Abstract
Maple syrup is a natural sweetener that is consumed worldwide. It has been previously reported that dark-colored maple syrup exerts an inhibitory effect on colorectal cancer (CRC) proliferation and invasion. In the present study, the underlying mechanism of CRC cell growth inhibition was examined with dark-colored maple syrup treatment using a shotgun liquid chromatography-tandem mass spectrometry-based global proteomic approach. Applying a semi-quantitative method based on spectral counting, 388 proteins were identified with expression changes of >1.5-fold following dark-colored maple syrup treatment. Gene Ontology analysis revealed that these proteins possessed cell cycle-associated functions. It was also indicated that CRC cells treated with dark-colored maple syrup exhibited decreased proliferating cell nuclear antigen (PCNA) expression and S-phase cell cycle arrest. Dark-colored maple syrup treatment also resulted in altered expression of cell cycle-associated genes, including cyclin-dependent kinase (CDK)4 and CDK6. In conclusion, these data suggested that dark-colored maple syrup induced S-phase cell cycle arrest in CRC cells by reducing the expression of PCNA and regulating cell cycle-associated genes. These findings suggest that dark-colored maple syrup may be a source of compounds for the development of novel drugs for colorectal cancer treatment.
Keywords: maple syrup, colorectal cancer, proteomics, cell cycle, proliferating cell nuclear antigen
Introduction
Colorectal cancer (CRC) is one of the most commonly diagnosed types of cancer and is the second leading cause of cancer-associated mortality worldwide (1,2). Etiological studies report that dietary factors serve an important role in CRC carcinogenesis (3–5). CRC risk is increased by a high intake of red and processed meats (6), as red meat is a contributing factor in the initiation of colorectal carcinogenesis (7). However, milk and other dairy products reportedly have a protective effect against CRC, due to their high calcium content and bioactive constituents, including vitamin D (8–10). Fermented dairy products, including yogurt, also exhibit protective effects against CRC, possibly due to lactic acid bacteria and their reported ability to inactivate intestinal carcinogens and therefore reduce CRC risk (11). Although the present epidemiological evidence is insufficient (12–16), diet-associated preventative measures may be an important strategy for CRC reduction.
Maple syrup is a natural sweetener produced by boiling down sap, which is collected from the sugar maple, Acer saccharum, and is consumed worldwide by individuals of all ages (17,18). The sugar maple is distributed throughout North America, and maple trees serve an important role in traditional medicine among Native Americans (19). A number of previous studies have examined the chemical composition and biological properties of maple-derived products, including maple syrup (20–26).
The climatic conditions during production season influence maple sap composition, including the color, the aroma and the taste of the maple syrup, which vary based on the season of sap collection (27–29). Maple syrup is primarily graded according to its flavor and transmittance, including visual color differences, ranging from light-colored and delicately flavored, to dark-colored and strongly flavored (18). Although the variation in composition may further lead to different grades of maple syrup, along with different biological effects, the differences in composition of ingredients among each grade of maple syrup remain unknown and require further investigation. In our previous study of the anticancer effects of different grades of maple syrup, it was reported that dark-colored maple syrup reduced AKT, also termed protein kinase B, activation, and therefore significantly inhibited proliferation and invasion in CRC cells (30). In addition, another previous study showed that it significantly inhibited growth in other types of gastrointestinal cancer cell (31). This suggests that dark-colored maple syrup may be a useful dietary factor for potentially preventing cancer progression.
In the present study, a shotgun liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based global proteomic analysis was performed on human CRC cells treated with different grades of maple syrup, in order to examine the underlying mechanism behind dark-colored maple syrup inhibiting CRC proliferation. Two types of maple syrup, which indicated the strongest and weakest anticancer effects in our previous study of colon cancer cells (30), were selected. A total of 388 proteins were identified that were differentially expressed in CRC cells treated with dark-grade maple syrup compared with extra-light grade maple syrup. The current study focused on the expression of proliferating cell nuclear antigen (PCNA), which is a key factor of cell cycle regulation. Therefore, further investigations were conducted on whether changes of PCNA expression following dark grade maple syrup treatment may be involved in cell cycle regulation in human CRC cells.
Materials and methods
Materials
Urea was purchased from GE Healthcare Life Sciences (Little Chalfont, UK), and thiourea and Triton X-100 were obtained from Nacalai Tesque, Inc., (Kyoto, Japan). All other chemicals and reagents were purchased from Wako Pure Chemical Industries, Ltd., (Osaka, Japan). Maple syrups were purchased at a local grocery store in Osaka, Japan, in March 2015.
Based on Canadian standards, maple syrup is classified into the following five grades: AA, extra light; grade A, light; grade B, medium; grade C, amber, and grade D dark. Since the present study was performed the maple syrup classification has changed according to the following: Golden, delicate Taste; amber, rich Taste; dark, robust taste; and very dark, strong taste (32). In Japan, these new grading maple syrup grades have been used since April 2017. Since the differences in ingredient composition among each grade of maple syrup are not yet fully understood, in the present study, two grades of maple syrup were selected: The extra light grade maple syrup, which has a slightly golden tint and a delicate flavor with >75% light transmission, and the dark grade maple syrup, which has a much darker brown color and a strong flavor with <25% light transmission.
The colorectal cancer DLD-1 cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). All cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in an atmosphere containing 5% CO2.
Protein preparation
The DLD-1 cells were plated at a density of 2×105 cells/60-mm dish with RPMI-1640 medium. The next day, the culture medium was replaced with culture medium with 1% (v/v) extra light grade maple syrup (extra), dark grade maple syrup (dark) or without syrup (control). This concentration was selected due to results from our previous study indicating a lack of cytotoxic effects against DLD-1 cells due to the high concentration of sucrose (30). After 72 h, the cells were solubilized in urea lysis buffer (7 M urea, 2 M thiourea, 5% 3-[(3-Cholamidopropyl) dimethylammonio] propanesulfonate and 1% Triton X-100), and the protein concentration was measured with the Bio-Rad Protein assay (cat. no. 5000006JA; Bio-Rad Laboratories Inc., Hercules, CA, USA), according to the manufacturer's protocols.
Gel-free digestion was subsequently performed, as described previously (33). Briefly, 10 µg protein extract from each sample was reduced by adding 45 mM dithiothreitol and 20 mM tris (2-carboxyethyl) phosphine. The proteins were subsequently alkylated with 100 mM iodoacetic acid. Following alkylation, the samples were digested at 37°C for 24 h using MS-grade trypsin gold (Promega Corporation, Madison, WI, USA). Finally, the digests were purified using PepClean C-18 Spin Columns (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Liquid chromatography tandem-mass spectrometry (LC-MS/MS) analysis for protein identification
Peptide samples (~2 µg) were injected into a peptide L-trap column (Chemicals Evaluation and Research Institute, Tokyo, Japan) with an HTC PAL autosampler (CTC Analytics AG, Zwingen, Switzerland). The samples were subsequently further separated through a Paradigm MS4 (AMR Inc., Tokyo, Japan) with a reverse-phase C18-column (L-column, 3-µm-diameter gel particles, 120 Å pore size, 0.2×150 mm; Chemicals Evaluation and Research Institute, Tokyo, Japan). The column flow rate was 1 µl/min, and the mobile phase consisted of 0.1% formic acid in water (solution A) and acetonitrile (solution B), with a concentration gradient of 5% solution B to 40% solution B over 120 min. Gradient-eluted peptides were introduced into the mass spectrometer through the nanoelectrospray ionization (NSI) interface that had a separation column outlet directly connected with an NSI needle. The peptides were analyzed with an LTQ ion-trap mass spectrometer (Thermo Fisher Scientific, Inc.). No sheath or auxiliary gas was used. The MS scan sequence used was full-scan MS in the normal/centroid mode and sequential MS/MS in the normal/centroid mode. The positive ion mass spectra were acquired in a data-dependent manner, with MS/MS fragmentation performed on the two most intense peaks of every full MS scan with an isolation width of 1.0 m/z and a collisional activation amplitude of 35% in the m/z range of 300–2,000.
All MS/MS spectral data were searched against the SwissProt Homo Sapiens database (https://www.uniprot.org/) using Mascot version 2.4.01 (Matrix Science, Ltd., London, UK). The search criteria were ‘enzyme’ and ‘trypsin’, with the following allowances: ≤2 missed cleavage peptides; mass tolerance, ± 2.0 Da; MS/MS tolerance, ± 0.8 Da; cysteine carbamidomethylation; and methionine oxidation modifications.
Semi-quantitative analysis of identified proteins
The fold-change in expression was calculated as the log2 ratio of protein abundance (Rsc), evaluated by spectral counting (34). For comparisons, the relative amounts of identified proteins were calculated using the normalized spectral abundance factor (NSAF) (35). Differentially expressed proteins were considered significant when the Rsc was >0.585 or <-0.585, corresponding to fold-changes of >1.5 or <0.66, respectively.
Gene ontology (GO) analysis
The functions of proteins that indicated altered expression with maple syrup treatment were additionally investigated. Their sequences were assigned to Kyoto Encyclopedia of Genes and Genomes (https://www.genome.jp/kegg/kegg_ja.html) signaling pathway terms to examine their functional annotations using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) version 6.8 (http://david.abcc.ncifcrf.gov/home.jsp) (36–38). P<0.05 was considered to indicate a significant category.
Western blot analysis
Total protein (5 µg) that had been prepared as aforementioned was mixed with loading buffer and boiled at 95°C for 10 min. The proteins were then separated on a 12% SDS-PAGE gel. The separated proteins were transferred to polyvinylidene fluoride membranes (Merck KGaA, Darmstadt, Germany) for 30 min at 15 V. Following blocking in TBS-Tween-20 (0.1%) buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) with 5% skimmed milk for 2 h at room temperature, the membranes were incubated with an anti- PCNA antibody (1:20,000; cat. no. 13110; Cell Signaling Technology, Inc.) at 4°C overnight. The membranes were subsequently washed and incubated with horseradish peroxidase-conjugated anti-rabbit immunoglobulin(Ig)G antibody (1:4,000; cat. no. A106PU; American Qualex, San Clemente, CA, USA) at room temperature for 1 h. The blots were washed and visualized with SuperSignal West Dura Extended Duration substrate (Thermo Fisher Scientific, Inc.). The bands were analyzed with the myECL Imager system 2.0 software (Thermo Fisher Scientific, Inc.). The membranes were subsequently stripped by Restore Western Blot Stripping buffer (Thermo Fisher Scientific, Inc.), and the same membranes were re-probed with an anti-β-actin antibody (1:5,000 dilution; cat. no. sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight, which served as the protein loading control. The relative quantities of PCNA over β-actin were used to evaluate PCNA expression under different conditions. All western blot analyses were performed as three independent experiments.
Cell cycle analysis by flow cytometry
To analyze cell cycle distribution, DNA was stained with propidium iodide (PI; Nacalai Tesque, Inc., Kyoto, Japan). Briefly, DLD-1 cells were plated at a density of 2×105 cells/100-mm dish in culture medium. The next day, the culture medium was replaced with 1% (v/v) extra light grade maple syrup (Extra), dark grade maple syrup (Dark) or without syrup (control). Following 72 h of incubation at 37°C, the cells were washed with PBS and fixed in ice-cold 70% ethanol at 4°C for 2 h. The cells were subsequently treated with 0.25 mg/ml RNase in PBS for 60 min at 37°C, followed by staining with 50 µg/ml PI in PBS for 30 min at 4°C in the dark. Cell proportion in different phases of the cell cycle was determined using a BD LSRFortessa flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using FACS DIVA software v8.0.1 (BD Biosciences).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) using TaqMan array analysis
Total RNA was extracted from treated DLD-1 cells using the GenElute Mammalian Total RNA Miniprep kit (cat. no. RTN70-1KT; Sigma-Aldrich; Merck KGaA), according to the manufacturer's protocols. From the extracted RNA, cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (cat. no. 4368814; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. Analysis was performed using TaqMan Array Human Cyclins & Cell Cycle Regulation 96-Well Plates (cat. no. 4414123; Thermo Fisher Scientific, Inc.) in the 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. The thermocycling conditions were as follows: Denaturation at 95°C for 20 sec, followed by 40 cycles of amplification at 95°C for 3 sec and 60°C for 30 sec. The relative gene expression was calculated using the 2−ΔΔCq method (39–43). The ΔΔCq method uses the normalized ΔCq value of each sample, which was calculated with 18S rRNA as the endogenous control gene. The ΔΔCq value is the difference between treated and control samples. Finally, the fold-change was determined as 2−ΔΔCq.
Statistical analysis
All experiments were repeated at a minimum of three times. All data are presented as the mean ± standard error of the mean (SEM). The data were analyzed by one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference. Computations were performed using GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Identification and semi-quantitative comparisons of differentially expressed proteins in maple syrup-treated DLD-1 cells
To investigate the inhibitory effects of maple syrup on CRC proliferation, shotgun proteomics was used to examine the molecular profile of proteins that were regulated by maple syrup treatment. Applying the aforementioned search parameters, a total of 575 proteins were identified in DLD-1 cells treated with extra light grade maple syrup, and 549 proteins in DLD-1 cells treated with dark-colored maple syrup. Proteins were categorized into the ‘Extra’ and ‘Dark’ groups accordingly.
The proteins expressed in maple syrup-treated DLD-1 cells were further evaluated using a label-free semi-quantitative method based on spectral counting. The Rsc values were calculated for the proteins identified in the Extra and Dark groups. A positive Rsc value indicated increased expression with dark-colored maple syrup treatment, and a negative value indicated reduced expression with dark-colored maple syrup treatment (Fig. 1; light grey area). The NSAF value was also calculated for each protein identified in the Extra and Dark groups. Proteins with a >0.585 and <-0.585 Rsc value were considered candidate dark-colored maple syrup-regulated proteins.
Figure 1.
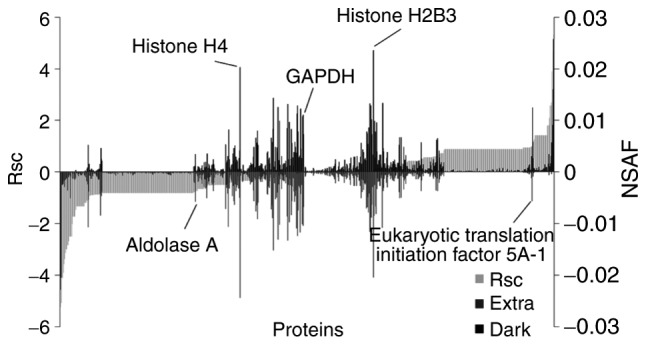
Semi-quantitative comparison of proteins differentially expressed in maple syrup-treated DLD-1 cells. For the identified proteins, Rsc and NSAF values were calculated to compare protein expression levels between DLD-1 cells treated with extra light maple syrup vs. dark-colored maple syrup. Proteins are positioned along the x-axis, according to Rsc value, increasing from left to right (light grey area). NSAF values are indicated for the Extra group (below the axis; grey bar) and for the Dark group (above the axis; black bar). Proteins highly expressed in the Extra and Dark groups, respectively, are near the left and right sides of the x-axis. Housekeeping proteins are located near the center of the x-axis. Rsc, log2 ratio of protein abundance; NSAF, normalized spectral abundance factor; Extra, extra light maple syrup; Dark, dark-colored maple syrup.
This semi-quantitative procedure resulted in the identification of 388 proteins that were differentially expressed with dark-colored maple syrup treatment (data not shown). Maple syrup treatment did not alter the expression of housekeeping proteins, including glyceraldehyde-3-phosphate dehydrogenase and histone H4 (Fig. 1).
Functional annotation of proteins regulated by maple syrup
GO analysis of the candidate dark-colored maple syrup-regulated proteins was performed. GO terms associated with ‘pathway’ were searched for in DAVID (Fig. 2), and the focus was on proteins classified as associated with the ‘cell cycle’ (Table I).
Figure 2.
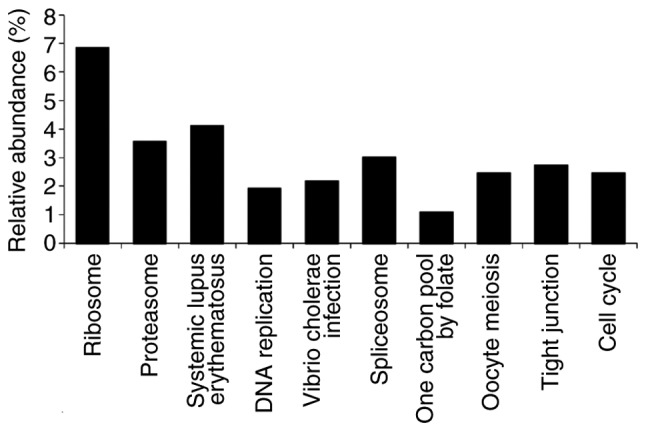
Gene Ontology analysis of identified proteins. Differentially-expressed proteins were assigned to Kyoto Encyclopedia of Genes and Genomes signaling pathway terms. Only significant categories are presented (P<0.05).
Table I.
Proteins categorized as associated with the ‘cell cycle’ in Gene Ontology analysis.
| Accession number | Description | Fold-change (Rsc) |
|---|---|---|
| P33992 | DNA replication licensing factor MCM5 | −0.812 |
| P49736 | DNA replication licensing factor MCM2 | −0.812 |
| P33991 | DNA replication licensing factor MCM4 | −0.812 |
| P12004 | Proliferating cell nuclear antigen | −0.781 |
| P62258 | 14-3-3 protein ε | −0.648 |
| Q14566 | DNA replication licensing factor MCM6 | 0.884 |
| Q13547N | Histone deacetylase 1 | 0.884 |
| P61981 | 14-3-3 protein γ | 2.107 |
| P27348 | 14-3-3 protein τ | 2.573 |
Rsc, log2 ratio of protein abundance.
Effects of maple syrup on PCNA expression in DLD-1 cells
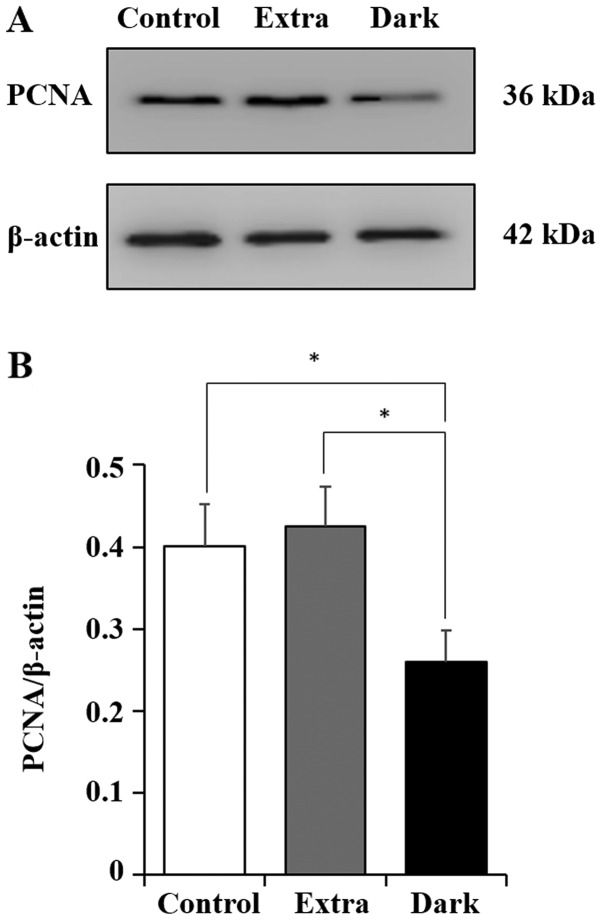
The expression of PCNA protein in maple syrup-treated DLD-1 cells was examined. The results of the present study indicated a significant decrease in PCNA expression with dark-colored maple syrup treatment (Dark) compared with that in the cells treated with extra light grade maple syrup treatment (Extra) and the untreated (control) cells (Fig. 3).
Figure 3.
PCNA expression in maple syrup-treated DLD-1 cells. (A) Western blot analysis indicated the expression levels of PCNA in DLD-1 cells treated in the control group (without maple syrup) and in the two maple syrup groups, the Extra and Dark groups. (B) Quantification of western blot analysis results. Data is presented as the mean ± standard error of the mean of three independent experiments in maple syrup-treated DLD-1 cells. *P<0.05. PCNA, proliferating cell nuclear antigen; Extra, extra light maple syrup; Dark, dark-colored maple syrup.
Effects of maple syrup on cell cycle progression of CRC cells
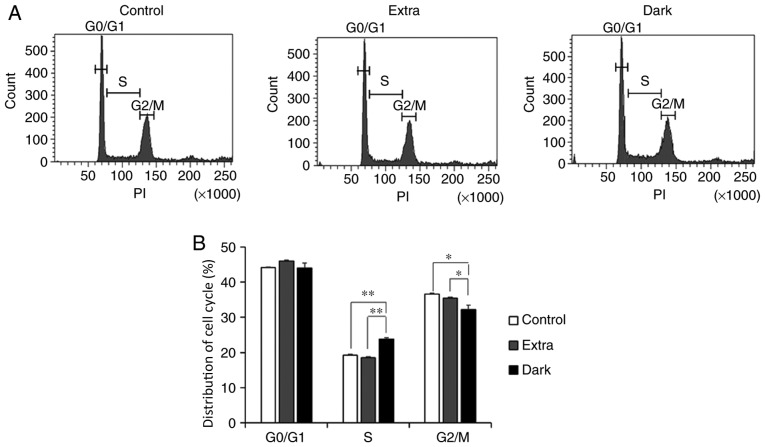
The present study also investigated whether the dark-colored maple syrup-induced decrease in PCNA expression affected cell cycle progression in DLD-1 cells. Flow cytometry analysis revealed a significantly increased cell population in the S phase (P<0.01), and a decreased population in the G2/M phase (P<0.05) in the Dark group compared with that in the Extra group and the control group (Fig. 4A and B).
Figure 4.
Flow cytometry analysis of cell cycle progression in maple syrup-treated DLD-1 cells. (A) Representative histograms of cell cycle distribution in maple syrup-treated DLD-1 cells. (B) Data is presented as the mean ± standard error of the mean of three independent experiments in maple syrup-treated DLD-1 cells. *P<0.05 and **P<0.01. PI, propidium iodide; Extra, extra light maple syrup; Dark, dark-colored maple syrup.
Effects of maple syrup on the expression of cell cycle-associated factors in CRC cells
Using the TaqMan Array Human Cyclins & Cell Cycle Regulation 96-Well Plate, qPCR was conducted to examine the molecular profile of cell cycle-associated mRNAs that were regulated by maple syrup treatment. RT-qPCR analysis was performed with DLD-1 cells treated with extra light or dark-colored maple syrup. In the Dark group, fold-changes were induced in the relative expression of cell cycle-associated factors. Expression levels in the Extra group were set as 1, and the fold-changes following dark-colored maple syrup treatment were evaluated using the DDCq method. Among the 44 tested cell cycle-associated genes, 12 genes indicated changes in expression of >2-fold in DLD-1 cells treated with dark-colored maple syrup treatment compared with extra light grade maple syrup (Fig. 5). It was indicated that dark-colored maple syrup treatment reduced cyclin-dependent kinase 4 (CDK4), CDK6 and transforming growth factor β1 (TGFB1) expression, and induced cyclin-dependent kinase inhibitor 2B (CDKN2B) expression (Fig. 5).
Figure 5.
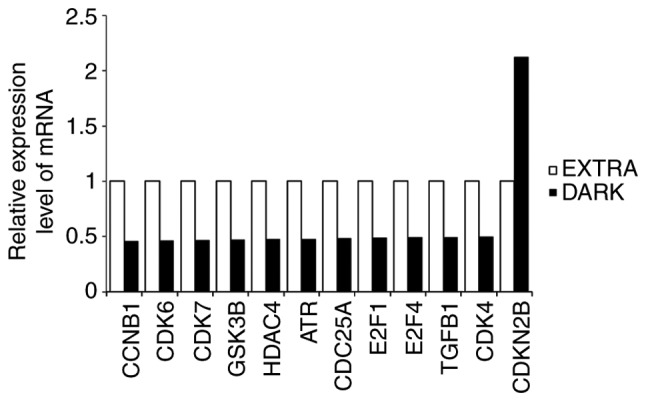
Expression profile of cell cycle-associated genes in maple syrup-treated DLD-1 cells, based on polymerase chain reaction array analysis. Extra, extra light maple syrup; Dark, dark-colored maple syrup; CCNB1, cyclin B1; CDK6, cyclin-dependent kinase 6; CDK7, cyclin-dependent kinase 7; GSK3B, glycogen synthase kinase 3 beta; HDAC4, histone deacetylase 4; ATR, ATR serine/threonine kinase; CDC25A, cell division cycle 25A; E2F1, E2F transcription factor 1; E2F4, E2F transcription factor 4; TGFB1, transforming growth factor, beta 1; CDK4, cyclin-dependent kinase 4; CDKN2B, cyclin-dependent kinase inhibitor 2B.
Discussion
In the present study, a gel-free LC-MS/MS-based proteomics approach was applied to examine the underlying mechanism of CRC cell growth inhibition by dark-colored maple syrup. Using a semi-quantitative method of spectral counting, a total of 388 proteins were identified that indicated >1.5-fold changes in expression following maple syrup treatment. The roles of these identified proteins were examined with GO analysis, focusing on the functions of proteins classified as associated with the ‘cell cycle’, since these proteins serve important roles in the proliferation system. The study also focused on PCNA, which is a member of this pathway. A western blot analysis was subsequently performed to validate the spectral counting results, and to determine whether dark-colored maple syrup treatment led to decreased PCNA expression in DLD-1 cells.
PCNA has been reported to exhibit different behaviors depending on the cell cycle phase, and serves important roles in DNA replication and DNA repair (44–47). PCNA also recruits chromatin remodeling and epigenetic modification factors (48). During the S phase of the cell cycle, PCNA is reportedly localized in the active replication site, and has the ability to differentiate between early, mid and late S phase (49,50). Therefore, PCNA expression is considered to be associated with S-phase cell cycle progression.
The present study observed that dark-colored maple syrup treatment decreased PCNA expression, which induced the S-phase cell cycle arrest of DLD-1 cells. Previous reports also indicate that induction of S-phase cell cycle arrest is accompanied by decreased PCNA expression in a number of tumor cells, including in CRC cells treated with functional compounds, such as resveratrol and oxoaporphine metal complexes (51,52). Previous data also indicate that the induction of S-phase cell cycle arrest is associated with changes in the expression of cyclins and CDKs in these cells. Therefore, maple syrup treatment may influence the cell cycle-associated gene expression in CRC cells. Accordingly, we hypothesized that dark-colored maple syrup induced S-phase cell cycle arrest in DLD-1 cells, which may affect the expression of cell cycle-associated genes. The study findings supported this hypothesis.
CDK4/6 is a key kinase in cell cycle promotion, and is currently considered a molecular target for anticancer drug development (53,54). The CDK4/6 inhibitor palbociclib is clinically used for the treatment of advanced breast cancer (55,56). The results of the present study support the possibility that the bioactive compounds in dark-colored maple syrup may be useful in the development of novel anticancer drugs for treatment of CRC and other types of advanced cancer. In addition, CDKN2B is also known as a cell-cycle regulator via its interaction with CDK4 and CDK6, and tumor suppressor genes, including p53 and p18 (57). Therefore, it can by hypothesized that the bioactive compounds, which have been indicated to upregulate CDKN2B expression may be present in dark grade maple syrup and may be a useful resource in developing novel anticancer drugs. In addition, these bioactive compounds may have an inhibitory effect on the TGF signaling pathway by suppressing TGF-β1 expression by upregulating CDNK2B expression, since previous reports demonstrated that downregulation of CDKN2B expression induced an increase of TGFβ1 expression in human smooth muscle cells and umbilical vein endothelial cells (58). A previous study reported that the ginnalins-polyphenols that are present in maple syrup inhibit proliferation through S-phase cell cycle arrest (59). However, the effects of ginnalins on PCNA, CDK4/6 and CDKN2B expression in CRC cells are not well understood. Further studies are required to identify the bioactive compounds in dark-colored maple syrup that are responsible for inhibiting the expression of PCNA, CDK4/6 and/or CDKN2B, and therefore, inducing cell cycle arrest. Further clarification is required in order to examine whether dark-colored maple syrup inhibits proliferation through S-phase cell cycle arrest by regulating cell cycle-associated gene expression in other types of cancer cells.
In conclusion, the present study indicated that dark-colored maple syrup induced S-phase cell cycle arrest in CRC cells by reducing PCNA expression and regulating cell cycle-associated genes. These findings suggest that dark-colored maple syrup may be a useful dietary factor, with a potential preventative effect against CRC. Compounds in dark-colored maple syrup may also be useful for the development of novel anticancer drugs for colorectal cancer treatment.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CRC
colorectal cancer
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NSAF
normalized spectral abundance factor
- PBS
phosphate-buffered saline
- SEM
standard error of measurement
- GO
Gene Ontology
- PCNA
proliferating cell nuclear antigen
- CDK
cyclin-dependent kinase
Funding
The present study was supported in part by the MEXT-Supported Program of the Strategic Research Foundation at Private Universities (grant no. S1411037).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
TY and AT designed the study and analyzed the data. TY and TN performed the experiments. TY drafted the manuscript. AT critically evaluated the study and the final version of the manuscript. All authors participated in discussion of the work and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Tariq K, Ghias K. Colorectal cancer carcinogenesis: A review of mechanisms. Cancer Biol Med. 2016;13:120–135. doi: 10.20892/j.issn.2095-3941.2015.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18(pii):E197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975;15:617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Peto R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. doi: 10.1093/jnci/66.6.1192. [DOI] [PubMed] [Google Scholar]
- 5.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–1260.e16. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS One. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao A, Thun MJ, Connell CJ, McCullough ML, Jacobs EJ, Flanders WD, Rodriguez C, Sinha R, Calle EE. Meat consumption and risk of colorectal cancer. JAMA. 2005;293:172–182. doi: 10.1001/jama.293.2.172. [DOI] [PubMed] [Google Scholar]
- 8.Holt PR, Atillasoy EO, Gilman J, Guss J, Moss SF, Newmark H, Fan K, Yang K, Lipkin M. Modulation of abnormal colonic epithelial cell proliferation and differentiation by low-fat dairy foods: A randomized controlled trial. JAMA. 1998;280:1074–1079. doi: 10.1001/jama.280.12.1074. [DOI] [PubMed] [Google Scholar]
- 9.Glinghammar B, Venturi M, Rowland IR, Rafter JJ. Shift from a dairy product-rich to a dairy product-free diet: Influence on cytotoxicity and genotoxicity of fecal water-potential risk factors for colon cancer. Am J Clin Nutr. 1997;66:1277–1282. doi: 10.1093/ajcn/66.5.1277. [DOI] [PubMed] [Google Scholar]
- 10.Norat T, Riboli E. Dairy products and colorectal cancer. A review of possible mechanisms and epidemiological evidence. Eur J Clin Nutr. 2003;57:1–17. doi: 10.1038/sj.ejcn.1601522. [DOI] [PubMed] [Google Scholar]
- 11.Wollowski I, Ji ST, Bakalinsky AT, Neudecker C, Pool-Zobel BL. Bacteria used for the production of yogurt inactivate carcinogens and prevent DNA damage in the colon of rats. J Nutr. 1999;129:77–82. doi: 10.1093/jn/129.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Kesse E, Boutron-Ruault MC, Norat T, Riboli E, Clavel-Chapelon F, E3N Group Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int J Cancer. 2005;117:137–144. doi: 10.1002/ijc.21148. [DOI] [PubMed] [Google Scholar]
- 13.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, et al. Dairy foods, calcium, and colorectal cancer: A pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–2397. [PubMed] [Google Scholar]
- 15.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP diet and health study. Arch Intern Med. 2009;169:391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SA, Shu XO, Yang G, Li H, Gao YT, Zheng W. Animal origin foods and colorectal cancer risk: A report from the Shanghai Women's health study. Nutr Cancer. 2009;61:194–205. doi: 10.1080/01635580802419780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball DW. The chemical composition of maple syrup. J Chem Educ. 2007;84:1647. doi: 10.1021/ed084p1647. [DOI] [Google Scholar]
- 18.Perkins TD, van den Berg AK. Maple syrup-production, composition, chemistry, and sensory characteristics. Adv Food Nutr Res. 2009;56:101–143. doi: 10.1016/S1043-4526(08)00604-9. [DOI] [PubMed] [Google Scholar]
- 19.Arnason T, Hebda RJ, Johns T. Use of plants for food and medicine by Native People's of eastern Canada. Can J Bot. 1981;59:2189–2325. doi: 10.1139/b81-287. [DOI] [Google Scholar]
- 20.Apostolidis E, Li L, Lee C, Seeram NP. In vitro evaluation of phenolic-enriched maple syrup extracts for inhibition of carbohydrate hydrolyzing enzymes relevant to type 2 diabetes management. J Funct Foods. 2011;3:100–106. doi: 10.1016/j.jff.2011.03.003. [DOI] [Google Scholar]
- 21.Legault J, Girard-Lalancette K, Grenon C, Dussault C, Pichette A. Antioxidant activity, inhibition of nitric oxide overproduction, and in vitro antiproliferative effect of maple sap and syrup from Acer saccharum. J Med Food. 2010;13:460–468. doi: 10.1089/jmf.2009.0029. [DOI] [PubMed] [Google Scholar]
- 22.González-Sarrías A, Li L, Seeram NP. Effects of maple (Acer) plant part extracts on proliferation, apoptosis and cell cycle arrest of human tumorigenic and non-tumorigenic colon cells. Phytother Res. 2012;26:995–1002. doi: 10.1002/ptr.3677. [DOI] [PubMed] [Google Scholar]
- 23.Theriault M, Caillet S, Kermasha S, Lacroix M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chem. 2006;98:490–501. doi: 10.1016/j.foodchem.2005.05.079. [DOI] [Google Scholar]
- 24.Hawco CL, Wang Y, Taylor M, Weaver DF. A maple syrup extract prevents β-amyloid aggregation. Can J Neurol Sci. 2016;43:198–201. doi: 10.1017/cjn.2015.270. [DOI] [PubMed] [Google Scholar]
- 25.Nagai N, Ito Y, Taga A. Comparison of the enhancement of plasma glucose levels in type 2 diabetes otsuka long-evans tokushima fatty rats by oral administration of sucrose or maple syrup. J Oleo Sci. 2013;62:737–743. doi: 10.5650/jos.62.737. [DOI] [PubMed] [Google Scholar]
- 26.Nagai N, Yamamoto T, Tanabe W, Ito Y, Kurabuchi S, Mitamura K, Taga A. Changes in plasma glucose in otsuka long-evans tokushima fatty rats after oral administration of maple syrup. J Oleo Sci. 2015;64:331–335. doi: 10.5650/jos.ess14075. [DOI] [PubMed] [Google Scholar]
- 27.Kim YT, Leech RH. Effects of climatic conditions on sap flow in sugar maple. Forest Chron. 1985;61:303–307. doi: 10.5558/tfc61303-4. [DOI] [Google Scholar]
- 28.Marvin JW, Erickson RO. A statistical evaluation of some of the factors responsible for the flow of sap from the sugar maple. Plant Physiol. 1956;31:57–61. doi: 10.1104/pp.31.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houle D, Paquette A, Côté B, Logan T, Power H, Charron I, Duchesne L. Impacts of climate change on the timing of the production season of maple syrup in Eastern Canada. PLoS One. 2015;10:e0144844. doi: 10.1371/journal.pone.0144844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Uemura K, Moriyama K, Mitamura K, Taga A. Inhibitory effect of maple syrup on the cell growth and invasion of human colorectal cancer cells. Oncol Rep. 2015;33:1579–1584. doi: 10.3892/or.2015.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto T, Sato K, Kubota Y, Mitamura K, Taga A. Effect of dark-colored maple syrup on cell proliferation of human gastrointestinal cancer cell. Biomed Rep. 2017;7:6–10. doi: 10.3892/br.2017.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maple products regulation. https://laws-lois.justice.gc.ca/eng/regulations/C.R.C.,_c._289/page-8.html#h-24
- 33.Bluemlein K, Ralser M. Monitoring protein expression in whole-cell extracts by targeted label- and standard-free LC-MS/MS. Nat Protoc. 2011;6:859–869. doi: 10.1038/nprot.2011.333. [DOI] [PubMed] [Google Scholar]
- 34.Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005;4:1487–1502. doi: 10.1074/mcp.M500084-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Zybailov B, Coleman MK, Florens L, Washburn MP. Correlation of relative abundance ratios derived from peptide ion chromatograms and spectrum counting for quantitative proteomic analysis using stable isotope labeling. Anal Chem. 2005;77:6218–6224. doi: 10.1021/ac050846r. [DOI] [PubMed] [Google Scholar]
- 36.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 37.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh P, Bai H, Swartz MF, Alfieris GM, Dean DA. Identification of differentially regulated genes in human patent ductus arteriosus. Exp Biol Med (Maywood) 2016;241:2112–2118. doi: 10.1177/1535370216661778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Carbotti G, Nikpoor AR, Vacca P, Gangemi R, Giordano C, Campelli F, Ferrini S, Fabbi M. IL-27 mediates HLA class I up-regulation, which can be inhibited by the IL-6 pathway, in HLA-deficient small cell lung cancer cells. J Exp Clin Cancer Res. 2017;36:140. doi: 10.1186/s13046-017-0608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adnan M, Morton G, Hadi S. Analysis of rpoS and bolA gene expression under various stress-induced environments in planktonic and biofilm phase using 2(−ΔΔCT) method. Mol Cell Biochem. 2011;357:275–282. doi: 10.1007/s11010-011-0898-y. [DOI] [PubMed] [Google Scholar]
- 43.Soejima M, Koda Y. TaqMan-based real-time polymerase chain reaction for detection of FUT2 copy number variations: Identification of novel Alu-mediated deletion. Transfusion. 2011;51:762–769. doi: 10.1111/j.1537-2995.2010.02895.x. [DOI] [PubMed] [Google Scholar]
- 44.Kelman Z. PCNA: Structure, functions and interactions. Oncogene. 1997;14:629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 45.Majka J, Burgers PM. The PCNA-RFC families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol Biol. 2004;78:227–260. doi: 10.1016/S0079-6603(04)78006-X. [DOI] [PubMed] [Google Scholar]
- 46.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Bártová E, Suchánková J, Legartová S, Malyšková B, Hornáček M, Skalníková M, Mašata M, Raška I, Kozubek S. PCNA is recruited to irradiated chromatin in late S-phase and is most pronounced in G2 phase of the cell cycle. Protoplasma. 2017;254:2035–2043. doi: 10.1007/s00709-017-1076-1. [DOI] [PubMed] [Google Scholar]
- 48.Budhavarapu VN, Chavez M, Tyler JK. How is epigenetic information maintained through DNA replication? Epigenetics Chromatin. 2013;6:32. doi: 10.1186/1756-8935-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celis JE, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: Subdivision of S phase. Proc Natl Acad Sci USA. 1985;82:3262–3266. doi: 10.1073/pnas.82.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schönenberger F, Deutzmann A, Ferrando-May E, Merhof D. Discrimination of cell cycle phases in PCNA-immunolabeled cells. BMC Bioinformatics. 2015;16:180. doi: 10.1186/s12859-015-0618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia J, Liu J, Chen N, Li M, Zhu R. Resveratrol inhibits proliferation in human colorectal carcinoma cells by inducing G1/S-phase cell cycle arrest and apoptosis through caspase/cyclin-CDK pathways. Mol Med Rep. 2014;10:1697–1702. doi: 10.3892/mmr.2014.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin JL, Shen WY, Chen ZF, Zhao LF, Qin QP, Yu YC, Liang H. Oxoaporphine metal complexes (CoII, NiII, ZnII) with high antitumor activity by inducing mitochondria-mediated apoptosis and S-phase arrest in HepG2. Sci Rep. 2017;7:46056. doi: 10.1038/srep46056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H, Hu X, Cao K, Zhang Y, Zhao K, Tang C, Feng B. Synthesis and SAR of 4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline derivatives as potent and selective CDK4/6 inhibitors. Eur J Med Chem. 2018;157:935–945. doi: 10.1016/j.ejmech.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 54.Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 56.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 57.Krimpenfort P, Ijpenberg A, Song JY, van der Valk M, Nawijn M, Zevenhoven J, Berns A. p15Ink4b is a critical tumour suppressor in the absence of p16Ink4a. Nature. 2007;448:943–946. doi: 10.1038/nature06084. [DOI] [PubMed] [Google Scholar]
- 58.Nanda V, Downing KP, Ye J, Xiao S, Kojima Y, Spin JM, DiRenzo D, Nead KT, Connolly AJ, Dandona S, et al. CDKN2B regulates TGFβ signaling and smooth muscle cell investment of hypoxic neovessels. Circ Res. 2016;118:230–240. doi: 10.1161/CIRCRESAHA.115.307906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.González-Sarrías A, Ma H, Edmonds ME, Seeram NP. Maple polyphenols, ginnalins A-C, induce S- and G2/M-cell cycle arrest in colon and breast cancer cells mediated by decreasing cyclins A and D1 levels. Food Chem. 2013;136:636–642. doi: 10.1016/j.foodchem.2012.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.