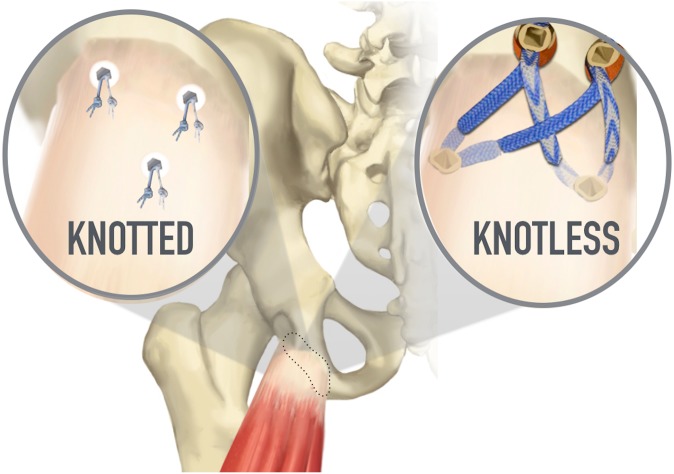
Abstract
Background:
Despite an abundance of literature regarding construct strength for a myriad of anchors and anchor configurations in the shoulder, there remains a paucity of biomechanical studies detailing the efficacy of these implants for proximal hamstring repair.
Purpose:
To biomechanically evaluate the ultimate failure load and failure mechanism of knotless and knotted anchor configurations for hamstring repair.
Study Design:
Controlled laboratory study.
Methods:
A total of 17 cadaveric specimens divided into 3 groups composed of intact hamstring tendons as well as 2 different anchor configurations (all-knotted and all-knotless) underwent first cyclic loading and subsequent maximal loading to failure. This protocol entailed a 10-N preload, followed by 100 cycles incrementally applied from 20 to 200 N at a frequency of 0.5 Hz, and ultimately followed by a load to failure with a loading rate of 33 mm/s. The ultimate failure load and mechanism of failure were recorded for each specimen, as was the maximal displacement of each bone-tendon interface subsequent to maximal loading. Analysis of variance was employed to calculate differences in the maximal load to failure as well as the maximal displacement between the 3 study groups. Holm-Sidak post hoc analysis was applied when necessary.
Results:
The all-knotless suture anchor construct failed at the highest maximal load of the 3 groups (767.18 ± 93.50 N), including that for the intact tendon group (750.58 ± 172.22 N). There was no statistically significant difference between the all-knotless and intact tendon groups; however, there was a statistically significant difference in load to failure when the all-knotless construct was compared with the all-knotted technique (549.56 ± 20.74 N) (P = .024). The most common mode of failure in both repair groups was at the suture-tendon interface, whereas the intact tendon group most frequently failed via avulsion of the tendon from its insertion site.
Conclusion:
Under biomechanical laboratory testing conditions, proximal hamstring repair using all-knotless suture anchors outperformed the all-knotted suture anchor configuration with regard to elongation during cyclic loading and maximal load to failure. Failure in the all-knotted repair group was at the suture-tendon interface in most cases, whereas the all-knotless construct failed most frequently at the musculotendinous junction.
Clinical Relevance:
No biomechanical studies have clearly identified the optimal anchor configuration to avert proximal hamstring repair failure. Delineating this ideal suture anchor construct and its strength compared with an intact hamstring tendon may alter the current standards for postoperative rehabilitation, which remain extremely conservative and onerous for these patients.
Keywords: hamstring, tendon repair, biomechanics, anchor, hamstring injury
Although a rare injury in the general population, athletes of all ages and competition levels are vulnerable to proximal hamstring injuries.10 An increasing body of systematic reviews and well-conducted clinical studies continue to strengthen the argument for operative intervention of complete proximal hamstring avulsions as well as partial tears refractory to conservative management.6,8 Approximately 80% of higher level athletes treated conservatively report persistent cramping and weakness.6,8 Both patient-reported outcomes and diminished failure rates for acute intervention in retracted tendon avulsions (≥2 cm) as well as for chronic symptomatic partial tears continue to markedly outperform those associated with nonoperative management.1,4
Several repair techniques, including both open and arthroscopic, have been reported in the literature.1 More anchors and sites of fixation incorporated into the repair technique have predictably yielded a more stable construct5; however, no optimal configuration of these anchors to prevent postoperative repair failure has been established. Moreover, an optimal construct would be equivalent or superior in strength to the native proximal hamstring insertion to enable orthopaedic surgeons to expedite an onerous and slowly progressive postoperative rehabilitation protocol that is currently advised.12
Constructs with an increased number of anchors and their biomechanical strength have been exhaustively detailed in the rotator cuff repair literature, and although it is difficult to directly extrapolate these findings to the hip, there is a valuable proof of concept that can be applied. Although controversy exists, multiple studies have suggested that double-row repair does provide a biomechanical strength advantage and potential enhancement of bone-tendon footprint healing compared with single-row repair.2,14 Further, some clinical studies have demonstrated a lower rerupture rate when a lateral row is employed.13 The forces present in the posterior hip surrounding the musculature place this repair site in a challenging healing environment in which the strength of repair is of the utmost importance.
The purpose of this study was to biomechanically evaluate the ultimate failure load and failure mechanism of knotless and knotted anchor configurations for hamstring repair. We hypothesized that the utilization of 4 knotless anchors in a double-row configuration that enhances compression at the anatomic footprint will exceed the strength of the knotted repair technique and most effectively prevent the failure of proximal hamstring tendon repair under maximal load conditions. Secondarily, we hypothesized that the majority of the failures will occur in the musculotendinous junction.
Methods
Study Design
Specimen selection for biomechanical testing began with 20 fresh-frozen human cadaveric hemipelvises (all male) that ranged in age from 52 to 89 years. Institutional review board approval was not required because the use of cadaveric specimens is exempt at our institution. The cadaveric specimens utilized in this study were donated to a tissue bank for the purpose of medical research and then purchased by our institution. The specimens were then randomly allocated to 1 of 3 groups for biomechanical testing: the intact tendon group (control), the all-knotted (AK) repair group, and the all-knotless (AS) repair group. Specimens were maintained at –20°C and thawed at room temperature for 24 hours before testing. Soft tissues were kept moist through the testing time to avoid changes in their histological properties.
Specimen Preparation
After securing the hemipelvises in the prone position, an open approach was carried out to isolate the proximal insertion of the semimembranosus, semitendinosus, and long head of the biceps tendon at the anatomic footprint just proximal to the ischial tuberosity. Meticulous dissection allowed us to ensure that no portion of the adductor magnus or surrounding soft tissue insertions along the ischiopubic ramus was inadvertently included in the repair site. After optimal exposure of the insertion site was achieved in these cadaveric specimens (Figure 1), we then sought to identify any bony deformities, prior injuries, or poor-integrity soft tissue that could potentially compromise the efficacy of our biomechanical analysis. Three specimens had obvious preexisting disease with significant gross anatomic changes at the attachments and thus were omitted from the study, but they were used to help calibrate our loading/testing apparatus as well as to help perfect our potting technique before carrying out the final study.
Figure 1.
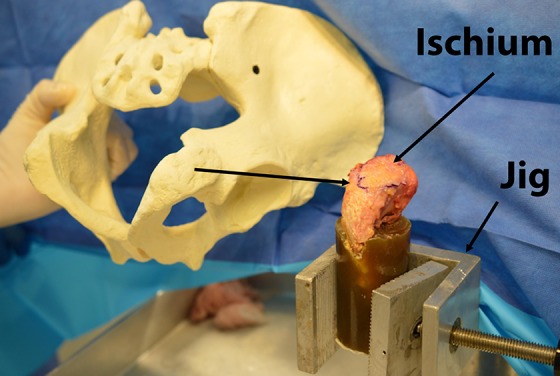
Cadaveric dissection demonstrating the testing setup for hamstring load to failure on a right ischium. A Sawbones model of the pelvis is included to facilitate 3-dimensional understanding of the position of the bone.
Osseous cuts were then completed at the level of the ischial spine and through the inferior pubic ramus to preserve the insertion site and to provide ample bone for preparation and potting of the specimen. Subsequent to the repairs, all specimens were potted using an identical technique in which six 4.0-mm cancellous shear screws were placed into the ischiopubic ramus to create a reinforced composite for better potting fixation. The osseous portion of each specimen was then suspended in a small plastic cylinder and then submerged in a polyester resin.
Surgical Technique
The 17 specimens randomly allocated to 1 of the 3 proximal hamstring states included (1) 6 intact tendons (control), (2) 5 AK repairs, and (3) 6 AS repairs. The hamstring insertion was subperiosteally elevated and completely detached before repair in the AK and AS groups to simulate a true acute avulsion. Repair was then carried out with 1 of 2 differing techniques.
Three 5.5-mm Corkscrew Anchor AK Repair
After detachment of the proximal hamstring insertion site, a 4.5-mm spade drill tip was utilized to drill 3 anchor tunnel sites perpendicular to the tendon insertion site, and these were subsequently tapped in all specimens. A fully threaded double-loaded 5.5 mm–diameter × 14.7 mm–long anchor (BioComposite Corkscrew; Arthrex) was then inserted into each of the tunnel sites. The suture material consisted of a 1.3 mm–wide ultra-high molecular weight polyethylene nonabsorbable suture (SutureTape; Arthrex). Previous marking of the bone-tendon interface with indelible blue marker allowed us to reapproximate our repair in an anatomic fashion. One limb of each suture pairing was run through the tendon in a Krackow running-locking fashion, and its matching limb was simply passed just proximal to the Krackow pattern in a deep to superficial trajectory to allow the tendon to slide and reduce to its bony insertion site along this suture strand. This was performed at the distal 2 anchor sites before completing the repair with the proximal suture anchor, all with the identical technique (Figure 2).
Figure 2.
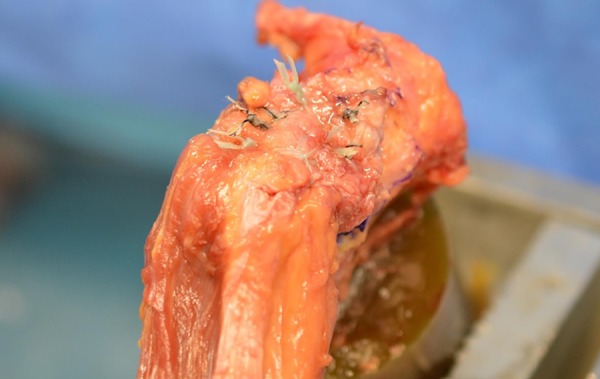
Cadaveric right ischium demonstrating the all-knotted technique (3 anchors) for hamstring repair.
Four 4.75-mm SwiveLock Anchor AS Repair
After detachment of the proximal hamstring insertion site, a 4.5-mm spade drill tip was utilized to drill 4 anchor tunnel sites in a rectangular configuration encompassing the entirety of the tendon insertion area, and these were subsequently tapped in all specimens. A double-loaded 4.75 mm–diameter × 19.1 mm–long anchor (BioComposite SwiveLock; Arthrex) was then inserted into the 2 distal tunnel sites. The suture material consisted of a 2.0 mm–wide ultra-high molecular weight polyethylene nonabsorbable suture (FiberTape; Arthrex) as well as an ultra-high molecular weight polyethylene nonabsorbable braided suture (No. 2 FiberWire; Arthrex). Previous marking of the bone-tendon interface with indelible blue marker allowed us to reapproximate our repair in an anatomic fashion. Utilizing first the No. 2 FiberWire, 1 limb of each suture pairing was run through the tendon in a Krackow running-locking fashion for 4 passes, and its matching limb was simply passed just proximal to the Krackow pattern in a deep to superficial trajectory to allow the tendon to slide and reduce to its bony insertion site along this suture strand. The 4 FiberTape suture strands were then passed in a simple deep to superficial manner, through the tendon, and crossed in a bridging pattern before fixating along with the FiberWire sutures in a double-row fashion utilizing swivel lock anchors (Figures 3 and 4).
Figure 3.

Cadaveric right ischium demonstrating the all-knotless technique (4 anchors) for hamstring repair.
Figure 4.
Schematic representation of the all-knotted and all-knotless configurations used in this cadaveric study.
Biomechanical Testing
A pilot study conducted before final biomechanical testing allowed us to refine and optimize our potting, tendon positioning, and loading techniques to re-create conditions nearly identical to the stresses applied in vivo.11 The osseous portion of the specimen (which had previously been potted in polyester resin) was first secured in a custom vise at the base of an Instron testing system; after this, the musculotendinous portion of the specimen was secured with a cryoclamp 2 cm distal to the musculotendinous transition. The specimen was oriented such that the vector of force applied would be anatomically aligned to simulate the in vivo trajectory of pull (traction was applied distally in the native physiological axis).
We established a loading protocol that reproduced in vivo forces9,11 encountered in the early postoperative phase with dynamic sawtooth cyclic testing and subsequently progressed to a maximal load, which was applied until construct failure. The assimilation of data provided in several previous gait analysis studies allowed the estimation of net forces endured by the proximal hamstring insertion during postoperative passive range of motion as well as during normal ambulation.11 A maximal force of 20 N/kg during ambulation and 9.6 N/kg experienced by the proximal hamstring during the swing and foot strike phases of the gait cycle was measured in these aforementioned studies,11 and thus, correlating with deductions performed by Hamming et al,5 an average 70-kg male would experience slightly less than 700 N with the knee in full extension during postoperative passive range-of-motion exercises. We therefore assumed that postoperative rehabilitation exercises in the first 2 weeks after surgery would not likely exceed this value.
With the primary objective of evaluating each construct’s maximal load endured until failure, we devised a loading protocol that dynamically stressed each specimen in a cyclical pattern with submaximal loads before applying a steadily increasing load until failure was observed. A 10-N preload was applied to all specimens before cyclic loading of 100 cycles using sinusoidal function between 20 and 200 N with a 0.5-Hz frequency. Tendon displacement was measured before postcyclic loading, which entailed a loading rate of 33 mm/s until failure of the construct.3 The loading rate was based on previous biomechanical studies.3 The maximal load as well as the mechanism of failure was then recorded for each specimen.
Statistical Analysis
The maximal load preceding construct failure and displacement of the tendon after conclusion of the cyclic loading phase were statistically analyzed. These variables were assessed utilizing 1-way analysis of variance (ANOVA) to compare the means of these parametric data between the 3 groups. Holm-Sidak post hoc analysis was performed for ANOVAs that demonstrated a statistically significant difference to examine for significant differences between each group. The statistical significance level was set at <.05, and SPSS v 20 (IBM) was used to complete all analyses.
Results
Mechanical Properties
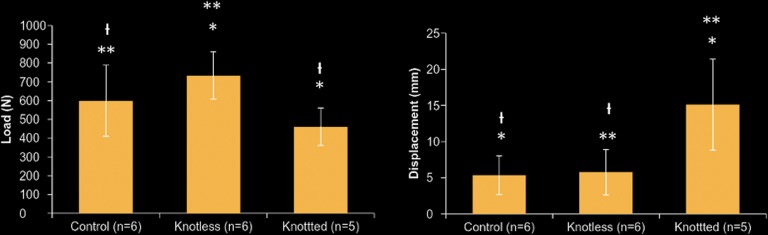
All specimens survived the cyclic loading phase of the testing protocol, and failure was observed during maximal loading. The AS suture anchor construct failed at the highest mean maximal load of the 3 groups (767.18 ± 93.50 N), including that for the intact tendon group (750.58 ± 172.22 N). There was no statistically significant difference between the AS and intact tendon groups (P = .248); however, there was a statistically significant difference in the mean load to failure when the AS construct was compared with the AK technique (549.56 ± 20.74 N) (P = .024). Displacement measurements of both the AS group (5.75 ± 3.13 mm) and the intact tendon group (5.33 ± 2.66 mm) were significantly less than that of the AK group (15.12 ± 6.30 mm) (P = .005) after cyclic loading. There was no statistically significant difference between the control and AS groups (P = .866) (Figure 5).
Figure 5.
Bar graphs demonstrating the load (left) and displacement (right) of the control (noninjured) group, all-knotted repair repair group, and all-knotless repair group. For the load bar graph: *P = .024, **P = .248, and  P = .140. For the displacement bar graph: *P = .005, **P = .005, and
P = .140. For the displacement bar graph: *P = .005, **P = .005, and  P = .866.
P = .866.
Failure Location
The most common mode of failure in both of the repair groups was at the suture-tendon interface, whereas the intact tendon group most frequently failed via avulsion of the tendon from its insertion site. The AK configuration failed at the footprint in 4 of the 5 specimens and via suture anchor pullout in the fifth specimen. The AS construct failed most frequently at the musculotendinous junction, with 5 of the 6 specimens failing at this location, with the remaining failure observed directly from the footprint (Figure 6).
Figure 6.
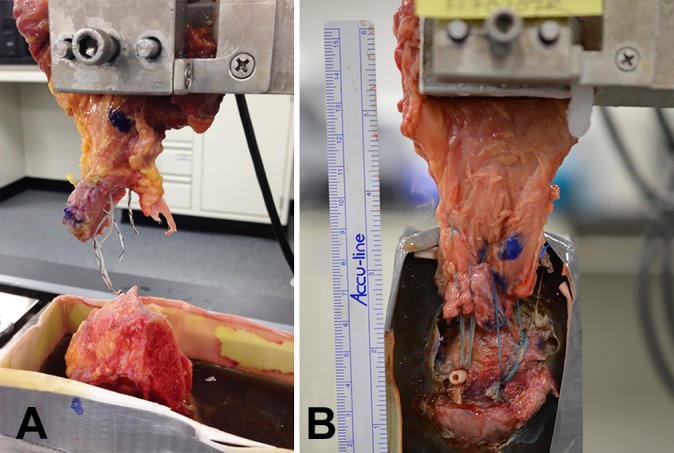
Failure in the (A) all-knotted and (B) all-knotless constructs at the suture-tendon interface and at the musculotendinous junction, respectively.
Discussion
The main findings of this study were that proximal hamstring repair using AS suture anchors outperformed the AK suture anchor configuration with regard to maximal load to failure under biomechanical laboratory testing conditions. Additionally, the displacement seen in the AS group and intact tendon group was one-third of the displacement of the AK group (P = .005) after cyclic loading. Failure in the AK repair group was at the suture-tendon interface in most cases, whereas the AS construct failed most frequently at the musculotendinous junction. This can mimic what happens in the shoulder when repair constructs that have sutures at the musculotendinous junction have introduced a new failure mechanism characterized by suture cutout at or near the musculotendinous junction.7 The intact tendon group most frequently failed via avulsion of the tendon from its insertion site. The AK configuration failed at the footprint in 4 of the 5 specimens and via suture anchor pullout in the fifth specimen. In the AS group, 5 of the 6 specimens failed at the musculotendinous junction, with the remaining failure observed directly from the footprint.
Although there has been an increase in both the identification and concomitant surgical management of proximal hamstring injuries, an optimal surgical construct remains poorly defined. More anchors intuitively provide a biomechanically superior construct; however, knotless fixation and alternate suture anchor configurations have been less thoroughly investigated. Hamming et al5 recently reported that 5 small anchors yielded similar results to the intact tendon and provided a stronger construct than repair using only 2 large or 2 small anchors for complete avulsions in a cadaveric study. The findings of the current laboratory evaluation provide a valuable and, to our knowledge, previously uninvestigated assessment of double-row proximal hamstring repair using AS suture anchors in comparison with both intact proximal hamstring tendons and a commonly performed AK repair technique frequently described in the literature.
The goal with proximal hamstring repair, as with any tendon-to-bone repair surgery, is to strive for maximal fixation strength so as to minimize the possibility of early failure and ideally to allow for an earlier and accelerated rehabilitation program. Theoretically, a stronger knotless repair, should allow for the introduction of an accelerated rehabilitation program with fewer restrictions compared with previous repair techniques. Based on these data, we have indeed changed our rehabilitation protocol. We have eliminated the requirement of postoperative bracing and have allowed our patients to eliminate weightbearing assistance at 2 weeks, which is 25% to 50% faster than the previous protocol. This type of accelerated rehabilitation protocol will need to be validated with future clinical studies.
Several studies have demonstrated the biomechanical strength advantage and potential enhancement of bone-tendon footprint healing with double-row AS repair compared with single-row AK repair for rotator cuff repair.2,14 Clinically, studies have had differing results on whether a lower rerupture rate is seen with double-row repair.13 Concordantly, our analysis demonstrated that the AS repair construct strength is statistically equivalent to the intact tendon controls in this cadaveric setting. The biomechanical data from this study demonstrated a reproducible construct that was equivalent to the intact tendon state.
This study is not without limitations that could have potentially affected the findings of this investigation. As with any cadaveric study, the inherent limitations of biologically inert bone and its diminished quality as well as the tendinous tissue quality are inferior to in vivo conditions. Procuring a large number of specimens to increase the sample size and strengthen the results reported also is challenging. Although the double-row construct was biomechanically superior in our evaluation, the necessary surgical exposure of the ischial tuberosity to complete this repair could potentially be more demanding than a standard AK repair, and this was unable to be controlled for in the laboratory setting as well. Additionally, the number of anchors was different (3 AK and 4 AS), and the sutures in both groups were not equal; the AK group used 1.3-mm suture tape, and the AS group used 2.0-mm suture tape, which could have had an impact on our final outcomes.
Conclusion
Proximal hamstring repair using AS suture anchors outperformed the AK suture anchor configuration with regard to elongation during cyclic loading and maximal load to failure under biomechanical laboratory testing conditions. Failure in the AK repair group was at the suture-tendon interface in most cases, whereas the AS construct failed most frequently at the musculotendinous junction.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: M.B.G. is a paid consultant for Arthrex, Medacta, and Ferring Pharmaceuticals; receives royalties from Arthrex; has received research grants from Arthrex; has received hospitality payments from Arthrex and Stryker; and is a shareholder in the Cedars-Sinai Kerlan-Jobe institute. B.S.A. has received research support from Smith & Nephew, Arthrex, DJO, Stryker, CDC Medical, and Zimmer Biomet. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Ahmad CS, Redler LH, Ciccotti MG, Maffulli N, Longo UG, Bradley J. Evaluation and management of hamstring injuries. Am J Sports Med. 2013;41(12):2933–2947. [DOI] [PubMed] [Google Scholar]
- 2. Baums MH, Kostuj T, Klinger HM, Papalia R. [Rotator cuff repair: single- vs double-row. Clinical and biomechanical results]. Orthopade. 2016;45(2):118–124. [DOI] [PubMed] [Google Scholar]
- 3. Campbell KA, Quirno M, Hamula M, et al. Suture anchor repair of complete proximal hamstring ruptures: a cadaveric biomechanical evaluation. Bull Hosp Jt Dis (2013). 2017;75(4):241–247. [PubMed] [Google Scholar]
- 4. Chu SK, Rho ME. Hamstring injuries in the athlete: diagnosis, treatment, and return to play. Curr Sports Med Rep. 2016;15(3):184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamming MG, Philippon MJ, Rasmussen MT, et al. Structural properties of the intact proximal hamstring origin and evaluation of varying avulsion repair techniques: an in vitro biomechanical analysis. Am J Sports Med. 2015;43(3):721–728. [DOI] [PubMed] [Google Scholar]
- 6. Harris JD, Griesser MJ, Best TM, Ellis TJ. Treatment of proximal hamstring ruptures: a systematic review. Int J Sports Med. 2011;32(7):490–495. [DOI] [PubMed] [Google Scholar]
- 7. Ladermann A, Christophe FK, Denard PJ, Walch G. Supraspinatus rupture at the musclotendinous junction: an uncommonly recognized phenomenon. J Shoulder Elbow Surg. 2012;21(1):72–76. [DOI] [PubMed] [Google Scholar]
- 8. Lempainen L, Banke IJ, Johansson K, et al. Clinical principles in the management of hamstring injuries. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2449–2456. [DOI] [PubMed] [Google Scholar]
- 9. Magnusson SP, Aagaard P, Simonsen EB, Bojsen-Moller F. Passive tensile stress and energy of the human hamstring muscles in vivo. Scand J Med Sci Sports. 2000;10(6):351–359. [DOI] [PubMed] [Google Scholar]
- 10. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209–226. [DOI] [PubMed] [Google Scholar]
- 11. Schache AG, Kim HJ, Morgan DL, Pandy MG. Hamstring muscle forces prior to and immediately following an acute sprinting-related muscle strain injury. Gait Posture. 2010;32(1):136–140. [DOI] [PubMed] [Google Scholar]
- 12. Sherry MA, Johnston TS, Heiderscheit BC. Rehabilitation of acute hamstring strain injuries. Clin Sports Med. 2015;34(2):263–284. [DOI] [PubMed] [Google Scholar]
- 13. Tudisco C, Bisicchia S, Savarese E, et al. Single-row vs. double-row arthroscopic rotator cuff repair: clinical and 3 Tesla MR arthrography results. BMC Musculoskelet Disord. 2013;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wall LB, Keener JD, Brophy RH. Double-row vs single-row rotator cuff repair: a review of the biomechanical evidence. J Shoulder Elbow Surg. 2009;18(6):933–941. [DOI] [PubMed] [Google Scholar]