Abstract
Pristimerin is an active compound isolated from the traditional Chinese herbs Celastraceae and Hippocrateaceae. It has been reported to exert antitumor effects under experimental and clinical conditions; however, the antitumor effects and underlying mechanisms of pristimerin in oral cancer cells have not yet been identified. In the present study, the anticancer potential of pristimerin was investigated in two oral squamous cell carcinoma (OSCC) cell lines, CAL-27 and SCC-25. Results demonstrated that pristimerin was toxic against the two cell lines, and exhibited inhibitory effects against proliferation. Furthermore, pristimerin exhibited a more potent anti-proliferative activity in CAL-27 and SCC-25 cells than the common chemotherapy drugs cisplatin and 5-fluorouracil. In addition, cell cycle distribution analysis revealed that G0/G1 phase arrest was induced following pristimerin treatment in CAL-27 and SCC-25 cells, which was strongly associated with upregulation of p21 and p27, coupled with downregulation of cyclin D1 and cyclin E. Meanwhile, pristimerin induced significant apoptosis of CAL-27 and SCC-25 cells, alongside decreased levels of caspase-3 and specific cleavage of poly (ADP-ribose) polymerase. These effects were associated with inhibition of the mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 and protein kinase B signaling pathways. With regards to these results, pristimerin may be considered a potent novel active substance for the treatment of OSCC.
Keywords: pristimerin, oral squamous cell carcinoma, proliferation, apoptosis, antitumor
Introduction
Oral carcinoma is an aggressive malignant disease, with ~640,000 new cases being detected annually worldwide; in addition, it is responsible for ~145,000 cases of mortality every year (1). Five-year survival rates of patients with early oral cancer are between 55 and 60%, and decrease to 30–40% in cases of advanced oral cancer. The development of oral cancer is mainly associated with the consumption of areca nut, tobacco and alcohol (2–4). There are various types of oral cancer; however, >90% are squamous cell carcinomas. Because it is very invasive, the prognosis of oral squamous cell carcinoma (OSCC) is poor (5). Currently, surgical removal, chemotherapy and radiotherapy are the most common and effective treatments for patients with oral carcinoma. However, because of distant metastasis, chemotherapeutic resistance and poor tolerance, these treatments can fail. Therefore, the development of novel chemotherapeutics with low toxicity and high efficiency for oral carcinoma is crucial.
Interest has grown in natural compounds for the development of anticancer drugs, due to their low toxicity and higher tolerance in patients (6). Pristimerin, a quininemethide triterpenoid compound, is isolated from several plant species belonging to the Celastraceae and Hippocrateaceae families. It is commonly used as an antioxidant, anti-malarial, insecticidal, anti-inflammatory and anti-fungal agent (7–9). Pristimerin has also been reported to induce apoptosis of various human cancer cells, including in multiple myeloma (10), breast (11), liver (12), pancreatic (13) and prostate cancer (14). In addition to apoptosis induction (11), the mechanisms involved in the anticancer effects of pristimerin include stimulation of reactive oxygen species generation (15), blocking of nuclear factor-κB (16) and proteasome inhibition (10).
To the best of our knowledge, the anticancer effects of pristimerin on OSCC have rarely been reported. In the present study, the potent antitumor effects of pristimerin on OSCC cells were investigated. Pristimerin exhibited potent anti-proliferative and apoptosis-inducing effects on the OSCC cell lines CAL-27 and SCC-25. The underlying mechanisms of these effects were primarily mediated by G1 phase cell cycle arrest and inhibition of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulate kinase 1/2 (Erk 1/2) and protein kinase B (Akt) signaling pathways.
Materials and methods
Reagents and cell culture
Pristimerin, 5-fluorouracil, cisplatin and propidium iodide (PI) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Pristimerin (molecular structure shown in Fig. 1A) was prepared as a 20 mM stock solution in dimethyl sulfoxide. The CAL-27 and SCC-25 cells, which were initially isolated from the epidermal tongue tissue of patients with OSCC, were kindly donated by Professor Hongzhang Huang (Department Oral & Maxillofacial Surgery, Sun Yat Sen University, Guangzhou, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) F-12 (Biological Industries, Shanghai, China) supplemented with 10% fetal bovine serum (FBS; Biological Industries), at 37°C in a humidified atmosphere containing 5% CO2.
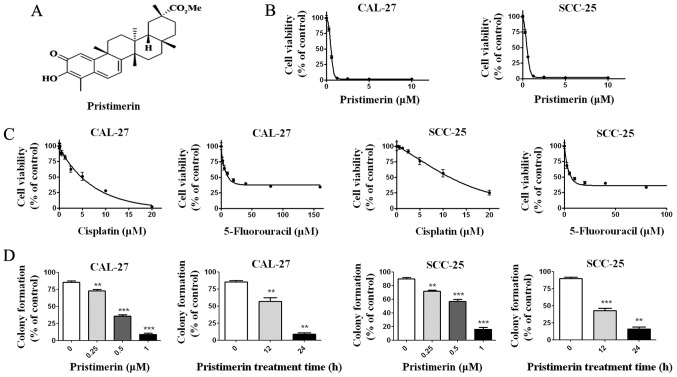
Figure 1.
Pristimerin inhibits proliferation of CAL-27 and SCC-25 cells. (A) Molecular structure of pristimerin compound. (B) Cells were treated with increasing concentrations of pristimerin for 72 h, and cell viability was determined by MTS assay. (C) Cell viability was measured after cells were treated with cisplatin and 5-fluorouracil. (D) Colony formation ability of CAL-27 and SCC-25 cells was determined following treatment with the indicated concentrations of pristimerin for 24 h or with 1 µM for various durations. **P<0.01 and ***P<0.001 vs. control.
Cell viability assay
Cell viability was measured by MTS assay (CellTiter 96 AQueous MTS Reagent; Promega Corporation, Madison, WI, USA), according to the manufacturer's protocol. Briefly, CAL-27 and SCC-25 cells (5×103 cells/well) were seeded into a 96-well plate for 12 h to allow cell attachment, and were then treated with increasing concentrations of pristimerin (0, 0.15625, 0.3125, 0.625, 1.25, 2.5, 5, 10 µM), cisplatin (0, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20 µM) or 5-fluorouracil (0, 1.25, 2.5, 5, 10, 20, 40, 80, 160 µM) at 37°C for 68 h. Thereafter, 20 µl MTS/PMS (20:1 in volume) was added and incubated for an additional 4 h. Cell viability was finally determined using a microplate reader (Detie, Nanjing, China) at 490 nm. The half maximal inhibitory concentration (IC50) of pristimerin was calculated.
Clonogenicity assay
CAL-27 and SCC-25 cells (2×105/ml) were treated with increasing concentrations of pristimerin (0, 0.25, 0.5, 1 µM) at 37°C for 12 or 24 h. They were then collected, washed three times with PBS and seeded in a 12-well plate (103/well) in DMEM F-12 medium containing 0.3% agar and 20% FBS. After a further 10–14 days of culture at 37°C, colonies containing >50 cells were counted under an inverted phase-contrast microscope.
Flow cytometric analysis of cell apoptosis
CAL-27 and SCC-25 cells (2×105/ml) were treated with various concentrations of pristimerin (0, 0.25, 0.5, 1 µM) at 37°C for 12 or 24 h. After collection, cells were washed with PBS and stained with Annexin V-fluorescein isothiocyanate (FITC)/PI (Annexin V-FITC Apoptosis Detection kit; Sigma-Aldrich; Merck KGaA) according to the manufacturer's protocol. The number of viable, necrotic and apoptotic cells were assessed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Assessment of cell cycle distribution
CAL-27 and SCC-25 cells (2×105/ml) were treated with various concentrations of pristimerin (0, 0.25, 0.5, 1 µM) at 37°C for 12 or 24 h. After collection, they were washed twice with cold PBS and fixed with cold 75% ethanol at 4°C overnight. Ethanol was eventually discarded, cells were resuspended in PBS containing PI (50 µg/ml) and were incubated in a water bath (37°C) for 1 h. The cell cycle distribution was finally examined by flow cytometry.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Following treatment with pristimerin (0, 0.25, 0.5, 1 µM for 24 h and 1 µM for 12 h), total cellular RNA was extracted from CAL-27 and SCC-25 cells with TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. RNA was reverse transcribed into cDNA (MMLV reverse transcriptase; Promega Corporation), according to the manufacturer's protocol, and the mRNA expression levels were measured by GoTaq qPCR Master Mix (Promega, Corporation) using the ABI7000 cycler (Applied Biosystems; Thermo Fisher Scientific Inc.). The primers for RT-qPCR were designed as follows: p21, forward 5′-TCTTGTACCCTTGTGCCTCG-3′, reverse 5′-GAAGATCAGCCGGCGTTTG-3′; p27, forward 5′-GTCAAACGTAAACAGCTCGAAT-3′, reverse 5′- TGCATAATGCTACATCCAACG-3′; p53, forward 5′- GAGGTTGGCTCTGACTGTACC-3′, reverse 5′- TCCGTCCCAGTAGATTACCAC-3′; cyclin D1, forward 5′-GTGCTGCGAAGTGGAAACC-3′, reverse 5′-ATCCAGGTGGCGACGATCT-3′; cyclin E, forward 5′-GTTATAAGGGAGACGGGGAGC-3′, reverse 5′-TGCTCTGCTTCTTACCGCTC-3′; and GAPDH, forward 5′-CGACCACTTTGTCAAGCTCA-3′; and reverse 5′-AGGGGTCTACATGGCAACTG-3′. PCR was performed at 94°C for 5 min, followed by 40 cycles at 94°C for 30 sec and 56°C for 30 sec. Relative quantification of gene expression was performed using the threshold cycle difference 2−ΔΔCq method (17), and the geometric mean of GAPDH levels was used as an internal control to normalize the variability in expression level.
Western blot analysis
Cells were washed with cold PBS and lysed with lysis buffer (1X PBS, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) supplemented with freshly added 1 mM phenylmethylsulfonyl fluoride, 1X Roche complete Mini protease inhibitor cocktail (Roche Diagnostics, Shanghai, China), 10 mM glycerophosphate, 10 mM NaF, and 1 mM sodium orthovanadate. DNA contained in the lysate was sheared by sonication with 10 1-sec bursts with a 3 sec interval at medium power. Following quantification using the bicinchoninic acid method, proteins (60 µg) were separated by 10–15% SDS-PAGE. Thereafter, proteins were transferred to polyvinylidene difluoride membrane and blocked with 5% non-fat milk in PBS-Tween (PBST) at 37°C for 1 h. Antibodies against poly (ADP-ribose) polymerase (PARP) (cat. no. 9532), caspase-3 (cat. no. 9665), p21 (cat. no. 2947), Erk1/2 (cat. no. 4695), phosphorylated (p)-Erk1/2 (cat. no. 4370), Akt (cat. no. 4691) and p-Akt (cat. no. 4060) were purchased from CST Biological Reagents Co., Ltd. (Shanghai, China). Horseradish peroxidase-conjugated goat antibodies against mouse and rabbit (cat. no. 31430, cat. no. 31460) were purchased from Thermo Fisher Scientific Inc. Antibodies was prepared in PBST with dilutions of 1:500 (p-Erk1/2 and p-Akt), 1:8,000 (β-actin) and the rest of the antibodies were 1:1,000. Primary antibody was added to the membrane and incubated at 4°C overnight. Following washing in triplicate with PBST, the secondary antibody was added (1:5,000) and incubated at room temperature for 1 h, subsequently washed 2 times with PBST and 1 time with PBS. Enhanced chemiluminescence reagent (Immobilon Western; cat. no. WBKLS0500, EMD Millipore, Billerica, MA, USA) was added and the x-ray film was exposed. Protein bands were quantified and normalized to β-actin by using Image-Pro Plus 6.0 system (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Each experiment was performed at least three times. GraphPad 5.0 Software (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis. Data are expressed as the means ± standard deviation, and differences between groups were assessed by one-way analysis of variance with post-hoc intergroup comparisons using Turkey test. P<0.05 was considered to indicate a statistically significant difference.
Results
Pristimerin inhibits OSCC cell proliferation
To investigate the possible effects of pristimerin on OSCC cells, CAL-27 and SCC-25 cells were treated with increasing concentrations of pristimerin for 68 h, prior to determining cell viability with the MTS assay. Results demonstrated that pristimerin effectively inhibited the growth of CAL-27 and SCC-25 cells in a dose-dependent manner with IC50 values of 0.70 and 0.73 µM, respectively (Fig. 1B). The toxic effects of pristimerin on CAL-27 and SCC-25 viability were compared to the ones observed after 72 h treatment with the commonly used antitumor drugs cisplatin and 5-fluorouracil. The results demonstrated that the IC50 values of cisplatin and 5- fluorouracil in CAL-27 cells were 7.69 and 11.98 µM, respectively. The IC50 values of cisplatin and 5-fluorouracil in SCC-25 cells were 15.96 and 11.45 µM, respectively (Fig. 1C). Compared with cisplatin and 5-fluorouracil, the IC50 values of pristimerin in oral cancer cells were lowest (~0.7 µM). Because clonogenicity is believed to better reflect the malignant behavior of tumor cells, colony formation was determined after CAL-27 and SCC-25 cells were treated either with increasing concentrations of pristimerin for 24 h, or with 1 µM pristimerin for various durations. The results demonstrated that pristimerin potently inhibited the colony formation o CAL-27 and SCC-25 cells in a dose- and time-dependent manner (Fig. 1D).
Pristimerin induces G0/G1 phase arrest and modulates the expression levels of cell cycle-associated molecules
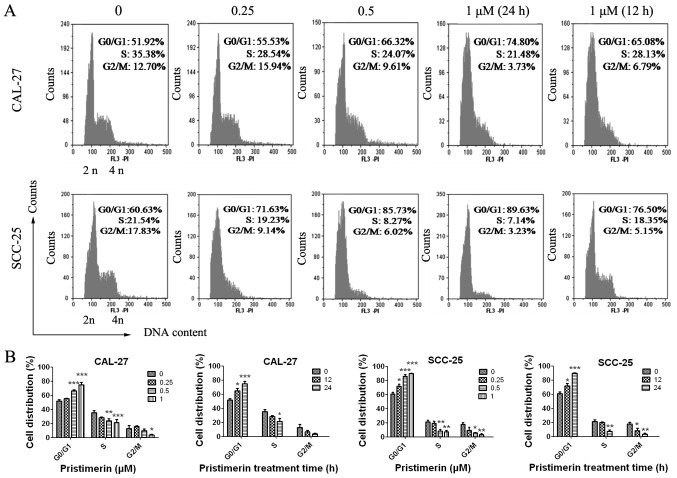
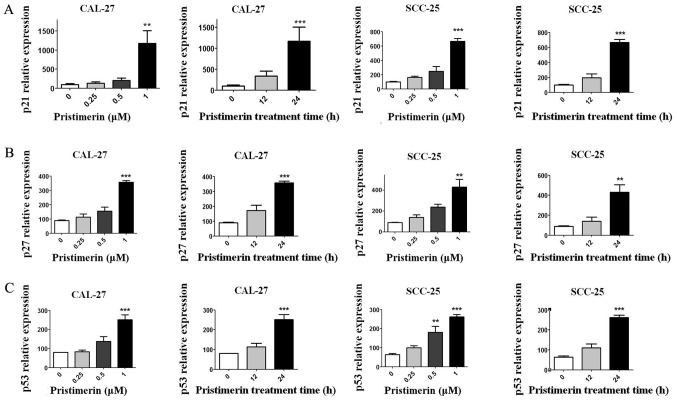
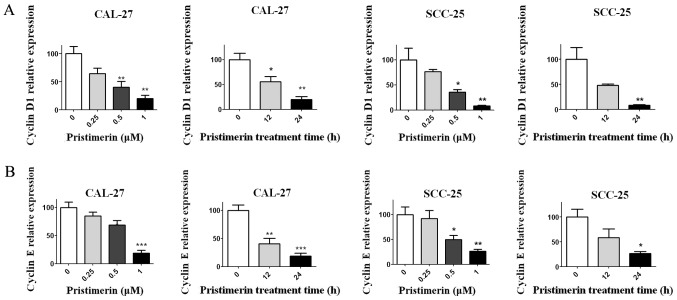
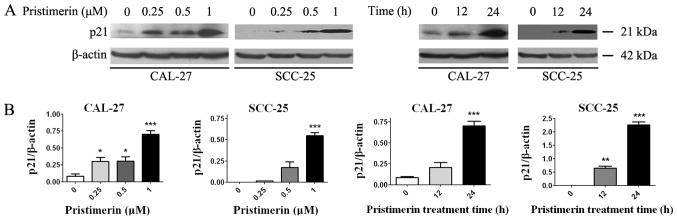
To explore the underlying mechanism of pristimerin-induced inhibition of cell proliferation, the effects of pristimerin on cell cycle distribution were examined by flow cytometry (Fig. 2A). The results revealed that the proportion of G0/G1 phase CAL-27 cells treated with 0, 0.25, 0.5 and 1 µM pristimerin for 24 h were 51.92, 55.53, 66.32 and 74.80%, respectively. Similarly, in SCC-25 cells, G0/G1 phase distributions were 60.63, 71.63, 85.73 and 89.63% following treatment with 0, 0.25, 0.5 and 1 µM pristimerin, respectively, for 24 h. In addition, the proportion of G0/G1 phase CAL-27 cells was 65.08% and of G0/G1 phase SCC-25 cells was 76.50% following treatment with 1 µM pristimerin for 12 h (Fig. 2A). In parallel, the S and G2/M phase distributions were reduced. These data revealed that pristimerin significantly increased the proportion of G0/G1 phase CAL-27 and SCC-25 cells, thus suggesting that G0/G1 phase arrest may be induced following pristimerin treatment. Furthermore, the expression levels of molecules involved in the cell cycle process, including cyclin D1, cyclin E, cyclin-dependent kinase (CDK) inhibitors (CDKIs) p21 and p27, and p53, were assessed by RT-qPCR. Results demonstrated that p21, p27 and p53 expression levels were significantly increased following pristimerin treatment (Fig. 3A-C), whereas cyclin D1 and cyclin E expression levels were significantly decreased under the same conditions (Fig. 4A and B). These modulating effects of pristimerin were observed in a dose- and time-dependent manner in both cell lines. Moreover, western blot analysis exhibited increased levels of p21 protein in a dose- and time-dependent manner in both cell lines (Fig. 5A and B). These results were consistent with the ones obtained from RT-qPCR analysis of p21 expression (Fig. 3).
Figure 2.
Pristimerin disturbs cell cycle distribution in CAL-27 and SCC-25 cells. (A) CAL-27 and SCC-25 cells were treated with the indicated concentrations of pristimerin for 24 h or with 1 µM for 12 h, and were stained with PI. Subsequent cell cycle distributions were assessed by flow cytometry. (B) Statistical analysis of cell cycle distributions in CAL-27 and SCC-25 cells (=3). PI, propidium iodide. *P<0.05; **P<0.01 and ***P<0.001 vs. control.
Figure 3.
Pristimerin upregulates the expression levels of p21, p27 and p53. The expression levels of (A) p21, (B) p27 and (C) p53 were determined by reverse transcription-quantitative polymerase chain reaction analysis after CAL-27 and SCC-25 cells were treated with the indicated concentrations of pristimerin for 24 h or with 1 µM for various durations. **P<0.01 and ***P<0.001 vs. control.
Figure 4.
Pristimerin reduces cyclin D1 and cyclin E expression levels. CAL-27 and SCC-25 cells were treated with the indicated concentration of pristimerin for 24 h or with 1 µM for various durations. Thereafter, the expression levels of (A) cyclin D1 and (B) cyclin E were determined by reverse transcription-quantitative polymerase chain reaction. *P<0.05; **P<0.01 and ***P<0.001 vs. control.
Figure 5.
Pristimerin increases p21 protein expression levels. (A) CAL-27 and SCC-25 cells were treated with the indicated concentrations of pristimerin for 24 h or with 1 µM for various durations, and p21 protein expression was detected by western blot analysis. (B) Statistical analysis of p21 protein density normalized to the internal control β-actin. *P<0.05; **P<0.01 and ***P<0.001 vs. control.
Pristimerin induces apoptosis of CAL-27 and SCC-25 cells
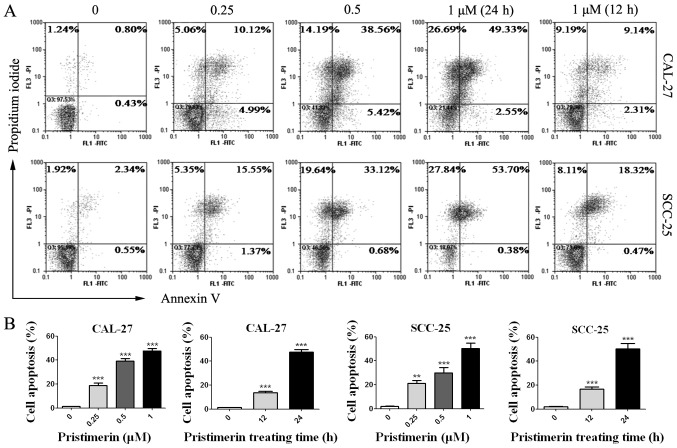
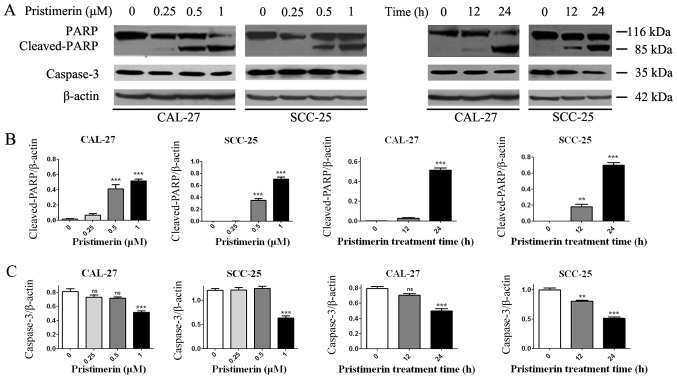
The effects of pristimerin on cancer cell apoptosis were assessed by flow cytometry. CAL-27 and SCC-25 cells were treated with various concentrations of pristimerin for various durations (Fig. 6), and were double-stained with Annexin V-FITC/PI. Results demonstrated that apoptotic cells were significantly increased by pristimerin in a dose- and time-dependent manner (Fig. 6A and B). The mean apoptotic cell rates from three independent experiments were 1.29, 18.81, 39.1 and 47.7% in CAL-27 cells, and were 2.1, 21.1, 30.05 and 50.23% in SCC-25 cells following treatment with pristimerin at 0, 0.25, 0.5 and 1 µM, respectively for 24 h. Following cell treatment with 1 µM pristimerin for 12 h, the mean apoptotic cell rates were 13.61% in CAL-27 cell and 16.67% in SCC-25 cells. Additionally, PARP and caspase-3, the hallmark proteins in apoptosis, were examined by western blot analysis. As presented in Fig. 7, caspase-3 expression levels were significantly reduced in the two cell lines in a dose- and time-dependent manner following pristimerin treatment. In addition, cleaved-PARP (85 kDa) levels were substantially increased in a dose- and time-dependent manner following pristimerin treatment in CAL-27 and SCC-25 cells (Fig. 7). The upregulated cleaved-PARP level and downregulated caspase-3 level further suggested that apoptosis was induced following pristimerin treatment in OSCC cells (Fig. 7).
Figure 6.
Pristimerin induces apoptosis of oral squamous cell carcinoma. (A) CAL-27 and SCC-25 cells were exposed to the indicated concentrations of pristimerin for 24 h or with 1 µM for 12 and 24 h, and cell death was assessed after Annexin V/PI double staining. (B) Statistical analysis of flow cytometry analysis in CAL-27 and SCC-25 cells (=3). FITC, fluorescein isothiocyanate; PI, propidium iodide. **P<0.01 and ***P<0.001 vs. control.
Figure 7.
Pristimerin increases cleaved-PARP and reduces caspase-3 protein levels. (A) CAL-27 and SCC-25 cells were exposed to the indicated concentrations of pristimerin for 24 h or with 1 µM for 12 or 24 h, and cleaved-PARP and caspase-3 expression levels were detected by western blot analysis. (B) Statistical analysis of cleaved-PARP and (C) caspase-3 protein density normalized to the internal control β-actin. PARP, poly (ADP-ribose) polymerase; ns, no significance. **P<0.01 and ***P<0.001 vs. control.
Pristimerin inhibits the MAPK/Erk1/2 and phosphoinositide 3-kinase (PI3K)/Akt signaling pathways
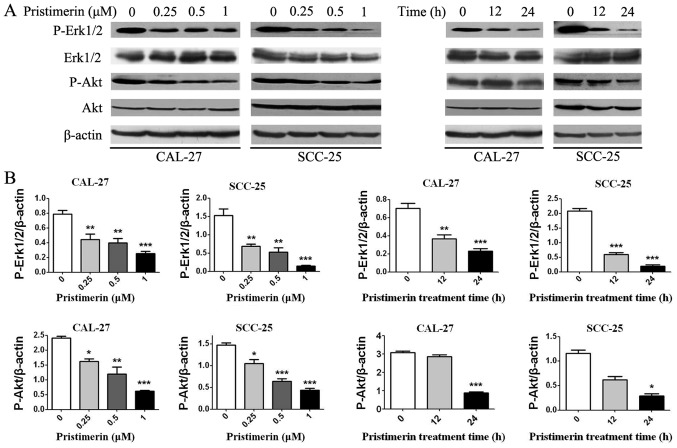
To determine whether the MAPK/Erk1/2 and PI3K/Akt pathways were involved in the anticancer effects of pristimerin in OSCC cells, p-Akt, Akt, p-Erk1/2 and Erk1/2 levels were detected by western blotting (Fig. 8). Results demonstrated that p-Akt and p-Erk1/2 expression levels were decreased in a dose- and time-dependent manner; however, there were no significant differences in the expression levels of total Akt and Erk1/2.
Figure 8.
Pristimerin inhibits Akt and Erk1/2 signaling. CAL-27 and SCC-25 cells were treated with the indicated concentrations of pristimerin for 24 h or with 1 µM for various durations. (A) Expression levels of p-Akt, Akt, p-Erk1/2 and Erk1/2 were detected by western blot analysis. (B) Statistical analysis of p-Akt and p-Erk1/2 protein density normalized to the internal control β-actin. Akt, protein kinase B; Erk1/2, extracellular signal-regulated kinase 1/2; p, phosphorylated. *P<0.05; **P<0.01 and ***P<0.001 vs. control.
Discussion
There has been growing interest in the last 30 years in natural products derived from plants for the development of novel anticancer therapies (6). Pristimerin is a quinine methyltriterpenoid extracted from Chinese plants; it has been reported to possess potential anticancer effects in various types of cancer (9). To the best of our knowledge, the present study is the first to determine the cytotoxic potency of pristimerin against two OSCC cell lines, CAL-27 and SCC-25, in a time- and dose-dependent manner. Furthermore, the effects of pristimerin on cell proliferation were greater than those observed by the conventional drugs cisplatin and 5-fluorouracil. In the present study, the antitumor activities of pristimerin were only detected in CAL-27 and SCC-25 cells; our future studies aim to test the antitumor activities of pristimerin in other OSCC cells.
The disruption of cell cycle regulation is a main cause for the proliferation of tumor cells. Therefore, modulation of cell cycle progression in cancer cells is considered a target for the treatment of human malignancies (18–21). Cyclins, CDKs and CDKIs are three important modulators of cell cycle progression. Abnormal expression of these molecules leads to aberrant cell proliferation that can stimulate tumor development. Cyclins bind to CDKs and promote cell cycle progression, whereas CDKIs inhibit CDK activity by binding to cyclin-CDKs, cyclins or CDKs, ultimately blocking cell proliferation (22–25). In this study, the upregulation of p21 and p27, and downregulation of cyclin D1 and cyclin E expression levels were observed and responsible for the pristimerin-induced G0/G1 phase arrest, eventually leading to cell proliferation suppression in CAL-27 and SCC-25 cells. Previous studies reported likewise, that pristimerin can induce G1 phase arrest, which is mediated by the upregulation of p21 and downregulation of cyclin D1 (10,26,27). Additionally, the tumor suppressor protein p53, which is known as a cell cycle regulator and a guardian of genetic integrity, was increased following pristimerin treatment in CAL-27 and SCC-25 cells.
Most anticancer drugs mediate their effects via cell apoptosis induction, which is the major mechanism involved in the treatment of cancer, including oral cancer (28). Apoptosis can be initiated either by the mitochondria (the intrinsic pathway) or through cell death receptors (the extrinsic pathway), leading to the activation of caspase cascades and resulting in apoptosis. Previous studies have reported that pristimerin may induce cell apoptosis via both the mitochondria and the death receptor-mediated extrinsic pathways in U87 glioma cells (29) and cervical cancer cells (30). Pristimerin likewise modulates the levels of B-cell lymphoma 2 (Bcl-2) family proteins, which are known to be involved in mitochondria-mediated apoptosis, in pancreatic cancer cells (31). In the present study, pristimerin induced significant apoptosis of CAL-27 and SCC-25 cells, which was mediated by PARP specific cleavage and caspase-3 downregulation. Lee et al (26) and Yousef et al (27) also reported that pristimerin induces PARP cleavage and apoptosis in breast and colorectal cancer cells, respectively. Specific cleavage of PARP indicates cell apoptosis. Caspase-3 acts as a junction between the exogenous apoptotic pathway and the endogenous apoptotic pathway, and its activation by cleavage itself ultimately induces cell apoptosis (32,33). The effects of pristimerin against apoptosis-associated Bcl-2 family proteins, and whether the intrinsic and/or extrinsic pathway(s) are involved in the apoptotic process in CAL-27 and SCC-25 cells requires further investigation.
The MAPK/Erk1/2 and PI3K/Akt pathways are important signaling pathways associated with the regulation of cell proliferation, differentiation, apoptosis and tumor pathogenesis (34–36). The downregulation of MAPK/Erk1/2 and PI3K/Akt signaling demonstrated in this study may account for the proliferation-suppressing and apoptosis-inducing effects of pristimerin in CAL-27 and SCC-25 cells. Furthermore, previous studies have reported that pristimerin inhibits MAPK/Erk1/2 and PI3K/Akt signaling in breast (26,37), colorectal (27) and pancreatic cancer cells (13). However, the complete regulatory mechanisms of pristimerin on MAPK/Erk1/2 and PI3K/Akt signaling pathways in OSCC cells requires further elucidation.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Fund of China (grant no. 81460032) and the Natural Science Fund of Kunming University of Science and Technology (grant no. KKSY201460068).
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Author's contributions
HW and LL conceived and designed the study. HW, LL, ZA and JY performed the experiments. HW and LL wrote the manuscript. LC reviewed and edited the manuscript and was also involved in the conception of the study. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: A meta-analysis with implications for cancer control. Int J Cancer. 2014;135:1433–1443. doi: 10.1002/ijc.28643. [DOI] [PubMed] [Google Scholar]
- 3.Pednekar MS, Gupta PC, Yeole BB, Hebert JR. Association of tobacco habits, including bidi smoking, with overall and site-specific cancer incidence: Results from the Mumbai cohort study. Cancer Causes Control. 2011;22:859–868. doi: 10.1007/s10552-011-9756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radoï L, Luce D. A review of risk factors for oral cavity cancer: The importance of a standardized case definition. Community Dent Oral Epidemiol. 2013;41:97–109, e178-e191. doi: 10.1111/j.1600-0528.2012.00710.x. [DOI] [PubMed] [Google Scholar]
- 5.Wikner J, Gröbe A, Pantel K, Riethdorf S. Squamous cell carcinoma of the oral cavity and circulating tumour cells. World J Clin Oncol. 2014;5:114–124. doi: 10.5306/wjco.v5.i2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 7.Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68:732–766. doi: 10.1016/j.phytochem.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao JM, Wu WJ, Zhang JW, Konishi Y. The dihydro-beta-agarofuran sesquiterpenoids. Nat Prod Rep. 2007;24:1153–1189. doi: 10.1039/b601473a. [DOI] [PubMed] [Google Scholar]
- 9.Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: Natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65:2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiedemann RE, Schmidt J, Keats JJ, Shi CX, Zhu YX, Palmer SE, Mao X, Schimmer AD, Stewart AK. Identification of a potent natural triterpenoid inhibitor of proteosome chymotrypsin-like activity and NF-kappaB with antimyeloma activity in vitro and in vivo. Blood. 2009;113:4027–4037. doi: 10.1182/blood-2008-09-179796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR, Wu YC. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther. 2005;4:1277–1285. doi: 10.1158/1535-7163.MCT-05-0027. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Zhang W, Yan YY, Ma CG, Wang X, Wang C, Zhao JL. Triterpenoid pristimerin induced HepG2 cells apoptosis through ROS-mediated mitochondrial dysfunction. J BUON. 2013;18:477–485. [PubMed] [Google Scholar]
- 13.Deeb D, Gao X, Liu YB, Pindolia K, Gautam SC. Pristimerin, a quinonemethide triterpenoid, induces apoptosis in pancreatic cancer cells through the inhibition of pro-survival Akt/NF-kappaB/mTOR signaling proteins and anti-apoptotic Bcl-2. Int J Oncol. 2014;44:1707–1715. doi: 10.3892/ijo.2014.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YB, Gao X, Deeb D, Arbab AS, Gautam SC. Pristimerin induces apoptosis in prostate cancer cells by down-regulating Bcl-2 through ROS-dependent Ubiquitin-proteasomal degradation pathway. J Carcinog Mutagen Suppl. 2013;6:005. doi: 10.4172/2157-2518.S6-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byun JY, Kim MJ, Eum DY, Yoon CH, Seo WD, Park KH, Hyun JW, Lee YS, Lee JS, Yoon MY, Lee SJ. Reactive oxygen species-dependent activation of Bax and poly(ADP-ribose) polymerase-1 is required for mitochondrial cell death induced by triterpenoid pristimerin in human cervical cancer cells. Mol Pharmacol. 2009;76:734–744. doi: 10.1124/mol.109.056259. [DOI] [PubMed] [Google Scholar]
- 16.Lu Z, Jin Y, Chen C, Li J, Cao Q, Pan J. Pristimerin induces apoptosis in imatinib-resistant chronic myelogenous leukemia cells harboring T315I mutation by blocking NF-kappaB signaling and depleting Bcr-Abl. Mol Cancer. 2010;9:112. doi: 10.1186/1476-4598-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Call JA, Eckhardt SG, Camidge DR. Targeted manipulation of apoptosis in cancer treatment. Lancet. Oncol. 2008;9:1002–1011. doi: 10.1016/S1470-2045(08)70209-2. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif. 2000;33:261–274. doi: 10.1046/j.1365-2184.2000.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi M, Shimada M, Niida H. Genetic instability in cancer cells by impaired cell cycle checkpoints. Cancer Sci. 2006;97:984–989. doi: 10.1111/j.1349-7006.2006.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball KL. p21: Structure and functions associated with cyclin-CDK binding. Prog Cell Cycle Res. 1997;3:125–134. doi: 10.1007/978-1-4615-5371-7_10. [DOI] [PubMed] [Google Scholar]
- 23.Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin-dependent kinase-1: Linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 2002;9:1287–1293. doi: 10.1038/sj.cdd.4401130. [DOI] [PubMed] [Google Scholar]
- 24.Guo H, Ray RM, Johnson LR. RhoA stimulates IEC-6 cell proliferation by increasing polyamine-dependent Cdk2 activity. Am J Physiol Gastrointest Liver Physiol. 2003;285:G704–G713. doi: 10.1152/ajpgi.00044.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 26.Lee JS, Yoon IS, Lee MS, Cha EY, Thuong PT, Diep TT, Kim JR. Anticancer activity of pristimerin in epidermal growth factor receptor 2-positive SKBR3 human breast cancer cells. Biol Pharm Bull. 2013;36:316–325. doi: 10.1248/bpb.b12-00685. [DOI] [PubMed] [Google Scholar]
- 27.Yousef BA, Guerram M, Hassan HM, Hamdi AM, Zhang LY, Jiang ZZ. Pristimerin demonstrates anticancer potential in colorectal cancer cells by inducing G1 phase arrest and apoptosis and suppressing various pro-survival signaling proteins. Oncol Rep. 2016;35:1091–1100. doi: 10.3892/or.2015.4457. [DOI] [PubMed] [Google Scholar]
- 28.Wang WH, Hsuan KY, Chu LY, Lee CY, Tyan YC, Chen ZS, Tsai WC. Anticancer effects of Salvia miltiorrhiza alcohol extract on oral squamous carcinoma cells. Evid Based Complement Alternat Med. 2017;2017:5364010. doi: 10.1155/2017/4695951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan YY, Bai JP, Xie Y, Yu JZ, Ma CG. The triterpenoid pristimerin induces U87 glioma cell apoptosis through reactive oxygen species-mediated mitochondrial dysfunction. Oncol Lett. 2013;5:242–248. doi: 10.3892/ol.2012.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eum DY, Byun JY, Yoon CH, Seo WD, Park KH, Lee JH, Chung HY, An S, Suh Y, Kim MJ, Lee SJ. Triterpenoid pristimerin synergizes with taxol to induce cervical cancer cell death through reactive oxygen species-mediated mitochondrial dysfunction. Anticancer Drugs. 2011;22:763–773. doi: 10.1097/CAD.0b013e328347181a. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Zhou Y, Zhou H, Jia G, Liu J, Han B, Cheng Z, Jiang H, Pan S, Sun B. Pristimerin causes G1 arrest, induces apoptosis, and enhances the chemosensitivity to gemcitabine in pancreatic cancer cells. PLoS One. 2012;7:e43826. doi: 10.1371/journal.pone.0043826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ba XH, Cai LP, Han W. Effect of cilostazol pretreatment on the PARP/AIF-mediated apoptotic pathway in rat cerebral ischemia-reperfusion models. Exp Ther Med. 2014;7:1209–1214. doi: 10.3892/etm.2014.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan Y, Xin Y, Zhang C, Wu D, Ding D, Tang L, Owusu L, Bai J, Li W. Fermentation supernatants of Lactobacillus delbrueckii inhibit growth of human colon cancer cells and induce apoptosis through a caspase 3-dependent pathway. Oncol Lett. 2014;7:1738–1742. doi: 10.3892/ol.2014.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Zhu G, Getzenberg RH, Veltri RW. The upregulation of PI3K/Akt and MAP kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J Cell Biochem. 2015;116:1341–1349. doi: 10.1002/jcb.25091. [DOI] [PubMed] [Google Scholar]
- 36.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie G, Yu X, Liang H, Chen J, Tang X, Wu S, Liao C. Pristimerin overcomes adriamycin resistance in breast cancer cells through suppressing Akt signaling. Oncol Lett. 2016;11:3111–3116. doi: 10.3892/ol.2016.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.