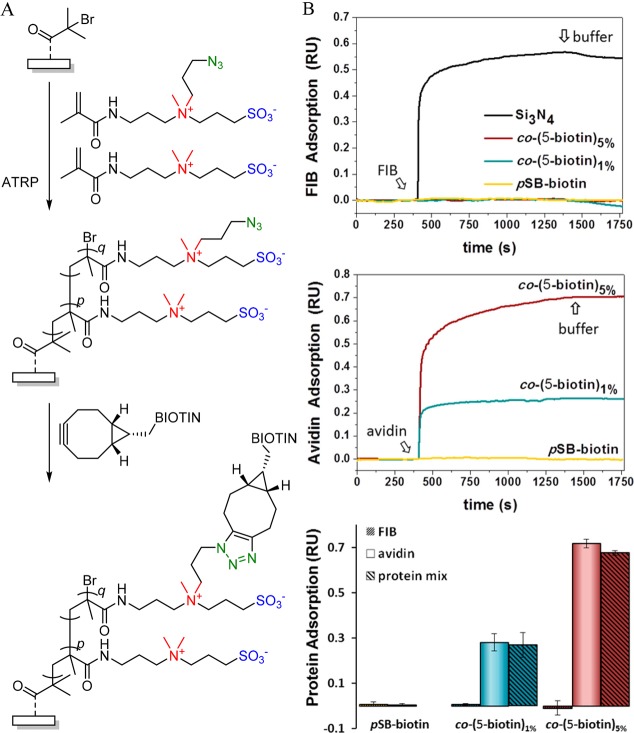
In the examples discussed above with antifouling copolymer brushes, two monomers were needed to impart the overall polymer brush coating with “romantic features”. That is, one monomer, typically zwitterionic in nature, provides the antifouling character to the brush, while a second monomer, equipped with a reactive handle, is used to immobilize the biorecognition element (e.g., protein or antibody). While this second monomer is not necessarily present with a high fraction inside the brush to bind the typically large biomolecule, there is still an inherent trade-off between good antifouling performance and biofunctionalizability. To overcome this trade-off, our group designed and synthesized an intrinsically zwitterionic sulfobetaine monomer that is also equipped with a reactive azide group (Figure 10).73 As a result, when this monomer is used to grow a polymer brush, the introduction of reactive groups inside the brush does not occur at the expense of the zwitterionic character of the brush. In contrast, regardless of the loading of this functionalizable sulfobetaine monomer, all monomer units in the copolymer brush are permanently zwitterionic, maintaining the overall charge neutrality of the brush and thus minimizing charge effects in the potential physisorption of unwanted proteins.
Figure 10.
(A) Random surface-initiated copolymerization of an azide-containing SBMA monomer and a nonfunctional SBMA monomer (q = 0, 1, or 5%), followed by a SPAAC reaction of the azides to immobilize biotin inside the brush. (B) Protein adsorption and binding onto bare and polymer-brush-coated (as described in A) surfaces as measured by reflectometry: Top, fibrinogen adsorption; middle, avidin adsorption; bottom, bar plot summarizing fibrinogen adsorption, the avidin binding of pure avidin, and a mixed protein solution of avidin and fibrinogen.73
(Random) copolymerization of the azide-containing sulfobetaine (typically 1–5%) together with a standard sulfobetaine monomer resulted in antifouling copolymer brushes that performed equally well in antifouling experiments as the corresponding unfunctionalizable poly(sulfobetaine) brush, as could be concluded from fibrinogen fouling experiments, probed by reflectometry (Figure 10). However, upon reacting the azide groups in the copolymer brushes with a biotin-linked bicyclooctyne derivative via a strain-promoted click reaction, the biotin-containing copolymer brushes were able to selectively bind streptavidin from a protein mixture.73 Overall, the availability of a functionalizable and zwitterionic monomer that can be (co)polymerized in a brush allows for a high degree of loading of the desired biorecognition element, without loss of the antifouling performance.
Independent from our work on a zwitterionic sulfobetaine with an azide-based “click handle”, Hu and Emrick reported a phosphorylcholine-based zwitterionic monomer equipped with an alkyne “click handle” (Figure 11).74 While the authors did not specifically address the use of this monomer for the synthesis of biofunctionalizable antifouling brush coatings, they do note—and demonstrate—that this monomer provides access to functional zwitterions, hydrogels, fluorophores, and prodrugs.
Figure 11.
Phosphorylcholine-based zwitterionic monomer with an alkyne “click handle”.74
Their use of an alkyne handle in the zwitterionic monomer means that an azide–alkyne cycloaddition can only be catalyzed by Cu(I) and cannot be strain-promoted (as was possible in the case of the azide-containing sulfobetaine (Figure 10)). In the former case, undesired interactions between the charged Cu(I) ion and the zwitterionic brush might interfere with the CuAAC reaction, or—in contrast—subsequent binding of Cu ions to the brush might take place, yielding, e.g., undesired copper-induced effects on living cells in the case of the use of such romantic surfaces where they matter most: in living matter. To eliminate the need for a Cu(I) catalyst, the thiol–yne click reaction could be an alternative functionalization strategy.75
Outlook
The rise of biospecifically interacting surfaces over the past decade holds great promise for the development of future diagnostics, in which instrumental developments toward very high signals can be coupled to a reduction of the (bio)chemical noise. A wider application of these romantic surfaces is therefore to be rapidly expected. Such applications are only further facilitated by the development of light-induced living polymerization methods, which allow for an easy patterning of surfaces.32,76 Wide-spread adoption will also be further catalyzed by emerging polymerization methods that are not as sensitive to oxygen as standard SI-ATRP reactions.77,78 This will enable the large-scale production of bioactive antifouling biomedical devices—for example, for point-of-care diagnostics.
The zwitterionic polymers also hold great potential for in vivo applications because of their low immunoresponse. This, for example, improves the circulation time of drug-carrying nanoparticles.79 Nanoparticles that show longer circulating times have a higher probability to reach their target, which improves their therapeutic efficacy. However, the nonfouling character of these particles also reduces their cellular uptake.80 Decorating these particles with biorecognition elements could overcome this limitation and even induce specific targeting of the nanoparticles. Research in this direction will contribute to development of effective targeted drug-delivery systems. Similarly, antifouling surfaces have been shown to be very effective as the outermost part of, e.g., hip implants.81−83 The combination of such minimally immunogenic, antifouling brushes with specific wound-healing components would further increase the effectiveness of implants in humans.
The concept of generically protein-repelling surfaces has now taken firm hold, but the background for this is still not fully understood. As a point in case, the group of Rodriguez-Emmenegger developed a non-zwitterionic class of brushes based on N-(2-hydroxypropyl)methacrylamide (HPMA) that is nevertheless highly antifouling.84 Since the water-binding capacity of HPMA is evidently lower than that of zwitterionic brushes, yet the antifouling capacity is of a comparably high quality, there are likely more factors involved. A further optimization of such antifouling surfaces85 can only take place upon deepening our knowledge into these factors, which then, we hope, can be followed up by “romanticizing” such surfaces as well.
Finally, a next step would be to combine biospecific interactions with self-healing characteristics. As a first step, our group developed a series of self-healing antifouling surfaces, which can withstand various sorts of mechanical and chemical damage to recover the antifouling characteristics.86−88 Implementation of biospecific recognition in such coatings would be a logical next step, which is currently under investigation in our laboratories.
Acknowledgments
The authors acknowledge financial support (in alphabetical order) from Aquamarijn Inc. (to J.B.), Chinese National Science Foundation (973 National Basic Research Program of China (2015CB856500, to H.Z.), MicroNed (H.Z.), NanoNextNL (program 3E, H.Z.), The Netherlands Organization for Scientific Research (NWO Veni grant 722.012.005 and Vidi grant 016.Vidi.189.031, both to M.M.J.S.), Tianjin University (H.Z.), and Wageningen University (all). We furthermore thank all the co-workers and colleagues mentioned in the references for extensive discussions and input sine qua non.
Biographies

Jacob Baggerman obtained his Ph.D. from the University of Amsterdam (The Netherlands), working on the photochemistry and photophysics of photoactive hydrogen-bonded rotaxanes. After a postdoc with Han Zuilhof focusing on surface science at Wageningen University (The Netherlands), he joined Aquamarijn (Zutphen, The Netherlands), where he is now Chief Technological Officer, while he is also a part-time Senior Scientist at Wageningen University. His current research interests are in surface functionalization for the development of biosensors and microfluidics.

Maarten Smulders obtained his Ph.D. in supramolecular chemistry with Bert Meijer (Eindhoven University of Technology). After postdoctoral stays with Jonathan Nitschke (Cambridge University) and Jeroen Cornelissen (Twente University), he joined Wageningen University in 2013 as an assistant professor, starting his independent research, funded by an NWO Veni grant. His current research interests are in functional antifouling polymer brushes and dynamic-covalent (polymer) chemistry. In 2018 he was awarded an NWO Vidi grant, to fortify his research program in the latter area.

Han Zuilhof is the Chair of Organic Chemistry at Wageningen University. His interests focus on surface-bound (bio)organic and analytical chemistry. He obtained both an M.Sc. in Chemistry and an M.A. in Philosophy. After earning a Ph.D. in Chemistry (Leiden University, 1994; highest honors) and doing postdoctoral work at the University of Rochester (New York) and Columbia University, he joined the faculty at Wageningen University. He is a Perennial Distinguished Guest Professor at Tianjin University (Tiajin, China) and a Distinguished Adjunct Professor of Chemical Engineering at King Abdulaziz University (Jeddah, Saudi Arabia), is a Senior Editor of Langmuir, serves on the Editorial Advisory Boards of Advanced Materials Interfaces and Applied Surface Science, and loves watching cloudless night skies.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Vaisocherová H.; Brynda E.; Homola J. Functionalizable low-fouling coatings for label-free biosensing in complex biological media: advances and applications. Anal. Bioanal. Chem. 2015, 407 (14), 3927–3953. 10.1007/s00216-015-8606-5. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rexach E.; Meaurio E.; Sarasua J.-R. Recent developments in drug eluting devices with tailored interfacial properties. Adv. Colloid Interface Sci. 2017, 249, 181–191. 10.1016/j.cis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Harding J. L.; Reynolds M. M. Combating medical device fouling. Trends Biotechnol. 2014, 32 (3), 140–146. 10.1016/j.tibtech.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Turner A. P. F. Biosensors: sense and sensibility. Chem. Soc. Rev. 2013, 42 (8), 3184–3196. 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Zhang Y.; Wang H.; Brash J.; Chen H. Anti-fouling bioactive surfaces. Acta Biomater. 2011, 7 (4), 1550–1557. 10.1016/j.actbio.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Joshi S.; Zuilhof H.; van Beek T. A.; Nielen M. W. F. Biochip Spray: Simplified Coupling of Surface Plasmon Resonance Biosensing and Mass Spectrometry. Anal. Chem. 2017, 89 (3), 1427–1432. 10.1021/acs.analchem.6b04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. SPR Biosensors: Historical Perspectives and Current Challenges. Sens. Actuators, B 2016, 229, 110–130. 10.1016/j.snb.2016.01.118. [DOI] [Google Scholar]
- Smolsky J.; Kaur S.; Hayashi C.; Batra K. S.; Krasnoslobodtsev V. A. Surface-Enhanced Raman Scattering-Based Immunoassay Technologies for Detection of Disease Biomarkers. Biosensors 2017, 7 (4), 7. 10.3390/bios7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszykowski C.; Sheikh S.; Thompson M. A survey of state-of-the-art surface chemistries to minimize fouling from human and animal biofluids. Biomater. Sci. 2015, 3 (10), 1335–1370. 10.1039/C5BM00085H. [DOI] [PubMed] [Google Scholar]
- Prime K. L.; Whitesides G. M. Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): a model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115 (23), 10714–10721. 10.1021/ja00076a032. [DOI] [Google Scholar]
- Seigel R. R.; Harder P.; Dahint R.; Grunze M.; Josse F.; Mrksich M.; Whitesides G. M. On-line detection of nonspecific protein adsorption at artificial surfaces. Anal. Chem. 1997, 69 (16), 3321–3328. 10.1021/ac970047b. [DOI] [PubMed] [Google Scholar]
- Rosso M.; Nguyen A. T.; de Jong E.; Baggerman J.; Paulusse J. M. J.; Giesbers M.; Fokkink R. G.; Norde W.; Schroën K.; van Rijn C. J. M.; Zuilhof H. Protein-Repellent Silicon Nitride Surfaces: UV-Induced Formation of Oligoethylene Oxide Monolayers. ACS Appl. Mater. Interfaces 2011, 3 (3), 697–704. 10.1021/am100985c. [DOI] [PubMed] [Google Scholar]
- Norde W.; Gage D. Interaction of bovine serum albumin and human blood plasma with PEO-tethered surfaces: Influence of PEO chain length, grafting density, and temperature. Langmuir 2004, 20 (10), 4162–4167. 10.1021/la030417t. [DOI] [PubMed] [Google Scholar]
- Hamburger R.; Azaz E.; Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm. Acta Helv. 1975, 50 (1–2), 10–7. [PubMed] [Google Scholar]
- Crouzet C.; Decker C.; Marchal J. Caractérisation de réactions primaires de dégradation oxydante au cours de l’autoxydation des poly(oxyéthylène)s à 25°C: Étude en solution aqueuse avec amorçage par radiolyse du solvant, 8. Étude cinétique en fonction du ph compris entre 1 et 13. Makromol. Chem. 1976, 177 (1), 145–157. 10.1002/macp.1976.021770112. [DOI] [Google Scholar]
- Zhang P.; Sun F.; Liu S.; Jiang S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Controlled Release 2016, 244, 184–193. 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni E.; Chapman R. G.; Holmlin R. E.; Takayama S.; Whitesides G. M. A Survey of Structure–Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17 (18), 5605–5620. 10.1021/la010384m. [DOI] [Google Scholar]
- Chapman R. G.; Ostuni E.; Takayama S.; Holmlin R. E.; Yan L.; Whitesides G. M. Surveying for Surfaces that Resist the Adsorption of Proteins. J. Am. Chem. Soc. 2000, 122 (34), 8303–8304. 10.1021/ja000774f. [DOI] [Google Scholar]
- Kane R. S.; Deschatelets P.; Whitesides G. M. Kosmotropes Form the Basis of Protein-Resistant Surfaces. Langmuir 2003, 19 (6), 2388–2391. 10.1021/la020737x. [DOI] [Google Scholar]
- Holmlin R. E.; Chen X. X.; Chapman R. G.; Takayama S.; Whitesides G. M. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17 (9), 2841–2850. 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- Kitano H.; Tada S.; Mori T.; Takaha K.; Gemmei-Ide M.; Tanaka M.; Fukuda M.; Yokoyama Y. Correlation between the structure of water in the vicinity of carboxybetaine polymers and their blood-compatibility. Langmuir 2005, 21 (25), 11932–11940. 10.1021/la0515571. [DOI] [PubMed] [Google Scholar]
- Murphy E. F.; Lu J. R.; Lewis A. L.; Brewer J.; Russell J.; Stratford P. Characterization of Protein Adsorption at the Phosphorylcholine Incorporated-Polymer Water Interface. Macromolecules 2000, 33 (12), 4545–4554. 10.1021/ma991642d. [DOI] [Google Scholar]
- Murphy E. F.; Lu J. R.; Brewer J.; Russell J.; Penfold J. The Reduced Adsorption of Proteins at the Phosphoryl Choline Incorporated Polymer–Water Interface. Langmuir 1999, 15 (4), 1313–1322. 10.1021/la9813580. [DOI] [Google Scholar]
- Ishihara K.; Iwasaki Y. Reduced Protein Adsorption on Novel Phospholipid Polymers. J. Biomater. Appl. 1998, 13 (2), 111–127. 10.1177/088532829801300203. [DOI] [PubMed] [Google Scholar]
- Hayward J. A.; Chapman D. Biomembrane surfaces as models for polymer design - the potential for hemocompatibility. Biomaterials 1984, 5 (3), 135–142. 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Chao T.; Chen S.; Jiang S. Superlow Fouling Sulfobetaine and Carboxybetaine Polymers on Glass Slides. Langmuir 2006, 22 (24), 10072–10077. 10.1021/la062175d. [DOI] [PubMed] [Google Scholar]
- Jiang S.; Cao Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22 (9), 920–932. 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- Nguyen A. T.; Baggerman J.; Paulusse J. M. J.; van Rijn C. J. M.; Zuilhof H. Stable Protein-Repellent Zwitterionic Polymer Brushes Grafted from Silicon Nitride. Langmuir 2011, 27 (6), 2587–2594. 10.1021/la104657c. [DOI] [PubMed] [Google Scholar]
- Schönemann E.; Laschewsky A.; Rosenhahn A. Exploring the Long-Term Hydrolytic Behavior of Zwitterionic Polymethacrylates and Polymethacrylamides. Polymers 2018, 10 (6), 639. 10.3390/polym10060639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Cao Z.; Bai T.; Carr L.; Ella-Menye J.-R.; Irvin C.; Ratner B. D.; Jiang S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553. 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y.; Ishihara K. Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces. Sci. Technol. Adv. Mater. 2012, 13 (6), 064101. 10.1088/1468-6996/13/6/064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppe J. O.; Ataman N. C.; Mocny P.; Wang J.; Moraes J.; Klok H.-A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117 (3), 1105–1318. 10.1021/acs.chemrev.6b00314. [DOI] [PubMed] [Google Scholar]
- Lunn D. J.; Discekici E. H.; Read de Alaniz J.; Gutekunst W. R.; Hawker C. J. Established and emerging strategies for polymer chain-end modification. J. Polym. Sci., Part A: Polym. Chem. 2017, 55 (18), 2903–2914. 10.1002/pola.28575. [DOI] [Google Scholar]
- Yao Y.; Ma Y.-Z.; Qin M.; Ma X.-J.; Wang C.; Feng X.-Z. NHS-ester functionalized poly(PEGMA) brushes on silicon surface for covalent protein immobilization. Colloids Surf., B 2008, 66 (2), 233–239. 10.1016/j.colsurfb.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Kuzmyn A. R.; de los Santos Pereira A.; Pop-Georgievski O.; Bruns M.; Brynda E.; Rodriguez-Emmenegger C. Exploiting end group functionalization for the design of antifouling bioactive brushes. Polym. Chem. 2014, 5 (13), 4124–4131. 10.1039/C4PY00281D. [DOI] [Google Scholar]
- Nguyen A. T.; Baggerman J.; Paulusse J. M. J.; Zuilhof H.; van Rijn C. J. M. Bioconjugation of Protein-Repellent Zwitterionic Polymer Brushes Grafted from Silicon Nitride. Langmuir 2012, 28 (1), 604–610. 10.1021/la2031363. [DOI] [PubMed] [Google Scholar]
- Li Y.; Giesbers M.; Gerth M.; Zuilhof H. Generic Top-Functionalization of Patterned Antifouling Zwitterionic Polymers on Indium Tin Oxide. Langmuir 2012, 28 (34), 12509–12517. 10.1021/la3022563. [DOI] [PubMed] [Google Scholar]
- Ishihara K.; Fukuda Y.; Konno T.; Inoue Y. Molecular integration on phospholipid polymer-coated magnetic beads for gene expression analysis in cells. React. Funct. Polym. 2017, 119, 125–133. 10.1016/j.reactfunctpolym.2017.08.011. [DOI] [Google Scholar]
- Inoue Y.; Onodera Y.; Ishihara K. Initial Cell Adhesion onto a Phospholipid Polymer Brush Surface Modified with a Terminal Cell Adhesion Peptide. ACS Appl. Mater. Interfaces 2018, 10 (17), 15250–15257. 10.1021/acsami.8b01906. [DOI] [PubMed] [Google Scholar]
- van Andel E.; de Bus I.; Tijhaar E. J.; Smulders M. M. J.; Savelkoul H. F. J.; Zuilhof H. Highly Specific Binding on Antifouling Zwitterionic Polymer-Coated Microbeads as Measured by Flow Cytometry. ACS Appl. Mater. Interfaces 2017, 9 (44), 38211–38221. 10.1021/acsami.7b09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtory D.; Sen R.; Kuzmyn A. R.; Escorihuela J.; Zuilhof H. Strain-Promoted Cycloaddition of Cyclopropenes with o-Quinones: A Rapid Click Reaction. Angew. Chem., Int. Ed. 2018, 57 (32), 10118–10122. 10.1002/anie.201800937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H.; Suzuki H.; Matsuura K.; Ohno K. Molecular Recognition at the Exterior Surface of a Zwitterionic Telomer Brush. Langmuir 2010, 26 (9), 6767–6774. 10.1021/la904111r. [DOI] [PubMed] [Google Scholar]
- Tajima N.; Takai M.; Ishihara K. Significance of Antibody Orientation Unraveled: Well-Oriented Antibodies Recorded High Binding Affinity. Anal. Chem. 2011, 83 (6), 1969–1976. 10.1021/ac1026786. [DOI] [PubMed] [Google Scholar]
- Trilling A. K.; Beekwilder J.; Zuilhof H. Antibody orientation on biosensor surfaces: a minireview. Analyst 2013, 138 (6), 1619–1627. 10.1039/c2an36787d. [DOI] [PubMed] [Google Scholar]
- Trilling A. K.; Hesselink T.; Houwelingen A. v.; Cordewener J. H. G.; Jongsma M. A.; Schoffelen S.; Hest J. C. M. v.; Zuilhof H.; Beekwilder J. Orientation of llama antibodies strongly increases sensitivity of biosensors. Biosens. Bioelectron. 2014, 60, 130–136. 10.1016/j.bios.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Sakaki S.; Iwasaki Y.; Nakabayashi N.; Ishihara K. Water-Soluble 2-Methacryloyloxyethyl Phosphorylcholine Copolymer as a Novel Synthetic Blocking Reagent in Immunoassay System. Polym. J. 2000, 32, 637. 10.1295/polymj.32.637. [DOI] [Google Scholar]
- Zhang P.; Sun F.; Tsao C.; Liu S.; Jain P.; Sinclair A.; Hung H.-C.; Bai T.; Wu K.; Jiang S. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (39), 12046. 10.1073/pnas.1512465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe A. J.; Jiang S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat. Chem. 2012, 4, 59. 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata R.; Satoh R.; Iwasaki Y.; Akiyoshi K. Covalent immobilization of antibody fragments on well-defined polymer brushes via site-directed method. Colloids Surf., B 2008, 62 (2), 288–298. 10.1016/j.colsurfb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Nishizawa K.; Konno T.; Takai M.; Ishihara K. Bioconjugated Phospholipid Polymer Biointerface for Enzyme-Linked Immunosorbent Assay. Biomacromolecules 2008, 9 (1), 403–407. 10.1021/bm7010246. [DOI] [PubMed] [Google Scholar]
- Park J.; Kurosawa S.; Takai M.; Ishihara K. Antibody immobilization to phospholipid polymer layer on gold substrate of quartz crystal microbalance immunosensor. Colloids Surf., B 2007, 55 (2), 164–172. 10.1016/j.colsurfb.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Goto Y.; Matsuno R.; Konno T.; Takai M.; Ishihara K. Polymer Nanoparticles Covered with Phosphorylcholine Groups and Immobilized with Antibody for High-Affinity Separation of Proteins. Biomacromolecules 2008, 9 (3), 828–833. 10.1021/bm701161d. [DOI] [PubMed] [Google Scholar]
- Akkahat P.; Kiatkamjornwong S.; Yusa S.-i.; Hoven V. P.; Iwasaki Y. Development of a Novel Antifouling Platform for Biosensing Probe Immobilization from Methacryloyloxyethyl Phosphorylcholine-Containing Copolymer Brushes. Langmuir 2012, 28 (13), 5872–5881. 10.1021/la204229t. [DOI] [PubMed] [Google Scholar]
- Wiarachai O.; Vilaivan T.; Iwasaki Y.; Hoven V. P. Clickable and Antifouling Platform of Poly[(propargyl methacrylate)-ran-(2-methacryloyloxyethyl phosphorylcholine)] for Biosensing Applications. Langmuir 2016, 32 (4), 1184–1194. 10.1021/acs.langmuir.5b02727. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y.; Omichi Y.; Iwata R. Site-Specific Dense Immobilization of Antibody Fragments on Polymer Brushes Supported by Silicone Nanofilaments. Langmuir 2008, 24 (16), 8427–8430. 10.1021/la801327a. [DOI] [PubMed] [Google Scholar]
- Song L.; Zhao J.; Luan S.; Ma J.; Liu J.; Xu X.; Yin J. Fabrication of a Detection Platform with Boronic-Acid-Containing Zwitterionic Polymer Brush. ACS Appl. Mater. Interfaces 2013, 5 (24), 13207–13215. 10.1021/am404206v. [DOI] [PubMed] [Google Scholar]
- Trapiella-Alfonso L.; Pons T.; Lequeux N.; Leleu L.; Grimaldi J.; Tasso M.; Oujagir E.; Seguin J.; d’Orlyé F.; Girard C.; Doan B.-T.; Varenne A. Clickable-Zwitterionic Copolymer Capped-Quantum Dots for in Vivo Fluorescence Tumor Imaging. ACS Appl. Mater. Interfaces 2018, 10 (20), 17107–17116. 10.1021/acsami.8b04708. [DOI] [PubMed] [Google Scholar]
- Li W.; Chu K.; Liu L. Zwitterionic Gel Coating Endows Gold Nanoparticles with Ultrastability. Langmuir 2018, 10.1021/acs.langmuir.8b01600. [DOI] [PubMed] [Google Scholar]
- Li W.; Liu Q.; Zhang P.; Liu L. Zwitterionic nanogels crosslinked by fluorescent carbon dots for targeted drug delivery and simultaneous bioimaging. Acta Biomater. 2016, 40, 254–262. 10.1016/j.actbio.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Chen S.; Jiang S. Dual-Functional Biomimetic Materials: Nonfouling Poly(carboxybetaine) with Active Functional Groups for Protein Immobilization. Biomacromolecules 2006, 7 (12), 3311–3315. 10.1021/bm060750m. [DOI] [PubMed] [Google Scholar]
- Vaisocherová H.; Yang W.; Zhang Z.; Cao Z.; Cheng G.; Piliarik M.; Homola J.; Jiang S. Ultralow Fouling and Functionalizable Surface Chemistry Based on a Zwitterionic Polymer Enabling Sensitive and Specific Protein Detection in Undiluted Blood Plasma. Anal. Chem. 2008, 80 (20), 7894–7901. 10.1021/ac8015888. [DOI] [PubMed] [Google Scholar]
- Gao C.; Li G.; Xue H.; Yang W.; Zhang F.; Jiang S. Functionalizable and ultra-low fouling zwitterionic surfaces via adhesive mussel mimetic linkages. Biomaterials 2010, 31 (7), 1486–1492. 10.1016/j.biomaterials.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Yang W.; Xue H.; Carr L. R.; Wang J.; Jiang S. Zwitterionic poly(carboxybetaine) hydrogels for glucose biosensors in complex media. Biosens. Bioelectron. 2011, 26 (5), 2454–2459. 10.1016/j.bios.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Kirk J. T.; Brault N. D.; Baehr-Jones T.; Hochberg M.; Jiang S.; Ratner D. M. Zwitterionic polymer-modified silicon microring resonators for label-free biosensing in undiluted humanplasma. Biosens. Bioelectron. 2013, 42, 100–105. 10.1016/j.bios.2012.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F.; Wu K.; Hung H.-C.; Zhang P.; Che X.; Smith J.; Lin X.; Li B.; Jain P.; Yu Q.; Jiang S. Paper Sensor Coated with a Poly(carboxybetaine)-Multiple DOPA Conjugate via Dip-Coating for Biosensing in Complex Media. Anal. Chem. 2017, 89 (20), 10999–11004. 10.1021/acs.analchem.7b02876. [DOI] [PubMed] [Google Scholar]
- Lin X.; Jain P.; Wu K.; Hong D.; Hung H.-C.; O’Kelly M. B.; Li B.; Zhang P.; Yuan Z.; Jiang S. Ultra-low Fouling and Functionalizable Surface Chemistry Based on Zwitterionic Carboxybetaine Random Copolymers. Langmuir 2018, 10.1021/acs.langmuir.8b02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson J.-F.; Battaglia T. M.; Davidson M. J.; Kim Y.-C.; Prakash A. M. C.; Beaudoin S.; Booksh K. S. Biocompatible polymers for antibody support on gold surfaces. Talanta 2005, 67 (5), 918–925. 10.1016/j.talanta.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Huang C.-J.; Li Y.; Jiang S. Zwitterionic Polymer-Based Platform with Two-Layer Architecture for Ultra Low Fouling and High Protein Loading. Anal. Chem. 2012, 84 (7), 3440–3445. 10.1021/ac3003769. [DOI] [PubMed] [Google Scholar]
- Brault N. D.; Sundaram H. S.; Huang C.-J.; Li Y.; Yu Q.; Jiang S. Two-Layer Architecture Using Atom Transfer Radical Polymerization for Enhanced Sensing and Detection in Complex Media. Biomacromolecules 2012, 13 (12), 4049–4056. 10.1021/bm301335r. [DOI] [PubMed] [Google Scholar]
- de los Santos Pereira A.; Riedel T.; Brynda E.; Rodriguez-Emmenegger C. Hierarchical antifouling brushes for biosensing applications. Sens. Actuators, B 2014, 202, 1313–1321. 10.1016/j.snb.2014.06.075. [DOI] [Google Scholar]
- Lísalová H.; Brynda E.; Houska M.; Víšová I.; Mrkvová K.; Song X. C.; Gedeonová E.; Surman F.; Riedel T.; Pop-Georgievski O.; Homola J. Ultralow-Fouling Behavior of Biorecognition Coatings Based on Carboxy-Functional Brushes of Zwitterionic Homo- and Copolymers in Blood Plasma: Functionalization Matters. Anal. Chem. 2017, 89 (6), 3524–3531. 10.1021/acs.analchem.6b04731. [DOI] [PubMed] [Google Scholar]
- Vaisocherová-Lísalová H.; Surman F.; Víšová I.; Vala M.; Špringer T.; Ermini M. L.; Šípová H.; Šedivák P.; Houska M.; Riedel T.; Pop-Georgievski O.; Brynda E.; Homola J. Copolymer Brush-Based Ultralow-Fouling Biorecognition Surface Platform for Food Safety. Anal. Chem. 2016, 88 (21), 10533–10539. 10.1021/acs.analchem.6b02617. [DOI] [PubMed] [Google Scholar]
- Lange S. C.; van Andel E.; Smulders M. M. J.; Zuilhof H. Efficient and Tunable Three-Dimensional Functionalization of Fully Zwitterionic Antifouling Surface Coatings. Langmuir 2016, 32 (40), 10199–10205. 10.1021/acs.langmuir.6b02622. [DOI] [PubMed] [Google Scholar]
- Hu G.; Emrick T. Functional Choline Phosphate Polymers. J. Am. Chem. Soc. 2016, 138 (6), 1828–1831. 10.1021/jacs.5b13156. [DOI] [PubMed] [Google Scholar]
- Lowe A. B.; Hoyle C. E.; Bowman C. N. Thiol-yne click chemistry: A powerful and versatile methodology for materials synthesis. J. Mater. Chem. 2010, 20 (23), 4745–4750. 10.1039/b917102a. [DOI] [Google Scholar]
- Chen M.; Zhong M.; Johnson J. A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116 (17), 10167–10211. 10.1021/acs.chemrev.5b00671. [DOI] [PubMed] [Google Scholar]
- Yeow J.; Chapman R.; Gormley A. J.; Boyer C. Up in the air: oxygen tolerance in controlled/living radical polymerisation. Chem. Soc. Rev. 2018, 47 (12), 4357–4387. 10.1039/C7CS00587C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D.; Hung H.-C.; Wu K.; Lin X.; Sun F.; Zhang P.; Liu S.; Cook K. E.; Jiang S. Achieving Ultralow Fouling under Ambient Conditions via Surface-Initiated ARGET ATRP of Carboxybetaine. ACS Appl. Mater. Interfaces 2017, 9 (11), 9255–9259. 10.1021/acsami.7b01530. [DOI] [PubMed] [Google Scholar]
- Yang W.; Liu S.; Bai T.; Keefe A. J.; Zhang L.; Ella-Menye J.-R.; Li Y.; Jiang S. Poly(carboxybetaine) nanomaterials enable long circulation and prevent polymer-specific antibody production. Nano Today 2014, 9 (1), 10–16. 10.1016/j.nantod.2014.02.004. [DOI] [Google Scholar]
- Yuan Y.-Y.; Mao C.-Q.; Du X.-J.; Du J.-Z.; Wang F.; Wang J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24 (40), 5476–5480. 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- Kyomoto M.; Moro T.; Yamane S.; Watanabe K.; Hashimoto M.; Tanaka S.; Ishihara K. Hydrated Phospholipid Polymer Gel-Like Layer for Increased Durability of Orthopedic Bearing Surfaces. Langmuir 2018, 10.1021/acs.langmuir.8b01494. [DOI] [PubMed] [Google Scholar]
- Moro T.; Takatori Y.; Tanaka S.; Ishihara K.; Oda H.; Kim Y. T.; Umeyama T.; Fukatani E.; Ito H.; Kyomoto M.; Oshima H.; Tanaka T.; Kawaguchi H.; Nakamura K. Clinical safety and wear resistance of the phospholipid polymer-grafted highly cross-linked polyethylene liner. J. Orthop. Res. 2017, 35 (9), 2007–2016. 10.1002/jor.23473. [DOI] [PubMed] [Google Scholar]
- Kyomoto M.; Moro T.; Yamane S.; Hashimoto M.; Takatori Y.; Ishihara K. Effect of UV-irradiation intensity on graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on orthopedic bearing substrate. J. Biomed. Mater. Res., Part A 2014, 102 (9), 3012–3023. 10.1002/jbm.a.34973. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Emmenegger C.; Brynda E.; Riedel T.; Houska M.; Šubr V.; Alles A. B.; Hasan E.; Gautrot J. E.; Huck W. T. S. Polymer Brushes Showing Non-Fouling in Blood Plasma Challenge the Currently Accepted Design of Protein Resistant Surfaces. Macromol. Rapid Commun. 2011, 32 (13), 952–957. 10.1002/marc.201100189. [DOI] [PubMed] [Google Scholar]
- van Andel E.; Lange S. C.; Pujari S. P.; Tijhaar E. J.; Smulders M. M. J.; Savelkoul H. F. J.; Zuilhof H. Systematic Comparison of Zwitterionic and Non-Zwitterionic Antifouling Polymer Brushes on a Bead-Based Platform. Langmuir 2018, 10.1021/acs.langmuir.8b01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Zuilhof H. Self-healing fluoropolymer brushes as highly polymer-repellent coatings. J. Mater. Chem. A 2016, 4 (7), 2408–2412. 10.1039/C5TA09209D. [DOI] [PubMed] [Google Scholar]
- Wang Z.; van Andel E.; Pujari S. P.; Feng H.; Dijksman J. A.; Smulders M. M. J.; Zuilhof H. Water-repairable zwitterionic polymer coatings for anti-biofouling surfaces. J. Mater. Chem. B 2017, 5 (33), 6728–6733. 10.1039/C7TB01178D. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Fei G.; Xia H.; Zuilhof H. Dual water-healable zwitterionic polymer coatings for anti-biofouling surfaces. J. Mater. Chem. B 2018, 6, 6930. 10.1039/C8TB01863D. [DOI] [PubMed] [Google Scholar]