Abstract
Background:
We performed a meta-analysis to evaluate the benefit of patent foramen ovale closure in stroke prevention.
Methods:
We searched Medline/PubMed, EMBASE, Web of Science and Cochrane central database for randomized control trials assessing the incidence of recurrent stroke after patent foramen ovale closure when compared to medical therapy. Pooled odds ratio and 95% confidence intervals were calculated using a random effects model. The heterogeneity among studies was tested using the χ2 test and inconsistency was quantified using the I2 statistic.
Results:
Our search strategy yielded 71 articles. We included five studies with a total of 3440 patients. Median age in the device group was 45 (43, 5.5) years and in the medical group was 45 (44.5, 46) years; 52% were male, 27.7% of patients had an atrial septal aneurysm, 25% had hypertension, and 20.5% had diabetes mellitus. The median follow-up time was 44 (34.5–50) months. The pooled odds ratio of recurrent stroke, transient ischemic attack and composite end point of stroke + transient ischemic attack + peripheral embolism in the patent foramen ovale closure versus medical therapy group were 0.4 (95% confidence interval 0.25–0.63, I2 = 57.5%), 0.93 (95% confidence interval 0.61–1.42, I2 = 0%), and 0.6 (95% confidence interval 0.44–0.82, I2 = 0%), respectively. The incidence of atrial fibrillation was found to be significantly higher in the patent foramen ovale closure group with odds ratio of 6 (95% confidence interval 3.13–11.4, I2 = 33.5%). On subgroup analysis, patent foramen ovale closure appeared to benefit males and patients with a large shunt. Number needed to treat to prevent one recurrent stroke with patent foramen ovale closure is 42. Number needed to harm to cause one atrial fibrillation with patent foramen ovale closure is 39.
Conclusion:
This meta-analysis of randomized trials concludes that percutaneous patent foramen ovale closure is effective in recurrent stroke prevention especially in males and in those with a large shunt.
Keywords: Atrial fibrillation, cryptogenic stroke, patent foramen ovale, transient ischemic attack
Background
In United States, the incidence of stroke is approximately 800,000/year.1 Ischemic stroke is the fifth leading cause of death in the United States. Common causes of ischemic stroke are cardio embolism, large vessel atherosclerosis, and small vessel disease. Following standard neurologic workup, about 1 in 4 symptomatic ischemic strokes does not have well-defined etiology. This subgroup is defined as cryptogenic stroke.2 Cryptogenic stroke can occur due to cardiac causes such as intra cardiac shunts, occult atrial fibrillation, and aortic atherosclerosis. In studies conducted by Overell et al.,3 it was found that patent foramen ovale (PFO) is commonly seen in patients with cryptogenic stroke. In patients with PFO, the putative mechanism is paradoxical embolism, where thrombus in the venous circulation travels through the PFO into the systemic circulation. Once in the systemic circulation, it can cause cerebral arterial occlusion, resulting in stroke.
After the first episode of cryptogenic stroke, patients are at increased risk for recurrent strokes. Thus, secondary prevention is essential. Percutaneous device closure and medical management (antiplatelet and/or anticoagulant medications) are the common treatment modalities for PFO. The efficacy of PFO closure and medical therapy (MT) versus MT alone as secondary prevention of stroke was compared in several randomized controlled trials (RCTs), but results are conflicting.4–6 The American Heart Association/American Stroke Association guidelines offer closure of PFO in a patient with previous cryptogenic stroke as a class III recommendation; however, in patients with evidence of deep venous thrombosis, it is considered a class IIb recommendation.7
Several RCTs comparing PFO closure to MT have been published. The aim of our study is to evaluate the efficacy of percutaneous closure of a PFO after a cryptogenic stroke through meta-analysis of RCT data and systematic literature review.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses(PRISMA) statement, a systematic literature review was conducted.8 We searched Medline/PubMed, EMBASE, Web of Science and Cochrane central database for randomized control trials assessing recurrence of recurrent stroke after PFO closure when compared to MT. The search terms included “patent foramen ovale,” “PFO,” “stroke,” “percutaneous closure” and “trans catheter closure” (see Supplementary data). The inclusion criteria for study selection were as follows: (1) RCT, (2) patients >18 years with cryptogenic stroke, (3) reported outcomes including stroke, transient ischemic attack (TIA), cardiac and all-cause death, and peripheral embolism. Exclusion criteria included (1) studies which assessed the impact of PFO closure without MT groups, (2) abstracts which were published, but do not have full-text publication, and (3) studies which lack end point measurements such as stroke and/or TIA. Methodological and descriptive data, procedural success and complications were abstracted in duplicate from each study independently and agreement was tested.
RCTs fulfilling the predefined inclusion and exclusion criteria and which reported recurrent stroke and/or TIA in patients with PFO closure were included in the systematic review and quantitative analysis. Studies which included the following criteria: (1) RCTs which compared PFO closure with MT, (2) adult subject population (>18 years of age) and (3) with a diagnosis of PFO and a history of stroke and/or TIA.
Using a standardized data extraction form, two authors (P.A. and C.Y.) independently performed the study selection and data extraction. By consensus differences were resolved. The Jadad scale was used for quality assessment.9
Using the data extracted from selected studies, risk ratio (RR) and 95% confidence interval (CI) were estimated. As per intention-to-treat analysis, event rates for each outcome were calculated. The number needed to treat (NNT) and number needed to harm (NNH) were calculated based on estimated odds ratio (OR) and CI from meta-analysis.10 With random effects model using Mantel–Haenszel weighting, meta-analysis was conducted. By visually inspecting the funnel plot, publication bias was analyzed (see Supplementary data). Based on the presence or absence of atrial septal aneurysm, the size of the shunt, and the type of MT, subgroup analysis was done. For all analyses, a two-sided p value of <0.05 was considered as statistically significant. StatsDirect statistical software (Version 3.0.0; StatsDirect Ltd, Cheshire, UK) has been used for analysis. To calculate the means and the standard deviation for age, excel 2013 (Microsoft, Redmond, United States) was used.
Pooled OR and 95% CIs were calculated using a fixed and random effects model. The heterogeneity among studies was tested using the X2 test and inconsistency was quantified using the I2 statistic. According to I2 statistics, >25% is considered as low, >50% as moderate and 75% as high heterogeneity.11 Using age, gender, co-morbidities, interatrial septal aneurysm, closure device, the use of antiplatelet and anticoagulation therapy, baseline patient characteristics were collected.
Results
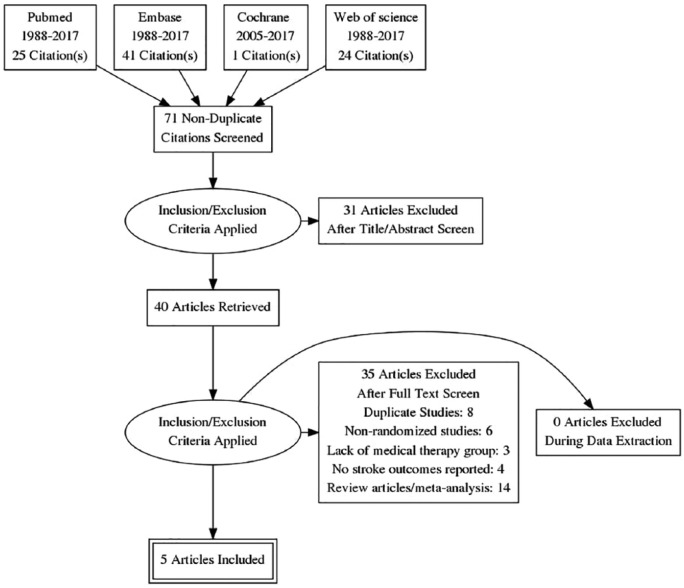
Our search strategy yielded 71 articles and 5 RCTs which met the inclusion criteria (Figure 1). Among the included RCTs, significant heterogeneity was noted in design, device choice and medical regimens across the included RCTs (Tables 1 and 2). The median follow-up duration was 3.6 years (interquartile range (IQR) 1.35). Median age in device group was 45 (43, 5.5) years and medical group was 45 (44.5, 46) years. No significant difference was noted among risk factors for stroke including hypertension, diabetes mellitus, smoking and coronary artery disease among the PFO closure and MT groups. Lowest rate of cardiovascular risk factors and the highest rate of high-risk PFO features were noted in CLOSE trial.12 Lowest risk of bias (high risk in two of seven categories) was seen in CLOSE trial,12 whereas highest risk of bias (high risk in five of seven categories) was seen in RESPECT trial.6 In all the trails, stroke is defined as acute focal neurological event which is positive on neuroimaging or which lasts >24 h without neuroimaging.
Figure 1.
PRISMA statement.
Table 1.
Baseline patient characteristics of included trials.
| Characteristics | CLOSE (2017)12 |
REDUCE (2017)13 |
RESPECT (2017)6 |
PC trial (2013)5 |
CLOSURE I (2012)4 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFO closure | MT | PFO closure | MT | PFO closure | MT | PFO closure | MT | PFO closure | MT | |
| Device used | Amplatzer | Helex/GSO | Amplatzer | Amplatzer | StarFLEX | |||||
| Number | 238 | 235 | 441 | 223 | 499 | 481 | 204 | 210 | 447 | 462 |
| Duration of follow-up (years) | 5.2 | 5.4 | 3.2 | 3.2 | 6.2 | 5.6 | 4.1 | 4 | 2 | 2 |
| Age: mean (years) | 42.9 | 43.8 | 45.4 | 44.8 | 45.7 | 46.2 | 44.3 | 44.6 | 45.3 | 45.7 |
| Male sex | 58% | 60% | 59% | 62% | 54% | 56% | 45% | 54% | 52% | 52% |
| Cardiovascular risk factors | ||||||||||
| Smoking (%) | 29 | 29 | 14 | 11 | 15 | 11 | 26 | 22 | 22 | 23 |
| Diabetes mellitus (%) | 1 | 4 | 4 | 5 | 7 | 9 | 3 | 3 | NR | NR |
| Hyperlipidemia (%) | 13 | 15 | NR | NR | 39 | 41 | 25 | 30 | 47 | 41 |
| Hypertension (%) | 11 | 10 | 25 | 26 | 32 | 32 | 24 | 28 | 34 | 28 |
| Coronary artery disease (%) | NA | NA | NA | NA | 3.8 | 1.9 | 2 | 1.9 | 1.3 | 0.9 |
| Two or more prior strokes (%) | 4.2 | 3 | 9.5 | 5.8 | 10.6 | 10.6 | 37.3 | 37.6 | NA | NA |
| Interatrial septal aneurysm (%) | 34 | 31.5 | 20.4 | NA | 36.1 | 35.3 | 23 | 24.3 | 37.6 | 35.7 |
| PFO characteristics | ||||||||||
| Atrial septal aneurysm (%) | 34 | 31 | 20 | NR | 36 | 35 | 23 | 24 | 38 | 36 |
| Outcomes | ||||||||||
| Atrial fibrillation (%) | 4.84 | 0.85 | 7.03 | 0.45 | 0.8 | 0.63 | 3.3 | 0.96 | 6.07 | 0.66 |
| Stroke (%) | 0 | 6.33 | 1.37 | 5.68 | 1.83 | 3.44 | 0.49 | 2.43 | 2.76 | 2.89 |
| Transient ischemic attacks (%) | 3.47 | 3.52 | 4.01 | 3.72 | 1.22 | 0.84 | 2.51 | 3.45 | 2.99 | 3.82 |
PFO: patent foramen ovale; MT: medical therapy; NR: Not reported; NA: Not available; GSO: Gore Septal occluder.
Table 2.
Details of included randomized control trials.
| Study | Inclusion criteria | PFO closure |
Medical therapy regimen | Total new AF cases | Transient or persistent AF | |
|---|---|---|---|---|---|---|
| PFO device | Antiplatelet drug and duration | |||||
| CLOSE, 201712 | ASA or large shunt, few cardiovascular risk factors, standardized stroke workup | Multiplea | DAPT for 3 months, then aspirin or clopidogrel for rest of the trial | Aspirin, clopidogrel or aspirin + aggrenox | 11 cases | 11 transient AF, no recurrence |
| RESPECT, 20176 | Moderate–large shunt, controlled CV risk factors | Amplatzer | DAPT for 1 month, then aspirin for 5 months, then AP drug at discretion of site | Aspirin, clopidogrel, aspirin + aggrenox, DAPT or warfarin | 7 cases | 7 per-procedural AF which were self-resolved |
| REDUCE, 201713 | Any size shunt | Helex, Cardioform | Aspirin, clopidogrel or aspirin + aggrenox for remainder of trial | Aspirin alone, aspirin + aggrenox or clopidogrel alone | 29 cases | 17 transient AF, 12 persistent AF |
| PC trial, 20135 | Any shunt size, stroke or peripheral embolism | Amplatzer | DAPT for 1–6 months | AP or AC at the discretion of physician | 6 cases | 2 transient AF, 4 persistent AF |
| CLOSURE I, 20124 | Any size shunt | STARFlex | DAPT for 6 months, then aspirin 81–325 mg for 18 months | Aspirin, warfarin or both | 23 cases | 17 transient AF, 6 persistent AF |
AC: anticoagulation; AP: antiplatelet; ASA: atrial septal aneurysm; CV: cardiovascular; DAPT: dual antiplatelet therapy; m: months; MT: medical therapy; PFO: patent foramen ovale; AF: atrial fibrillation.
Amplatzer used in 51% of the patients.
In one study, magnetic resonance imaging (MRI) was mandated as the imaging modality, whereas in other trials computed tomography (CT) or MRI was used. One of the trials used STARFlex device (NMT Medical, Inc., Boston, United States),4 three trials used the Amplatzer device (AGA Medical Corporation, Minnesota, United States)5,12,14 and one trial used GORE septal occluder/Helex (W.L. Gore and Associates, Inc., Phoenix, Arizona, United States).13 For MT, in one trial warfarin, aspirin or both were used,4 one trial was as per physician discretion,5 and in the remaining, the three trials,12–14 combinations of aspirin, clopidogrel, dipyridamole, or anticoagulation, were used.
Using Jadad scale, trial quality was assessed. Bias resistant features such as randomization and reporting of all pre-specified outcomes were performed by all trials included in our meta-analysis. Since blinded assessment of outcomes is the most important potential bias in trials, CLOSE,12 CLOSURE 1,4 and REDUCE13 trials were considered to be of intermediate quality as they did not specify independent, blinded verdict of clinical events. Whereas PC5 and RESPECT6 trials were considered as high-quality trials since they specifically mentioned independent verdict of clinical events by assessors unaware of treatment allocation.
Recurrent stroke and TIA
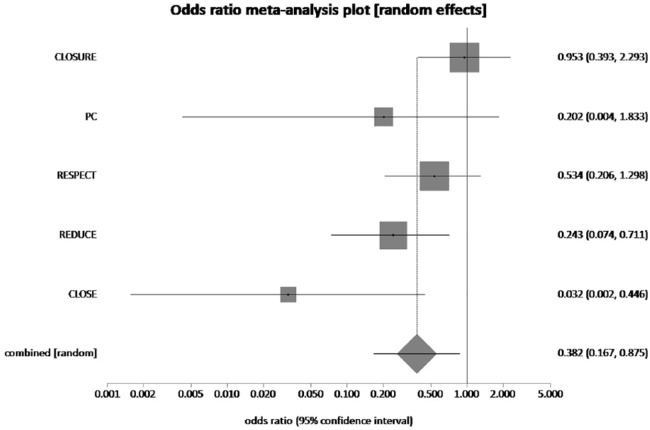
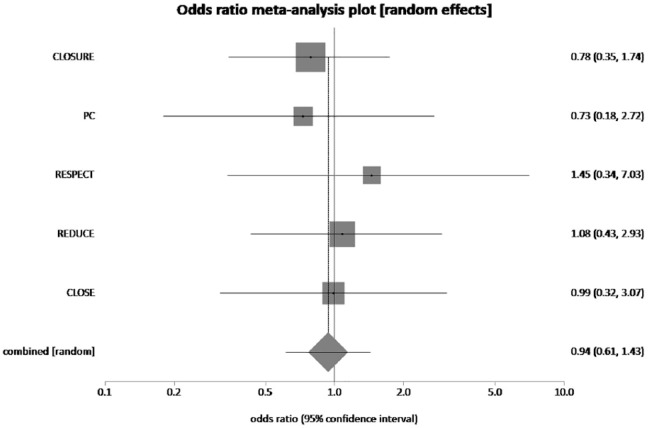
The pooled rate of recurrent stroke in patients who received PFO closure plus MT was 1.55% and 3.9% in patients who received MT alone. PFO closure plus MT reduced the risk of recurrent stroke by 33% when compared to MT alone (pooled OR = 0.38, 95% CI = 0.17–0.88, p = 0.02, I2 = 57.5%) (Figure 2). The pooled rate of TIA in PFO closure plus MT group and MT alone group was 2.75% and 2.81%, respectively. PFO closure plus MT reduced the risk of TIA by 2.13% when compared to MT alone, which was not statistically significant (pooled OR = 0.94, 95% CI = 0.61–1.43, p = 0.76, I2 = 0%) (Figure 3). NNT with PFO closure to prevent 1 recurrent stroke is 42.
Figure 2.
The forest plot of odds ratios (ORs) of recurrent stroke with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke was recorded with 95% confidence interval (95% CI) and p value. OR = 0.38, 95% CI = 0.17–0.88, p = 0.02, I2 = 57.5%.
Figure 3.
The forest plot of odds ratios (ORs) of recurrent TIA with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent TIA was recorded with 95% confidence interval (95% CI) and p value. OR = 0.94, 95% CI = 0.61–1.43, p = 0.76, I2 = 0%.
Composite outcome of recurrent stroke, TIA and peripheral embolism
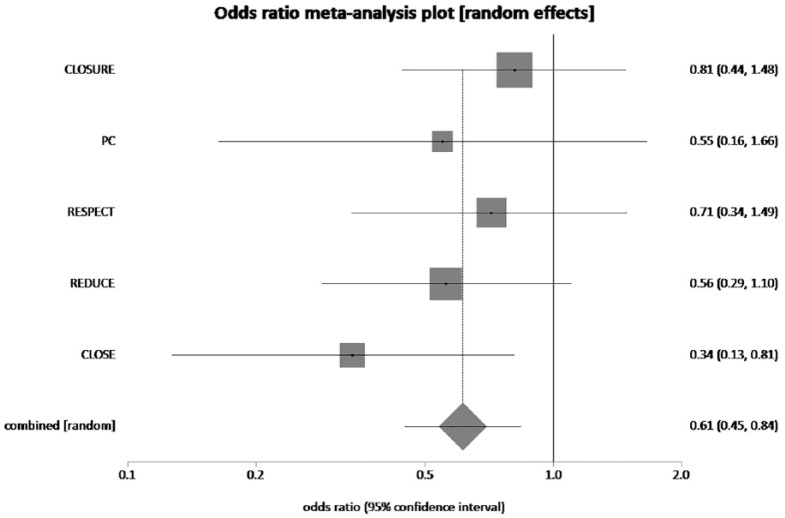
The pooled rate of composite of stroke, TIA and peripheral embolism was 4.3% in PFO closure and MT group and 6.8% in MT alone group. PFO plus MT reduced composite of TIA, stroke and peripheral embolism by 36.6% compared to MT alone, which was statistically significant (pooled OR = 0.61, 95% CI = 0.45–0.84, p = 0.01, I2 = 0%) (Figure 4).
Figure 4.
The forest plot of odds ratios (ORs) of recurrent TIA, stroke and peripheral embolism with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent TIA, stroke and peripheral embolism was recorded with 95% confidence interval (95% CI) and p value. OR = 0.61, 95% CI = 0.45–0.84, p = 0.01, I2 = 0%.
Atrial fibrillation
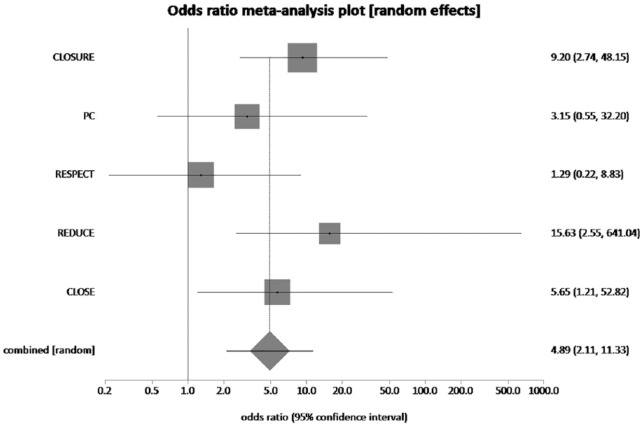
The pooled rate of newly detected atrial fibrillation in PFO closure plus MT group and in MT alone group was 4.1% and 0.69%, respectively. Higher incidence of atrial fibrillation was noted in the PFO closure which was statistically significant (pooled OR = 4.89, 95% CI = 2.11–11.33, p = 0.0002, I2 = 33.5%) (Figure 5). The NNH to cause one atrial fibrillation with PFO closure is 39.
Figure 5.
The forest plot of odds ratios (ORs) of new onset atrial fibrillation with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for new onset atrial fibrillation was recorded with 95% confidence interval (95% CI) and p value. OR = 4.89, 95% CI = 2.11–11.33, p = 0.0002, I2 = 33.5%.
Major bleeding
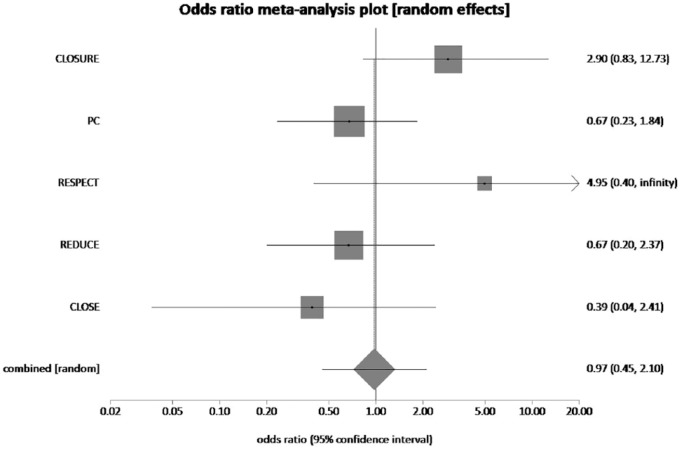
The pooled rates of major bleeding in PFO closure plus MT group and the MT alone group were 1.71% and 1.71%, respectively. No significant difference was noted between both groups (pooled OR = 0.97, 95% CI = 0.45–2.1, p = 0.56, I2 = 40.5%) (Figure 6).
Figure 6.
The forest plot of odds ratios (ORs) of major bleeding with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for major bleeding was recorded with 95% confidence interval (95% CI) and p value. OR = 0.97, 95% CI = 0.45–2.1, p = 0.56, I2 = 40.5%.
Subgroup analysis
Age
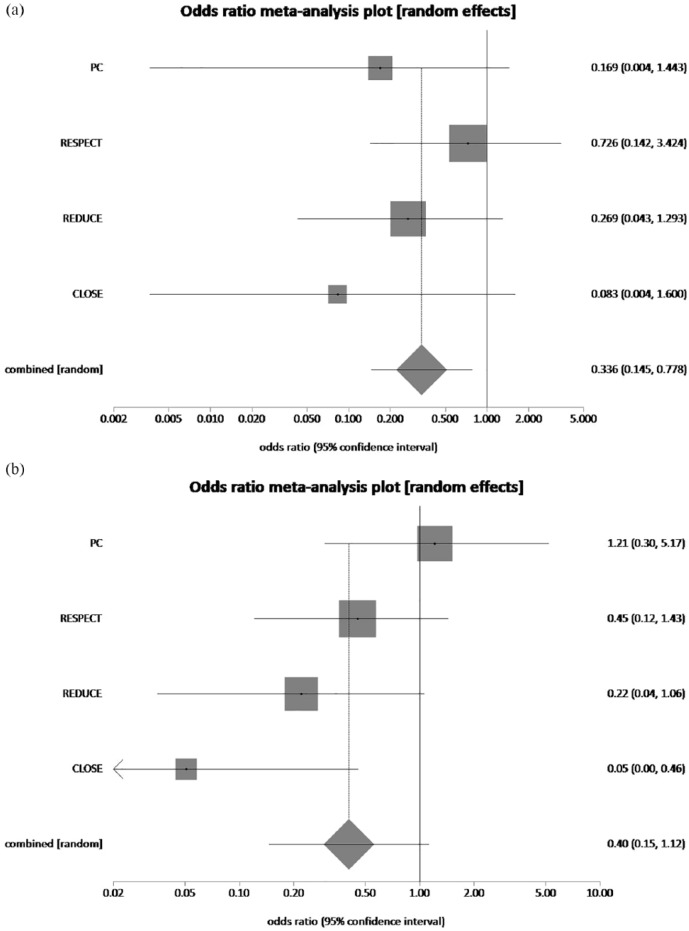
The pooled rate of recurrent stroke in patients with age <45 years who had underwent PFO closure plus MT and MT alone was 1.25% and 4.3%, respectively. PFO closure plus MT reduced recurrent stroke rate by 70.9% in patients with age <45 years, which was statistically significant (pooled OR = 0.33, 95% CI = 0.14–0.78, p = 0.01, I2 = 0%) (Figure 7(a)). The pooled rate of recurrent stroke in patients with age >45 years who had underwent PFO closure plus MT and MT alone was 1.97% and 5.34%, respectively. PFO closure plus MT resulted in recurrent stroke reduction by 63.1% in patients with age >45 years, which was not statistically significant (pooled OR = 0.40, 95% CI = 0.15–1.12, p = 0.08, I2 = 49.3%) (Figure 7(b)).
Figure 7.
(a) The forest plot of odds ratios (ORs) of recurrent stroke (age < 45) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (age < 45) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.33, 95% CI = 0.14–0.78, p = 0.01, I2 = 0%. (b) The forest plot of ORs of recurrent stroke (age > 45) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (age > 45) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.40, 95% CI = 0.15–1.12, p = 0.08, I2 = 49.3%.
Gender
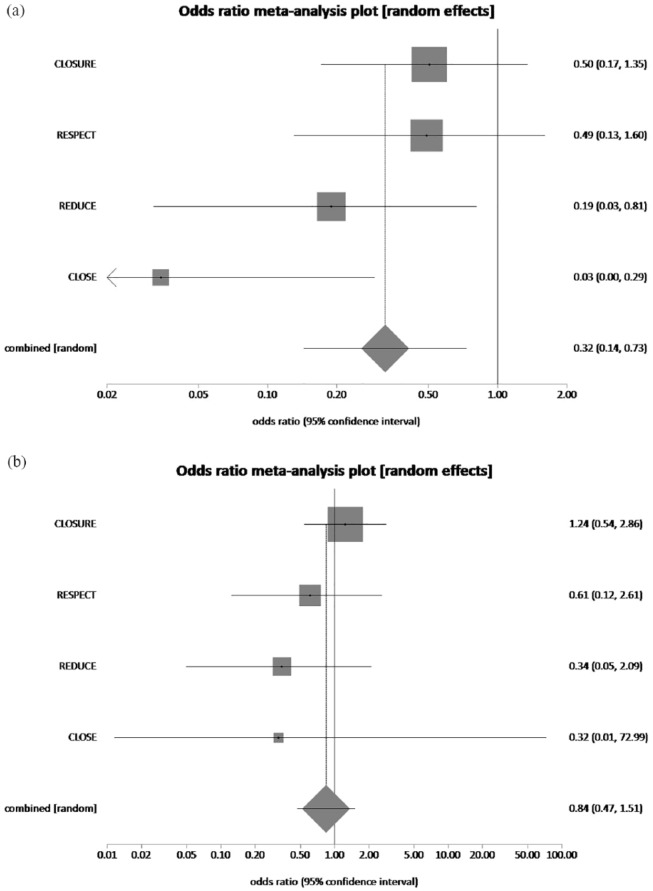
The pooled rate of recurrent stroke in males who had underwent PFO closure plus MT and MT alone was 1.75% and 6.26%, respectively. PFO closure plus MT reduced recurrent stroke rate by 72% in male patients, which was statistically significant (pooled OR = 0.32, 95% CI = 0.14–0.73, p = 0.01, I2 = 35.9%) (Figure 8(a)). The pooled rate of recurrent stroke in females who had underwent PFO closure plus MT and MT alone was 3.22% and 4.27%, respectively. PFO closure plus MT reduced recurrent stroke rate by 24.5% in female patients, which was not statistically significant (pooled OR = 0.84, 95% CI = 0.47–1.51, p = 0.56, I2 = 0%) (Figure 8(b)).
Figure 8.
(a) The forest plot of odds ratios (ORs) of recurrent stroke (male patients) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (male patients) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.32, 95% CI = 0.14–0.73, p = 0.01, I2 = 35.9%. (b) The forest plot of ORs of recurrent stroke (female patients) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (female patients) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.84, 95% CI = 0.47–1.51, p = 0.56, I2 = 0%.
Degree of shunt and atrial septal aneurysm
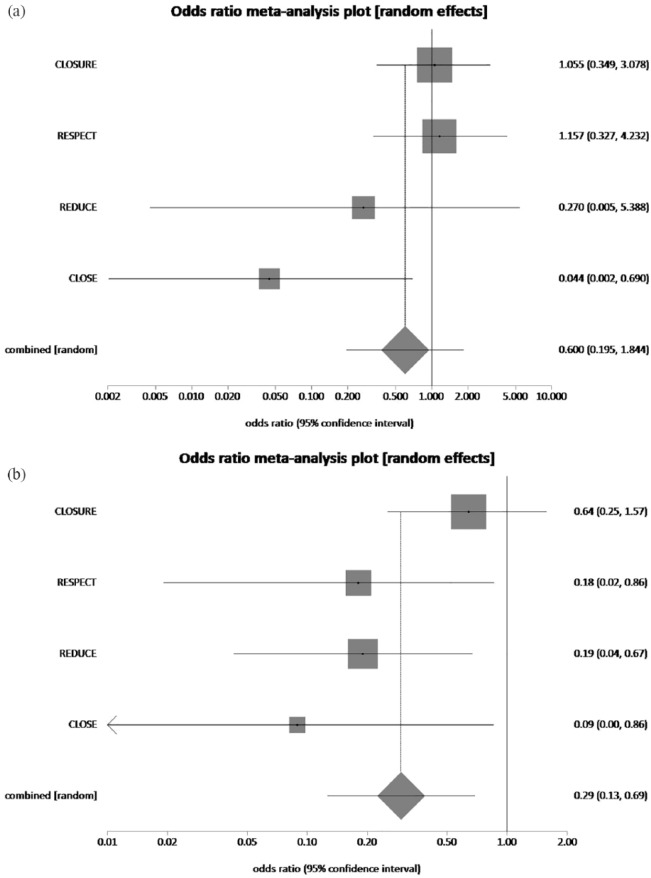
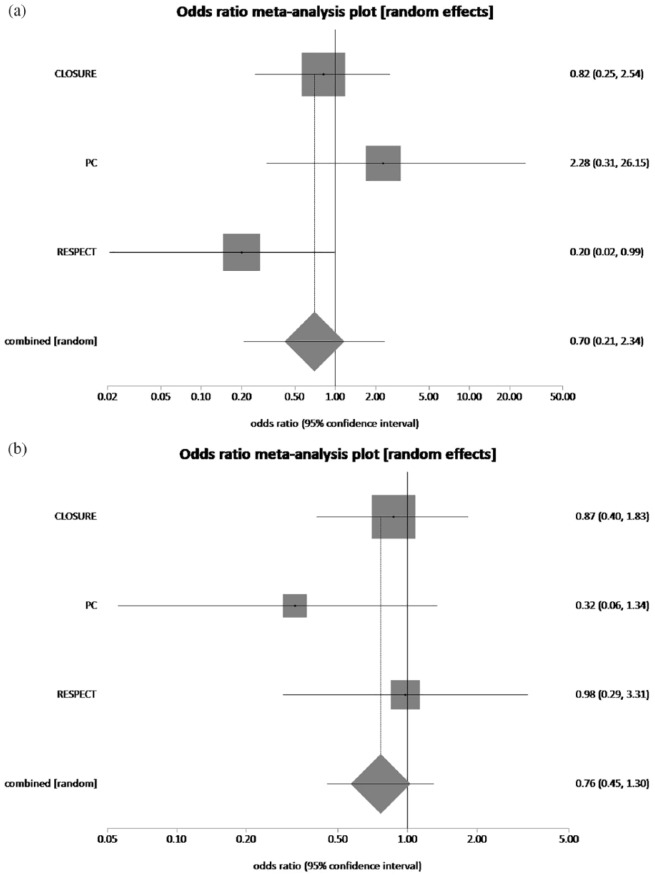
PFO having a moderate to large right-to-left shunt was defined using agitated saline contrast echocardiography if more than 20–30 contrast bubbles appeared in the left atrium. The pooled rate of recurrent stroke in patients with none to trace right-to-left shunt who had underwent PFO closure plus MT and MT alone was 3.1% and 5.52%, respectively. PFO closure plus MT reduced recurrent stroke rate by 43.8% in patients with none to trace right-to-left shunt, which was not statistically significant (pooled OR = 0.60, 95% CI = 0.19–1.84, p = 0.37, I2 = 51.8%) (Figure 9(a)). The pooled rate of recurrent stroke in patients with moderate to large right-to-left shunt who had underwent PFO closure plus MT and MT alone was 1.6% and 5.31%, respectively. Thus, PFO closure plus MT reduced recurrent stroke rate by 68.9% in patients with moderate to substantial right-to-left shunt, which was statistically significant (pooled OR = 0.29, 95% CI = 0.13–0.69, p = 0.37, I2 = 37.1%) (Figure 9(b)). Presence or absence of an atrial septal aneurysm had no statistically significant effect on recurrence of stroke (Figure 10(a) and (b)).
Figure 9.
(a) The forest plot of odds ratios (ORs) of recurrent stroke (none/trace right to left shunt) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (none-trace right to left shunt) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.60, 95% CI = 0.19–1.84, p = 0.37, I2 = 51.8%. (b) The forest plot of ORs of recurrent stroke (moderate/large right to left shunt) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (moderate/large right to left shunt) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.29, 95% CI = 0.13–0.69, p = 0.37, I2 = 37.1%.
Figure 10.
(a) The forest plot of odds ratios (ORs) of recurrent stroke (presence of atrial septal aneurysm) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (presence of atrial septal aneurysm) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.70, 95% CI = 0.21–2.34, p = 0.56, I2 = 54.6%. (b) The forest plot of ORs of recurrent stroke (absence of atrial septal aneurysm) with device closure versus medical therapy. Sizes of data markers are proportional to the weight of each study in the meta-analysis. Horizontal bars represent the 95% confidence interval of individual trials. OR measured by random effects for recurrent stroke (absence of atrial septal aneurysm) was recorded with 95% confidence interval (95% CI) and p value. OR = 0.76, 95% CI = 0.45–1.30, p = 0.32, I2 = 0%.
Discussion and limitations
In this meta-analysis of five published RCTs, it was found that PFO closure with MT significantly reduced the risk of recurrent stroke compared with MT alone with a NNT to prevent one stroke being 42. NNH to cause one atrial fibrillation with PFO closure is 39.
With regard to secondary efficacy end points, PFO closure did not result in significant reduction of risk of TIA or improvement in overall survival. One of the possible reasons could be difficulty in detecting TIA cases. Results of our meta-analyses are consistent with the results of recent meta-analysis published evaluating the efficacy of PFO closure in cryptogenic stroke.15–20
DEFENSE-PFO trial21 included only patients with high-risk PFO which includes presence of atrial septal aneurysm, hypermobility or PFO > 2 mm. The study showed lower rates of recurrent stroke and vascular death. We have not included this trial for our analysis because (1) it was published after we finished our initial analysis; (2) the trial only included patients with high-risk PFO and it did not meet our inclusion criteria. Some of the recent meta-analysis included this trial and showed results which were congruent with our findings.22–25 Subgroup analysis shows that PFO closure resulted in significant relative risk reduction in younger age and large size shunts compared to older age and small shunt subgroups.
Results of our study also show significant risk reduction in male patients compared to female patients. However, numerically the positive result is also confirmed in females and closure should by no means be restricted to males. Results of observational and retrospective studies show higher prevalence of PFO with cryptogenic stroke in men compared to women.26 However, the underlying mechanism supporting significant reduction in stroke rate in males who underwent PFO closure was not found in the literature. Since the confidence intervals of OR between men and women overlap, potentially null hypothesis for gender disparity can be expressed.
However, smaller sample size and non-homogeneous reporting of outcomes act as the major limiting factors for this subgroup analysis. Degree of shunt was defined separately among different studies and thus acts as one of the limiting factors. Rather than conclusive subgroup analysis interpretation should be hypothesis generating.
In REDUCE trial,13 which found 77% risk reduction of recurrent stroke with PFO closure over 3.2 years, patients with moderate–large PFO shunts, no previous lacunar strokes and no uncontrolled vascular risk factors were selected.13
In CLOSE trial,12 with PFO closure, 97% risk reduction was found in recurrent stroke for a follow-up period of 5.3 years. Patients with large right to left PFO shunts or atrial septal aneurysms, few vascular risk factors and who underwent a standardized stroke workup before enrollment were enrolled.12
In RESPECT trial,6 62% risk reduction with PFO closure found. Patients with any PFO shunt size were included and pre-enrollment stroke workup was not standardized.14 A significant increased risk of newly detected atrial fibrillation of 4.6% with PFO closure, compared to 0.9% with MT (p = 0.02), was noted in CLOSE trial.12
In REDUCE trial,13 a 6.6% increased risk of atrial fibrillation with PFO closure was noted when compared to 0.4% with MT (p < 0.001). How much of this atrial fibrillation was “new-onset” versus “newly detected” is debatable. Data from previous studies show that up to 16% of patients with cryptogenic stroke have latent paroxysmal atrial fibrillation which goes undetected.27 However, if this is the fact, then rates of “newly detected” atrial fibrillation should be similar in each study arm. Moreover, atrial fibrillation was mostly detected in the immediate post-procedure period in the PFO closure could be driven by an organic mechanisms related to device deployment.
This meta-analysis is being done at study data level, not at individual patient data level, raising the probability for bias. Lack of individual participant data limited our ability to perform extensive subgroup analysis. In many studies, no standard protocol was used for MT and varied PFO closure devices were used. For instance, the antithrombotic regimens such as antiplatelet therapy (single or dual agents), anticoagulation therapy and combination of therapies or none have been used in MT among trials. In some trials, the choice of therapy was not equally distributed among treatment arms and was left at the discretion of treating physician. This acted as a major confounding factor which had adverse effects on the results of earlier trials. Antiplatelet and anticoagulation were identified as the clinical end points in MT subgroups in our study. Compared to antiplatelet therapy, PFO closure demonstrated clear benefit, but no proven results were found comparing PFO closure and anticoagulation therapy. Patients who received anticoagulation therapy in the CLOSE trial12 had an event rate of 1.6% compared to 5.1% in patients who had received only antiplatelet therapy.
Paradoxical embolism from the venous system acts as the proposed mechanism for PFO-related strokes. But in most of the trials, the prevalence of deep venous thrombosis was not mentioned. While in most of the trials, PFO closure was compared to antiplatelet therapy, which is not an optimal therapy in patients with suspected venous thrombosis. For cryptogenic stroke, results of current studies do not show superiority of anticoagulation therapy compared to antiplatelet therapy.7 No significant difference in the risk of stroke was noted in trials comparing PFO± oral anticoagulants versus anticoagulants alone.4,14 The primary limitation of RESPECT,6 PC,5 and CLOSURE 14 trials was relatively small numbers of events and short duration follow-up.
The STARFlex (NMT Medical, Inc., Boston, United States) occluder device, which is now considered inferior due to its high complication rate and low success rate, was used in CLOSURE 1 trial.4 These findings have led to the approval of Amplatzer PFO occluder (AGA Medical Corporation, Minnesota, United States) for percutaneous closure of PFO in October 2016 by Food and Drug Administration (FDA). Even the type of device might have an impact on the risk of post procedural atrial fibrillation. In CLOSURE 1 trial,4 where STARFlex device was used for PFO closure resulted in significant higher rate of atrial fibrillation than MT alone.
In REDUCE trial13 (where HELEX and CARDIOFORM septal occluders were used), risk for atrial fibrillation was higher in the device group than with the MT alone. Conversely, in both the PC5 and RESPECT6 trails (where Amplatzer PFO occluder was used), no statistically significant risk for atrial fibrillation was noticed between device therapy group and MT alone group. These variations led to moderate heterogeneity between studies, which could have significant impact on this meta-analysis result.
Based on our analysis, we have shown that more patients develop atrial fibrillation compared to the number of strokes prevented. This would affect subsequent cardiovascular care as most patients enrolled in the study have a CHA2DS2-VASc score of at least 2 if they develop atrial fibrillation, which based on the current guidelines is an indication for anticoagulation.28,29 Prior studies have shown no difference in ischemic stroke or death between PFO closure and anticoagulation with a mildly elevated risk of major bleeds with anticoagulation.30 However, the incidence of atrial fibrillation associated with PFO closure is predominantly early, self-limited and rarely portends an increased risk of future stroke or need for anticoagulation. Therefore, PFO closure must never be withheld as a therapeutic option for patients with cryptogenic stroke and patent PFO. Future studies must evaluate the long-term rate of recurrence of atrial fibrillation in patients undergoing PFO closure to further substantiate the low-risk nature of the procedure in long term.
Endorsement of PFO closure for cryptogenic stroke is variable among different professional societies as evidenced by existing guidelines. However, overwhelming majority of them does not support routine use of device closure in patients with cryptogenic stroke and PFO (Table 3). We hope that the results of our meta-analysis will help update the current guidelines and suggest that PFO closure should be preferred strategy patients at low risk of atrial fibrillation, high risk of bleeding, age < 45 years, with moderate to large right to left shunt and history of recurrent cerebral events.
Table 3.
Guideline-based recommendations for patent foramen ovale closure.
| Guideline | Year | Recommendation |
|---|---|---|
| American Academy of Neurology | 2016 | Clinicians should not routinely offer percutaneous PFO closure to patients with cryptogenic ischemic stroke outside of a research setting. For recurrent strokes despite adequate medical therapy with no other mechanism identified, AMPLATZER PFO may be recommended based on availability |
| American Heart Association/American Stroke Association (AHA/ASA) | 2014 | For patients with TIA or cryptogenic ischemic stroke and a PFO (without any evidence of DVT), benefit from PFO closure was supported from available data. In the setting of DVT and PFO, based on the risk of recurrent DVT, PFO closure by a transcatheter device might be considered |
| National Institute for Health and Care Excellence (NICE) | 2013 | Evidence on the safety of percutaneous closure of patent foramen ovale to prevent recurrent cerebral embolic events shows infrequent but serious complications |
| American College of Chest Physicians (ACCP) | 2012 | In patients with cryptogenic stroke and atrial septal aneurysm or PFO, who had experienced recurrent events despite aspirin therapy, treatment with VKA therapy (target INR 2.5; range 2–3) and consideration of device closure over aspirin therapy are recommended |
PFO: patent foramen ovale; DVT: deep venous thrombosis; INR: International normalized ratio; TIA: transient ischemic attack; VKA: vitamin K antagonist.
Conclusion
Thus, based on the data from five RCTs, we conclude that PFO closure plus MT results in significant reduction in risk of recurrent stroke compared to MT alone in patients with previous cryptogenic stroke. This meta-analysis of randomized trials concludes that percutaneous PVO closure is effective in recurrent stroke prevention at the cost of increased incidence of atrial fibrillation, especially in males and those with large shunt. NNT to prevent one recurrent stroke with PFO closure is 42. NNH to cause one atrial fibrillation with PFO closure is 39.
Supplemental Material
Supplemental material, Supplementary_Data for Are we there yet with patent foramen ovale closure for secondary prevention in cryptogenic stroke? A systematic review and meta-analysis of randomized trials by Pradyumna Agasthi, Kantha Ratnam Kolla, Charan Yerasi, Sibghat Tullah, Venkata Siva Pulivarthi, Boshra Louka, Reza Arsanjani, Eric H Yang, Farouk Mookadam and F David Fortuin in SAGE Open Medicine
Acknowledgments
The authors acknowledge and appreciate our librarian Ms Diana Almader-Douglas M.L.S. who extended her expertise in performing a through and extensive search of all the relevant databases. P.A., K.R.K. and C.Y. were responsible for design, conception, acquisition of data, analysis and interpretation of data, drafting of initial manuscript. S.T., B.L., R.A., E.H.Y., F.M. and F.D.F. critically revised the manuscript. All authors critically revised the manuscript and gave final approval.
Footnotes
Availability of data and materials: All data and materials used in this research are freely available in electronic databases (PubMed, EMBASE, Cochrane database and Web of Science). References have been provided.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: IRB waiver was given by Mayo clinic IRB board due to the nature of the study being a meta-analysis of published data.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Pradyumna Agasthi  https://orcid.org/0000-0003-3067-6979
https://orcid.org/0000-0003-3067-6979
References
- 1. Guercini F, Acciarresi M, Agnelli G, et al. Cryptogenic stroke: time to determine aetiology. J Thrombos Haemostas 2008; 6(4): 549–554. [DOI] [PubMed] [Google Scholar]
- 2. Saver JL. CLINICAL PRACTICE. Cryptogenic stroke. New Engl J Med 2016; 374(21): 2065–2074. [DOI] [PubMed] [Google Scholar]
- 3. Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000; 55(8): 1172–1179. [DOI] [PubMed] [Google Scholar]
- 4. Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. New Engl J Med 2012; 366(11): 991–999. [DOI] [PubMed] [Google Scholar]
- 5. Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. New Engl J Med 2013; 368(12): 1083–1091. [DOI] [PubMed] [Google Scholar]
- 6. Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. New Engl J Med 2013; 368(12): 1092–1100. [DOI] [PubMed] [Google Scholar]
- 7. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45(7): 2160–2236. [DOI] [PubMed] [Google Scholar]
- 8. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark HD, Wells GA, Huet C, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trial 1999; 20(5): 448–452. [DOI] [PubMed] [Google Scholar]
- 10. Cates CJ. Simpson’s paradox and calculation of number needed to treat from meta-analysis. BMC Medical Research Methodol 2002; 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. New Engl J Med 2017; 377(11): 1011–1021. [DOI] [PubMed] [Google Scholar]
- 13. Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. New Engl J Med 2017; 377(11): 1033–1042. [DOI] [PubMed] [Google Scholar]
- 14. Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. New Engl J Med 2017; 377(11): 1022–1032. [DOI] [PubMed] [Google Scholar]
- 15. Alvarez C, Siddiqui WJ, Aggarwal S, et al. Reduced stroke after transcatheter patent foramen ovale closure: a systematic review and meta-analysis. American J Med Sci 2018: 356(2): 103–113. [DOI] [PubMed] [Google Scholar]
- 16. Garg L, Haleem A, Varade S, et al. Patent foramen ovale closure in the setting of cryptogenic stroke: a meta-analysis of five randomized trials. J Stroke Cerebrovasc Dis 2018; 27(9): 2484–2493. [DOI] [PubMed] [Google Scholar]
- 17. Mojadidi MK, Elgendy AY, Elgendy IY, et al. Transcatheter patent foramen ovale closure after cryptogenic stroke: an updated meta-analysis of randomized trials. JACC Cardiovasc Interv 2017; 10(21): 2228–2230. [DOI] [PubMed] [Google Scholar]
- 18. Ntaios G, Papavasileiou V, Sagris D, et al. Closure of patent foramen ovale versus medical therapy in patients with cryptogenic stroke or transient ischemic attack: updated systematic review and meta-analysis. Stroke 2018; 49(2): 412–418. [DOI] [PubMed] [Google Scholar]
- 19. Smer A, Salih M, Mahfood Haddad T, et al. Meta-analysis of randomized controlled trials on patent foramen ovale closure versus medical therapy for secondary prevention of cryptogenic stroke. Am J Cardiol 2018; 121(11): 1393–1399. [DOI] [PubMed] [Google Scholar]
- 20. Vaduganathan M, Qamar A, Gupta A, et al. Patent foramen ovale closure for secondary prevention of cryptogenic stroke: updated meta-analysis of randomized clinical trials. Am J Med 2018; 131(5): 575–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee PH, Song JK, Kim JS, et al. Cryptogenic Stroke and High-Risk Patent Foramen Ovale: the DEFENSE-PFO Trial. J Am Coll Cardiol 2018; 71(20): 2335–2342. [DOI] [PubMed] [Google Scholar]
- 22. Ma Y, Li D, Bai F, et al. Patent foramen ovale closure or medical therapy for secondary prevention of cryptogenic stroke: an update meta-analysis of randomized controlled trials. Medicine 2018; 97(34): e11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah R, Nayyar M, Jovin IS, et al. Device closure versus medical therapy alone for patent foramen ovale in patients with cryptogenic stroke: a systematic review and meta-analysis. Ann Intern Med 2018; 168(5): 335–342. [DOI] [PubMed] [Google Scholar]
- 24. Sitwala P, Khalid MF, Khattak F, et al. Percutaneous closure of patent foramen ovale in patients with cryptogenic stroke—an updated comprehensive meta-analysis. Cardiovasc Revasc Med 2018; 18: 30412–30413. [DOI] [PubMed] [Google Scholar]
- 25. Turc G, Calvet D, Guerin P, et al. Closure, anticoagulation, or antiplatelet therapy for cryptogenic stroke with patent foramen ovale: systematic review of randomized trials, sequential meta-analysis, and new insights from the CLOSE study. J Am Heart Assoc 2018; 7(12): e008356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nedeltchev K, Wiedmer S, Schwerzmann M, et al. Sex differences in cryptogenic stroke with patent foramen ovale. Am Heart J 2008; 156(3): 461–465. [DOI] [PubMed] [Google Scholar]
- 27. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. New Engl J Med 2014; 370(26): 2467–2477. [DOI] [PubMed] [Google Scholar]
- 28. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64(21): e1–e76. [DOI] [PubMed] [Google Scholar]
- 29. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg 2016; 50(5): e1–e88. [DOI] [PubMed] [Google Scholar]
- 30. Kuijpers T, Spencer FA, Siemieniuk RAC, et al. Patent foramen ovale closure, antiplatelet therapy or anticoagulation therapy alone for management of cryptogenic stroke? A clinical practice guideline. BMJ 2018; 362: k2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Data for Are we there yet with patent foramen ovale closure for secondary prevention in cryptogenic stroke? A systematic review and meta-analysis of randomized trials by Pradyumna Agasthi, Kantha Ratnam Kolla, Charan Yerasi, Sibghat Tullah, Venkata Siva Pulivarthi, Boshra Louka, Reza Arsanjani, Eric H Yang, Farouk Mookadam and F David Fortuin in SAGE Open Medicine