Abstract
Background
Lysine is used widely in livestock production due to the shortage of feed protein resources. L-lysine·H2SO4 contains L-lysine sulphate as well as fermentation co-products which contain other amino acids and phosphorus. However, there are few articles about L-lysine·H2SO4 product regarding intestinal morphology and liver pathology of broiler chickens. In this article, we focus on the absorption and metabolism of L-lysine·H2SO4 revealed in the variation of intestinal morphology and liver pathology to determine the tolerance of chicks for L-lysine·H2SO4.
Methods
To evaluate the tolerance of broilers for L-lysine·H2SO4, 240 one day old broilers were allocated randomly to one of five dietary treatments which included corn-soybean diets containing 0, 1%, 4%, 7% or 10% L-lysine·H2SO4 (L-lysine content = 55%).
Results
Supplementation of 1% L-lysine·H2SO4 in the diet had no negative effects. However, 4%, 7% or 10% L-lysine·H2SO4 supplementation produced negative responses on broiler performance, carcass characteristics, blood biochemistry, and particularly on intestinal morphology and liver pathology compared with broilers fed the control diet.
Conclusion
Our results show that supplementation with 1% L-lysine·H2SO4 had no negative effects on performance, carcass characteristics, blood biochemistry, intestinal morphology and liver pathology in broilers, but supplementation with 4%, 7% or 10% L-lysine·H2SO4 produced a negative response, particularly with respect to intestinal morphology and liver pathology.
Keywords: Broilers, Intestinal morphology, Liver pathology, L-lysine·H2SO4
Background
Lysine is a limiting amino acid that is commonly supplemented in the form of L-lysine·HCl in diets fed to pigs [1, 2], rainbow trout [3], and broiler chickens [4]. In recent years, an alternative source of lysine in the form of L-lysine·H2SO4 (55% lysine) has been developed with its use being highly attractive for both ecological and economic reasons. This occurs because the production of L-lysine·HCl involves a costly, multiple-stage process requiring the handling of hazardous materials such as ammonia solution (elution of the Ion exchange column) and hydrochloric acid (neutralization of the L-lysine base) which generates residual solutions that are harmful to the environment [5, 6].
L-lysine·H2SO4 is a new source of lysine containing L-lysine·sulphate and fermentation co-products which contain other amino acids and phosphorus. This new source of lysine has been shown to be equally efficacious compared with L-lysine·HCl in diets fed to broilers [6, 7]. Furthermore, the mortality of broiler chickens fed L-lysine·H2SO4 was lower from d 1 to d 42 compared with birds fed L-lysine·HCl, which suggests that L-lysine·H2SO4 can improve the bird’s immune status.
Lysine is an indispensable amino acid for non-ruminant animals and has been widely used as a supplement in livestock diets [8, 9]. However, excessive concentrations of dietary lysine can produce negative effects in chickens, such as decreased weight gain, and increased incidence of severe leg problems [10–12]. Furthermore, there are no reports about the effects of high levels of L-lysine·H2SO4 on performance, carcass characteristics, blood biochemistry, intestinal morphology or liver pathology of broiler chickens. Therefore, we designed this experiment to determine the tolerance of broiler chickens to L-lysine·H2SO4 and observe the effect of this product on intestinal morphology and liver pathology when used as a source of lysine in diets.
Methods
Experimental material
L-lysine·H2SO4 (L-lysine content = 55%) was manufactured by the Changchun Dacheng Industrial Group (Changchun, China).
Birds and experimental design
All management and experimental procedures followed the animal care protocol approved by the Animal Care and Use Committee of China Agricultural University (Beijing, China). This study was conducted on Arbor Acres broiler chickens (Beijing, China) for a 35-day period with a completely randomized design. One day old (n = 240) broiler chickens were housed in a standard broiler house with separate feed and drinking facilities. These broilers were weighed individually, wing banded, and allocated randomly to 1 of 5 treatments with 8 replicates per treatment and 6 birds per replicate.
The corn-soybean meal diets were formulated as shown in Table 1. The experimental diets were comprised of a control diet with no supplemental lysine and 4 diets supplemented with 1%, 4%, 7% or 10% L-lysine·H2SO4 in two periods (first period: d 1 to 21, second period: d 22 to 35). According to the “European Food Safety Authority” [13], “Guidelines for the Evaluation of Tolerance of Feed and Feed Additives on Livestock and Poultry Target Animals” (2011) and “Feeding Standard for Poultry” (2004), the multidose group is generally 10 times as the effective dose for evaluating new feed products. As prescribed, the general addition of lysine in feed was 1%. Thus, 1% was selected as the initial concentration, and increased by 3% points in turn, reaching the highest concentration of 10%. Meanwhile, 10% as the highest dose for lysine was also consistent with the standard. The control diet provided 3.04 Mcal/kg according to the nutrient requirements for chickens.
Table 1.
Ingredient composition and nutrient levels of the experimental diets (% as fed)
| Items | Dietary L-lysine·H2SO4 levels (%) fed from d 1 to 21 | Dietary L-lysine·H2SO4 levels (%) fed from d 22 to 35 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | 0 | 1 | 4 | 7 | 10 | |
| Ingredient, % | ||||||||||
| Corn | 59.12 | 59.75 | 62.41 | 65.21 | 68.85 | 60.67 | 61.27 | 63.67 | 67.77 | 71.58 |
| Soybean meal | 31.00 | 29.40 | 24.00 | 19.95 | 14.65 | 31.75 | 30.10 | 24.45 | 18.34 | 12.43 |
| Fish meal | 3.25 | 3.15 | 2.95 | 1.55 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Soybean meal | 3.00 | 2.97 | 2.80 | 2.00 | 1.00 | 4.00 | 4.00 | 3.95 | 2.70 | 1.70 |
| Limestone | 1.40 | 1.46 | 1.35 | 1.30 | 1.25 | 1.10 | 1.15 | 1.20 | 1.25 | 1.20 |
| Dicalcium phosphate | 1.10 | 1.12 | 1.20 | 1.50 | 1.60 | 1.40 | 1.40 | 1.52 | 1.55 | 1.55 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mineral and vitamin premix1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| L-lysine·H2SO4 | 0.00 | 1.00 | 4.00 | 7.00 | 10.00 | 0.00 | 1.00 | 4.00 | 7.00 | 10.00 |
| DL-methionine | 0.15 | 0.16 | 0.20 | 0.25 | 0.28 | 0.10 | 0.10 | 0.15 | 0.20 | 0.21 |
| L-tryptophan | 0.00 | 0.00 | 0.00 | 0.04 | 0.07 | 0.00 | 0.00 | 0.00 | 0.02 | 0.06 |
| L-threonine | 0.00 | 0.01 | 0.11 | 0.22 | 0.32 | 0.00 | 0.00 | 0.08 | 0.19 | 0.29 |
| Choline chloride | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Antioxidant | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Calculated nutritional value | ||||||||||
| ME, Mcal/kg | 3.04 | 3.05 | 3.10 | 3.10 | 3.10 | 3.10 | 3.11 | 3.16 | 3.15 | 3.16 |
| Analyzed nutritional value, %2 | ||||||||||
| Dry matter | 88.24 | 88.33 | 88.62 | 88.85 | 88.82 | 89.10 | 89.11 | 89.28 | 89.47 | 90.03 |
| Crude protein | 21.70 | 22.82 | 22.59 | 23.13 | 22.71 | 20.02 | 21.20 | 21.58 | 20.40 | 20.31 |
| Total calcium | 1.05 | 1.14 | 0.73 | 0.71 | 1.01 | 0.96 | 1.20 | 0.97 | 0.82 | 1.10 |
| Total phosphorus | 0.49 | 0.55 | 0.49 | 0.61 | 0.57 | 0.57 | 0.55 | 0.54 | 0.55 | 0.58 |
| Lysine | 1.30 | 1.89 | 3.18 | 4.19 | 5.78 | 1.25 | 1.69 | 3.57 | 4.61 | 6.47 |
| Tryptophan | 0.23 | 0.23 | 0.20 | 0.21 | 0.19 | 0.23 | 0.22 | 0.19 | 0.18 | 0.20 |
| Methionine | 0.49 | 0.49 | 0.49 | 0.48 | 0.43 | 0.36 | 0.38 | 0.42 | 0.42 | 0.39 |
| Cystine | 0.30 | 0.29 | 0.28 | 0.25 | 0.21 | 0.28 | 0.29 | 0.28 | 0.25 | 0.21 |
| Threonine | 0.81 | 0.80 | 0.83 | 0.83 | 0.80 | 0.78 | 0.75 | 0.73 | 0.75 | 0.77 |
1The mineral and vitamin premix provided the following per kg of feed: zinc, 75 mg; iron, 200 mg; copper, 15 mg; iodine, 0.88 mg; selenium, 0.35 mg; manganese, 100 mg; vitamin A, 9000 IU; vitamin D3, 3750 IU; vitamin E, 25 IU; vitamin K3, 2 mg; thiamine, 1.5 mg; vitamin B12, 120 μg; riboflavin, 6 mg; nicotinic acid, 40 mg; pantothenic acid, 10 mg; pyridoxine, 3 mg; biotin, 0.1 mg
2Values are the means of a chemical analysis conducted in duplicate, n = 6/diet
Experimental management
The broiler chickens were kept in zinc-plated cages (90 cm × 70 cm × 50 cm) with plastic slatted flooring. The chicks were housed in cages with 24 h constant-lighting and had free access to feed and water. All broilers were vaccinated with live, combined Newcastle disease and infectious bronchitis vaccine on d 7 and d 21 and inactivated infectious bursal disease vaccine on d 14 and d 28.
Dietary analysis
Diets were analyzed for dry matter, crude protein, calcium, total phosphorus and amino acids according to AOAC [14] procedures. Lysine and threonine were determined by Ion-Exchange Chromatography using an AA Analyzer (Hitachi L-8800, Tokyo, Japan) after acid hydrolysis with 6 mol/L HCl (refluxed for 24 h at 110 °C). Methionine and cystine were determined after oxidation with performic acid and subsequent hydrolysis with 6 mol/L HCl at 120 °C for 16 h and separated by Reversed-Phase HPLC (Agilent 1200, Santa Clara, CA). Tryptophan was measured after LiOH hydrolysis for 22 h at 110 °C using High-Performance Liquid Chromatography (Agilent 1200 Series, Santa Clara, CA).
Growth performance
Broilers were weighed on d 1, 21, and 35. Feed consumption on a pen was measured every week. Average daily feed intake (ADFI), average daily gain (ADG), and feed conversion ratio (FCR, ADFI: ADG) were calculated from d 1 to 21, d 22 to 35 and d 1 to 35, respectively.
Carcass characteristics
On the d 35 of the trial, one broiler chicken was selected randomly from each cage after an overnight fast. The broilers were slaughtered by exsanguination and eviscerated manually. Carcass weight, semi-eviscerated weight, eviscerated weight, liver weight, abdominal fat weight and pH45 min of the breast and thigh muscle were measured. Dressing percentage, semi-eviscerated percentage, eviscerated percentage, abdominal fat percentage and drip loss were calculated according to the Performance Terms and Measurement for Poultry (NY/T 823–2004, China). Drip loss was calculated by hanging a section (breast and thigh muscle sample) in an inflated and closed plastic bag for 24 h at 4 °C.
Intestinal morphology and liver pathology
Samples of the duodenum, jejunum, ileum, and liver were dissected from the slaughtered broilers and immediately fixed in 4% (v/v) paraformaldehyde solution for histological examination. The fixed tissues were processed routinely, embedded in paraffin and cut into 5 μm serial sections, and sections from each tissue sample were selected and stained with hematoxylin–eosin for identification [15]. Five well-oriented villi (determined as the distance between the crypt openings and the end of the villi) and their associated crypt (measured from the crypt villous junction to the base of the crypt) per section were selected and measured under a light microscope (CK-40, Olympus, Tokyo, Japan) at 40 × magnification and analyzed with an Image Analyzer (Lucia Software. Lucia, Za Drahou, Czechoslovakia). The sections of liver were prepared for microscopic examination and representative samples were photographed.
Blood biochemistry
Blood was collected in uncoated serum tubes and EDTA-coated tubes at 35 days of age from 8 birds in each treatment by puncturing the brachial vein. Serum was collected and stored at − 20 °C. Serum amino acid concentrations were determined with an Amino Acid Analyzer (S-433D AA Analyzer, Sykam, Germany). Individual serum samples were analyzed for albumin, total protein, globulin, total bilirubin, creatinine and uric acid. The activities of alanine amino transferase, aspartate amino transferase and lactic dehydrogenase were analyzed at the Beijing Sino-UK Institute of Biological Technology Company (Beijing, China). Hematology parameters such as white blood cell count, red blood cell count, hemoglobin, hematocrit, corpuscular volume, corpuscular hemoglobin concentration, red cell distribution width, platelet distribution width, platelet volume and proportion of large platelets were measured by corresponding commercial kits (BioSino Bio-Technology and Science Company, Beijing, China) using an Automatic Biochemical Analyzer (Hitachi 7160, Hitachi High-Technologies Corporation, Tokyo, Japan).
Statistical analysis
Data were analyzed by the GLM procedure of SAS (Statistical Analysis System, Version 9.2) using a randomized design. Means are expressed as the least squares means with pen as the experimental unit. An alpha level of P < 0.05 was the criterion for statistical significance. Polynomial contrasts were performed to determine linear and quadratic relationships of lysine levels.
Results
Growth performance
Increasing dietary L-lysine·H2SO4 linearly and quadratically decreased both ADG and ADFI and increased FCR from d 1 to 21, d 22 to 35, and d 1 to 35 (P < 0.05) (Table 2). No significant difference in performance was observed between broiler chicks fed the control and 1% L-lysine·H2SO4 diets during any phase of the experiment (Table 2). When the L-lysine·H2SO4 level in the diet increased to 4%, 7% or 10%, the performance of broilers was negatively affected compared with broiler chicks fed the control and 1% L-lysine·H2SO4 diets during all phases of the experiment (P < 0.05) (Table 2).
Table 2.
Effect of dietary L-lysine·H2SO4 levels on performance of broiler chickens1
| Items | Dietary L-lysine·H2SO4 levels, % | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | ANOVA | Linear | Quadratic | ||
| D 1 to 21 | |||||||||
| Average daily feed intake, g | 43.0a | 42.7a | 32.9b | 13.5c | 6.5d | 0.66 | < 0.01 | < 0.01 | < 0.01 |
| Average daily gain, g | 32.7a | 32.7a | 22.6b | 6.0c | 1.5d | 0.77 | < 0.01 | < 0.01 | < 0.01 |
| Feed conversion ratio | 1.3d | 1.3d | 1.5c | 2.3b | 4.4a | 0.06 | < 0.01 | < 0.01 | < 0.01 |
| D 22 to 35 | |||||||||
| Average daily feed intake, g | 111.3a | 109.2a | 56.5b | 24.7c | 10.8d | 2.00 | < 0.01 | < 0.01 | < 0.01 |
| Average daily gain, g | 67.0a | 65.6a | 19.5b | 6.3c | 1.9d | 1.52 | < 0.01 | < 0.01 | < 0.01 |
| Feed conversion ratio | 1.7d | 1.7d | 3.0c | 4.1b | 6.0a | 0.26 | <0.01 | <0.01 | <0.01 |
| D 1 to 35 | |||||||||
| Average daily feed intake, g | 70.3a | 69.3a | 42.4b | 18.0c | 8.2d | 1.00 | < 0.01 | < 0.01 | < 0.01 |
| Average daily gain, g | 46.4a | 45.9a | 21.4b | 6.1c | 1.6d | 0.83 | <0.01 | < 0.01 | < 0.01 |
| Feed conversion ratio | 1.5d | 1.5d | 2.1c | 3.0b | 5.1a | 0.12 | < 0.01 | <0.01 | < 0.01 |
1Means in the same row with different superscripts significantly differ from one another (P < 0.05). SEM standard error of the mean; n = 8/diet
Carcass characteristics
No significant difference in carcass characteristics was observed between broilers fed the control and 1% L-lysine·H2SO4 diets (Table 3). Increased dietary L-lysine·H2SO4 linearly and quadratically decreased eviscerated percentage and abdominal fat percentage of broilers (P < 0.05), and increased liver index and drip loss (breast and thigh muscle) of broiler chickens (P < 0.05). There were no notably changes in pH value among different levels of L-lysine·H2SO4 treatments.
Table 3.
Effect of dietary L-lysine·H2SO4 levels on carcass characteristics of broiler chickens1
| Items | Dietary L-lysine·H2SO4 levels, % | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | ANOVA | Linear | Quadratic | ||
| Dressing percentage, % | 91.5 | 90.2 | 90.9 | 91.4 | 0.83 | 0.66 | 0.97 | 0.54 |
| Semi-eviscerated percentage, % | 86.0 | 84.2 | 83.4 | 81.9 | 1.11 | 0.12 | 0.01 | 0.53 |
| Eviscerated percentage, % | 73.4a | 71.9a | 67.6b | 62.7c | 1.30 | < 0.01 | < 0.01 | < 0.01 |
| Abdominal fat percentage, % | 1.1ab | 1.2a | 0.8b | 0.08c | 1.13 | < 0.01 | < 0.01 | < 0.01 |
| Liver index, % | 2.1b | 2.1b | 2.8b | 4.3a | 0.19 | < 0.01 | < 0.01 | < 0.01 |
| Breast muscle | ||||||||
| pH 45 min | 6.2 | 6.2 | 6.5 | 6.4 | 0.09 | 0.11 | 0.05 | 0.11 |
| Drip loss, % | 2.1b | 2.2b | 3.3b | 6.7a | 0.43 | < 0.01 | < 0.01 | < 0.01 |
| Thigh muscle | ||||||||
| pH 45 min | 6.5 | 6.5 | 6.6 | 6.7 | 0.06 | 0.05 | 0.01 | 0.02 |
| Drip loss, % | 3.3b | 4.4b | 4.2b | 12.7a | 0.91 | < 0.01 | < 0.01 | < 0.01 |
1Broiler chickens fed with 10% L-lysine·H2SO4 supplemented diet were too small to collect carcass characteristics in this experiment. Means in the same row with different superscripts significantly differ from one another (P < 0.05). SEM standard error of the mean; n = 6/diet
Intestinal morphology
Intestinal morphology was influenced by high levels of dietary L-lysine·H2SO4 (Fig. 1), but no significant differences were observed between broilers fed control and 1% L-lysine·H2SO4 diets. Villous height and crypt depth in broilers fed the 7% and 10% L-lysine·H2SO4 diets were lower than those of the broilers fed 0, 1% and 4% L-lysine·H2SO4 diets (P < 0.05). Villous height, crypt depth and villous height to crypt depth ratio in the duodenum, jejunum, and ileum were not affected when comparing to broilers fed control with those fed 1% L-lysine·H2SO4 diets (P > 0.05).
Fig. 1.
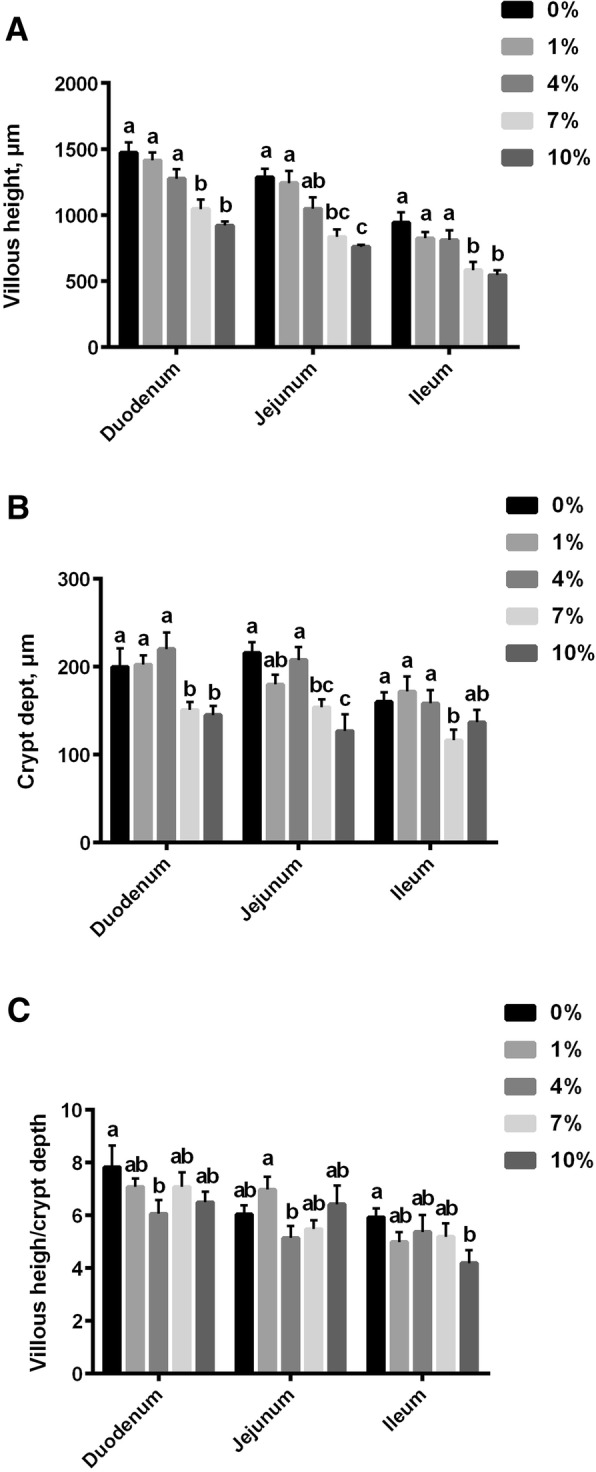
Effect of dietary L-lysine·H2SO4 levels (%) on intestinal morphology of broiler chickens. a, villous height (μm) of broiler chickens fed different diets; b, crypt depth (μm) of broiler chickens fed different diets; c, ratio of villous height to crypt depth of broiler chickens fed different diets. Data were means ± SEM (n = 6). Different letters indicate a difference (P < 0.05)
Liver pathology
No differences pathology of livers were observed between broilers fed the control and 1% L-lysine·H2SO4 diets (Fig. 2). Hepatocytes were arranged compactly and orderly. There were a few lipid droplets in hepatocytes of broilers fed the 4% L-lysine·H2SO4 diets. When broilers were fed 7% L-lysine·H2SO4, obvious pathological changes were present in those livers. Liver cells were dissociated from each other in hepatic cords, cytoplasmic fat vacuoles. Pushed hepatocyte nuclei to the cell periphery in some places and reduction in size of a few hepatocytes was also seen. There was diffuse infiltration of fat vacuoles indicating vacuolar degeneration in livers of broilers fed the 10% L-lysine·H2SO4 diet. Furthermore, liver cells were dissociated from each other and cytoplasmic fat vacuoles pushed hepatocyte nuclei to the cell periphery in many cells. Tissue edema was obvious in livers.
Fig. 2.
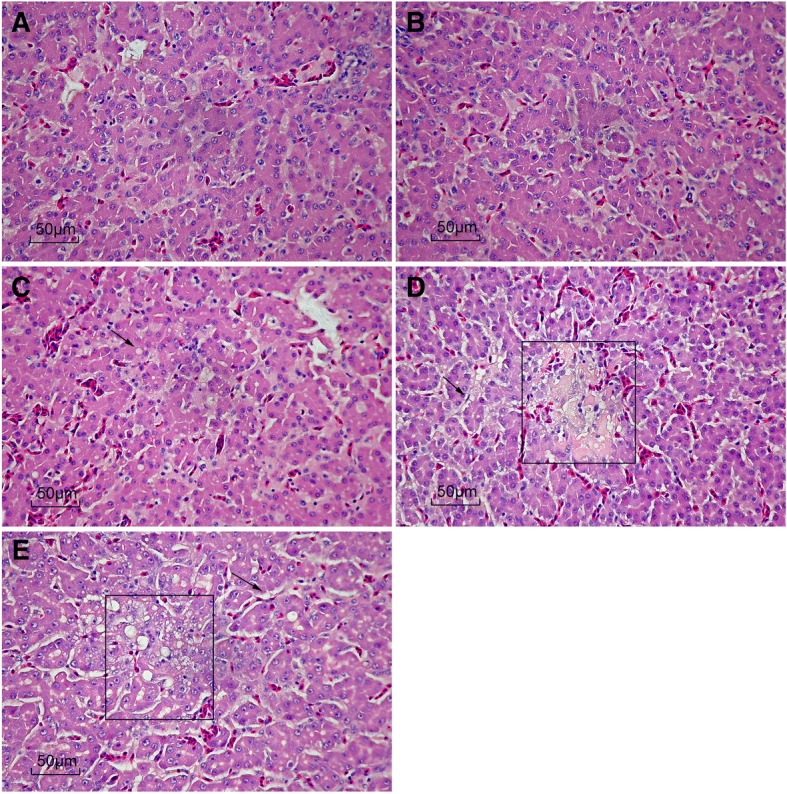
Effect of dietary L-lysine·H2SO4 levels on liver morphology of broiler chickens. a, Broilers fed on basal diets; b, Broilers supplemented with 1% L-lysine·H2SO4; c, Broilers supplemented with 4% L-lysine·H2SO4; d, Broilers supplemented with 7% L-lysine·H2SO4; e, Broilers supplemented with 10% L-lysine·H2SO4
Serum amino acid concentrations
No significant differences were observed among broilers fed control, 1%, 4%, 7% and 10% L-lysine·H2SO4 diets for serum concentrations of arginine, histidine, methionine, cysteine, serine and tyrosine (Table 4). Serum lysine and threonine concentrations of broilers were linearly and quadratically increased with increasing dietary L-lysine·H2SO4 level (P < 0.05). Serum glycine concentration of broilers fed 1% L-lysine·H2SO4 diet was higher than that of broilers fed control diets (P < 0.05), while serum proline concentration of the broilers fed 1% L-lysine·H2SO4 diet was higher than that of broilers fed the 4% L-lysine·H2SO4 diet (P < 0.05). Serum isoleucine, leucine and valine concentrations of the broilers fed the 7% L-lysine·H2SO4 diet was higher than that of broilers fed the control diet (P < 0.05).
Table 4.
Effect of dietary L-lysine·H2SO4 levels on serum amino acid concentrations of broilers 1
| Items | Dietary L-lysine·H2SO4 levels, % | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | ANOVA | Linear | Quadratic | ||
| Essential AA, nmol/mL | |||||||||
| Lysine | 402.0c | 576.5 c | 1031.4b | 1395.3a | 1521.8a | 105.18 | < 0.01 | < 0.01 | < 0.01 |
| Arginine | 374.9 | 310.3 | 281.7 | 319.4 | 284.4 | 43.55 | 0.58 | 0.21 | 0.37 |
| Histidine | 114.9 | 145.6 | 137.3 | 144.7 | 157.7 | 14.91 | 0.36 | 0.07 | 0.20 |
| Isoleucine | 112. 9b | 170.9ab | 189.0ab | 231.9a | 156.4ab | 21.39 | < 0.01 | 0.10 | < 0.01 |
| Leucine | 175.8b | 258.0ab | 229.0ab | 283.0a | 228.3ab | 24.59 | 0.04 | 0.12 | 0.04 |
| Methionine | 68.9 | 80.8 | 79.4 | 81.5 | 76.7 | 6.93 | 0.69 | 0.44 | 0.37 |
| Phenylalanine | 121.5b | 166.7a | 178.8a | 176.5a | 138.7ab | 11.96 | < 0.01 | 0.30 | < 0.01 |
| Threonine | 623.5b | 667.7b | 776.7b | 1070.5b | 1453.0a | 117.63 | < 0.01 | < 0.01 | < 0.01 |
| Valine | 169.4c | 260.9b | 312.9b | 408.6a | 310.1b | 27.71 | < 0.01 | < 0.01 | < 0.01 |
| Non-essential AA, nmol/mL | |||||||||
| Alanine | 1043.5b | 1797.6ab | 806.1b | 2167.4a | 848.9b | 258.68 | < 0.01 | 0.92 | 0.23 |
| Asparagine | 79.9b | 182.1ab | 118.8ab | 221.3a | 143.4ab | 30.28 | 0.02 | 0.11 | 0.07 |
| Glycine | 764.9b | 1058.2a | 918.5ab | 911.0ab | 845.5ab | 66.07 | 0.05 | 0.95 | 0.09 |
| Cysteine | 304.9 | 403.0 | 296.5 | 415.1 | 238.6 | 53.94 | 0.12 | 0.57 | 0.22 |
| Proline | 1926.8ab | 2622.0a | 1464.6b | 1881.1ab | 2415.6a | 204.41 | < 0.01 | 0.80 | 0.50 |
| Serine | 647.1 | 764.4 | 601.3 | 656.5 | 751.3 | 58.40 | 0.27 | 0.63 | 0.71 |
| Tyrosine | 109.6 | 153.1 | 137.4 | 137.9 | 143.2 | 12.26 | 0.17 | 0.21 | 0.21 |
1Means in the same row with different superscripts significantly differ from one another (P < 0.05). AA amino acid, SEM standard error of the mean; n = 6/diet
Blood chemistry and hematology
No differences in blood chemistry were observed between broilers fed the control and 1% L-lysine·H2SO4 diets (P > 0.05) (Table 5). Both albumin and total protein of broilers fed the 7% and 10% L-lysine·H2SO4 diets were higher than those of the broilers fed the control diet (P < 0.05), meanwhile increased dietary L-lysine·H2SO4 linearly and quadratically increased albumin and total protein of broiler chickens (P < 0.05). Total bilirubin of the broilers fed the 7% L-lysine·H2SO4 diet was higher than that of broilers fed 0, 1%, 4% and 10% L-lysine·H2SO4 diets (P < 0.05).
Table 5.
Effect of dietary L-lysine·H2SO4 levels on serum chemistry of broiler chickens1
| Items | Dietary L-lysine·H2SO4 levels, % | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | ANOVA | Linear | Quadratic | ||
| Albumin, g/L | 11.1c | 11.3c | 12.3bc | 14.5a | 13.0b | 0.38 | <0.01 | < 0.01 | < 0.01 |
| Total protein, g/L | 23.7c | 25.4bc | 28.2ab | 29.1a | 27.7ab | 0.79 | < 0.01 | < 0.01 | < 0.01 |
| Globulin, g/L | 12.6 | 14.2 | 15.9 | 14.6 | 14.8 | 0.85 | 0.13 | 0.09 | 0.05 |
| Alanine amino transferase, U/L | 21.3ab | 22.9ab | 16.8b | 31.6a | 23.8ab | 2.78 | 0.02 | 0.15 | 0.36 |
| Aspartate amino transferase, U/L | 65.3 | 86.9 | 69.6 | 68.6 | 95.1 | 7.24 | 0.06 | 0.18 | 0.33 |
| Total bilirubin, μmol/L | 19.4b | 22.2b | 22.7b | 42.5a | 20.6b | 2.90 | < 0.01 | 0.04 | 0.02 |
| Creatinine, μmol/L | 24.0 | 25.3 | 26.5 | 25.2 | 21.6 | 1.18 | 0.12 | 0.36 | 0.03 |
| Uric acid, μmol/L | 332.2 | 356.1 | 291.7 | 250.5 | 365.5 | 46.66 | 0.43 | 0.66 | 0.55 |
| Lactic dehydrogenase, U/L | 1508.0 | 1443.8 | 1567.5 | 1146.0 | 1792.7 | 182.12 | 0.24 | 0.84 | 0.52 |
1Means in the same row with different superscripts differ from one another (P < 0.05). SEM standard error of the mean; n = 6/diet
No significant differences in hematology were observed between broilers fed the control and 1% L-lysine·H2SO4 diets (Table 6). Hemoglobin and corpuscular hemoglobin concentration of the broilers fed the 10% L-lysine·H2SO4 diet were lower than those of the broilers fed the control and 1% L-lysine·H2SO4 diets (P < 0.05). Increasing dietary L-lysine·H2SO4 linearly and quadratically decreased the corpuscular hemoglobin content of broilers (P < 0.05).
Table 6.
Effect of dietary L-lysine·H2SO4 levels on blood hematology of broiler chickens1
| Items | Dietary L-lysine·H2SO4 levels, % | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 7 | 10 | ANOVA | Linear | Quadratic | ||
| White blood cells, 109/L | 49.0ab | 37.9b | 48.6ab | 55.8a | 54.1ab | 4.20 | 0.05 | 0.07 | 0.11 |
| Red blood cells, 1012/L | 2.6ab | 2.6ab | 2.7ab | 2.8a | 2.5b | 0.07 | 0.04 | 0.91 | 0.11 |
| Hemoglobin, g/L | 86.5a | 86.6a | 83.6a | 89.1a | 73.0b | 3.30 | 0.02 | 0.06 | 0.04 |
| Hematocrit, % | 33.1ab | 33.7ab | 33.0ab | 34.9a | 30.1b | 0.99 | 0.05 | 0.26 | 0.08 |
| Corpuscular volume, fL | 127.7ab | 130.4a | 121.7b | 124.7ab | 122.4b | 1.81 | 0.01 | 0.01 | 0.05 |
| Corpuscular hemoglobin concentration, g/L | 261.1a | 258.4a | 252.3ab | 255.4ab | 242.2b | 4.07 | 0.03 | < 0.01 | 0.01 |
| Red cell distribution width, fL | 35.7 | 37.5 | 36.3 | 37.3 | 38.5 | 1.40 | 0.66 | 0.22 | 0.47 |
| Platelet distribution width, fL | 6.8 | 6.1 | 7.1 | 6.6 | 6.5 | 0.59 | 0.80 | 0.91 | 0.99 |
| Platelet volume, fL | 8.2 | 8.8 | 9.2 | 9.1 | 8.3 | 0.49 | 0.49 | 0.65 | 0.18 |
| Proportion of large platelets, % | 15.2 | 20.5 | 20.4 | 18.7 | 15.4 | 3.20 | 0.62 | 0.98 | 0.28 |
1Means in the same row with different superscripts significantly differ from one another (P < 05); SEM standard error of the mean; n = 6/diet
Discussion
L-lysine·H2SO4, as a new source of lysine, contains L-lysine·sulphate and fermentation co-products which contain other amino acids and phosphorus [7]. We evaluated effects of L-lysine·H2SO4 on intestinal morphology and liver pathology when used as a source of lysine in broilers diets. We showed that excessive L-lysine·H2SO4 addition in corn-soybean meal diets have negative effects in broilers.
The high proportion of L-lysine·H2SO4 (4%, 7% and 10%) in diets had different effects on performance and carcass characteristics when compared to the control and 1% L-lysine·H2SO4 supplementation diets. These results are consistent with previous studies that reported excessive lysine supplementation affected feed intake, weight gain, carcass traits, and feed conversion ratio [16–18]. The content and ratio of dietary amino acids affect the efficiency of amino acid utilization for animal growth [19–21]. For example, 1.95% lysine decreased feed intake and growth rate through reducing arginine efficacy in chicks [22]. In young pigs, three to four times the recommended lysine level (1.15% for young pigs) also decreased feed intake and weight gain through aggravating an arginine deficiency [23]. These results indicated that high levels of L-lysine·H2SO4 that reduced growth performance in broilers may down so by lowering the utilization ratio of other amino acids. In addition, excess L-lysine·H2SO4 caused a high level of dietary sulphate which can influence broiler performance via alterations in acid-base balance.
It is generally known that blood plasma proteins and amino acids play key roles in the maintenance of colloid osmotic pressure, by keeping the balance of amino acid through a rapid substitute for indispensable amino acids, assuring steady glucose levels through gluconeogenesis, forming various functional enzymes and enhancing the immune system in the organism [24–26]. Therefore, blood plasma proteins and amino acids have an exceptional significance in maintenance of homeostasis. Changes in serum levels of lysine, threonine, phenylalanine, valine, isoleucine, and leucine in the present experiment were possibly mediated through a change in dietary amino acid levels in the experimental diets.
Blood chemistry is a common parameter utilized to estimate avian body condition. Blood albumin serves as the most favorable source of amino acids for synthesis of tissue proteins in the period of quick somatic growth of birds, especially under feed restricted conditions [27, 28]. Experiments on chickens revealed a relatively constant level of albumin in blood over a period from the 2 to 42 days of age [28]. In our study, content of total protein and albumin in serum were higher with supplementation of 4%, 7% or 10% L-lysine·H2SO4 than the control group, which is probably a direct consequence of a high demand for amino acids for somatic growth in birds fed excess L-lysine diets.
The chemistry profile and hematological count in blood of animals fed different diets can indicate the chemical and nutritional value of the diets [29, 30]. Addition of lysine in poultry diets improved immunity of birds and resulted in immunological regulatory action [31, 32]. However, the number of white blood cells in blood was higher for broilers fed the diet with 7% L-lysine·H2SO4 compared with broiler chicks fed the 1% L-lysine·H2SO4 diets, which could lead to the resulting in the secretion of much more cytokine and led to inflammatory reactions [33, 34]. Therefore, the growth performance of broilers supplemented with 4%, 7% or 10% L-lysine·H2SO4 was negatively affected compared with broiler chicks fed control diets during all phases of the experiment. In addition, the corpuscular hemoglobin concentration was also markedly decreased when the diet was supplemented with 4%, 7% or 10% L-lysine·H2SO4. This response suggests that there were toxic effects on the hematological system particularly for red blood cells [35, 36].
The folds in the intestine called villi are an important exchange area for digestion and absorption [37]. Villous height and crypt depth are critical factors affecting the animal’s ability to absorb nutrients [38]. In the current study, there was a significant reduction of villous height and a decline of crypt depth in the duodenum, jejunum and ileum in broilers supplemented with 7% or 10% L-lysine·H2SO4. Similar findings were reported for weanling pigs fed a lysine-deficient diet [39]. The reduction in villus height would reduce the surface area available for absorption of nutrients and this may partially explain the reduced growth performance and reduced feed efficiency in pigs fed the excessive L-lysine·H2SO4 diet.
The liver is the main size for protein synthesis, and liver histology is important in understanding pathological alterations in response to nutritional sources. Studies have shown that the addition of excessive protein in feed can reduce physiological function of the liver, so the lipoprotein in the liver cells cannot be transported in time, resulting in the accumulation of lipoprotein in the liver and liver lesions [40, 41]. In the present study, we found that high levels of L-lysine·H2SO4 might induce hepatic steatosis in broilers. The total protein content of the serum can reflect the ability of the liver to synthesize protein. The present study showed serum total protein content increased with the addition of L-lysine·H2SO4 in poultry diets, and more than 4% L-lysine·H2SO4 groups were significantly higher than the control group. Hence, excessive L-lysine·H2SO4 could cause a certain degree of damage to the liver cells, which is consistent with the results of liver histology in this study.
Conclusions
Our results show that supplementation with 1% L-lysine·H2SO4 had no negative effects on broilers, while supplementation with 4%, 7% or 10% L-lysine·H2SO4 negatively influenced growth performance, intestinal morphology and liver pathology. Our data indicates that doses higher than 1% dietary L-lysine·H2SO4 resulted in a negative response for broilers.
Acknowledgements
We appreciate the technological support from Beijing Advanced Innovation Center for Food Nutrition and Human Health, China Agricultural University, and the Modern Agro-Industry Technology Research System of Beijing. And we thank Dacheng Group (Changchun, China) for providing the crystalline amino acids.
Funding
The present study was supported by the 111 Project (B16044) of China.
Availability of data and materials
All the data were presented in the main manuscript and available to readers.
Abbreviations
- AA
Amino acids
- ADFI
Average daily feed intake
- ADG
Average day gain
- AOAC
Association of Official Analytical Chemists
- FCR
The ratio of average daily feed intake to average daily gain
- GLM
General liner model
- HCl
Hydrogen chloride
- HPLC
High performance liquid chromatography
- ME
Metabolic energy
- SAS
Statistical Analysis System
Authors’ contributions
The article was mainly conceived and designed by SQ and XM. Experimental data were collected and analyzed by HJ and WM. The manuscript was mainly written by HJ, assisting revised the draft by TH, JZ and Lee Johnston; and edited by TH, SZ, JZ, HY and SQ. XZ and SQ resourced the project. All the authors contributed to, read and approved the final manuscript.
Ethics approval and consent to participate
All procedures used in this study were performed according to the guidelines for the ethical treatment of animals by the Institutional Animal Care and Use Committee of China Agricultural University (Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Hongmin Jia, Email: jiahongmin@126.com.
Ting He, Email: ht920819@cau.edu.cn.
Haitao Yu, Email: 15600660793@163.com.
Xiangfang Zeng, Email: zengxf@cau.edu.cn.
Shihai Zhang, Email: zhangshihai@scau.edu.cn.
Wenfeng Ma, Email: a113boy@163.com.
Jie Zhang, Email: 71819@bvca.edu.cn.
Shiyan Qiao, Email: qiaoshiyan@cau.edu.cn.
Xi Ma, Email: maxi@cau.edu.cn.
References
- 1.Smiricky-Tjardes MR, Mavromichalis I, Albin DM, Wubben JE, Rademacher M, Gabert VM. Bioefficacy of L-lysine sulfate compared with feed-grade L-lysine·HCl in young pigs. J Anim Sci. 2004;82:2610–2614. doi: 10.2527/2004.8292610x. [DOI] [PubMed] [Google Scholar]
- 2.Jones CK, Tokach MD, Usry JL, Neill CR, Patience JF. Evaluating lysine requirements of nursery pigs fed low protein diets with different sources of nonessential amino acids. J Anim Sci. 2014;92:3460–3470. doi: 10.2527/jas.2014-7018. [DOI] [PubMed] [Google Scholar]
- 3.Rodehutscord M, Borchert F, Gregus Z, Pfeffer E. Availability and utilisation of free lysine in rainbow trout (Oncorhynchus mykiss): 2 comparison of L-lysine·HCl and L-lysine·sulphate. Aquaculture. 2000;187:177–83.
- 4.Waguespack AM, Powell S, Bidner TD, Payne RL, Southern LL. Effect of incremental levels of L-lysine and determination of the limiting amino acids in low crude protein corn-soybean meal diets for broilers. Poult Sci. 2009;88:1216–1226. doi: 10.3382/ps.2008-00452. [DOI] [PubMed] [Google Scholar]
- 5.Kircher M, Pfefferle W. The fermentative production of L-lysine as an animal feed additive. Chemosphere. 2001;43:27–31. doi: 10.1016/S0045-6535(00)00320-9. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad G, Mushtaq T, Mirza MA, Ahmed Z. Comparative bioefficacy of lysine from L-lysine hydrochloride or L-lysine sulfate in basal diets containing graded levels of canola meal for female broiler chickens. Poult Sci. 2007;86:525–530. doi: 10.1093/ps/86.3.525. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZR, You JM, Qiao SY, Wang X. Bioefficacy of L-lysine·H2SO4 relative to L-lysine·HCl in broiler chickens, estimated by slope-ratio model. Br Poult Sci. 2007;48:381–388. doi: 10.1080/00071660701370517. [DOI] [PubMed] [Google Scholar]
- 8.Leibholz J, Love RJ, Mollah Y, Carter RR. The absorption of dietary L-lysine and extruded L-lysine in pigs. Anim Feed Sci Tech. 1986;15:141–148. doi: 10.1016/0377-8401(86)90021-0. [DOI] [Google Scholar]
- 9.Jackson M. A closer look at lysine sources: L-lysine sulfate plus fermentation co-products. Feed Inter. 2001;22:18–20. [Google Scholar]
- 10.Latshaw JD. Dietary lysine concentrations from deficient to excessive andthe effects on broiler chicks. Br Poult Sci. 1993;34:951–958. doi: 10.1080/00071669308417655. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama Y, Hisanaga N, Nishii T, Yoshida A. Effects of some potassium compounds on liver lipid accumulation induced by excess dietary lysine. Comp Biochem Physiol A Comp Physiol. 1994;107:227–231. doi: 10.1016/0300-9629(94)90298-4. [DOI] [Google Scholar]
- 12.Henry MH, Pesti GM, Bakalli R, Lee J, Toledo RT, Eitenmiller RR, et al. The performance of broiler chicks fed diets containing extruded cottonseed meal supplemented with lysine. Poult Sci. 2001;80:762–8. [DOI] [PubMed]
- 13.EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Scientific opinion on the safety and efficacy of L-lysine sulphate produced by fermentation with Escherichia coli CGMCC 3705 for all animal species. EFSA J. 2015;13:4155. [Google Scholar]
- 14.George W, Jr L. Official methods of analysis of AOAC international. 20. Gaithersburg, Maryland: AOAC international; 2016. [Google Scholar]
- 15.Wang WW, Zeng XF, Mao XB, Wu GY, Qiao SY. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J Nutr. 2010;140:981–986. doi: 10.3945/jn.109.118497. [DOI] [PubMed] [Google Scholar]
- 16.Jones J. Lysine toxicity in the chick. J Nutr. 1961;73:107–112. doi: 10.1093/jn/73.2.107. [DOI] [Google Scholar]
- 17.Sauberlich H. Studies on the toxicity and antagonism of amino acids for weanling rats. J Nutr. 1961;75:61–72. doi: 10.1093/jn/75.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Kidd MT. Nutritional modulation of immune function in broilers. Poult Sci. 2004;83:650–657. doi: 10.1093/ps/83.4.650. [DOI] [PubMed] [Google Scholar]
- 19.Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci. 2018;19(4):954. doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad T, Mushtaq T, Khan MA, Babar ME, Yousaf M, Hasan ZU, et al. Influence of varying dietary electrolyte balance on broiler performance under tropical summer conditions. J Anim Physiol Anim Nutr. 2009;93:613–21. [DOI] [PubMed]
- 21.Zhang GJ, Xie CY, Thacker PA, Htoo JK, Qiao SY. Estimation of the ideal ratio of standardized ileal digestible threonine to lysine for growing pigs (22-50 kg) fed low crude protein diets supplemented with crystalline amino acids. Anim Feed Sci Technol. 2013;180:83–91. doi: 10.1016/j.anifeedsci.2013.01.006. [DOI] [Google Scholar]
- 22.Allen NK, Baker DH, Scott HM, Norton HW. Quantitative effect of excess lysine on the ability of arginine to promote chick weight gain. J Nutr. 1972;102:171–180. doi: 10.1093/jn/102.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Edmonds MS, Baker DH. Failure of excess dietary lysine to antagonize arginine in young pigs. J Nutr. 1987;117:1396–1401. doi: 10.1093/jn/117.8.1396. [DOI] [PubMed] [Google Scholar]
- 24.Ma N, Guo P, Zhang J, He T, Kim SW, Zhang G, et al. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front Immunol. 2018;9:5. doi: 10.3389/fimmu.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He L, Han M, Farrar S, Ma X. Impacts and regulation of dietary nutrients on gut microbiome and immunity. Protein Pept Lett. 2017;24:380–381. doi: 10.2174/092986652405170510214715. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Han M, Rezaei A, Li D, Wu G, Ma X. L-arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr Protein Pept Sci. 2017;18:599–608. doi: 10.2174/1389203717666160627074017. [DOI] [PubMed] [Google Scholar]
- 27.Yaman MA, Kita K, Okumura J. Different responses of protein synthesis to refeeding in various muscles of fasted chicks. Br Poult Sci. 2000;41:224–228. doi: 10.1080/00071660050022317. [DOI] [PubMed] [Google Scholar]
- 28.Filipović N, Stojević Z, Milinković-Tur S, Ljubić BB, Zdelar-Tuk M. Changes in concentration and fractions of blood serum proteins of chickens during fattening. Vet Arhiv. 2007;77:319–326. [Google Scholar]
- 29.Nalle CL, Ravindran V, Ravindran G. Nutritional value of faba beans (Vicia faba L.) for broilers: apparent metabolizable energy, ileal amino acid digestibility and production performance. Anim Feed Sci Technol. 2010;156:104–11.
- 30.Usayran NN, Sha'ar H, Barbour GW, Yau SK, Maalouf F, Farran MT. Nutritional value, performance, carcass quality, visceral organ size, and blood clinical chemistry of broiler chicks fed 30% tannin-free fava bean diets. Poult Sci. 2014;93:2018–2027. doi: 10.3382/ps.2014-03872. [DOI] [PubMed] [Google Scholar]
- 31.Kidd MT, Kerr BJ, Anthony NB. Dietary interactions between lysine and threonine in broilers. Poult Sci. 1997;76:608–614. doi: 10.1093/ps/76.4.608. [DOI] [PubMed] [Google Scholar]
- 32.Panda AK, Rao SV, Raju MV, Niranjan MV, Niranjan MR, Reddy MR. Effect of nutrient density on production performance, egg quality and humoral immune response of brown laying (Dahlem red) hens in the tropics. Trop Anim Health Prod. 2012;44:293–299. doi: 10.1007/s11250-011-0017-9. [DOI] [PubMed] [Google Scholar]
- 33.Siegel HS. Blood cells and chemistry of young chickens during daily ACTH and cortisol administration. Poult Sci. 1968;47:1811–1817. doi: 10.3382/ps.0471811. [DOI] [PubMed] [Google Scholar]
- 34.Post J, Rebel JM, Huurne AA. Physiological effects of elevated plasma corticosterone concentrations in broiler chickens. An alternative means by which to assess the physiological effects of stress. Poult Sci. 2003;82:1313–1318. doi: 10.1093/ps/82.8.1313. [DOI] [PubMed] [Google Scholar]
- 35.Kubena LF, Harvey RB, Huff WE, Corrier DE, Philips TD, Rottinghaus GE. Influence of ochratoxin a and T-2 toxin singly and in combination on broiler chickens. Poult Sci. 1989;68:867–872. doi: 10.3382/ps.0680867. [DOI] [PubMed] [Google Scholar]
- 36.Ledoux DR, Broomhead JN, Bermudez AJ, Rottinghaus GE. Individual and combined effects of the fusarium mycotoxins fumonisin B1 and moniliformin in broiler chicks. Avian Dis. 2003;47:1368–1375. doi: 10.1637/7028. [DOI] [PubMed] [Google Scholar]
- 37.Pluske JR, Thompson MJ, Atwood CS, Bird PH, Williams IH, Hartmann PE. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br J Nutr. 1996;76:409–422. doi: 10.1079/BJN19960046. [DOI] [PubMed] [Google Scholar]
- 38.Kelly D, Smyth J, McCracken K. Digestive development of the early-weaned pig. Brit J Nutr. 1991;65:181–188. doi: 10.1079/BJN19910079. [DOI] [PubMed] [Google Scholar]
- 39.He LQ, Yang HS, Hou YQ, Li TJ, Fang J, Zhou XH, et al. Effects of dietary L-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids. 2013;45:383–391. doi: 10.1007/s00726-013-1514-0. [DOI] [PubMed] [Google Scholar]
- 40.Robaina L, Lquierdo MS, Moyano FJ, Socorro J, Vergara JM, Montero D, et al. Soybean and lupin seed meals as protein sources in diets for gilthead seabream (Sparus aurata): nutritional and histological implications. Aquaculture. 1995;130:219–33.
- 41.Caballeroa MJ, López-Calerob G, Socorrob J, Roob FJ, Izquierdoa MS, Férnandezc AJ. Combined effect of lipid level and fish meal quality on liver histology of gilthead seabream (Sparus aurata). Aquaculture. 1999;179:277–90.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data were presented in the main manuscript and available to readers.


