Abstract
Background
Indwelling urinary catheters are commonly used for patients undergoing general and orthopaedic surgery. Despite infectious and non-infectious harms of urinary catheters, there is limited guidance available to surgery teams regarding appropriate perioperative catheter use.
Objective
Using the RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Method, we assessed the appropriateness of indwelling urinary catheter placement and different timings of catheter removal for routine general and orthopaedic surgery procedures.
Methods
Two multidisciplinary panels consisting of 13 and 11 members (physicians and nurses) for general and orthopaedic surgery, respectively, reviewed the available literature regarding the impact of different perioperative catheter use strategies. Using a standardised, multiround rating process, the panels independently rated clinical scenarios (91 general surgery, 36 orthopaedic surgery) for urinary catheter placement and postoperative duration of use as appropriate (ie, benefits outweigh risks), inappropriate or of uncertain appropriateness.
Results
Appropriateness of catheter use varied by procedure, accounting for procedure-specific risks as well as expected procedure time and intravenous fluids. Procedural appropriateness ratings for catheters were summarised for clinical use into three groups: (1) can perform surgery without catheter; (2) use intraoperatively only, ideally remove before leaving the operating room; and (3) use intraoperatively and keep catheter until postoperative days 1–4. Specific recommendations were provided by procedure, with postoperative day 1 being appropriate for catheter removal for first voiding trial for many procedures.
Conclusion
We defined the appropriateness of indwelling urinary catheter use during and after common general and orthopaedic surgical procedures. These ratings may help reduce catheter-associated complications for patients undergoing these procedures.
Keywords: healthcare quality improvement, nosocomial infections, patient safety
Introduction
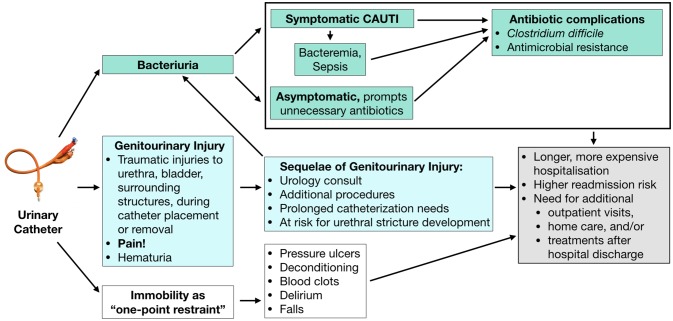
Urinary catheters (commonly known as Foley catheters) are frequently placed in patients undergoing surgical procedures. They serve to prevent bladder distention or incontinence in the anaesthetised patient, as well as facilitate the measurement of urine output during and after surgery. Yet urinary catheters are increasingly recognised as having the potential to harm patients, as illustrated in figure 1.1 Infectious harms include catheter-associated urinary tract infection2 and the potential of drug-resistant infections and orthopaedic prosthetic infections. There are also non-infectious harms, such as urethral pain, trauma and mobility restrictions caused by a 1-point restraint to a urinary drainage device, which are as common as infectious complications and lengthen hospitalisations.3–6 Furthermore, catheter use beyond 48 hours following surgery has been associated with an increase in hospital-acquired urinary tract infections and 30-day mortality, and a decreased likelihood of discharge home.7 8 Unfortunately, significant variation in catheter use exists across routine surgical procedures, often varying by local training and clinician preferences.9
Figure 1.
Infectious and non-infectious urinary catheter complications. CAUTI, catheter-associated urinary tract infection.
Perioperative urinary catheter use has previously been considered routine, but more recent guidelines recommend using urinary catheters only when required and removing them as soon as possible postoperatively.10–18 Some procedure-specific literature reviews and protocols promoting enhanced recovery after surgery provide guidance on when a urinary catheter can be avoided or removed after brief use.10–18 More recently, institutions are developing guidelines for urinary catheter use based on consensus agreement among an institution’s own surgeons,19 20 which can vary by procedure types and case complexity seen at each institution. The currently available guidelines are not defined enough to be both broadly applicable to a large variety of surgical procedures, as well as to account for procedural complexities that only some surgeons and institutions encounter (eg, complex colon surgery).
To address this gap, we applied the RAND Corporation/University of California Los Angeles (RAND/UCLA) Appropriateness Method 21 to formally rate the appropriateness of urinary catheter placement and timing for removal across routine general and orthopaedic surgical procedures in adults, as rated by clinicians who practise in different clinical settings across the USA and informed by the available literature involving perioperative urinary catheter use. We sought to provide guidance for and promote standardisation of urinary catheter use during and after these common procedures in order to appropriately minimise catheter use and related complications, and to be applicable across many institutions — yielding the Michigan Appropriate Perioperative (MAP) criteria for urinary catheter use in common general and orthopaedic surgeries.
Methods
The RAND/UCLA Appropriateness Method21 combines a review of the available scientific evidence for a given practice, in this case urinary catheterisation in the perioperative setting for specific routine procedures, with clinical judgement using multidisciplinary panels of experienced clinicians to produce clinically relevant guidance statements regarding the practice’s appropriateness. Our team previously applied this method to define appropriateness criteria for urinary catheter use in hospitalised medical patients.22
Literature review methods
For each panel, we performed a systematic literature search in Web of Science, Cumulative Index to Nursing and Allied Health Literature, Embase, Cochrane and PubMed/MEDLINE. Searches identified studies involving procedures of interest with respect to urinary catheter use and related outcomes (eg, urinary retention, need for recatheterisation, haematuria, length of stay). Search details are provided in online supplementary appendix figures 1–2 and online supplementary appendix text 1–2. We chose common procedures for general surgery (hernia repair, bariatric procedures, appendectomy, cholecystectomy and colorectal surgery) and orthopaedic surgery (hip fracture repair, elective hip arthroplasty and elective knee arthroplasty). Our final literature searches for the general and orthopaedic surgery procedures were conducted in February and March of 2015, respectively. An expert in urinary catheter use who cares for hospitalised adults reviewed the records meeting the criteria by title, abstract and full-text review to select the final articles.
bmjqs-2018-008025supp001.pdf (1.4MB, pdf)
For each search, we categorised articles into groups based on relevance to perioperative catheter strategies and outcomes. The most relevant articles (group 1) reported at least one infectious or non-infectious outcome of interest for procedures with respect to specific urinary catheter strategies. We also identified key review articles that assessed perioperative catheter use for the procedures of interest. Group 2 articles reported relevant patient outcomes without assessing a particular type of urinary catheter strategy, such as urinary retention rates after cholecystectomy. For the orthopaedic search, we also included studies involving bladder scanner protocols in orthopaedic populations (group 3). We prepared summary tables of the group 1–3 articles, organised by procedure type, study design and type of catheter (eg, indwelling catheter, intermittent straight catheter), to provide panellists with infectious and/or non-infectious outcomes reported.
Panellist selection and rating process
For each panel (general surgery, orthopaedics) we recruited experienced, practising experts from academic, private and government organisations from across the USA to participate by sending an introductory email describing the panel and processes. Panellists included surgeons who performed the procedure of interest, perioperative nurses, a urologist and a hospitalist experienced in comanagement of these surgical patients (tables 1–2).
Table 1.
Characteristics of the general surgery panellists for urinary catheter appropriateness panel
| Name | Title | Affiliation, at time of panel participation | Specialty |
| Hailey Allen, BSN, RN, CBN | Bariatric Program Assistant/Circulator, Weight Management/Surgical Services | Mercy Health Saint Mary’s Hospital, Grand Rapids, Michigan | Nursing |
| Philip Chang, MD, FACS | Medical Director, Perioperative Services; Associate Chief Medical Officer; Section Chief, Trauma and Surgical Critical Care | University of Kentucky, Lexington, Kentucky | General surgery |
| E Patchen Dellinger, MD | Professor of Surgery | University of Washington, Seattle, Washington | General surgery |
| Daniel Eiferman, MD, FACS | Assistant Professor of Surgery, Associate Director of Surgical Intensive Care Unit | Ohio State University, Columbus, Ohio | Acute care surgery and surgical critical care |
| Jonathan F Finks, MD | Associate Professor of Surgery; Associate Director, Michigan Bariatric Surgery Collaborative | University ofMichigan Ann Arbor, Michigan; Michigan Bariatric Surgery Collaborative, Ann Arbor, Michigan |
Bariatric surgery |
| John L Gore, MD, MSHS, FACS | Associate Professor, Department of Urology; Adjunct Associate Professor, Department of Surgery |
University of Washington, Seattle, Washington | Urology |
| Jon Hourigan, MD, FACS, FASCRS | Associate Professor of Surgery | University of Kentucky, Lexington, Kentucky | General surgery |
| Lillian Kao, MD, MS | Professor of Surgery | University of Texas Health Science Center at Houston, Houston, Texas | General surgery and critical care |
| Efren Manjarrez, MD, SFHM | Hospitalist | University of Miami, Miller School of Medicine, Miami, Florida | Hospitalist |
| Shawn Obi, DO, FACS | Chief of Surgery | Allegiance Health, Jackson, Michigan | General surgery |
| Amanda Stricklen, BSN, MS | Senior Project Manager for Bariatric Collaborative, Nurse | Michigan Bariatric Surgery Collaborative, Ann Arbor, Michigan | Nursing |
| Amber Wood, MSN, RN, CNOR, CIC | Perioperative nursing specialist | Association of periOperative Registered Nurses (AORN) | Nursing |
| Marilyn Woodruff, BSN, MSN, ANP-BC | Nurse practitioner specialising in bariatric and general surgery | VA Ann Arbor Healthcare System, Ann Arbor, Michigan | Bariatric and general surgery |
Table 2.
Characteristics of the orthopaedic surgery panellists for urinary catheter appropriateness panel
| Name | Title | Affiliation, at time of panel participation | Specialty |
| Hany Bedair, MD | Assistant Professor, Orthopaedic Surgery | Harvard Medical School, Boston, Massachusetts | Orthopaedic surgery |
| Michael B Cross, MD | Assistant Attending Orthopaedic Surgeon | Hospital for Special Surgery, New York, New York | Orthopaedic surgery |
| Adam Fonrouge, BS, RN | Operating Room Supervisor | Saint Joseph Hospital, Bangor, Maine | Nursing |
| Paul Grant, MD | Assistant Professor of Medicine; Director, Perioperative and Consultative Medicine | University of Michigan, Ann Arbor, Michigan | Hospitalist |
| Paul Holtom, MD | Professor of Medicine and Orthopaedics; Hospital Epidemiologist | Keck School of Medicine, University of Southern California, Los Angeles, California | Infectious diseases |
| Nathan Houchens, MD, FACP | Associate Staff Physician | Cleveland Clinic Hospital, Cleveland, Ohio | Hospitalist |
| Benjamin Miller, MD, MS | Assistant Professor; Staff Physician | University of Iowa, Iowa City, Iowa; VA Iowa City Healthcare System, Iowa City, Iowa | Orthopaedic surgery |
| Nicolas Noiseux, MD, MS, FRCSC | Vice Chair, Clinical Affairs; Assistant Professor, Orthopaedic Surgery | University of Iowa, Iowa City, Iowa | Orthopaedic surgery |
| J Kellogg Parsons, MD, MHS | Associate Professor of Surgery | UC San Diego Moores Cancer Center, La Jolla, California | Urology |
| Thomas Scharschmidt, MD, FACS | Associate Professor | Wexner Medical Center at The Ohio State University, Columbus, Ohio | Orthopaedic surgery |
| Charles Washington, MSN, RN, ACNS-BC | Unit Based Educator: Medical Surgical Unit | VA Ann Arbor Healthcare System, Ann Arbor, Michigan | Nursing education |
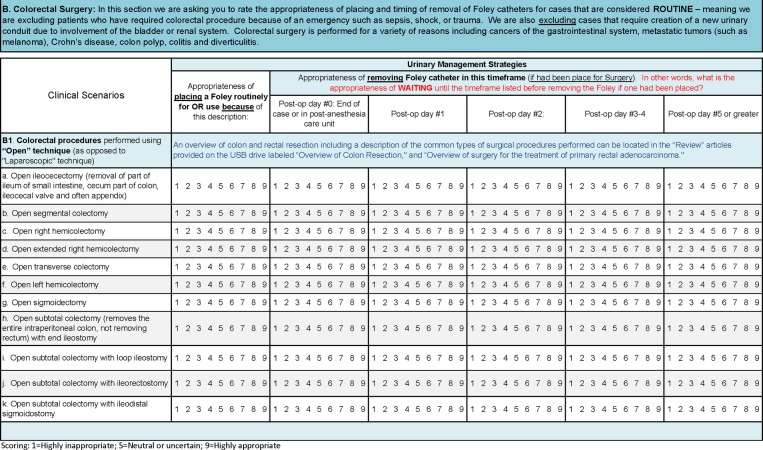
In Spring 2015, panellists were mailed materials that included instructions, printed literature summary tables, articles in electronic form on flash drive, printed key review articles assessing surgical outcomes with respect to urinary catheter strategies, and a round 1 scoring document (example section in figure 2). We included clinical scenarios for panellists to rate the appropriateness of catheter placement (on a scale of 1–9) for intraoperative use and when the first voiding trial should occur after a given surgery. Catheter placement and the timing of removal were considered appropriate if ‘The expected health benefit (eg, relief of pain, reduction in anxiety, improved functional capacity) exceeds the expected negative consequences (eg, mortality, morbidity, anxiety, pain, time lost from work) by a sufficiently wide margin that the procedure is worth doing, exclusive of cost’.23 24 A rating of 1 indicated the harms significantly outweigh the benefits (ie, inappropriate), whereas a rating of 9 indicated the benefits significantly outweigh the harms (ie, appropriate). A central rating of 5 indicated the benefits or harms were considered equal by the participant, or that the participant was unable to make an informed rating of the clinical scenario.
Figure 2.
Example of clinical scenarios from the round 1 rating document.
The scenarios focused on adult patients undergoing routine surgery in acute care inpatient or outpatient settings. We instructed panellists to use their best clinical judgement in combination with evidence from the literature review, assuming no other relevant patient characteristics or clinical indications for a urinary catheter than provided in the scenario. Panellists were instructed to focus on the appropriateness of catheter use with respect to the surgery performed, but without respect to catheter use specifically due to anaesthesia options of spinal or epidural anaesthesia.
After the round 1 ratings were completed, we conducted a 1-hour conference call with the panellists to clarify the clinical scenarios, aiming to reduce disagreement or uncertainty in ratings. The expert panel was then brought together for a face-to-face round 2 meeting, where preliminary scores and rating differences were discussed for each clinical scenario. These meetings occurred in April 2015 (general surgery) and May 2015 (orthopaedics). Panellists rerated each clinical scenario after the inperson discussion. The median round 2 scores were used to classify each scenario as appropriate (panel median score of 7–9); uncertain or neutral (panel median score of 4–6); or inappropriate (panel median score of 1–3) if there was no disagreement among the panel. If four or more panellists rated a scenario as appropriate (score of 7–9) and four or more rated it as inappropriate (score of 1–3), the scenario was rated as uncertain or neutral due to disagreement. Clinical scenario ratings from each panel were reviewed, and converted into a single, one-page, clinician-friendly table of recommendations to consider implementing to reduce catheter use.
Of note, the RAND/UCLA Appropriateness Method is designed to rate the appropriateness of a therapy (in this case, perioperative urinary catheter use) with respect to specific clinical settings. We applied this method to rate the appropriateness of placing a urinary catheter for use during the surgery, as well as the appropriateness of different timings for removal of the urinary catheter for the first voiding attempt. This method deliberately does not require consensus, because it recognises and allows for differences in clinical practice for which variation is acceptable, particularly when the medical literature does not clearly demonstrate one superior strategy of care. Therefore, for some procedures, the recommendations that are provided yield a single recommendation for catheter avoidance or use. However, in other procedures, more than one strategy of care was assessed as appropriate. For example, it may be appropriate to remove a catheter for a specific procedure either on the day of surgery or on the morning after surgery. Thus, this method yields both recommendations for the soonest a catheter could be removed for the first trial of urination without a catheter, as well as guidance for when two strategies of catheter removal are both appropriate, which allows the catheter use criteria to be applied to medical charts to assess appropriate use.
Results
General surgery procedures
General surgery literature summary highlights
Forty-five group 1 studies25–68 (online supplementary appendix table 1) including 18 randomised controlled trials (RCTs) and 1 systematic review13 involving catheter use in colorectal surgery were identified. The procedures studied included colorectal surgery,34–57 bariatric procedures,25–27 cholecystectomy,28–33 herniorrhaphy58–66 and other abdominal surgery types.56 67 68 Surgical approaches included laparoscopic and open procedures of varying levels of complexity, surgery for malignant and benign diagnoses, ‘fast track’ or enhanced recovery after surgery protocols, and surgeries with varied anaesthesia type. Many group 1 studies (online supplementary appendix table 1) using protocols with catheter avoidance or early postoperative catheter removal studies reported equivalent or fewer episodes of retention, recatheterisation or infection; however, several cohort studies reporting catheter use rates did not include a control group.
General surgery panel findings
The final detailed ratings of urinary catheter appropriateness for each of the 91 general surgery clinical scenarios for routine procedures are provided in online supplementary appendix table 2. Table 3 provides the overall summary of perioperative urinary catheter use recommendations, categorising the procedures into three categories: (A) procedures for which indwelling urinary catheter placement should be avoided, (B) procedures to consider removing indwelling urinary catheters before leaving the operating room (OR), and (C) procedures in which urinary catheter use in the OR and until at least postoperative day 1 is appropriate, with guidance on when the first trial of void is appropriate. For a few procedures, as explained above in the Methods section, the RAND/UCLA Appropriateness Method assessed two different timings of the first trial of void as appropriate, such as removal at the end of the case or on postoperative day 1. In these cases, the procedures are listed in table 3 in the earliest trial of void timing (category A, B or C) that was deemed appropriate, with a footnote indicating when later first trials of void were also felt to be appropriate. There was less confidence in removing catheters on postoperative day 0 for some procedures if no postoperative bladder scanner was available as reflected in several median scores compared with appropriate ratings with available bladder scanner. By discussion it was clear that bladder scanner availability and use were now common; table 3 reflects the panel’s recommendations assuming a bladder scanner is available to use in the postoperative setting.
Table 3.
Summary of perioperative urinary catheter use recommendations*
| A. Avoid placing indwelling urinary catheters for these routine procedures: these are procedures for which it is considered inappropriate to place a catheter for the procedure, as the catheter risk is considered to outweigh the benefits for the patient.†‡ | |
General surgery
|
Orthopaedic surgery
|
| B. Procedures to consider removing indwelling urinary catheter before leaving the OR | |
General surgery
|
Orthopaedic surgery
|
| C. Procedures in which urinary catheter use in the OR and until at least postoperative day 1 is appropriate, with the timing for the first trial of void detailed below by procedure | |
General surgery
|
Orthopaedic surgery
|
*These are recommendations for perioperative urinary catheter use for patients without another indication for urinary catheter use (eg, not needed to address a medical indication such as critical illness for which hourly urine output is being used to guide therapy such as vasopressors). For all procedures, using a postoperative protocol to monitor and address urinary retention symptoms is recommended; bladder scanners are increasingly common tools to verify retention in patients with symptoms to avoid unnecessary catheterisations.
†Routine urinary catheter use is not appropriate for these procedures when less than 2 hours of OR time and less than 2 L of intravenous fluids anticipated in the OR. Experts indicated that routine catheter use during the OR case could be appropriate for procedures >3 hours in duration or with >3 L of intraoperative fluids.
‡Patients are recommended to void before surgery. If concerned about postvoid residual, use of bladder scanner protocol with intermittent straight catheter as needed before surgery is an appropriate alternative to routine indwelling catheter use in patients with urinary retention.
§For these procedures, it was assessed also as clinically appropriate to remove catheter on postoperative day 1.
¶For this procedure, there was uncertainty about appropriateness of routinely removing on the same day of surgery; therefore, it could be clinically appropriate to remove earlier than postoperative day 1 by surgeon’s discretion.
**For open low anterior resection, removal before postoperative day 3 is appropriate, but there was uncertainty for whether removal was more appropriate on postoperative day 1 compared to postoperative day 2.
††For laparoscopic abdominal perineal resection, removal by postoperative day 4 is appropriate, but there was uncertainty for whether a particular day within the range of postoperative days 1–4 was more appropriate than others.
‡ ‡For open or laparoscopic total proctocolectomy with or without ileal pouch anal anastamosis, removal by postoperative day 4 is appropriate, but there was uncertainty for whether a particular day within the range of postoperative days 1-4 was more appropriate than others.
OR, operating room; TAPP, transabdominal preperitoneal; TEP, totally extraperitoneal.
Routine catheter placement was rated inappropriate for laparoscopic cholecystectomy, open appendectomy, laparoscopic appendectomy without a suprapubic port, open repair of reducible hernias (inguinal, femoral, umbilical, epigastric), and most laparoscopic repairs of reducible hernias of the same types if the patient voided preoperatively. One exception was laparoscopic reducible inguinal or femoral hernia repair by a totally extraperitoneal approach, for which there was some uncertainty on appropriateness of not using a catheter if the patient voided preoperatively; however, there was agreement on routine catheter placement for use in the OR as appropriate if the patient had not voided, with the recommendation to remove after the procedure, ideally before leaving the OR.
Four laparoscopic bariatric procedures (Roux-en-Y gastric bypass, adjustable gastric banding, sleeve gastrectomy, biliopancreatic diversion with duodenal switch) were discussed as sufficiently common to permit the rating of urinary catheter appropriateness. Routine urinary catheter use in the OR was inappropriate for adjustable gastric banding, of uncertain appropriateness for sleeve gastrectomy, appropriate for Roux-en-Y gastric bypass, and appropriate for biliopancreatic diversion with duodenal switch. For all four procedures, waiting until postoperative day 2 to remove the catheter for the first voiding trial if a catheter had been placed was inappropriate.
Catheter placement in the OR was rated highly appropriate for all routine colorectal procedures queried. For higher colorectal resections (ie, open or laparoscopic ileocecectomy, hemicolectomy, transverse colectomy, or sigmoidectomy), the time of catheter removal for the first voiding trial using a postoperative bladder scan protocol was rated as appropriate as early as postoperative day 0 (such as at the end of the case in the OR) or postoperative day 1, with waiting until postoperative day 2 or later rated as inappropriate. As summarised in table 3 and detailed in online supplementary appendix table 2, there was substantial variation in opinion regarding timing of catheter removal for some of the more complex lower colorectal procedures (eg, laparoscopic abdominal perineal resection), yet high agreement catheters should be removed for all of these procedures for the first trial of void by postoperative day 4. For one procedure, total proctocolectomy, the panellists agreed that removal for the first trial of void by postoperative day 4 was appropriate, but could not agree on a particular day within the range of postoperative days 1–4 as being more appropriate than others.
Panellists were also asked if a suprapubic port in laparoscopic surgery impacted the appropriateness of perioperative urinary drainage strategies. Panellists indicated it was appropriate to have the patient void preoperatively with the option of bladder scanning without placing an indwelling urinary catheter routinely, but were undecided about the appropriateness of routine indwelling urinary catheter placement in this setting. Discussion revealed this practice was highly influenced by how the surgeon trained and if the surgeon had experienced any bladder complications with suprapubic port procedures.
Orthopaedic procedures
Orthopaedic procedure literature summary
Twenty group 1 studies68–87 (online supplementary appendix table 3) including eight RCTs and one narrative review88 involving catheter use in joint replacement were identified. Most articles include both total hip and knee arthroplasty procedures, including both hip fracture and elective hip arthroplasty. Catheter strategies studied included performing surgery without indwelling catheters, removing catheters in the OR, removing early on postoperative day 1 or removal within 48 hours, with several studies involving spinal anaesthesia protocols. Many, but not all, studies demonstrated that avoiding or reducing catheter use was not associated with higher rates of adverse outcomes, including three studies69 75 79 supporting the avoidance of routine indwelling catheter use in patients receiving low-dose spinal analgesia.
Orthopaedic surgery panel findings
The final detailed ratings of urinary catheter appropriateness for each of the 36 orthopaedic surgery clinical scenarios are provided in online supplementary appendix table 4. Table 3 provides the overall summary of the perioperative urinary catheter use recommendations, categorising these routine hip and knee procedures into the same three categories used to categorise the general surgery procedures, as described above. In general, many common hip and knee procedures were assessed as appropriate to be performed without catheters, with other longer procedures appropriate to have a catheter placed but removed shortly after the surgery (such as before leaving the OR) or on postoperative day 1. Waiting until postoperative day 2 or later was assessed as inappropriate for routine knee or hip arthroplasty procedures, including repair of hip fracture.
For knee arthroplasty including unilateral, total or unicompartmental, or revision ≤2 hours, avoiding routine indwelling urinary catheter placement was recommended given it was appropriate to simply empty the bladder preoperatively by voiding or bladder scan with one intermittent straight catheterisation. Routine catheter placement was of uncertain appropriateness for bilateral total knee arthroplasty, meaning it could be used at the surgeon’s discretion. If placed, removal was appropriate on either the day of surgery or postoperative day 1.
For elective unilateral prosthetic hip replacement without hip fracture, routine indwelling urinary catheter use is not recommended. However, routine catheter use was recommended for bilateral or revision hip arthroplasty with expected duration of >2 hours. Panellists were undecided regarding routine catheter placement for revisions lasting 2 hours or less. If a catheter was placed for any of these hip procedures, removal was recommended on postoperative day 0 or 1, as detailed in table 3.
To begin the discussion of catheter use in patients with hip fracture, we first queried the appropriateness of placing an indwelling urinary catheter at the time of presentation to the emergency department in patients with hip fracture with uncontrolled pain. Indwelling urinary catheter placement was rated as inappropriate for male patients given the option of external ‘condom’ urinary catheters, and was ‘uncertain’ by neutral scores for female patients, though this is in the context of most clinicians not yet experienced with the more recently developed female external catheters to collect urine in women who cannot be easily turned. Voicing concern for prosthetic joint infection from urinary catheter-associated bacteriuria or infection, the orthopaedic panel also rated the use of indwelling urinary catheters as inappropriate in the postoperative hip surgery patient with incontinence, regardless of whether the wound was draining or not, either before or after removal of the occlusive dressing.
For unilateral closed reduction percutaneous pinning for femoral neck fracture, the panel ratings indicated that routine indwelling urinary catheter placement for OR use could be avoided, as having the patient void preoperatively or use of bladder scanner and a straight catheter to empy the bladder was appropriate. For other types of hip fracture repairs (open reduction and internal fixation, partial or total prosthetic hip arthroplasty), the panellists were uncertain about appropriateness for routine initial placement for OR use; if placed, catheter removal was appropriate on postoperative day 0 or 1.
Conclusions
We used the RAND/UCLA Appropriateness Method which combines a detailed review of the literature as well as multidisciplinary clinical expertise to inform a formal multiround rating method to determine appropriateness of placing indwelling urinary catheters for use in the OR for common general surgery and orthopaedic procedures, as well as appropriateness of the postoperative timing of urinary catheter removal, producing the Michigan Appropriate Perioperative (MAP) criteria for urinary catheter use.
Although we anticipate that several of the general surgical procedures, particularly when performed in the ambulatory setting, are becoming more commonly performed without urinary catheters, we expect that the panel’s recommendations to avoid catheter use in other common procedures such as several laparoscopic procedures (eg, bariatric, cholecystectomy, appendectomy) have the potential to change care for many patients as there remains tremendous variation in practice between surgeons. We also believe that the panel’s agreement that first voiding trials are appropriate on postoperative day 1, for even many complex colorectal procedures (eg, laparoscopic low anterior resection), and that it was inappropriate to wait more than 4 days postoperatively for a first voiding trial, will lead to more standardisation of early voiding trials for patients receiving these procedures, leading to fewer catheter-associated complications.
The literature and clinical experts were clear that the risk of infection from the urinary catheter needs to be taken very seriously for orthopaedic procedures, given the high morbidity of prosthetic joint infection. We anticipate the recommendations for reducing urinary catheter use in patients with hip fractures may change practice for many patients, both on surgical and medical services.
Our study has several limitations. Though our panels were diverse with broad representation from across the USA—and with significant clinical experience in the procedures evaluated—not every panellist performed every procedure type (or in the case of nurses, cared for every postoperative surgery patient type) that was described in the clinical scenarios for each panel. However, there was significant discussion at the inperson round 2 meeting to allow panellists to query each other’s expertise to inform their own rating of the clinical scenario, as well as the option of rating the catheter appropriateness as uncertain if outside their area of expertise.
Limitations notwithstanding, we believe these MAP criteria for urinary catheter use will inform expectations and interventions at the service/unit level, as well as large-scale interventions to avoid placement and support prompt removal of urinary catheters in surgical patients. In combination with the Ann Arbor Criteria for Appropriate Urinary Catheter Use in Hospitalised Medical Patients,22 these perioperative criteria are anticipated to improve the confidence of clinicians to know when catheters can be safely avoided for routine surgical procedures.
Acknowledgments
The authors thank Helen McGuirk for her assistance with coordination of the panels, and all of the panellists who participated in this project.
Footnotes
Contributors: JM and KEF had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: JM, TAS, KEF, SJB, JD, SS. Acquisition of data: JM, KEF, TAS, SJB, JDM. Analysis and/or interpretation of data: JM, KEF, SJB, JD, SS. Drafting of the manuscript: JM, KEF, JDM. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: KEF. Obtaining funding: JM, SS. Study supervision: JM, TAS, SS.
Funding: This project was funded by a contract from the Agency for Healthcare Research and Quality (AHRQ) (contract HHSA2902010000251/HHSA29032001T). Additional support was received from the University of Michigan and the Department of Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings' effort on this project was funded by concurrent support from AHRQ (K08 HS19767) and Dr. Skolarus' effort was funded by concurrent support from the Department of Veterans Affairs Health Services Research and Development (CDA 12-171).
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the Department of Veterans Affairs.
Competing interests: JM has reported receiving honoraria for lectures and teaching related to prevention and value-based purchasing policies involving catheter-associated urinary tract infection and hospital-acquired pressure ulcers. SS has reported receiving honoraria for lectures and teaching related to prevention of catheter-associated urinary tract infection, and is on the medical advisory boards of Doximity and Jvion.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are included in the online supplementary appendix.
References
- 1. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis 2011;52:1291–3. 10.1093/cid/cir195 [DOI] [PubMed] [Google Scholar]
- 2. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med 2016;374:2111–9. 10.1056/NEJMoa1504906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med 2018;178:1078–85. 10.1001/jamainternmed.2018.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saint S, Lipsky BA, Goold SD. Indwelling urinary catheters: a one-point restraint? Ann Intern Med 2002;137:125–7. 10.7326/0003-4819-137-2-200207160-00012 [DOI] [PubMed] [Google Scholar]
- 5. Aaronson DS, Wu AK, Blaschko SD, et al. National incidence and impact of noninfectious urethral catheter related complications on the Surgical Care Improvement Project. J Urol 2011;185:1756–60. 10.1016/j.juro.2010.12.041 [DOI] [PubMed] [Google Scholar]
- 6. Leuck AM, Wright D, Ellingson L, et al. Complications of Foley catheters–is infection the greatest risk? J Urol 2012;187:1662–6. 10.1016/j.juro.2011.12.113 [DOI] [PubMed] [Google Scholar]
- 7. Wald HL, Epstein AM, Radcliff TA, et al. Extended use of urinary catheters in older surgical patients: a patient safety problem? Infect Control Hosp Epidemiol 2008;29:116–24. 10.1086/526433 [DOI] [PubMed] [Google Scholar]
- 8. Wald HL, Ma A, Bratzler DW, et al. Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data. Arch Surg 2008;143:551–7. 10.1001/archsurg.143.6.551 [DOI] [PubMed] [Google Scholar]
- 9. Greene MT, Fakih MG, Fowler KE, et al. Regional variation in urinary catheter use and catheter-associated urinary tract infection: results from a national collaborative. Infect Control Hosp Epidemiol 2014;35(Suppl 3):S99–S106. 10.1086/677825 [DOI] [PubMed] [Google Scholar]
- 10. Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466–77. 10.1016/j.clnu.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 11. Forsmo HM, Erichsen C, Rasdal A, et al. Enhanced recovery after colorectal surgery (ERAS) in elderly patients is feasible and achieves similar results as in younger patients. Gerontol Geriatr Med 2017;3:233372141770629 10.1177/2333721417706299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg 2013;37:259–84. 10.1007/s00268-012-1772-0 [DOI] [PubMed] [Google Scholar]
- 13. Hendren S. Urinary catheter management. Clin Colon Rectal Surg 2013;26:178–81. 10.1055/s-0033-1351135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larsen K, Hvass KE, Hansen TB, et al. Effectiveness of accelerated perioperative care and rehabilitation intervention compared to current intervention after hip and knee arthroplasty. A before-after trial of 247 patients with a 3-month follow-up. BMC Musculoskelet Disord 2008;9:59 10.1186/1471-2474-9-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:240–58. 10.1007/s00268-012-1771-1 [DOI] [PubMed] [Google Scholar]
- 16. Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009;144:961–9. 10.1001/archsurg.2009.170 [DOI] [PubMed] [Google Scholar]
- 17. Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: enhanced recovery after surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:285–305. 10.1007/s00268-012-1787-6 [DOI] [PubMed] [Google Scholar]
- 18. Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 2010;31:319–26. 10.1086/651091 [DOI] [PubMed] [Google Scholar]
- 19. Sadeghi M, Leis JA, Laflamme C, et al. Standardisation of perioperative urinary catheter use to reduce postsurgical urinary tract infection: an interrupted time series study. BMJ Qual Saf 2019;28:32–8. 10.1136/bmjqs-2017-007458 [DOI] [PubMed] [Google Scholar]
- 20. Thakker A, Briggs N, Maeda A, et al. Reducing the rate of post-surgical urinary tract infections in orthopedic patients. BMJ Open Qual 2018;7:e000177 10.1136/bmjoq-2017-000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND, 2001. [Google Scholar]
- 22. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med 2015;162:S1–34. 10.7326/M14-1304 [DOI] [PubMed] [Google Scholar]
- 23. Brook RH, Chassin MR, Fink A, et al. A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care 1986;2:53–63. 10.1017/S0266462300002774 [DOI] [PubMed] [Google Scholar]
- 24. Park RE, Fink A, Brook RH, et al. Physician ratings of appropriate indications for six medical and surgical procedures. Am J Public Health 1986;76:766–72. 10.2105/AJPH.76.7.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campos GM, Ciovica R, Rogers SJ, et al. Spectrum and risk factors of complications after gastric bypass. Arch Surg 2007;142:969–75. 10.1001/archsurg.142.10.969 [DOI] [PubMed] [Google Scholar]
- 26. Capella JF, Capella RF. Is routine invasive monitoring indicated in surgery for the morbidly obese? Obes Surg 1996;6:50–3. 10.1381/096089296765557268 [DOI] [PubMed] [Google Scholar]
- 27. Schouten R, van Dijke JC, van ’t Hof G, et al. Prevalence and risk factors of urinary incontinence and bladder retention in gastric bypass surgery: a cross-sectional study. Obes Surg 2013;23:760–3. 10.1007/s11695-012-0863-1 [DOI] [PubMed] [Google Scholar]
- 28. Grbas H, Kunisek L, Zelić M, et al. Outcome evaluation of 10,317 laparoscopic cholecystectomies: a 17-year experience at a single center. Hepatogastroenterology 2013;60:1873–6. [PubMed] [Google Scholar]
- 29. Kotake S, Satoh W. Changes in lower urinary tract symptoms before and after using an indwelling urethral catheter. Japan Journal of Nursing Science 2004;1:99–106. 10.1111/j.1742-7924.2004.00014.x [DOI] [Google Scholar]
- 30. Kulaçoğlu H, Dener C, Kama NA. Urinary retention after elective cholecystectomy. Am J Surg 2001;182:226–9. 10.1016/S0002-9610(01)00703-6 [DOI] [PubMed] [Google Scholar]
- 31. Liu SK, Rassai H, Krasner C, et al. Urinary catheter in laparoscopic cholecystectomy: is it necessary? Surg Laparosc Endosc Percutan Tech 1999;9:184–6. 10.1097/00129689-199906000-00005 [DOI] [PubMed] [Google Scholar]
- 32. Majeed AW, Plura M, Priest S, et al. Is it necessary to catheterise the bladder before laparoscopy? Surg Laparosc Endosc 1998;8:157–8. 10.1097/00019509-199804000-00017 [DOI] [PubMed] [Google Scholar]
- 33. Mowschenson PM, Weinstein ME. Why catheterize the bladder for laparoscopic cholecystectomy? J Laparoendosc Surg 1992;2:215–7. 10.1089/lps.1992.2.215 [DOI] [PubMed] [Google Scholar]
- 34. Agrafiotis AC, Corbeau M, Buggenhout A, et al. Enhanced recovery after elective colorectal resection outside a strict fast-track protocol. A single centre experience. Int J Colorectal Dis 2014;29:99–104. 10.1007/s00384-013-1767-9 [DOI] [PubMed] [Google Scholar]
- 35. Baek SJ, Kim SH, Kim SY, et al. The safety of a "fast-track" program after laparoscopic colorectal surgery is comparable in older patients as in younger patients. Surg Endosc 2013;27:1225–32. 10.1007/s00464-012-2579-7 [DOI] [PubMed] [Google Scholar]
- 36. Baird G, Maxson P, Wrobleski D, et al. Fast-track colorectal surgery program reduces hospital length of stay. Clin Nurse Spec 2010;24:202–8. 10.1097/NUR.0b013e3181e3604c [DOI] [PubMed] [Google Scholar]
- 37. Basse L, Werner M, Kehlet H. Is urinary drainage necessary during continuous epidural analgesia after colonic resection? Reg Anesth Pain Med 2000;25:498–501. 10.1097/00115550-200009000-00010 [DOI] [PubMed] [Google Scholar]
- 38. Benoist S, Panis Y, Denet C, et al. Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery 1999;125:135–41. 10.1016/S0039-6060(99)70256-4 [DOI] [PubMed] [Google Scholar]
- 39. Bona S, Molteni M, Spinelli A, et al. Fast-track protocol in laparoscopic colorectal surgery: preliminary experience of a pilot study. Surg Endosc 2012;26:S29. [Google Scholar]
- 40. Cremona F, Pace U, Belli A, et al. Clinical benefit of fast-track protocol in frail elderly patients with colorectal cancer. Surg Endosc 2011;13:58. [Google Scholar]
- 41. de Moya MA, Zacharias N, Osbourne A, et al. Colovesical fistula repair: is early foley catheter removal safe? J Surg Res 2009;156:274–7. 10.1016/j.jss.2009.03.094 [DOI] [PubMed] [Google Scholar]
- 42. Foster JD, Smart NJ, White P, et al. An early prediction model for deviation and failure of enhanced recovery after surgery (ERAS) following laparoscopic colorectal surgery. Surg Endosc 2013;27:S98. [DOI] [PubMed] [Google Scholar]
- 43. Hardt J, Schwarzbach M, Hasenberg T, et al. The effect of a clinical pathway for enhanced recovery of rectal resections on perioperative quality of care. Int J Colorectal Dis 2013;28:1019–26. 10.1007/s00384-013-1650-8 [DOI] [PubMed] [Google Scholar]
- 44. Khoury W, Dakwar A, Sivkovits K, et al. Fast-track rehabilitation accelerates recovery after laparoscopic colorectal surgery. JSLS 2014;18:e2014.00076 10.4293/JSLS.2014.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kolozsvari NO, Capretti G, Kaneva P, et al. Impact of an enhanced recovery program on short-term outcomes after scheduled laparoscopic colon resection. Surg Endosc 2013;27:133–8. 10.1007/s00464-012-2446-6 [DOI] [PubMed] [Google Scholar]
- 46. Lee SM, Jang JH, Kim DW, et al. Comparison of early mobilization and diet rehabilitation program with conventional care after laparoscopic low anterior resection: A prospective randomized controlled trial. Surg Endosc 2012;26:S190. [DOI] [PubMed] [Google Scholar]
- 47. Mahajna A, Masarwee A, Bishara B, et al. Laparoscopy and nullfast tracknull rehabilitation in colorectal surgery - Does it improve the patients' outcome (our initial results). Tech Coloproctol 2010;14:91. [Google Scholar]
- 48. Mahajna A, Serkovich K, Assalia A, et al. Laparoscopy and ’fast track' rehabilitation in colorectal surgery-does it improve the patients' outcome. Surg Endosc 2012;26:S92. [Google Scholar]
- 49. Mahajna A, Wissam A, Bishara B, et al. Laparoscopy and fast-track rehabilitation in colorectal surgery-does it improve patient outcome. Tech Coloproctol 2011;15:123. [Google Scholar]
- 50. Nagle D, Curran T, Anez-Bustillos L, et al. Reducing urinary tract infections in colon and rectal surgery. Dis Colon Rectum 2014;57:91–7. 10.1097/DCR.0000000000000019 [DOI] [PubMed] [Google Scholar]
- 51. Nakagoe T, Sawai T, Tsuji T, et al. Use of minilaparotomy in the treatment of colonic cancer. Br J Surg 2001;88:831–6. 10.1046/j.1365-2168.2001.01765.x [DOI] [PubMed] [Google Scholar]
- 52. Patel GN, Rammos CK, Patel JV, et al. Further reduction of hospital stay for laparoscopic colon resection by modifications of the fast-track care plan. Am J Surg 2010;199:391–5. discussion 94-5 10.1016/j.amjsurg.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 53. Scatizzi M, Kröning KC, Boddi V, et al. Fast-track surgery after laparoscopic colorectal surgery: is it feasible in a general surgery unit? Surgery 2010;147:219–26. 10.1016/j.surg.2009.09.035 [DOI] [PubMed] [Google Scholar]
- 54. Smart NJ, White P, Allison AS, et al. Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis 2012;14:e727–34. 10.1111/j.1463-1318.2012.03096.x [DOI] [PubMed] [Google Scholar]
- 55. Stubbs BM, Badcock KJ, Hyams C, et al. A prospective study of early removal of the urethral catheter after colorectal surgery in patients having epidural analgesia as part of the Enhanced Recovery After Surgery programme. Colorectal Dis 2013;15:733–6. 10.1111/codi.12124 [DOI] [PubMed] [Google Scholar]
- 56. Thompson EG, Gower ST, Beilby DS, et al. Enhanced recovery after surgery program for elective abdominal surgery at three Victorian hospitals. Anaesth Intensive Care 2012;40:450–9. [DOI] [PubMed] [Google Scholar]
- 57. Zmora O, Madbouly K, Tulchinsky H, et al. Urinary bladder catheter drainage following pelvic surgery–is it necessary for that long? Dis Colon Rectum 2010;53:321–6. 10.1007/DCR.06013e3181c7525c [DOI] [PubMed] [Google Scholar]
- 58. Antonescu I, Baldini G, Watson D, et al. Impact of a bladder scan protocol on discharge efficiency within a care pathway for ambulatory inguinal herniorraphy. Surg Endosc 2013;27:4711–20. 10.1007/s00464-013-3119-9 [DOI] [PubMed] [Google Scholar]
- 59. Ferzli G, Sayad P, Huie F, et al. Endoscopic extraperitoneal herniorrhaphy. A 5-year experience. Surg Endosc 1998;12:1311–3. [DOI] [PubMed] [Google Scholar]
- 60. Hudak KE, Frelich MJ, Rettenmaier CR, et al. Surgery duration predicts urinary retention after inguinal herniorrhaphy: a single institution review. Surg Endosc 2015;29:3246–50. 10.1007/s00464-015-4068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mazeh H, Beglaibter N, Grinbaum R, et al. Laparoscopic inguinal hernia repair on a general surgery ward: 5 years' experience. J Laparoendosc Adv Surg Tech A 2008;18:373–6. 10.1089/lap.2007.0108 [DOI] [PubMed] [Google Scholar]
- 62. O’Connell JE, Kearney DE, Kukaswadia S, et al. Incidence of and risk factors for post-operative urinary retention in patients undergoing laparoscopic inguinal hernia repair. Colorectal Dis 2014;16:186.24267200 [Google Scholar]
- 63. Pavlin DJ, Pavlin EG, Gunn HC, et al. Voiding in patients managed with or without ultrasound monitoring of bladder volume after outpatient surgery. Anesth Analg 1999;89:90–7. [DOI] [PubMed] [Google Scholar]
- 64. Ryan JA, Adye BA, Jolly PC, et al. Outpatient inguinal herniorrhaphy with both regional and local anesthesia. Am J Surg 1984;148:313–6. 10.1016/0002-9610(84)90461-6 [DOI] [PubMed] [Google Scholar]
- 65. Sivasankaran MV, Pham T, Divino CM. Incidence and risk factors for urinary retention following laparoscopic inguinal hernia repair. Am J Surg 2014;207:288–92. 10.1016/j.amjsurg.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 66. Urbach KF, Lee WR, Sheely LL, et al. Spinal or general anesthesia for inguinal hernia repair?A comparison of certain complications in a controlled series. JAMA 1964;190:25–9. [DOI] [PubMed] [Google Scholar]
- 67. Greig JD, Mahadaven M, John TG, et al. Comparison of manual and ultrasonographic evaluation of bladder size in patients prior to laparoscopy. Surg Endosc 1996;10:432–3. 10.1007/BF00191633 [DOI] [PubMed] [Google Scholar]
- 68. Stéphan F, Sax H, Wachsmuth M, et al. Reduction of urinary tract infection and antibiotic use after surgery: a controlled, prospective, before-after intervention study. Clin Infect Dis 2006;42:1544–51. 10.1086/503837 [DOI] [PubMed] [Google Scholar]
- 69. Balderi T, Mistraletti G, D’Angelo E, et al. Incidence of postoperative urinary retention (POUR) after joint arthroplasty and management using ultrasound-guided bladder catheterization. Minerva Anestesiol 2011;77:1050–7. [PubMed] [Google Scholar]
- 70. Colon Cabassa S. Nurse-generated reminder system to reduce catheter associated urinary tract infection (Doctoral Dissertation): Fairleigh Dickinson University, 2010. [Google Scholar]
- 71. Emmett P, Faulkerson J, Gaudoin T. Reduction in duration of post-operative urinary catheters following implementation of an electronic reminder system. Am J Infect Control 2012;40:e62 10.1016/j.ajic.2012.04.108 [DOI] [Google Scholar]
- 72. Hozack WJ, Carpiniello V, Booth RE. The effect of early bladder catheterization on the incidence of urinary complications after total joint replacement. Clin Orthop Relat Res 1988:79–82. 10.1097/00003086-198806000-00009 [DOI] [PubMed] [Google Scholar]
- 73. Iorio R, Healy WL, Patch DA, et al. The role of bladder catheterization in total knee arthroplasty. Clin Orthop Relat Res 2000:80–4. 10.1097/00003086-200011000-00011 [DOI] [PubMed] [Google Scholar]
- 74. Johansson I, Athlin E, Frykholm L, et al. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs 2002;11:651–6. 10.1046/j.1365-2702.2002.00646.x [DOI] [PubMed] [Google Scholar]
- 75. Karason S, Olafsson TA. Avoiding bladder catheterisation in total knee arthroplasty: patient selection criteria and low-dose spinal anaesthesia. Acta Anaesthesiol Scand 2013;57:639–45. 10.1111/aas.12089 [DOI] [PubMed] [Google Scholar]
- 76. Knight RM, Pellegrini VD. Bladder management after total joint arthroplasty. J Arthroplasty 1996;11:882–8. 10.1016/S0883-5403(96)80127-6 [DOI] [PubMed] [Google Scholar]
- 77. Lampe HI, Sneller ZW, Rijnberg WJ. [Urination problems following total hip arthroplasty: insertion or not of an indwelling catheter?]. Ned Tijdschr Geneeskd 1992;136:827–31. [PubMed] [Google Scholar]
- 78. Michelson JD, Lotke PA, Steinberg ME. Urinary-bladder management after total joint-replacement surgery. N Engl J Med 1988;319:321–6. 10.1056/NEJM198808113190601 [DOI] [PubMed] [Google Scholar]
- 79. Miller AG, McKenzie J, Greenky M, et al. Spinal anesthesia: should everyone receive a urinary catheter?: a randomized, prospective study of patients undergoing total hip arthroplasty. J Bone Joint Surg Am 2013;95:1498–503. 10.2106/JBJS.K.01671 [DOI] [PubMed] [Google Scholar]
- 80. Hälleberg Nyman M, Gustafsson M, Langius-Eklöf A, et al. Intermittent versus indwelling urinary catheterisation in hip surgery patients: a randomised controlled trial with cost-effectiveness analysis. Int J Nurs Stud 2013;50:1589–98. 10.1016/j.ijnurstu.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 81. Oishi CS, Williams VJ, Hanson PB, et al. Perioperative bladder management after primary total hip arthroplasty. J Arthroplasty 1995;10:732–6. 10.1016/S0883-5403(05)80067-1 [DOI] [PubMed] [Google Scholar]
- 82. Ritter MA, Faris PM, Keating EM. Urinary tract catheterization protocols following total joint arthroplasty. Orthopedics 1989;12:1085–7. [DOI] [PubMed] [Google Scholar]
- 83. Schneider MA. Prevention of catheter-associated urinary tract infections in patients with hip fractures through education of nurses to specific catheter protocols. Orthop Nurs 2012;31:12–18. 10.1097/NOR.0b013e3182419619 [DOI] [PubMed] [Google Scholar]
- 84. Slappendel R, Weber EW. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopaedic surgery and its effect on urinary tract infections. Eur J Anaesthesiol 1999;16:503–6. 10.1097/00003643-199908000-00001 [DOI] [PubMed] [Google Scholar]
- 85. Uberoi V, Calixte N, Orlando R, et al. A strategy to reduce foley days, post operative urinary retention, and catheter-associated urinary tract infections. J Urol 2012;187:e111–2. 10.1016/j.juro.2012.02.333 [DOI] [Google Scholar]
- 86. van den Brand IC, Castelein RM. Total joint arthroplasty and incidence of postoperative bacteriuria with an indwelling catheter or intermittent catheterization with one-dose antibiotic prophylaxis: a prospective randomized trial. J Arthroplasty 2001;16:850–5. 10.1054/arth.2001.25547 [DOI] [PubMed] [Google Scholar]
- 87. Wiley MJ, Tran TA. Perioperative urinary catheterisation in conjunction with epidural anaesthesia for hip and knee arthroplasty. Is it safe? Int J Surg Investig 1999;1:157–60. [PubMed] [Google Scholar]
- 88. Edmond L. A brief literature search and clinical audit of postoperative urinary retention following total joint replacement. J Orthop Nurs 2006;10:67–72. 10.1016/j.joon.2006.03.001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqs-2018-008025supp001.pdf (1.4MB, pdf)