Abstract
Background:
Lumacaftor/ivacaftor combination therapy is efficacious and generally safe for patients with cystic fibrosis (CF) homozygous for the F508del-CF transmembrane conductance regulator (CFTR) mutation. However, long-term survival benefits of lumacaftor/ivacaftor (LUM/IVA) cannot yet be quantified. Simulation models can provide predictions about long-term health outcomes. In this study, we aimed to project long-term health outcomes of LUM/IVA plus standard care (SC) in patients with CF homozygous for F508del-CFTR.
Methods:
This modeling study was an individual patient simulation in US patients aged ⩾6 years with CF, homozygous for F508del-CFTR. The primary outcome was projected survival among (a) a cohort of patients who ever initiated LUM/IVA, accounting for treatment discontinuations, and (b) a cohort of patients who remain on continuous LUM/IVA. Patient characteristics and model parameters were derived from clinical trials: VX14-809-109, VX13-809-011B, TRAFFIC/TRANSPORT, and PROGRESS; published literature; and the US CF Foundation Patient Registry.
Results:
Lumacaftor/ivacaftor + SC is expected to increase median survival by 6.1 years versus SC alone, accounting for treatment discontinuations. The incremental median predicted survival versus SC assuming initiation of LUM/IVA at ages 6, 12, 18, and 25 years was 17.7, 12.6, 8.0, and 3.8 years, respectively. Assuming lifetime treatment with LUM/IVA, incremental median survival was predicted to be 7.8 years longer in the LUM/IVA + SC cohort. Initiating LUM/IVA at ages 6, 12, 18, and 25 years and assuming lifetime treatment resulted in incremental median predicted survival of 23.4, 18.2, 11.0, and 4.8 years, respectively.
Conclusions:
Lumacaftor/ivacaftor is projected to increase survival for patients with CF. Initiation at an early age and treatment persistence result in further increments in projected survival.
Keywords: cystic fibrosis, ivacaftor, lumacaftor, percent predicted forced expiratory volume in 1 second (ppFEV1), simulation model, survival, survival projection
Introduction
The survival of patients with cystic fibrosis (CF) in the US has increased as standard care (SC) has improved over the past 3 decades; median predicted survival for patients in the US increased from 27 years in 1985 to 47.7 years in 2016.1
SC for CF in the US includes physical airway clearance therapy, bronchodilators, inhaled mucolytics and antibiotics, and a high-calorie, high-fat diet.2,3 Recently, modulators of the CF transmembrane conductance regulator (CFTR) protein have been introduced in clinical practice. Ivacaftor is a CFTR modulator that facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein at the cell surface.4,5 The US Food and Drug Administration (FDA) approved ivacaftor monotherapy in January 2012 for patients with at least one copy of the G551D-CFTR mutation. The FDA extended approval to other ivacaftor-responsive mutations,6 but ivacaftor monotherapy is not effective in patients homozygous for the F508del-CFTR mutation.7 Lumacaftor is a CFTR corrector that acts directly on the F508del-CFTR protein to improve its cellular processing and trafficking, thereby increasing the quantity of functional CFTR at the cell surface.8 The combined effect of lumacaftor and ivacaftor increases the quantity and function of F508del-CFTR at the cell surface, resulting in increased chloride ion transport.9
Lumacaftor/ivacaftor (LUM/IVA) combination therapy was approved by the FDA in July 2015 for patients with CF who are aged 12 years and older and homozygous for the F508del-CFTR mutation; approval was based on two 24-week randomized placebo-controlled clinical trials demonstrating efficacy, safety, and tolerability (TRAFFIC and TRANSPORT trials).10 The FDA expanded the approval to include children aged 6–11 years in 2016 (VX13-809-011B).11,12 Data from the PROGRESS extension study demonstrated that the benefits of LUM/IVA were maintained for up to an additional 96 weeks in patients aged 12 years and older.13
CF progresses over many years, and long-term follow up is therefore needed to assess the impact of newly introduced CFTR modulators such as LUM/IVA on survival. In the absence of such data earlier in a product’s lifecycle, simulation models can provide projections of the survival impact. Simulation models have been used in a range of diseases to predict population-level effects of treatment on health outcomes including survival and may be used to guide health policy and disease management decisions when long-term clinical data and real-world data are not yet available.14–17
Methods
Study design
This analysis models the clinical outcomes and lifetime survival of patients with CF in the US who are aged ⩾ 6 years, homozygous for the F508del-CFTR mutation, and treated with LUM/IVA in addition to the current SC, compared with those treated with SC alone.
Key model inputs were derived from randomized clinical trials of LUM/IVA in patients aged 6–11 years11,18 and patients aged ⩾ 12 years,10 and an analysis in which patients aged ⩾ 12 years and treated with LUM/IVA for up to 120 weeks were compared with matched controls from the US Cystic Fibrosis Foundation Patient Registry (US CFFPR).13 Additional inputs on disease progression and other parameters predictive of survival were derived from the US CFFPR from 2006 to 201419,20 and other published literature.21–24
The clinical outcomes of interest included median predicted survival, mean residual life-years, mean time spent in different lung-function categories [percent predicted forced expiratory volume in 1 second (ppFEV1) > 90, 70 to <90, 40 to <70, and <40], lung transplantation rates, and time to transplantation among those who were transplanted.
Model overview
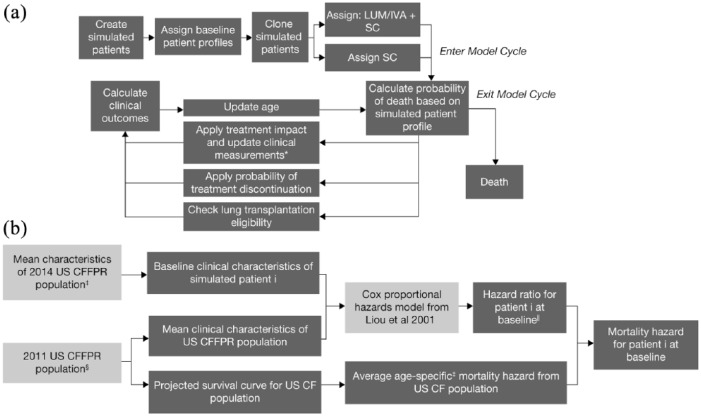
The conceptual framework for the model is illustrated in Figure 1(a). A total of 2000 patient profiles were derived by assigning each modeled patient baseline values for the predictors of survival identified by a published Cox model.21
Figure 1.
Model structure.
(a) Model schematic for patient-level simulations; and (b) steps for deriving patient-level mortality.$
*Clinical measurements include ppFEV1, the occurrence of PEx, incidence of diabetes, and weight-for-age Z score.
$For detailed calculations, see Appendix 2.
‡Patients had at least one study visit in 2014, were homozygous for the F508del-CFTR mutation, age ⩾ 6 years, and who had not received a lung transplant. For parameters that were not available from 2014 US CFFPR Report, alternate assumptions were used. Please see Table 1 for details on the sources for each parameter.
§All ages and genotypes. For parameters that were not available from 2011 US CFFPR Report, alternate assumptions were used; see Appendix 2.
||Specific to the age of patient i at baseline.
CF, cystic fibrosis; LUM/IVA, lumacaftor/ivacaftor; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 s; SC, standard care; US CFFPR, United States Cystic Fibrosis Foundation Patient Registry.
Patients are duplicated to form two identical simulated treatment cohorts receiving LUM/IVA + SC, or SC alone. The model tracks and updates clinical outcomes and survival over time divided into cycles. Each cycle involves three components. First, individualized predictions of the probability of death are calculated at the beginning of the cycle, based on the patient’s age and clinical characteristics. If the patient survives past that model cycle, the effect of natural disease progression on clinical outcomes are updated (e.g. ppFEV1, risk of infections); for patients in the LUM/IVA + SC cohort, the effects of both treatment and disease progression on clinical outcomes are updated in each cycle. Finally, patient age is updated by one cycle length, and patients are moved to the next cycle. This process is repeated until death for each patient, with each model cycle representing 4 weeks for the first 26 cycles, and 1 year thereafter. Once all patients have progressed through the model, health outcomes are averaged across each cohort (LUM/IVA + SC, and SC alone).
Prediction of mortality
The model estimates individual patient mortality using background mortality hazards derived from the US CFFPR,25 adjusted to account for individual patient characteristics that are predictors of survival in patients with CF.
A lifetime survival curve was extrapolated from partial survival curves from US CFFPR life table output using parametric survival analysis techniques.25,26 Multiple parametric curves were fitted to the observed US CFFPR data to find a parametric distribution with the best clinical plausibility and statistical fit. A Gompertz curve with a median predicted survival of 39.7 years was selected (Appendix 1). A Cox proportional hazards (CPH) model is applied to the above Gompertz curve for each individual patient at baseline to calculate the mortality hazard based on the individual patient’s clinical characteristics [Figure 1(b)].27
The mortality hazard is recalculated in each cycle of the model by adjusting for changes in clinical characteristics using the same CPH model.10,18,21 The mortality hazard was bounded by the general US population mortality.28
This mortality hazard is used to determine if a patient dies in any given cycle by converting to a probability of death and comparing that probability to a random number (Appendix 1). Each patient is simulated until death. In order to derive a population survival curve from the individual patient survival in the model, the Kaplan–Meier product-limit formula was used.29
Model inputs
Baseline characteristics
Baseline characteristics for the ‘registry matched’ modeled patient cohort, including age, sex, ppFEV1, weight-for-age Z score, pancreatic sufficiency status, and diabetes mellitus were derived from patients in the US CFFPR who had at least one study visit in 2014, were homozygous for the F508del-CFTR mutation, were aged ⩾ 6 years, and who had not received a lung transplant.20 The prevalence of Staphylococcus aureus, Burkholderia cepacia and diabetes, derived from the 2015 US CFFPR Report,2 was used to assign baseline comorbidity status. All patients were assumed to be pancreatic insufficient at baseline, as most patients with CF who are homozygous for the F508del-CFTR mutation develop pancreatic insufficiency at a young age.30,31 A summary of baseline characteristics used in the model is shown in Table 1. Alternative baseline characteristics tested in scenario analyses were derived by pooling individual patient-level baseline data collected in four clinical trials of LUM/IVA,10,11,18 weighted to match the age distribution of patients in the US CFFPR. These data were used to assign baseline age, sex, ppFEV1, and weight-for-age Z score for the ‘trial based’ cohort.
Table 1.
Model inputs; cohort baseline characteristics.
| Characteristic | Registry matched (base case) |
Trial based (scenario) |
||
|---|---|---|---|---|
| Input | Source | Input | Source | |
| Age, years (mean) | 21.0 | US CFFPR20 | 22.2 | VX14-809-109, VX13-809-011B, and TRAFFIC/TRANSPORT* |
| Male, % | 52.2 | US CFFPR20 | 49.1 | VX14-809-109, VX13-809-011B, and TRAFFIC/TRANSPORT* |
| Weight-for-age Z score (mean) | −0.4 | US CFFPR20 | −0.4 | VX14-809-109, VX13-809-011B, and TRAFFIC/TRANSPORT* |
| ppFEV1 (mean) | 74.4 | US CFFPR20 | 66.5 | VX14-809-109, VX13-809-011B, and TRAFFIC/TRANSPORT*$ |
| Annual rate of PEx (mean) | 0.7 | Whiting et al.;24 Goss and Burns32 | 0.8 | Whiting et al.;24 Goss and Burns32 |
| Pancreatic sufficiency, % | 0.0 | Assumption | 0.0 | Assumption |
| Diabetes, % | 19.3 | US CFFPR report2 | 21.3 | US CFFPR report2 |
| Burkholderia cepacia, % | 2.5 | US CFFPR report2 | 2.5 | US CFFPR report2 |
| Staphylococcus aureus, % | 69.7 | US CFFPR report2 | 69.7 | US CFFPR report2 |
A cohort of 2000 patients was sampled with replacement using VX14-809-109, VX13-809-011B and TRAFFIC/TRANSPORT baseline data.
ppFEV1 inclusion criteria at screening: VX13-809-011B: ppFEV1 ≥ 40%; VX14-809-109: ppFEV1 ≥ 70%; TRAFFIC/TRANSPORT: ppFEV1 40-90%.
PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second; US CFFPR, United States Cystic Fibrosis Foundation Patient Registry.
Change in ppFEV1
It was assumed that during the first 24 weeks, patients receiving SC alone experienced no change in ppFEV1, whereas there was an assumed age-dependent increase in the LUM/IVA + SC cohort based on clinical trial results.10,11,13,18 After week 24, an age-dependent annual decline in ppFEV1 was assumed with SC alone,22,23 reduced by 42% in the LUM/IVA + SC cohort (Appendix 2).
PEx rate
Pulmonary exacerbation (PEx) rate was predicted, contingent on patients’ ppFEV1 and age, using a published relationship derived from the 2004 US CFFPR.24,32 This published relationship and the Liou survivorship model were both derived from the US CFFPR, which identifies PEx as those treated with intravenous (IV) antibiotics. For this reason, only exacerbations requiring IV antibiotics were included in the simulation model, and the corresponding treatment effects reflect the impact of LUM/IVA on these specific types of exacerbations. Specifically, for patients aged ⩾ 12 years, it was assumed that LUM/IVA + SC treatment reduced the PEx rate by 56% applied over the lifetime of the simulation, based on data from the phase III clinical studies.10,18,32 For patients aged 6–11 years, no treatment effect of LUM/IVA + SC on PEx was assumed based on the lack of outcome data in this age range from clinical studies.
Change in weight-for-age Z score
During the first 2 years, weight-for-age Z scores were assumed to decline by 0.030 per year in the cohort receiving SC alone, and increase by 0.033 per year in the cohort treated with LUM/IVA + SC.13 After 2 years, weight-for-age Z score was assumed to remain constant.
Lung transplantation
Patients were assumed to be eligible for a lung transplant when their ppFEV1 fell below 30%33 in the model. The proportion of these eligible patients who went on to receive a transplant was assumed to be 26.8% based on US CFFPR data.2 Post-transplant mortality risk was predicted based on an analysis from the International Society for Heart and Lung Transplantation (ISHLT) International Registry for Heart and Lung Transplantation,34 which found the risk of death in the first year after transplant to be 15.2% and 5.7% in subsequent years.
Treatment discontinuation
The model evaluates the average benefits in a cohort of patients initiating LUM/IVA + SC, assuming that a proportion of patients discontinue LUM/IVA. For weeks 1–24, the LUM/IVA treatment discontinuation rate was derived from 24-week randomized controlled trials of patients aged 6–11 years and ⩾12 years.10,11,18 For weeks 25–96, the discontinuation rate was derived from the first 72 weeks of an open-label study of LUM/IVA in patients aged ⩾ 12 years;13 cumulative discontinuation over the full 96-week period for patients aged 6–11 years and ⩾12 years was 23.4% and 24.4%, respectively. Upon discontinuation of LUM/IVA, patients were assumed to transition to SC alone (Appendix 2). After week 96, no further discontinuation of LUM/IVA was assumed. All analyses were repeated to evaluate the impact of LUM/IVA among patients who remain on therapy, assuming 100% treatment persistence.
Model inputs are shown in Table 2. Detailed explanations of each parameter and its assumptions are provided in Appendix 2.
Table 2.
Clinical inputs in the simulation model.
| Clinical inputs | Time period | Initiate LUM/IVA + SC at: |
SC | Source | |
|---|---|---|---|---|---|
| Aged 6–11 years | Aged⩾12 years | ||||
| Treatment effects | |||||
| ppFEV1 mean change from baseline | Weeks 1–24 | 2.4 | 2.8* | 0.0 | Wainwright et al.;10 Ratjen et al.18 |
| PEx event rate ratio versus SC | Lifetime | 1.00 for aged 6–11; 0.44 for aged ⩾12 | 0.44 | – | Assumption, Wainwright et al.10 |
| Weight-for-age Z score mean change from baseline | Weeks 1–104 | 0.066** | 0.066** | −0.060** | Konstan et al.13 |
| Annual change in absolute ppFEV1 by age, years$ | Weeks 24+ | ||||
| 6–8 | −0.65 | N/A | −1.12 | Konstan et al.22 | |
| 9–12 | −1.39 | −1.39 | −2.39 | Konstan et al.22 | |
| 13–17 | −1.36 | −1.36 | −2.34 | Konstan et al.22 | |
| 18–24 | −1.11 | −1.11 | −1.92 | Konstan et al.23 | |
| 25+ | −0.84 | −0.84 | −1.45 | Konstan et al.23 | |
| Treatment discontinuation | |||||
| LUM/IVA discontinuation rate‡ | Weeks 1–24 | 0.13 | 0.15 | – | VX14-809-109 and Wainwright et al.10 |
| LUM/IVA discontinuation rate‡ | Weeks 24–96 | 0.14 | 0.14 | – | VX14-809-109 and Konstan et al.13 |
| Lung transplant | |||||
| ppFEV1 threshold | Lifetime | 30 | 30 | 30 | American Thoracic Society guidelines33 |
| Eligible patients who receive transplant, % | Lifetime | 26.8 | 26.8 | 26.8 | US CFFPR report2 |
| Postlung-transplant annual mortality risk, % | First year following transplant | 15.2 | 15.2 | 15.2 | ISHLT34 |
| Subsequent years | 5.7 | 5.7 | 5.7 | ISHLT34 | |
Applied at week 16 and held constant through week 24.
Patients receiving LUM/IVA + SC increase 0.033 per year for 2 years, whereas patients on SC decline by 0.030 per year for 2 years.
LUM/IVA treatment effect on ppFEV1 decline (i.e. 42% reduction) was reported by Konstan et al.13
Rate was measured as event rate per patient-year.
CFFPR, Cystic Fibrosis Foundation Patient Registry; ISHLT, International Society for Heart and Lung Transplantation; LUM/IVA, lumacaftor/ivacaftor; N/A, not applicable; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second; SC, standard care.
Model analyses
Base-case analysis
The base-case analysis was conducted using the inputs and assumptions described in Table 1 (registry matched) and Table 2. The median predicted survival, mean residual life-years (years of survival after model baseline), mean time spent in different lung-function categories, cumulative change in ppFEV1, proportion receiving a lung transplant, and mean time to transplant, were compared for the two treatment cohorts. All analyses were conducted in duplicate, assuming either discontinuation or 100% treatment persistence.
Scenario analyses
Scenario analyses were performed to explore the impact of model assumptions on survival projections.
Four cohorts of patients, each with a uniform baseline age, were tested using starting ages of 6, 12, 18, and 25 years.
To understand how the distribution of baseline characteristics for the simulated population affects results, the trial-based cohort was tested.
Alternate assumptions for ppFEV1 decline were tested using the single-age cohort of age 6 years. The first scenario evaluated the potential of LUM/IVA to further slow lung-function decline when initiating treatment earlier, and specifically assumed patients receiving LUM/IVA + SC experienced a reduction of 50% in lifetime ppFEV1 decline relative to SC alone (versus a 42% reduction in the base case). The other scenario conservatively assumed that patients receiving LUM/IVA + SC experienced ppFEV1 decline in line with SC alone until age 12 years, and then a reduction of 42% in ppFEV1 decline relative to SC alone for the remainder of the simulation.
Two additional scenarios were included to evaluate the impact of increased discontinuation both in the short and long term. Specifically, a scenario where 10% of patients discontinued in the first 24 weeks followed by base-case discontinuation through week 96 (i.e. cumulative discontinuation over the full 96-week period of 27.6%), and a separate scenario where base-case discontinuation was assumed for the initial 96 weeks followed by an additional 30% of patients discontinuing between week 96 and year 15 (cumulative discontinuation of 53.4% and 54.4% over 15 years for patients aged 6 to 11 years and ≥ 12 years, respectively).
Sensitivity analyses
One-way sensitivity analyses of the incremental residual life-years outcome were performed by systematically varying individual parameters from the base-case assumption. The analysis evaluated a lower and upper bound for each model parameter considered (Appendix 3). Probabilistic sensitivity analyses were used to generate the 95% credible intervals [95% confidence interval (CI)] on the point estimates of incremental residual life-years and median predicted survival (Appendix 4).
Model validation
To ensure that our model was able to replicate real-world survival among US patients with CF, the model was validated by running the simulation using a patient population with mean characteristics similar to those of patients of all ages and genotypes enrolled in the US CFFPR, and results were compared to real-world survival data from the US CFFPR for all genotypes (Appendix 5). The validation was conducted on all genotypes rather than on those homozygous for F508del-CFTR only, due to the lack of publicly available genotype-specific survival data for the US population with CF.
Results
Base-case results
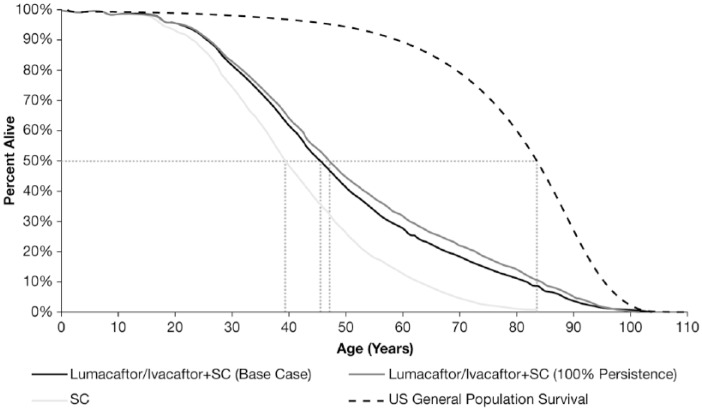
The projected survival curves for patients with CF who are aged ⩾ 6 years, homozygous for F508del-CFTR, and treated with LUM/IVA + SC, or SC alone, are shown in Figure 2. Patients with CF have a marked reduction in projected survival compared with the general US population. Accounting for discontinuation, median predicted survival in the LUM/IVA + SC cohort was 45.5 years (95% CI: 43.5–47.6) versus 39.4 years (95% CI: 38.1–40.8) for SC alone, an incremental gain of 6.1 years (95% CI: 4.3–8.2; Table 3). Patients in the LUM/IVA + SC cohort had a mean residual life expectancy (calculated as the number of years’ survival after the model start) of 30.8 life-years (95% CI: 27.7–34.0) versus 23.1 (95% CI: 21.8–24.6) for SC alone, a projected increase of 7.8 years (95% CI: 4.8–10.8).
Figure 2.
Projected survival for patients on lumacaftor/ivacaftor + SC or SC alone.*
*Dotted lines represent median survival.
SC, standard care; US, United States.
Table 3.
Projected lifetime outcomes of lumacaftor/ivacaftor + SC versus SC.
| Projected health outcomes | Base case |
100% persistence |
|||
|---|---|---|---|---|---|
| SC | LUM/IVA+SC | LUM/IVA + SC versus SC | LUM/IVA + SC | LUM/IVA + SC versus SC | |
| Median projected survival, years (95% CI) | 39.4 (38.1, 40.8) | 45.5 (43.5, 47.6) | 6.1 (4.3, 8.2) | 47.2 (44.9, 50.6) | 7.8 (5.7, 11.1) |
| Mean residual life-years (95% CI) | 23.1 (21.8, 24.6) | 30.8 (27.7, 34.0) | 7.8 (4.8, 10.8) | 32.9 (29.2, 36.8) | 9.8 (6.2, 13.6) |
| Mean time in ppFEV1 categories, years | |||||
| ⩾90% | 2.6 | 4.6 | 2.1 | 5.3 | 2.7 |
| 70 to <90% | 5.1 | 8.5 | 3.4 | 9.4 | 4.4 |
| 40 to <70% | 10.5 | 13.8 | 3.3 | 14.7 | 4.2 |
| <40% | 4.9 | 3.9 | −1.0 | 3.5 | −1.5 |
| Patients undergoing lung transplantation, % | 6.2 | 3.2 | −3.0 | 2.8 | −3.4 |
| Average time until lung transplantation, years | 28.0 | 40.1 | 12.1 | 43.5 | 15.5 |
CI, confidence interval; LUM/IVA, lumacaftor/ivacaftor; ppFEV1, percent predicted forced expiratory volume in 1 second; SC, standard care.
While the base-case estimates the average benefit in a cohort of patients initiating LUM/IVA + SC, some of whom are assumed to discontinue LUM/IVA, the persistence scenario estimates the benefit in those patients who remain on therapy over a lifetime. When perfect LUM/IVA treatment persistence was assumed for a cohort aged ⩾ 6 years, the model predicted a median increment of 7.8 years (95% CI: 5.7–11.1) for the LUM/IVA + SC patients versus SC alone; this represents an increase in median projected survival compared with the base case, which includes discontinuation [7.8 years (95% CI: 5.7–11.1) versus 6.1 years (95% CI: 4.3–8.2)].
The time spent in specific lung-function categories (ppFEV1 ⩾ 90, 70 to <90, 40 to <70, and <40) was evaluated. Patients in the LUM/IVA + SC cohort spent a greater number of years with higher lung function (i.e. ppFEV1 ⩾ 90, 70 to <90, and 40 to <70 categories) than those receiving SC alone (Table 3).
Fewer patients were projected to require lung transplantation when treated with LUM/IVA + SC versus SC alone (Table 3). Furthermore, among those receiving lung transplants, the average time to transplantation was approximately 12 years longer in the LUM/IVA + SC cohort versus the SC-alone cohort.
Scenario analyses
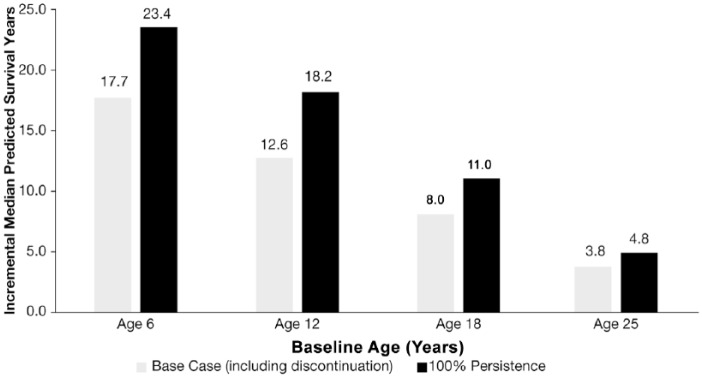
The model predicts that initiating LUM/IVA + SC at an earlier age will lead to increased survival benefit. When simulating patients who initiate treatment at age 6, 12, 18, and 25 years, LUM/IVA + SC increased median predicted survival by 17.7, 12.6, 8.0, and 3.8 years, respectively (Figure 3). Furthermore, assuming full treatment persistence in the same simulated cohorts, the incremental median predicted survival increased to 23.4, 18.2, 11.0, and 4.8 years for patients initiating at age 6, 12, 18, and 25 years, respectively.
Figure 3.
Incremental median predicted survival (years) by baseline age of lumacaftor/ivacaftor initiation.
A scenario analysis using patient profiles derived from trials of LUM/IVA showed a lower survival benefit for LUM/IVA + SC compared with the US registry-matched population (base case), with an incremental median predicted survival of 5.0 years (base case: 6.1 years), and an incremental mean residual life expectancy of 7.2 years (base case: 7.8 years); assuming full persistence, the median predicted survival was 7.0 years. The average ppFEV1 in the simulated cohort derived from the clinical trials was lower than that of the registry-matched cohort (66.5 versus 74.4, respectively) due to trial inclusion criteria that excluded patients with ppFEV1 >90%.
Based on simulations of patients aged 6 years from the registry-matched cohort, LUM/IVA + SC was associated with an incremental median predicted survival of 21.1 years when assuming a 50% reduction in ppFEV1 decline relative to SC alone over a lifetime (longer than 17.7 years assuming the 42% reduction in ppFEV1), and 15.6 years when assuming a delay in the 42% reduction in ppFEV1 decline relative to SC alone until age 12 years (shorter than 17.7 years assuming the reduction in ppFEV1 after week 24).
Additional scenario analyses confirm that discontinuation in the short and long term reduce the projected impact of LUM/IVA on median survival. Assuming 10% of patients discontinue in the first 24 weeks, LUM/IVA is associated with incremental median predicted survival of 5.7 years; assuming an additional 30% of patients discontinue between weeks 96 and year 15, on top of base-case discontinuation, the incremental survival gain is 5.2 years.
Sensitivity analyses and model validation results
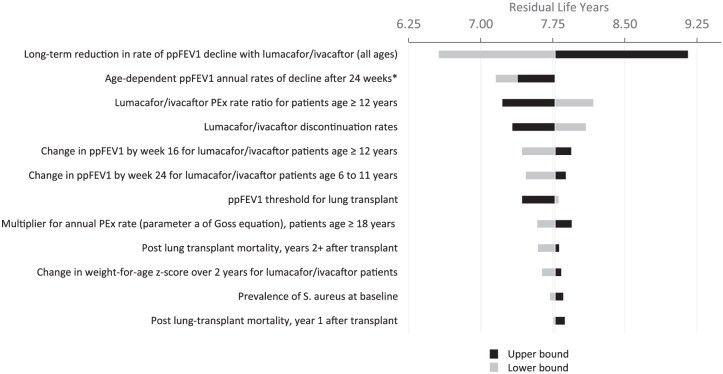
Variance in incremental residual life-year outcomes for LUM/IVA + SC versus SC alone as a result of one-way sensitivity analyses are presented in Appendix 3. Projected gain in life-years was most sensitive to individual changes in each of the following: long-term reduction in ppFEV1 decline for LUM/IVA + SC-treated patients, LUM/IVA treatment effect on rate of PEx, LUM/IVA discontinuation rates, and change in ppFEV1 by week 24 for LUM/IVA + SC. The most influential factors did not change when assuming 100% persistence. The survival curves produced from the model validation closely replicate real-world survival estimates from the US CFFPR and suggest that the model appropriately estimates survival for the CF population (Appendix 5).
Discussion
LUM/IVA demonstrated efficacy in patients aged ⩾ 6 years and homozygous for the F508del-CFTR mutation in clinical trials,10,11,13,18 however, due to the short duration of these studies (24–96 weeks) and newness as an available medication, its impact on long-term survival has not been fully assessed. Multiple analyses indicate that ppFEV1,21,30,34–39 PEx rate, and nutritional status40–44 predict survival in patients with CF. The model presented here estimates the extent to which improvements in ppFEV1, PEx rate, and nutritional status observed in clinical trials of LUM/IVA increase patient-relevant long-term outcomes. Our analysis projects that adding LUM/IVA to SC over a patient’s lifetime would substantially increase time spent in higher lung-function categories, reduce the rate of lung transplantation, and increase survival in treatment-eligible patients with CF. Model projections are stable across a range of realistic baseline conditions.
In the base-case analysis, the model predicted that LUM/IVA + SC will increase median survival by 6.1 years. Analyses investigating LUM/IVA + SC initiation at specific baseline ages found potentially greater survival benefits with earlier initiation. This trend is primarily driven by reducing the rate of lung-function decline among younger patients, since patients with CF have higher ppFEV1 earlier in life.
This model projects that LUM/IVA + SC will delay transplantation by reducing the rate of decline in ppFEV1 (independent of additional factors that influence the decision to proceed to transplantation). This has the potential to reduce the number of patients requiring lung transplantation, as a proportion of the treated population will die without ever reaching the severity of illness to trigger evaluation for lung transplantation.
The projected effects of initiating LUM/IVA at age 6 years are of particular interest, as patients initiating treatment in the future are likely to be those turning 6 years of age and newly eligible. While rate of reduction in lung-function decline associated with LUM/IVA has not been assessed in the patient population aged 6–11 years, clinical data from three recent studies11,13,18 showed that patients who initiated LUM/IVA between the ages of 6 and 11 years experienced significant improvements in lung-clearance index and nutritional status that continued to increase over the first 24 weeks of the follow-up open-label extension study.11,18 Several studies show a consistent linkage between these measures of early CF disease and longer-term ppFEV1 trajectory.43–46 To test a range of potential treatment effects for earlier LUM/IVA + SC treatment initiation, scenarios were modeled with higher or lower treatment effects on rate of ppFEV1 decline for patients initiating LUM/IVA + SC between the ages of 6 and 11 years (compared with initiating at age ⩾ 12 years). The scenario assuming a greater reduction in rate of ppFEV1 decline predicted greater survival benefits.
To ensure that our model can replicate real-world survival among US patients, it was tested by running the simulation using a patient population with mean characteristics similar to those of patients of all ages and genotypes enrolled in the US CFFPR. The model’s projected survival for this validation cohort, assuming patients received SC alone, was comparable with the real-world survival observed in the registry population. This ability of the model to match real-world survival data provides confidence in both the underlying survival prediction methodology and the assumptions used to model the natural history of the disease (Appendix 5).
For both the full registry-matched population and the single-age cohorts, dual scenarios were explored, assuming either some discontinuation of LUM/IVA or 100% persistence. For each simulated population examined, assuming 100% persistence predicted greater benefits compared with the corresponding scenario that included discontinuation, highlighting the clinical value of remaining on LUM/IVA over the long term.
Outcomes based on modeling have inherent limitations, including use of inputs from multiple data sources and extrapolation of observations from clinical trials over the longer term. Several assumptions were made or extrapolated in the model inputs due to the lack of existing clinical evidence. The functions developed to project survival, the rate of ppFEV1 decline for SC-treated patients, and the relationship between ppFEV1 and the PEx rate are derived from studies of the CF population that included all genotypes and are assumed to be applicable to patients homozygous for the F508del-CFTR mutation. Survival estimates were generated by combining two published sources [partial survival curves with SC (US CFFPR 1992–2011) and the CPH model obtained from Liou et al. (US CFFPR 1993–1997);21 they were shown as remaining stable from 1993 to 2015 (unpublished)], and assumed to be comparable in patients homozygous for the F508del-CFTR mutation. The survival model was originally designed to focus only on patient and disease characteristics rather than treatments. Treatment effects on survival are thus mediated via their effects on model covariates. And the model inherently assumes that the relationship between the clinical factors included in the CPH model proposed by Liou et al.21 and survival remains the same when treatment is introduced and that treatment impacts survival by changing the factors themselves. It is possible that treatment modifies these relationships (e.g. each one-unit increase in ppFEV1 with treatment is associated with more or less improvement in survival than a one-unit increase without treatment); however this is currently unknown. As real-world data continue to emerge on LUM/IVA, continued research in this area is warranted.
The model predicts that LUM/IVA will reduce the proportion of patients undergoing lung transplantation. The model utilizes crude assumptions about transplant eligibility and the probability of receiving a transplant once eligible, based on published literature. It should be noted that transplantation rates are influenced by various factors, including whether the patient meets the requirements for the waiting list, the availability of matching donor organ, and the patient’s health status, which are not accounted for in the model. Therefore, results may over- or underestimate actual transplant rates and the ability of LUM/IVA to impact transplant outcomes.
Clinical trial data for LUM/IVA used in this model are limited to 24 weeks for patients aged 6–11 years and up to 120 weeks (of which only 24 weeks were placebo controlled) for patients aged ⩾ 12 years. The long-term treatment effect of LUM/IVA on the rate of ppFEV1 decline is a major model driver and is derived from an analysis of patients aged ⩾ 12 years, treated with LUM/IVA and compared with matched controls from the US CFFPR. This treatment effect is assumed to apply to patients initiating LUM/IVA at age 6–11 years, and scenario analyses were conducted to evaluate the specific impact of this assumption on model results. Further observational research is needed to confirm the potential long-term benefits of LUM/IVA in patients with CF who are homozygous for the F508del-CFTR mutation.
Conclusion
This analysis predicts that treating patients with LUM/IVA will lead to increased survival, more years with greater lung function, and a lower risk of lung transplantation. Initiation of LUM/IVA at younger ages, when lung disease is mild, followed by uninterrupted treatment leads to increased survival gains among patients with CF.
Supplemental Material
Supplemental material, Lumacaftor-ivacaftor_survival_model_appendix for Modeling long-term health outcomes of patients with cystic fibrosis homozygous for F508del-CFTR treated with lumacaftor/ivacaftor by Jaime L. Rubin, Lasair O’Callaghan, Christopher Pelligra, Michael W. Konstan, Alexandra Ward, Jack K. Ishak, Conor Chandler and Theodore G. Liou in Therapeutic Advances in Respiratory Disease
Acknowledgments
The study sponsor (Vertex Pharmaceuticals Incorporated) designed the study in collaboration with the other authors. JLR and LOC led the model conceptualization and design, analysis plan development, validation of model framework and assumptions with clinical and economic experts, and interpretation of results. CGP, AW, KJI, and CC contributed to the background research, model conceptualization, design implementation, developing the model analysis plan, conducting analyses, and interpreting the results. MK and TGL provided input on the clinical-face validity of the model design and key assumptions, as well as providing guidance on the inputs, analysis scenarios, and interpretation. JLR and LOC guided the initial drafting of the manuscript, with input from all other authors. All authors participated in subsequent revisions and the decision to submit the manuscript for publication. Editorial assistance was provided under the direction of the authors by Yunyu Huang, PhD, of Excerpta Medica, with support from Vertex Pharmaceuticals Incorporated.
Appendix 1. Prediction of mortality
The model estimates individual patient mortality by adjusting background mortality hazards derived from the US CFFPR 1992–2011 birth cohorts to account for individual patient characteristics that predict survival based on a CPH model published by Liou et al.21 The projected mortality hazard for patients with CF estimated in this model is also capped by the sex-specific general US population mortality hazard at each age.
The US CFF provided the accompanying life table output for the Kaplan–Meier survival curves of five birth cohorts published in the 2011 US CFFPR (cohort groups: 1987–1991, 1992–1996, 1997–2001, 2002–2006, and 2007–2011). The life table output included number of deaths, as well as numbers at risk at yearly intervals. These were processed to generate virtual patient-level data by assigning deceased or living (i.e. censored) status at each age based on the available counts for each cohort.
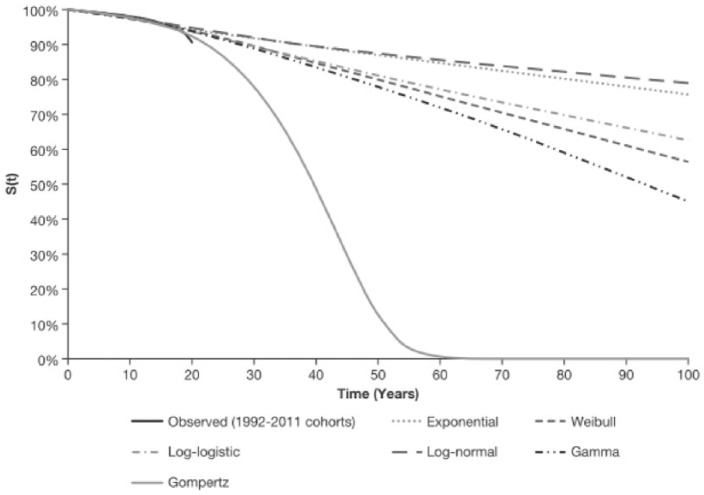
The data were analyzed to test various parametric distributions; these included the exponential, Weibull, Gompertz, log-normal, gamma, and log-logistic. The optimal parametric fit was selected based on statistical fit (Akaike information criterion and the Bayesian information criterion, closeness of fit in comparisons of observed and predicted curves), as well as based on the clinical plausibility of fits (based on median survival times, and shape of projected curves).
Data from each of the birth cohort groups were first analyzed separately to assess the possibility of accurate fitting in the most recent cohorts (e.g. 2007–2011), as these are more reflective of current survival expectations. Follow-up in these groups was very short, with curves dropping by a few percent only, which led to projections of implausibly long survival. Thus, the birth cohorts were grouped in order to overcome this issue. The final analyses were based on survival data from the 1992 to 2011 cohorts (Figure A1).
Figure A1.
Kaplan–Meier curve and parametric fits to the US CFF population (all genotypes): combined birth cohort 1992–2011.
CFF, Cystic Fibrosis Foundation; S(t), Gompertz survival function (survival time).
A Gompertz fit to the 1992–2011 birth cohorts produced the most plausible projection, with the curve reaching 0% near 65 years of age, and a predicted median of 39.7 years (Figure A1 and Table A1). The survival of birth cohorts for 2012 and after were not updated in the US CFFPR reports published after 2011, therefore, they were not included in the fitting exercise.
Table A1.
Parameters for Gompertz distribution used to derive CF survival projections based on US CFFPR population (all genotypes): birth cohort 1992–2011.
| Parameter | Value |
|---|---|
| λ | −6.7273 |
| γ | 0.1033 |
CF, cystic fibrosis; CFFPR, Cystic Fibrosis Foundation Patient Registry.
The Gompertz survival function used in the model is shown below:
The survival projections in the model are adjusted to reflect the characteristics of the simulated patients. For patients receiving LUM/IVA, the projections are further adjusted to account for the assumed treatment benefit gained by adding LUM/IVA to SC. Liou and colleagues developed the CPH model based on data collected from 1993 to 1997 by the US CFFPR on 11,630 individuals; the following nine characteristics of patients with CF were found to predict survival: age, ppFEV1, sex, weight-for-age Z score, pancreatic sufficiency, diabetes, S. aureus infection, B. cepacia infection, and number of acute exacerbations per year.21 Reference values for each of these characteristics were used to make the adjustment from the US CFFPR to an individual patient in the model at baseline. Covariates included in the Liou et al. model21 and the corresponding coefficients, as well as the reference values used in the model, are presented in Table A2.
Table A2.
Cox proportional hazards model coefficients and reference values.
| Covariate | Coefficient* |
Mean characteristics of reference population | |
|---|---|---|---|
| β | SE | ||
| Age (per year) | 0.011 | 0.0049 | 19.8$ |
| Sex (female = 1) | 0.15 | 0.074 | 0.48 |
| ppFEV1 (per %) | −0.042 | 0.0025 | 77.1 |
| Weight-for-age Z score | −0.28 | 0.041 | −0.85‡ |
| Pancreatic sufficiency (yes = 1) | −0.14 | 0.23 | 0.126 |
| Diabetes mellitus (yes = 1) | 0.44 | 0.098 | 0.19 |
| Staphylococcus aureus (yes = 1) | −0.25 | 0.09 | 0.68 |
| Burkholderia cepacia (yes = 1) | 1.41 | 0.19 | 0.03 |
| Annual number of acute exacerbations (maximum 5) | 0.35 | 0.024 | 0.7 |
| PEx B. cepacia | −0.28 | 0.06 | 0.0286§ |
Mean estimates obtained from US CFFPR 2011, except where indicated.
Unless specified, coefficients for each covariate are unitless.
Data not available from the US CFFPR 2011 report. Data reported in US CFFPR 2012 are used as proxy.
Liou et al. 2001.21
Assumed equal to mean B. cepacia mean* acute exacerbations.
PEx, pulmonary exacerbation; ppFEV1, percentage of predicted forced expiratory volume in 1 second; SE, standard error.
While the CPH model has not been updated since its publication, Liou and colleagues presented an updated analysis in 2015 of the logistic regression that was originally published alongside the CPH model in 2001.21 The updated logistic regression used US CFFPR data from 1993 to 2010. This analysis concluded that while there were some slight changes to coefficients, the factors predicting mortality in patients with CF have remained stable. These results support continued use of the 2001 CPH model in these simulations.
The probability of death at each cycle in the survival model (p) is calculated using the following formula:
where h is the annual mortality hazard calculated at that cycle and t is the cycle length (in years). Random numbers are used to determine in which cycle an individual patient would die. After death, the patient exits the model and the next patient is simulated through the model.
Appendix 2. Assumptions for clinical inputs
Percent predicted forced expiratory volume in 1 second
Over the first 24 weeks after LUM/IVA initiation, an increase in ppFEV1 was assumed, based on the placebo-controlled treatment effects from the relevant clinical trials.10,18 Patients initiating LUM/IVA + SC at ages of 6–11 years were assumed to experience an acute increase of 2.4 percentage points in ppFEV1 by week 24 of the simulation. Whereas, patients initiating at age ⩾ 12 years were assumed to experience an acute increase of 2.8 percentage points in in ppFEV1 over the first 24 weeks of the model, based on the placebo-controlled changes observed at the average of weeks 16 and 24 in TRAFFIC/TRANSPORT (primary clinical endpoint); simulated patients therefore receive an acute increase in ppFEV1 by week 16 and remain at that level until week 24 of the simulation. Patients receiving SC alone were assumed to have no change in ppFEV1 over the initial 24 weeks, since placebo-adjusted treatment effects were used for patients receiving LUM/IVA + SC.
It is well documented that lung function declines over time in patients with CF.19,22,23,46 After 24 weeks, the model assumed age-dependent annual ppFEV1 decline for the remainder of the simulation, based on the findings from Konstan et al.22,23 Patients receiving LUM/IVA + SC were assumed to have a reduced rate of ppFEV1 decline relative to SC alone.13 Based on findings from a matched analysis of data from TRAFFIC/TRANSPORT and PROGRESS studies comparing patients on LUM/IVA with a matched control cohort from the US CFFPR,13 the model assumed that while a patient was receiving LUM/IVA + SC, they had a 42% slower annual rate of decline in ppFEV1.
PEx rate
Occurrence of PEx per patient in each model cycle was predicted contingent on patient ppFEV1 and age from a relationship derived from the 2004 US CFFPR, based on a publication by Goss et al.32 PEx rates were found to increase with lower ppFEV1. The data reported were fitted to an exponential regression function, to provide a continuous relationship between the PEx rates and ppFEV1:24
Two equations were applied: for patients aged < 18 years (a = 8.594, b = 0 .035), and ⩾18 years (a = 3.789, b = 0.026). Since the PEx events tracked in this data source were likely those treated with intravenous antibiotics with or without hospitalization, it is this subset of PEx that is tracked in the model.
For patients aged 6–11 years, no treatment effect of LUM/IVA + SC was assumed on PEx, as the 809-109 study was not powered to detect a difference in PEx rate. For patients aged ⩾ 12 years, LUM/IVA + SC treatment was assumed to reduce the rate by 56%, the observed treatment effect on the rate of PExs treated with intravenous antibiotics from TRAFFIC/TRANSPORT study.10
Weight-for-age Z score
During the first 2 years of the simulation, patients on LUM/IVA + SC were assumed to experience a constant weight-for-age Z score increase of 0.033 per year based on the findings from the registry-matched analysis by Konstan et al.13 Patients on SC alone declined by 0.030 per year for the first 2 years after baseline.13 Weight-for-age Z score was updated during the first 2 years of treatment and was subsequently assumed to remain constant over time.
Lung transplantation
International guidelines suggest that patients with CF and a ppFEV1 of <30% should be evaluated for lung transplantation.31 Thus, in the model, patients were assumed to be eligible to receive a lung transplant when ppFEV1 fell below 30%. The percentage of eligible patients who went on to receive a transplant was estimated to be 26.8%, based on data from the 2015 US CFFPR report,2 this was implemented in the model as a one-time chance (26.8% risk) of receiving a lung transplant once ppFEV1 fell below 30%.
Lumacaftor/ivacaftor discontinuation
Discontinuation rates for weeks 1–24 of the simulation were derived from discontinuation data from either (a) TRAFFIC/TRANSPORT for patients who were aged ⩾12 years at baseline, or (b) the 809-109 study for patients aged 6–11 years at baseline. The discontinuation rate for weeks 25-96 was based on the discontinuation from the first 72 weeks of the PROGRESS study.13 While PROGRESS only included patients aged ⩾ 12 years who completed TRAFFIC/TRANSPORT, the model assumed these data were applicable for patients aged ⩾ 6 years in the absence of longer-term LUM/IVA discontinuation data for patients aged 6–11 years. Patients who discontinued LUM/IVA during the first 24 weeks of the model were assumed to retain the acute increase in ppFEV1, as the ppFEV1 treatment effect was derived from an intent-to-treat analysis and so included patients who discontinued. In contrast, for patients who discontinued LUM/IVA between in weeks 25–96, the acute ppFEV1 increase was removed in the cycle in which the patient discontinues. The model assumed no discontinuation of LUM/IVA after 96 weeks. Upon discontinuation of LUM/IVA, a patient was assumed to transition to SC alone.
Appendix 3. Parameter inputs in deterministic sensitivity analysis
The parameter inputs in deterministic sensitivity analysis (DSA) are given in Table A3 and Figure A2.
Table A3.
Lower and upper bounds for each model parameter in DSA.
| Parameter | INPUTS |
|||
|---|---|---|---|---|
| Base case | Lower bound | Upper bound | Bounds source | |
| Long-term reduction in rate of ppFEV1 decline with LUM/IVA+SC (all ages) | 42.0% | 33.9% | 52.6% | 95% CI (Konstan 2017,VXR-HQ-88-00035) (13) |
| LUM/IVA+SC PEx rate ratio for patients ⩾ 12 years | 0.44 | 0.33 | 0.60 | 95 % CI (Wainwright 2010) (10) |
| LUM/IVA discontinuation rates | Multiple Inputs | 20% lower | 20% greater | Assumption |
| Change in ppFEV1 by Week 16 for LUM/IVA+SC patients ⩾ 12 years | 2.8 | 1.8 | 3.8 | 95% CI (Wainwright 2010) (10) |
| Change in ppFEV1 by Week 24 for LUM/IVA+SC patients 6 to 11 years | 2.4 | 0.4 | 4.4 | 95% CI (Ratjen 2017) (18) |
| ppFEV1 threshold for lung transplant | 30 | 20 | 40 | Assumption |
| Multiplier for annual PEx rate (parameter a of Goss equation), patients ⩾ 18 years | 3.789 | 3.031 | 4.547 | Assumption (20% lower/higher) |
| Age-dependent ppFEV1 annual rates of decline after 24 weeks | Multiple Values | 20% lower (less negative) | 20% greater (more negative) | Assumption (20% lower/higher) |
| Post lung transplant mortality, years ⩾ 2 after transplant | 5.70% | 4.56% | 6.84% | Assumption (20% lower/higher) |
| Change in weight-for-age z-score over 2 years for LUM/IVA + SC patients | 0.066 | 0.012 | 0.122 | 95 % CI (Konstan 2017) (13) |
| Prevalence of S. aureus at baseline | 70.60% | 56.48% | 84.72% | Assumption (20% lower/higher) |
| Post lung-transplant mortality, year 1 after transplant | 15.18% | 12.14% | 18.22% | Assumption (20% lower/higher) |
| Percentage of eligible patients receiving lung transplantation | 26.81% | 21.45% | 32.17% | Assumption (20% lower/higher) |
| Change in weight-for-age z-score over 2 years for SC patients | −0.06 | −0.09 | −0.03 | 95 % CI (Konstan 2017) (13) |
| Multiplier for annual PEx rate (parameter a of Goss equation), patients <18 years | 8.594 | 6.875 | 10.313 | Assumption (20% lower/higher) |
| Minimum ppFEV1 | 15 | 12 | 18 | Assumption (20% lower/higher) |
| Prevalence of B. cepacia at baseline | 2.60% | 2.08% | 3.12% | Assumption (20% lower/higher) |
| Prevalence of diabetes at baseline | Multiple Values | 20% lower | 20% greater | Assumption (20% lower/higher) |
| Annual incidence rate of diabetes | Multiple Values | 20% lower | 20% greater | Assumption (20% lower/higher) |
CI, confidence interval; LUM/IVA, lumacaftor and ivacaftor; ppFEV1, percent predicted forced expiratory volume in 1 second; PEx, pulmonary exacerbation; SC, standard care.
Figure A2.
DSA tornado diagram: lumacaftor/ivacaftor + SC versus SC alone.
ppFEV1, percent predicted forced expiratory volume in 1 second; PEx, pulmonary exacerbation; SC, standard care.
*When all age-dependent ppFEV1 annual rates of decline are increased, both the LUM/IVA and SC rates of decline increase, leading to lower survival in both groups and reduced incremental survival vs. the base-case; when the rates are reduced, survival increases for both LUM/IVA and SC but in this case incremental survival is also reduced vs. the base-case.
Appendix 4. Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) was conducted to account for multivariate and stochastic uncertainty in the model. The PSA tests effect of statistical uncertainty of model parameters on model outcomes. The uncertainty in the individual parameters was characterized using probability distributions and analyzed using Monte Carlo simulation (1000 replications). In the PSA, the uncertainties around parameters were estimated as shown in Table A4. Output from the PSA was used to derive 95% credible intervals on the point estimates.
Table A4.
PSA assumptions.
| Parameter | Distribution | Mean | Standard error | Source |
|---|---|---|---|---|
| Change in ppFEV1 by week 16 for LUM/IVA + SC patients ⩾ 12 years | Normal, bounded by 0 | 2.80 | 0.52 | 95% CI (Ratjen et al.18) |
| Change in ppFEV1 by week 24 for LUM/IVA + SC patients 6–11 years | Normal, bounded by 0 | 2.40 | 1.00 | 95% CI (Wainwright et al.10) |
| Change in weight-for-age Z score over 2 years for LUM/IVA + SC patients | Normal, bounded by 0 | 0.066 | 0.028 | 95% CI (Konstan et al.13) |
| Change in weight-for-age Z score over 2 years for SC patients | Normal, bounded by 0 | −0.060 | 0.015 | 95% CI (Konstan et al.13) |
| Age-dependent ppFEV1 rates of decline after 24 weeks for SC 6–8 years | Normal, bounded by 0 | −1.12 | 0.22 | Assumed 20% of mean |
| Age-dependent ppFEV1 rates of decline after 24 weeks for SC 9–12 years | Normal, bounded by 0 | −2.39 | 0.48 | Assumed 20% of mean |
| Age-dependent ppFEV1 rates of decline after 24 weeks for SC 13–17 years | Normal, bounded by 0 | −2.34 | 0.47 | Assumed 20% of mean |
| Age-dependent ppFEV1 rates of decline after 24 weeks for SC 18–24 years | Normal, bounded by 0 | −1.92 | 0.38 | Assumed 20% of mean |
| Age-dependent ppFEV1 rates of decline after 24 weeks for SC 25+ years | Normal, bounded by 0 | −1.45 | 0.29 | Assumed 20% of mean |
| Long-term reduction in rate of ppFEV1 decline with LUM/IVA + SC (all ages) | Beta, bounded by 0 | 42.0% | 0.112 | 95% CI (Konstan et al.,13
VXR-HQ-88-00035) |
| LUM/IVA + SC PEx rate ratio for patients ⩾ 12 years | Log-normal, bounded by 0 | 0.440 | 0.152 | 95% CI (Wainwright et al.10) |
| Multiplier for annual PEx rate (parameter a of Goss equation), patients ⩾ 18 years | Normal, bounded by 0 | 8.594 | 1.719 | Assumed 20% of mean |
| Multiplier for annual PEx rate (parameter a of Goss equation), patients ⩾ 18 years | Normal, bounded by 0 | 3.789 | 0.758 | Assumed 20% of mean |
CI, confidence interval; LUM/IVA, lumacaftor and ivacaftor; ppFEV1, percent predicted forced expiratory volume in 1 second; PEx, pulmonary exacerbation; SC, standard care.
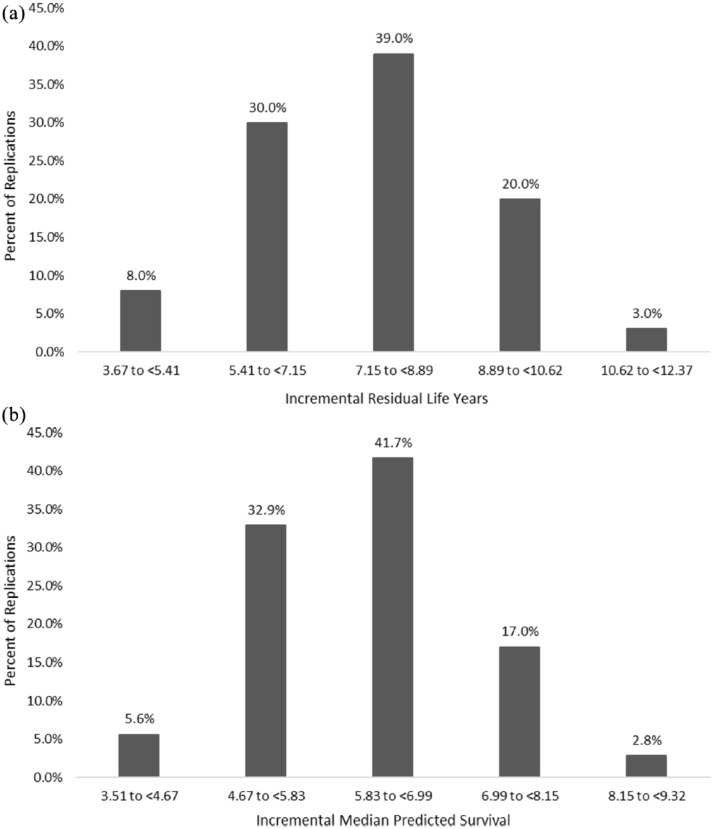
The empirical distribution of mean residual life-years and median predicted survival results from the PSA (Figures A3 and A4, respectively) delineate that treatment with LUM/IVA + SC yields additional survival benefits across all PSA replications, relative to SC.
Figure A3.
PSA histograms of incremental residual life-years and incremental median predicted survival of the base case.
(a) PSA histogram of incremental residual life-years (base case); (b) PSA histogram of incremental median predicted survival (base case).
PSA, probabilistic sensitivity analysis.
Figure A4.
PSA histograms of incremental residual life-years and incremental median predicted survival with 100% persistence.
(a) PSA histogram of incremental residual life-years (100% persistence); (b) PSA histogram of incremental median predicted survival (100% persistence).
PSA, probabilistic sensitivity analysis.
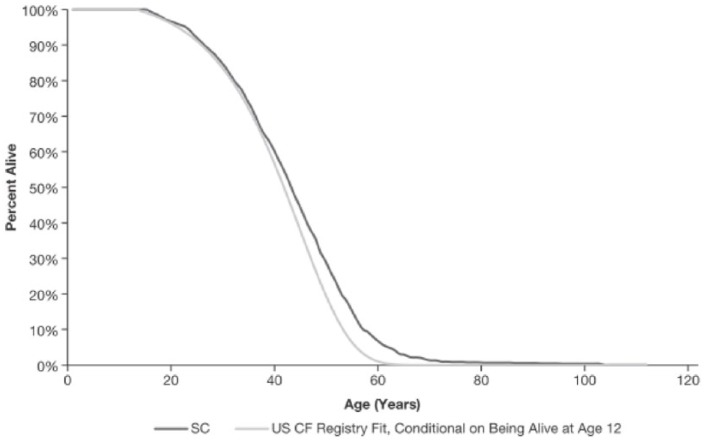
Appendix 5. Model validation
To validate the input cohort and natural disease history assumptions in the model, a cohort with baseline characteristics that resembled the population used to derive the registry reference curve detailed in Appendix 1 (i.e. patients with CF of all ages and all genotypes in the US CFFPR) was simulated. It would be expected that simulating such a cohort through the SC of the model would produce a survival curve similar to the reference curve derived from registry data using parametric survival analysis. A registry-matched cohort was generated with mean characteristics similar to those of the 2011 US CFFPR population, using the 2011 registry report.25. The model’s projected survival of this simulated cohort was compared to the curve fitted to the registry population. To create this validation cohort, it was necessary to deduce baseline risk profiles that collectively yielded the average profile. As such, a perfect match of the simulation to the registry cannot be expected. Nevertheless, the model output is a good fit to the registry survival curve, as shown in Figure A5.
Figure A5.
Validation of simulated cohort survival.
CF, cystic fibrosis; SC, standard care.
Footnotes
Funding: Vertex Pharmaceuticals Incorporated provided funding for this study and medical writing/ editorial support for development of the manuscript. The funder was involved in study design, data analysis, and data interpretation. The authors had full access to the data and had final responsibility for the decision to submit for publication.
Conflict of interest statement: JLR is an employee of Vertex Pharmaceuticals Incorporated and may hold stock or stock options in the company. LOC is a former employee of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company. CGP, AW, KJI, and CC are employed by Evidera, an independent research company that provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, they work with a variety of companies and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received payment from Vertex Pharmaceuticals Incorporated for the conduct of this study. MWK is employed by the Case Western Reserve University and UH Rainbow Babies and Children’s Hospital; UH Rainbow Babies and Children’s Hospital receives funding to perform clinical trials from sponsors including Vertex Pharmaceuticals Incorporated. MWK reports grant funding from the NIH and the Cystic Fibrosis Foundation (CFF), and has received consulting fees from Vertex Pharmaceuticals Incorporated, Albumedix, Anthera Pharmaceuticals, Celtaxsys, Chiesi USA Inc., Corbus Pharmaceuticals, Genentech, Laurent Pharmaceuticals, Merck, Novartis, Paranta Biosciences, Protalix Biotherapeutics, PTC Therapeutics, and Savara Pharmaceuticals, outside of the submitted work. MWK reports being a member of the CFF Clinical Research Executive Committee and the CFF Clinical Research Advisory Board. TGL is an employee of the University of Utah; the University of Utah receives funding to perform clinical trials, for which TGL is the local PI, from sponsors including Vertex Pharmaceuticals Incorporated. TGL reports grant funding for his work at the University of Utah from the NIH, NHLBI (R01 HL 125520), the CFF, the Ben B and Iris M Margolis Family Foundation of Utah and the Claudia Ruth Goodrich Stevens Endowment Fund. TGL additionally reports grant support for clinical trials from the foundation of the NIH, Gilead Sciences, Laurent Pharmaceuticals, Nivalis Therapeutics, Inc., Novartis, Proteostasis Therapeutics, Inc., Savara Pharmaceuticals, the Therapeutic Development Network of the CFF, CFF Therapeutics, and Vertex Pharmaceuticals. In addition, TGL has a provisional patent on a novel antibiotic, reports being a member of the Clinical Research Review Committee at the CFF, reviews grant applications and is a member of the editorial board for Chest, and reviews papers for various journals regarding CF and statistical analyses in medicine.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Jaime Rubin  https://orcid.org/0000-0003-1555-2070
https://orcid.org/0000-0003-1555-2070
Contributor Information
Jaime L. Rubin, Vertex Pharmaceuticals Incorporated, 50 Northern Ave, Boston, MA 02210, USA.
Lasair O’Callaghan, Vertex Pharmaceuticals Incorporated, Boston, MA, USA.
Christopher Pelligra, Evidera, Waltham, MA, USA.
Michael W. Konstan, Case Western Reserve University School of Medicine and Rainbow Babies and Children’s Hospital, Cleveland, OH, USA
Alexandra Ward, Evidera, Waltham, MA, USA.
Jack K. Ishak, Evidera, Montreal, QC, Canada
Conor Chandler, Evidera, Waltham, MA, USA.
Theodore G. Liou, University of Utah, Salt Lake City, UT, USA
References
- 1. Cystic Fibrosis Foundation. US Patient Registry: 2016 annual data report, https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf (2017, accessed 22 May 2018).
- 2. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2015, https://www.cff.org/Our-Research/CF-Patient-Registry/2015-Patient-Registry-Annual-Data-Report.pdf (accessed 22 May 2018).
- 3. Mogayzel PJ, Jr, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013; 187: 680–689. [DOI] [PubMed] [Google Scholar]
- 4. Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106: 18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. KALYDECO (Ivacaftor) label 2012, https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203188lbl.pdf (accessed 22 May 2018).
- 7. Flume PA, Liou G, Borowitz DS, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest 2012; 142: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011; 108: 18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cholon DM, Esther CR, Jr, Gentzsch M. Efficacy of lumacaftor-ivacaftor for the treatment of cystic fibrosis patients homozygous for the F508del-CFTR mutation. Expert Rev Precis Med Drug Dev 2016; 1: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373: 220–231. [DOI] [PubMed] [Google Scholar]
- 11. Milla CE, Ratjen F, Marigowda G, et al. Lumacaftor/ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR. Am J Respir Crit Care Med 2017; 195: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ORKAMBI (lumacaftor/ivacaftor) label 2015, https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206038Orig1s000lbl.pdf (accessed 22 May 2018).
- 13. Konstan MW, McKone EF, Moss RB, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017; 5: 107–118. [DOI] [PubMed] [Google Scholar]
- 14. Levy WC, Mozaffarian D, Linker DT, et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 15. Shen W, Sakamoto N, Yang L. Model to predict the survival benefit of radiation for patients with rhabdomyosarcoma after surgery: a population-based study. Int J Oncol 2014; 45: 549–557. [DOI] [PubMed] [Google Scholar]
- 16. Oberije C, De Ruysscher D, Houben R, et al. A validated prediction model for overall survival from stage III non-small cell lung cancer: toward survival prediction for individual patients. Int J Radiat Oncol Biol Phys 2015; 92: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Latimer N. NICE DSU technical support document 14: undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data, http://www.nicedsu.org.uk (2011, accessed 22 May 2018). [PubMed]
- 18. Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017; 5: 557–567. [DOI] [PubMed] [Google Scholar]
- 19. Cystic Fibrosis Data Network. Cystic Fibrosis Reports: United States 2012, http://www.cysticfibrosisdata.org/ReportsUS.html (accessed 22 May 2018).
- 20. Sawicki GS, Konstan MW, McKone EF, et al. Rate of lung function decline in patients with cystic fibrosis (CF) having a residual function gene mutation. Presented at the American Thoracic Society International Conference, 19–24 May 2017, Washington, DC. [Google Scholar]
- 21. Liou TG, Adler FR, Fitzsimmons SC, et al. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001; 153: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konstan MW, Morgan WJ, Butler SM, et al. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 2007; 151: 134–139. [DOI] [PubMed] [Google Scholar]
- 23. Konstan MW, Wagener JS, Vandevanter DR, et al. Risk factors for rate of decline in FEV1 in adults with cystic fibrosis. J Cyst Fibros 2012; 11: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whiting P, Al M, Burgers L, et al. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2014; 18: 1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2011, Annual Data Report, http://www.cysticfibrosisdata.org/LiteratureRetrieve.aspx?ID=132652 (accessed 22 May 2018).
- 26. Ishak J, Kreif N, Benedict A, et al. Overview of parametric survival analysis for health economic applications. Pharmacoeconomics 2013; 31: 663–675. [DOI] [PubMed] [Google Scholar]
- 27. Cox DR. Regression models and life-tables. J R Stat Soc B Met 1972; 34: 187–220. [Google Scholar]
- 28. Death rates, The Human Mortality Database www.mortality.org (2014, accessed 28 March 2018).
- 29. Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 30. McKone EF, Goss CH, Aitken ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest 2006; 130: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 31. Ahmed N, Corey M, Forstner G, et al. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut 2003; 52: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax 2007; 62: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. American Thoracic Society. International Guidelines for the Selection of Lung Transplant Candidates. Am J Respir Crit Care Med 1998;158:335–339. [DOI] [PubMed] [Google Scholar]
- 34. International Society for Heart and Lung Transplantation. Adult lung transplantation statistics, https://www.ishlt.org/registries/slides.asp?slides=heartLungRegistry (2016, accessed 22 May 2018).
- 35. Schluchter MD, Konstan MW, Davis PB. Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med 2002; 21: 1271–1287. [DOI] [PubMed] [Google Scholar]
- 36. Britto MT, Kotagal UR, Hornung RW, et al. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002; 121: 64–72. [DOI] [PubMed] [Google Scholar]
- 37. Taylor-Robinson D, Whitehead M, Diderichsen F, et al. Understanding the natural progression in %FEV1 decline in patients with cystic fibrosis: a longitudinal study. Thorax 2012; 67: 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aaron SD, Stephenson AL, Cameron DW, et al. A statistical model to predict one-year risk of death in patients with cystic fibrosis. J Clin Epidemiol 2015; 68: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 39. Flores JS, Rovedder PM, Ziegler B, et al. Clinical outcomes and prognostic factors in a cohort of adults with cystic fibrosis: a 7-year follow-up study. Respir Care 2016; 61: 192–199. [DOI] [PubMed] [Google Scholar]
- 40. Buzzetti R, Alicandro G, Minicucci L, et al. Validation of a predictive survival model in Italian patients with cystic fibrosis. J Cyst Fibros 2012; 11: 24–29. [DOI] [PubMed] [Google Scholar]
- 41. Stephenson AL, Tom M, Berthiaume Y, et al. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J 2015; 45: 670–679. [DOI] [PubMed] [Google Scholar]
- 42. Cogen J, Emerson J, Sanders DB, et al. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol 2015; 50: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zemel BS, Jawad AF, FitzSimmons S, et al. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr 2000; 137: 374–380. [DOI] [PubMed] [Google Scholar]
- 44. Peterson ML, Jacobs DR, Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics 2003; 112(3 Pt 1): 588–592. [DOI] [PubMed] [Google Scholar]
- 45. Aurora P, Stanojevic S, Wade A, et al. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med 2011; 183: 752–758. [DOI] [PubMed] [Google Scholar]
- 46. Vermeulen F, Proesmans M, Boon M, et al. Lung clearance index predicts pulmonary exacerbations in young patients with cystic fibrosis. Thorax 2014; 69: 39–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Lumacaftor-ivacaftor_survival_model_appendix for Modeling long-term health outcomes of patients with cystic fibrosis homozygous for F508del-CFTR treated with lumacaftor/ivacaftor by Jaime L. Rubin, Lasair O’Callaghan, Christopher Pelligra, Michael W. Konstan, Alexandra Ward, Jack K. Ishak, Conor Chandler and Theodore G. Liou in Therapeutic Advances in Respiratory Disease