Abstract
Overexpressed in lung cancer 1 (OLC1) is a potential oncogene overexpressed in human lung cancer and in other types of malignant tumor. The elevated expression of OLC1 contributes to tumor genesis and progression. However, the mechanisms regulating the expression of OLC1 remain unclear. In the present study, using lung and esophageal cancer cell lines, it was demonstrated that OLC1 was a short-lived, cell cycle-dependent protein regulated through the anaphase-promoting complex/cyclosome (APC/c)-ubiquitin pathway by directly interacting with the APC2 subunit. Through the action of two co activator proteins, cadherin 1 (Cdh1) and cell-division cycle protein 20 (Cdc20), the OLC1 protein was ubiquitinated and degraded. Following treatment with a proteasome inhibitor, OLC1 protein levels were elevated. Inversely, the upregulation of Cdh1 and Cdc20 facilitated OLC1 degradation. By inducing point mutations of the assumed degradation motif of OLC1, it was revealed that an intact destruction (D)-box was necessary. As expected, the D-box-mutated OLC1 exhibited a higher capacity for promoting cell growth and clone formation. Collectively, these findings indicate that the expression of the candidate oncogene OLC1 is cell cycle-dependent and is regulated by an APC/c mediated ubiquitin-proteasome pathway.
Keywords: overexpressed in lung cancer 1, degradation, anaphase-promoting complex/cyclosome, cell-division cycle protein 20, cadherin 1, destruction box
Introduction
The occurrence and development of malignant tumors is a complicated process, and numerous oncogenes and tumor suppressor genes are involved. Overexpressed in lung cancer 1 (OLC1) is a relatively novel candidate oncogene, which was originally discovered by Yuan et al (1) using suppression subtractive hybridization. It was identified that OLC1 was more highly expressed in squamous cell lung tumors compared with normal bronchial epithelial cells, and that it promoted tumor formation in nude mice. Similarly, it was also demonstrated that OLC1 was up regulated in human esophageal carcinoma and contributed to the proliferation of esophageal cancer cells (2).
The OLC1 gene was first designated as increased sodium tolerance 1 (IST1), which has also been identified and studied in non-lethal yeast mutants (3,4). It encodes a protein that participates in the disassembly of endosomal sorting complexes (5,6). It was reported that the human IST1 gene, now known as OLC1, may serve a significant function in cytokinesis in mitosis, as demonstrated in HeLa cells (7). As it is highly conserved and vital for organisms ranging from yeast to humans, studying the mechanisms that control OLC1 expression may provide a greater insight into its physiopathological function in human tumor genesis and progression.
A preliminary investigation indicated that OLC1 maybe degraded through the ubiquitin proteasome pathway and that cigarette smoke condensate (CSC) may affect this degradation (8). Through a bioinformatics analysis of the OLC1 protein, destruction (D)-box motif (amino acid sequence, RXXLXXXXN) was identified at amino acids 12–20. This motif is essential for the recognition and subsequent degradation of the protein by the anaphase-promoting complex/cyclosome (APC/c) (9,10). As the destruction of APC targets is regulated by two activators, cell-division cycle protein 20 (Cdc20) and cadherin 1 (Cdh1) (11–13), these activators are also predicted to govern the degradation of OLC1. Previous studies regarding this topic predominantly focused on the effects of CSC on OLC1 stability (8), whereas the present study explores the in-depth mechanism by which OLC1 is degraded through the ubiquitin proteasome pathway.
In the present study, it was revealed that OLC1 interacted with the APC/c through the APC2 subunit, assisted by two coactivator proteins, Cdh1 and Cdc20. The up regulation of Cdh1 and Cdc20 accelerated OLC1 degradation, whereas the down regulation of Cdh1/Cdc20 resulted in OLC1 protein accumulation. In addition, the D-box sequence was characterized, as this motif was a critical determinant of OLC1 degradation. Mutations of the D-box motif enhanced OLC1 protein stability and induced an increase in cell growth and colony formation.
Materials and methods
Cell lines, cell culture and synchronization procedures
A stable OLC1-overexpressing cell line KYSE150/GFP-OLC1 and its null control cell line KYSE150/GFP were constructed as previously described (2). H1299 human lung carcinoma cells were purchased from the American Type Culture Collection (Manassas, VA, USA). KYSE150/GFP, KYSE150/GFP-OLC1 and H1299 cells were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin, 100 µg/ml streptomycin and 400 µg/ml G418 in a humidified atmosphere with 5% CO2 at 37°C.
To synchronize the cells at the G0 phase, the cells were incubated in RPMI-1640 medium without serum for 72 h. The cells were transferred into fresh RPMI-1640 medium with serum for subsequent incubation. Then cells were collected every 2 h.
For G2/M phase arrest, cells were incubated with 400 ng/ml nocodazole (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 16 h. Following nocodazole treatment, cells were transferred into completeRPMI-1640 medium as described, and collected every 2 h.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from treated KYSE150/GFP cells with TRIzol reagent (Thermo Fisher Scientific, Inc.), and RNA (6 µg) was reverse-transcribed according to the manufacturer's protocol (Super Script™ First-Strand Synthesis System for RT-PCR kit; Thermo Fisher Scientific, Inc., Waltham, MA, USA). For PCR amplification, 1 µl cDNA solution was used as a template. The primer sequences used for the 435-bp OLC1 product were as follows: Forward, 5′-ACAGTGGGAGAGAGCACGTT-3′ and reverse, 5′-GCACCTTGTCCTTTCTCTGC-3′. The sequences for the 299-bp GAPDH product were as follows: Forward, 5′-GCTGAGAACGGGAAGCTTGT-3′ and reverse, 5′-GCCAGGGGTGCTAAGCAG-3′. The thermo cycling conditions for OLC1 are: 94°C for 5 min, 94°C for 30 sec, 58°C for 30 sec, 72°C for 1 min, 30 cycles, and 70°C for 7 min. The thermo cycling conditions for GAPDH are: 94°C for 4 min, 94°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec for 20 cycles, and 70°C for 4 min.
Protein stability experiments
In order to identify the half-life of OLC1, the protein synthesis inhibitor cycloheximide (CHX; Sigma-Aldrich and Merck KGaA, Darmstadt, Germany, 100 µg/ml) was added to the cell culture, and cells were collected at a range of time intervals. In order to ensure the effect of the proteasome on OLC1 degradation, MG132, a proteasome inhibitor, was added to the cell culture. The cells were incubated with CHX alone or with CHX and 20 µM MG132 for a range of durations (4, 8, 12 and 16 h), or with CHX and a range of MG132 concentrations (5, 10 and 20 µM). For each experiment, dimethyl sulfoxide (DMSO) was used as a control.
Immunoprecipitation and western blot analyses
Cells were harvested by washing twice with PBS and scraping away the cells, which were lysed in a lysis buffer on ice for 40 min. The composition of the lysis buffer was as follows: PBS, 2 µg/ml aprotinin, 2 µg/ml leupeptin, 1% NonidetP-40 and 100 µg/ml phenylmethylsulfonyl fluoride. The lysates were harvested via centrifugation at 4°C at 16,000 × g for 20 min, and the supernatants were extracted to collect complete protein samples.
For immunoprecipitation, 500 µg total cellular protein lysate was incubated with ~2 µg of the indicated antibodies, including OLC1 rabbit anti-human polyclonal antibody (ready for use), which was prepared and purified using the purified recombinant glutathione-S-transferase-OLC1 by Wuhan Sanying Biotechnology, Wuhan, China (1) and actin mouse anti-human monoclonal antibody (ready for use; cat. no., sc-8432; Santa Cruz Biotechnologies, Santa Cruz, CA, USA) at 4°C for 6 h and then incubated with 20 µl A/G-agarose beads (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Next, immune complexes was harvested via centrifugation at 4°C at 1,000 × g for 5 min and washed 7–8 times with 1% Nonidet P-40. The immunocomplexed proteins were then subjected to a western blot analysis.
For the western blot analysis, 80–100 µg cellular protein was resolved via 10% SDS-PAGE and transferred to Immobilon-P polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Then the membranes were incubated in PBS containing 5% nonfat milk at room temperature for 30 min to block nonspecific binding. Next the membranes were incubated at 37°C for 2 h with the following antibodies: OLC1 (dilution, 1:100; Wuhan Sanying Biotechnology, Wuhan, China), APC2 (dilution, 1:500; cat. no., sc-517022), cyclin A (dilution, 1:500; cat. no., sc-271682), cyclinD1 (dilution, 1:500; cat. no., sc-246), cyclin E (dilution, 1:500; cat. no., sc-247), actin (dilution, 1:1,000; cat. no., sc-8432), GFP (dilution, 1:500; cat. no., sc-9996) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Cdc20 (dilution, 1:2,000; cat. no., NB100-59828; Novus Biologicals, LLC, Littleton, CO, USA,) ubiquitin (dilution, 1:1,000; cat. no., U5379; Sigma Aldrich; Merck KGaA) and Cdh1 (dilution, 1:1,000; cat. no., C7855; Sigma Aldrich; Merck KGaA) and cyclin B1 (dilution, 1:250; cat. no., 554178; BD Biosciences, Franklin Lakes, NJ, USA). The membranes were washed in PBS with 0.1% Tween 20 five times and then incubated at room temperature for 1 h with a goat anti-mouse (cat. no., sc-2005) or goat anti-rabbit (cat. no., sc-2004) horseradish peroxidase-conjugated secondary antibody (the two antibodies were diluted at 1:1,000 and were purchased from Santa Cruz Biotechnology, Inc.). Next the membranes were washed five times, and antibody reactivity was visualized using a FUJIFILM LAS-4000 machine. Images were edited using Multi-Gauge Fujifilm (version 3; Fujifilm Life Science, Tokyo, Japan) and Photoshop CS software (Adobe Systems, Inc., San Jose, CA, USA).
Cell protein ubiquitination assay
Cultured cells were treated with 20 µM MG132 or DMSO. Then cells were collected andlysed on ice for 30 min with thelysis buffer. Then the lysates were centrifuged at 16,000 × g for 20 min at 4°C. In each immunoprecipitation reaction, as described previously, 500 µg total cellular protein lysate was incubated with 10 µl of the indicated antibody. Then the immune complexes were pulled down and analyzed with western blotting. Using an anti-ubiquitin antibody, the polyubiquitinated OLC1 protein was probed.
Plasmid mutation and cell transfection
pEGFP-N1 and pEGFP-N1-OLC1 plasmids were supplied by Professor Shujun Cheng of the Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). pCMV-Myc-Cdh1 and pCMV-Myc-Cdc20 plasmids were provided by Professor James Hsieh of Washington University (Seattle, WA, USA) and pCMV-3 plasmids were obtained from the State Key Laboratory of Molecular Oncology (Beijing, China).
In order to identify the specific sequences that acted as potential degradation signals for OLC1, a generated D-boxsite-directed mutation construct, pEGFP-N1-mut-OLC1, was produced in conjunction with Shanghai Gene Chem Co., Ltd. (Shanghai, China). For cell transfection, H1299 cells were seeded onto 35-mm plates 1 day prior to transfection to allow cells to reach 95% confluence at the time of transfection. To prepare each plate, 10 µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and 4 µg plasmid DNA were added to 500 µl RPMI-1640 medium without antibiotics. The solutions were mixed lightly, left to stand for 20 min at room temperature, diluted with 3 ml RPMI-1640 medium without antibiotics, and added to the plates to stand for 6 h at 37°C. Next, 4 ml RPMI-1640 medium from each plate was exchanged for an equal volume of RPMI-1640 medium supplemented with 10% FBS, and plates were incubated for an additional 48 h. Cells were then harvested.
Small interfering RNA (siRNA) construction and cell transfection
siRNA for Cdh1 and Cdc20 were constructed by Jikai Biotechnology, Inc. The Cdh1 siRNA sequence was 5′-UGAGAAGUCUCCCAGUCAGdTdT-3′ and the Cdc20 siRNA sequence was 5′-AAACCTGGCGGTGACCGCTAT-3′.
For cell transfection, H1299 cells were seeded onto 35-mm plates 1 day prior to transfection to allow the cells to reach 70% confluence at the time of transfection. To prepare each plate, 4 µg siRNA and 10 µl Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) were added to 500 µl RPMI-1640 medium without antibiotics. The solutions were mixed gently, sitting for 20 min at room temperature, diluted with 3 ml RPMI-1640 medium without antibiotics and added to the plates to sit for 6 h at 37°C. Next, 4 ml RPMI-1640 medium from each plate was exchanged for an equal volume of medium with 10% FBS, and plates were incubated for 48 h. Cells were then harvested.
Cell proliferation assay and colony formation
Cells in the exponential growth phase were seeded at a density of 3,000 cells per well in 12-well plates, in triplicate. Cells were then counted every 24 h for 5 days to produce a growth curve. All experiments were repeated three times.
At 24 h after transfection, cells transfected with each specific plasmid were seeded in RPMI-1640 with 10% FBS and 400 µg/ml G418 at a density of 1,000 cells per well in six-well plates in triplicate. After 14 days, the cells were washed with PBS, fixed in cold methanol and stained with 0.5% crystal violet for 10 min at room temperature. Colonies with more than 50 cells were counted. All experiments were repeated three times.
Statistical analysis
SPSS version 11.5.0 (SPSS, Inc., Chicago, IL, USA) was used to perform all statistical analyses. A Student's t-test was used for comparison between two groups. The differences between multiple groups were analyzed using a one-way analysis of variance and Fisher's least significant difference test as a post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
OLC1 expression is cell cycle-dependent
To explore whether the expression of OLC1 is associated with cell cycle progression, two cell cycle synchronization approaches were adopted in order to detect the OLC1 protein expression in KYSE150/GFP cells. By means of serum starvation, cells were synchronized at the G0 phase. Cells were arrested at the mitotic phase with the microtubule inhibitor nocodazole. Cells were subsequently collected every 2 h, and the expression of OLC1 protein was analyzed with western blot assays. The expression of cyclin B1, D1, A and E were analyzed to assess cell cycle progression. As presented in Fig. 1A, following serum starvation, OLC1 protein was highly expressed during most of the G0/G1 phase, remained at relatively low levels at the S phase and demonstrated a slightly elevated level in the G2/M phase. Similar results were obtained in the experiments performed using no codazole to synchronize cells at the mitotic phase (Fig. 1B). In an additional experiment, the mRNA expression of OLC1 was examined, which demonstrated relatively little change throughout the whole cell cycle (Fig. 1C). In conclusion, these observations indicate that OLC1 protein expression is cell cycle-dependent and that it is primarily regulated via post-translational, and not transcriptional, modification.
Figure 1.
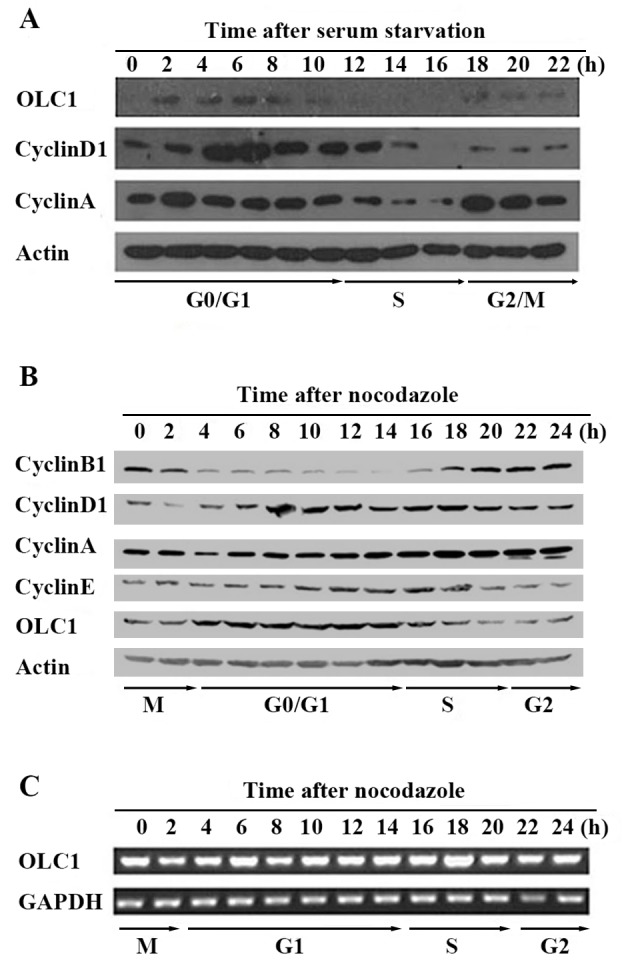
Expression of OLC1 throughout the cell cycle. (A) Using serum starvation, KYSE150/GFP cells were synchronized at the G0 phase. Following release, cells were collected every 2 for 24 h. The protein expression of OLC1, cyclin D1, cyclin A and actin was analyzed using western blot analysis. (B) Using 0.4 µg/ml nocodazole, KYSE150/GFP cells were synchronized at the mitotic phase. Following release, cells were collected every 2 for 24 h. The protein expression of OLC1, cyclin B1, cyclin D1, cyclin A, cyclin E and actin was analyzed using western blot analysis. (C) Using reverse transcription polymerase chain reaction, the mRNA expression of OLC1 and GAPDH were detected. OLC1, overexpressed in lung cancer 1.
OLC1 protein is short-lived and its stability is regulated by the ubiquitin proteasome pathway
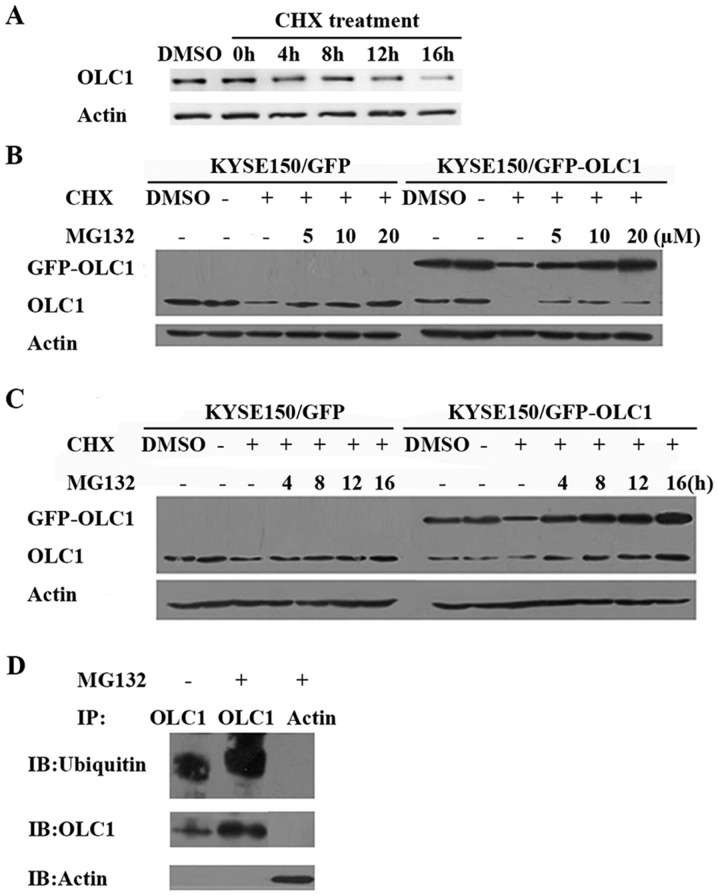
Considering that the OLC1 protein expression was determined to be cell cycle-dependent, similar to the cyclins, the half-life of OLC1 protein was not expected to be long. Following treatment with the protein synthesis inhibitor CHX for a range of times, the half-life of OLC1 was analyzed. OLC1 protein expression decreased to ~50% at 8 h of CHX treatment compared with the expression observed at 0 h and in the untreated control cells (Fig. 2A), suggesting that OLC1 protein has a rapid turnover rate. Given that 80–90% of intracellular proteins are degraded by the ubiquitin proteasome pathway, it was predicted that OLC1 protein stability may be regulated though this mechanism. Therefore, the selective proteasome inhibitor MG132 was used to treat KYSE150/GFP and KYSE150/GFP-OLC1 cells with or without CHX, which was followed by an analysis of endogenous and exogenous OLC1 protein expression. The addition of different concentrations of MG132 resulted in an increase in OLC1 expression compared with cells treated only with CHX. Similarly, with increasing MG132 treatment times, OLC1 protein expression levels were increased accordingly (Fig. 2B and C). This suggests that OLC1 protein degradation is regulated by the ubiquitin proteasome pathway. Subsequently, immune precipitation as says with an anti-OLC1 antibody was performed to further confirm this. Ubiquitin was detected in the OLC1 immuno complex, and as expected, the amount present was increased following the addition of MG132 (Fig. 2D).
Figure 2.
OLC1 protein expression with CHX and MG132 treatment. (A) Following treatment with 100 µg/ml CHX, KYSE150/GFP cells were collected for western blot analysis of OLC1 protein expression at the indicated times. KYSE150/GFP and KYSE150/GFP-OLC1 cells were co-treated with CHX (100 µg/ml) and MG132 at (B) different concentrations (5, 10 and 20 µM MG132) or (C) for different lengths of time (4, 8, 12 and 16 h) with 20 µM MG132, with the DMSO, negative control and MG132-negative groups collected for analysis after 16 h of treatment. Results are representative of three independent experiments. (D) With (+) or without (−) treatment with 20 µM MG132, KYSE150/GFP-OLC1 cells were incubated for 16 h, collected and lysed for IP with an anti-OLC1 antibody. Ubiquitins were detected in the immunocomplex, and the presence of OLC1 was verified. IP with an anti-actin antibody was used as a negative control. OLC1, overexpressed in lung cancer 1; CHX, cycloheximide; IP, immunoprecipitation.
OLC1 protein is degraded by APC/c
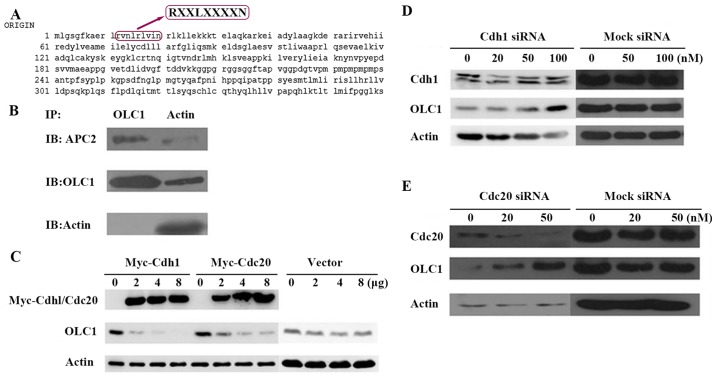
The OLC1 protein sequence was analyzed and a D-box motif was identified (amino acids 12–20; Fig. 3A). In the process of ubiquitination and degradation of proteins, D-box motifs are often the recognition sites for the E3 ligase of the APC/c (7,8). Thus, it was hypothesized that the E3 ligase of APC/c may facilitate OLC1 ubiquitination. In the APC/c, APC2 is the most important subunit, as it acts as the connector with the target protein. Therefore, the interaction between OLC1 with APC2 was examined. The results demonstrated that OLC1 directly interacts with APC/c through its APC2 component (Fig. 3B), which indicates that OLC1 protein ubiquitination and degradation are mediated by the APC/c pathway.
Figure 3.
OLC1 protein is degraded by the APC/c. (A) Analysis of the OLC1 protein sequence indicated that it contains a destruction box at the site of amino acids 12–20, which may be recognized by APC/c. (B) To investigate whether OLC1 may bind directly to the components of the APC/c, KYSE150/GFP-OLC1 cells were collected, lysed and subjected to IP. The presence of actin, OLC1 and APC2 in the immunocomplex were verified. (C) Different concentrations (2, 4 and 8 µg) of Myc-Cdh1/Cdc20 expression or mock vectors were transiently transfected into H1299 cells for 48 h. Then OLC1, Myc-Cdh1 and Myc-Cdc20 protein expression were evaluated. Actin was included as a loading control. Different concentrations (20, 50 and 100 nM) of (D) Cdh1 or (E) Cdc20 or mock siRNA were transiently transfected into H1299 cells for 48 h. Then OLC1, Cdh1 and Cdc20 protein expressions were evaluated. Actin was included as a loading control. OLC1, overexpressed in lung cancer 1; APC/c, anaphase-promoting cyclosome complex; IP, immunoprecipitation; Cdh1, cadherin 1; Cdc20, cell-division cycle protein 20; siRNA, small interfering RNA.
The activation of APC/c also requires the association with proteins containing tryptophan aspartate. Cdc20 (termed Fizzy in Drosophila) and Cdh1 (Fizzy-associated in Drosophila) have been identified as two of these activators, and have been demonstrated to activate APC/c E3 ligase and stimulate substrate degradation. These two proteins may directly interact with the APC/c and serve important functions as limiting, substrate-specific activators of APC-dependent proteolysis (11,12). To confirm whether Cdh1 or Cdc20 were required for OLC1 protein degradation, Cdh1 and Cdc20 expression was transiently up regulated individually in H1299 cells; it was revealed that the increase of each protein resulted in a reduction in OLC1 protein expression (Fig. 3C). On the contrary, the down regulation of the endogenous Cdh1 or Cdc20 via siRNAs resulted in the increased expression of OLC1 (Fig. 3D and E). From the above results, it was posited that Cdh1 and Cdc20 are involved in the process of OLC1 degradation.
Mutated D-box domains interfere with OLC1 protein degradation and accelerate cell growth and colony formation
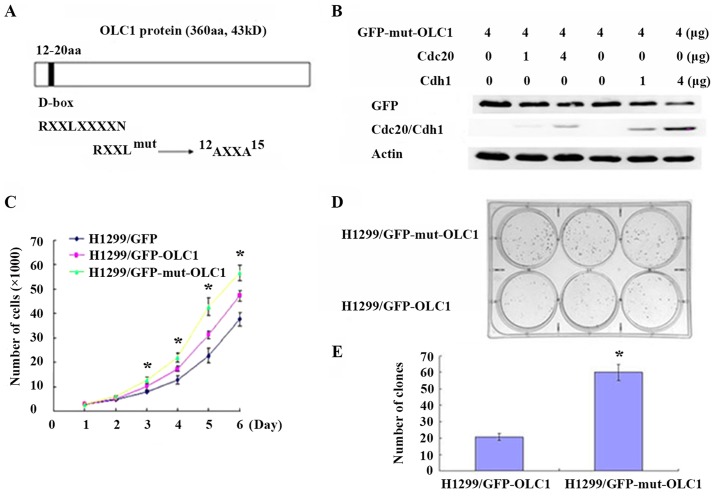
In the present study, the analysis of the OLC1 protein sequence revealed that it contained a D-box (amino acids 12–20). Generally, substrates containing a D-box or KEN-box domain are recognized by the APC/c E3 ligase, assisted by Cdc20 and Cdh1 (11,12). Therefore, it was hypothesized that the D-box domain mediated the ubiquitination-dependent destruction of OLC1 proteins. The conserved D-box motif was point-mutated, as presented in Fig. 4A, and it was assessed whether this mutation had an effect on OLC1 protein stability. H1299 cells were co-transfected with D-box-mutated GFP-OLC1 (GFP-mut-OLC1) and different concentrations of Cdh1 or Cdc20 expression vectors. As expected, the increased expression of Cdh1 and Cdc20 did not promote the degradation of the mutated OLC1, suggesting that the identified D-box motif was the critical sequence required for OLC1 ubiquitination and the subsequent degradation (Fig. 4B).
Figure 4.
Mutated D-box domains interfere with OLC1 protein degradation and accelerate cell growth and colony formation. (A) A schematic representation of the D-box degradation signal within OLC1. Numbers denote amino acid numbers where the D-box lies, and the conserved sequence motif is annotated. The mutation scheme for the OLC1 D-box is indicated. (B) H1299 cells were co-transfected with 4 µg OLC1 (D-box mutant) plasmids and different concentrations of human Myc-Cdh1/Cdc20 expression vectors for 48 h. OLC1, Cdh1 and Cdc20 protein expression were evaluated using western blot analysis. Actin was probed as a loading control. (C) H1299 cells were transfected with 4 µg pEGFP-N1, pEGFP-N1-OLC1 or pEGFP-N1-mut-OLC1. Mean values with standard deviations were calculated to produce a cell proliferation curve. The results were obtained in three independent experiments. *P<0.05 vs. wild-type OLC1. (D) A colony formation assay of H1299 cells was performed following transfections of a mutated or wild-typeOLC1 expression vector for 24 h and (E) the number of colonies were counted. The results were obtained from three independent experiments. *P<0.05 vs. control cells. OLC1, overexpressed in lung cancer 1; D-box, destruction box; Cdh1, cadherin 1; Cdc20, cell-division cycle protein 20.
Previous studies have demonstrated that OLC1 is an oncogenic protein, as the induced overexpression of OLC1 results in anchorage-independent growth in vitro and malignant transformation in vivo. Additionally, OLC1 has been identified as overexpressed in multiple types of malignant tumor, including in lung cancer and esophageal squamous carcinoma (1,2). Thus, additional experiments were performed to confirm if the cells with mutated OLC1 may exhibit more malignant characteristics. First, a cell growth assay was performed; the growth curve revealed that H1299 cells expressing OLC1 with a mutated D-box grew significantly faster compared with those expressing wild-type OLC1 (Fig. 4C). This indicated that the D-box mutated OLC1 exhibited a greater capacity to facilitate cell growth. Colony formation assays were also conducted; the cells expressing mutant OLC1 developed a significantly higher number of colonies compared with the control cells (Fig. 4D and E). These results indicated that the mutated D-box motif was not recognized by APC/c E3 ligase, affecting the subsequent degradation of the OLC1 protein. Overall, non-degradable OLC1 presents a greater oncogenic capacity compared with wild-type OLC1.
Discussion
The OLC1 gene is located in chromosome 16q22.2. When it was first identified in non-lethal yeast mutants in 1999, it was named the IST1 gene (14). In yeast, IST1 participates in the multi vesicular body (MVB) sorting pathway, in which certain membrane proteins are sorted into the lumen of the vacuole for their eventual degradation (15,16). The gene product of IST1/OLC1 in different organisms is highly conserved, including between yeast and humans. In humans, a previous study identified that OLC1 was essential for cytokinesis, another membrane scission event that is topologically similar to MVB formation, and that the depletion of OLC1 resulted in the accumulation of multinucleated cells (7). Cytokinesis is the last stage of the cell cycle, where a cell divides into two daughter cells, passing the same amount of genetic material to each daughter cell. Therefore, abnormal cytokinesis will result in the uneven distribution of the chromosomes in the cell, inducing cell genome instability and potentially leading to the development of a tumor (17,18).
A previous study regarding OLC1 studied it from another angle; the OLC1 gene was identified as a potential oncogene, that was highly expressed in lung cancer, in 2008 (1). It was identified that the over expression of OLC1 was associated with smoking history in patients with lung cancer, and that this overexpression induced tumor formation in athymic mice, whereas the knockdown of OLC1 increased apoptosis and decreased colony formation. It was also revealed that cigarette smoke may increase OLC1 protein expression at the cellular level (8). Previous studies have revealed that the OLC1 protein is highly expressed in numerous malignant tumor types, including esophageal squamous cancer, colorectal cancer, breast cancer and ovarian cancer. Furthermore, high expression levels of OLC1 are associated with a poor prognosis in a number of these cancer types (2,19–21).
However, until the present study, few studies focused on the molecular regulatory mechanism controlling OLC1 expression. Given the critical function of OLC1 from yeast to humans, studying how OLC1 is regulated may provide further insight into human tumor genesis. In the present study, it was demonstrated that OLC1 protein may be cell-cycle-dependent with a short half-life, and that it may be degraded through the APC/c-mediated proteasome pathway.
Two approaches for cell cycle synchronization were conducted in order to study the expression pattern of OLC1 during the entire cell cycle. Given that the expression of numerous other cyclins fluctuates in different phases of the cell cycle, cyclins A, B1, D1 and E were examined to determine the dynamic changes in OLC1 expression in the cell cycle. The OLC1 protein exhibited higher expression levels during the G0/G1 phase compared with the other phases, whereas the mRNA expression levels of OLC1 demonstrated little change across the entire cell cycle. In conclusion, the expression of OLC1 is regulated through post-transcriptional mechanisms during the cell cycle.
Mammalian cells contain two distinct major proteolytic pathways. One important non-lysosomal mechanism for the degradation of intracellular proteins is the ubiquitin-proteasome pathway (22,23). Ubiquitin and ubiquitin ligase post-translationally modify the abundance of target proteins, including oncogenes or tumor suppressor genes, and thereby alter their effect. For example, the Akt signaling pathway has many important biological functions, while its deregulation is associated to the development of numerous types of malignant tumors in humans. Although previous studies have primarily focused on Akt phosphorylation, other post-translational modifications to Akt, including ubiquitination, have been demonstrated to serve an important function in Akt activation. A previous study revealed that a cancer-associated Akt mutation within the Akt PH domain (E17K) was identified in a range of human cancer types, including colon and breast cancer. Akt E17K mutants displayed enhanced Akt ubiquitination, contributing to Akt hyperactivation and constitutive Akt membrane recruitment, suggesting a potential role for Akt ubiquitination in cancer (24). MDM2 is an E3 ubiquitin ligase with strong clinical relevance due to its ability to regulate the tumor suppressor p53. By recruiting an E2 ubiquitin-conjugating enzyme, MDM2 facilitates the export of p53 from the nucleus to the cytoplasm, and targets p53 for ubiquitin-dependent proteasome degradation (25).
Similar to the processes revealed for other oncogenes and tumor suppressor genes, ubiquitin-mediated proteolysis was determined as an important regulator of OLC1 protein expression. In the present study, it was demonstrated that the expression of OLC1 was elevated following treatment with MG132, a proteasome inhibitor. OLC1 protein degradation decreased, and increased ubiquitin was ligated to OLC1 proteins following MG132 treatment. All these results indicate that OLC1 degradation must be regulated by the ubiquitin-proteasome pathway.
A previous study has demonstrated that the interactions between OLC1 and other proteins are involved in a range of biological processes, including MVB biogenesis, cytokinesis and enveloped virus budding (16). In mammalian cells, the complete function of OLC1 is required for efficient abscission during cytokinesis (5). Additionally, in the process of cytokinesis, the E3 ligase APC/c mediates the ubiquitin-dependent proteolysis of cell-cycle-regulating proteins (26,27). Thus, we hypothesized that APC/c may function as an E3 ligase for OLC1 ubiquitination. In a further study, it was revealed that there was a conserved D-box motif of RVNLRLVINR within the OLC1 protein sequence, which is a conserved and well-recognized site for E3 ligase APC/c in a number of ubiquitinated substrates (28). Additionally, co activators containing tryptophan aspartate are also required to assist the activation of APC/c. Two of these proteins have been identified as Cdc20 and Cdh1 (13,29). Usually, Cdc20 targets D-box-containing substrates, whereas Cdh1 may interact with either the D-box or KEN box of proteins (11,30). Consistent with this, in the present study, immune precipitation as says revealed that OLC1 may directly bind to APC/c through the subunit APC2, and either Cdc20 or Cdh1 up regulation induced decreased OLC1 expression. Likewise, cells with Cdc20 and Cdh1 down regulated via siRNA exhibited OLC1 protein stabilization. Furthermore, mutations to the OLC1 D-box significantly reduced OLC1 degradation. Collectively, it was confirmed that the APC/c mediated ubiquitin-proteasome pathway regulated the degradation of OLC1.
Using constructed wild-type and D-box-mutated OLC1 plasmids, functional experiments were performed. Growth curve and colony formation assays demonstrated that the overexpression of the non-degradable OLC1 protein not only facilitated cell growth, but also enhanced the clone-forming capability of the cells. These findings reveal that the destruction of the degradation domain results in the abnormal accumulation of the OLC1 mutant, which could not be degraded through the APC/c-mediated ubiquitin-proteasome pathway. These malignant cell phenotypes support the idea that OLC1 exhibits on cogenic properties.
In conclusion, these findings have the potential to make important contributions in clarifying the mechanism of the APC/c-mediated destruction of the candidate oncogene OLC1.
Acknowledgements
The authors would like to thank Dr Shimada of Kyoto University (Kyoto, Japan) for providing the KYSE150 cells and Professor Shujun Cheng of the Chinese Academy of Medical Sciences and Peking Union Medical College, Cancer Institute and Cancer Hospital (Beijing, China), for providing the pEGFP-N1-OLC1 and pEGFP-N1 plasmids. pCMV-Myc-Cdh1 and pCMV-Myc-Cdc20 plasmids were generously provided by Professor James Hsieh of Washington University (Seattle, WA, USA).
Funding
This research was supported by Grants from the National High Technology Research and Development Program of China (grant no., 2006AA02A403).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author contributions
QMZ and TT obtained funding and participated in the study coordination. ZXJ and WC were responsible for study design. ZXJ, WC and NY performed the experimental procedures. LJD and MF performed data analysis, and ZXJ, WC and TT were responsible for editing and review of the manuscript. TT was also responsible for study design.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yuan J, Ma J, Zheng H, Shi T, Sun W, Zhang Q, Lin D, Zhang K, He J, Mao Y, et al. Overexpression of OLC1, cigarette smoke, and human lung tumorigenesis. J Natl Cancer Inst. 2008;100:1592–1605. doi: 10.1093/jnci/djn379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Suo J, Shao S, Xue L, Chen W, Dong L, Shi J, Fu M, Lu N, Zhan Q, Tong T. Overexpression of OLC1 promotes tumorigenesis of human esophageal squamous cell carcinoma. PLoS One. 2014;9:e90958. doi: 10.1371/journal.pone.0090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimaano C, Jones CB, Hanono A, Curtiss M, Babst M. Ist1 regulates Vps4 localization and assembly. Mol Biol Cell. 2008;19:465–474. doi: 10.1091/mbc.e07-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rue SM, Mattei S, Saksena S, Emr SD. Novel Ist1-Did2 complex functions at a late step in multivesicular body sorting. Mol Biol Cell. 2008;19:475–484. doi: 10.1091/mbc.e07-07-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agromayor M, Carlton JG, Phelan JP, Matthews DR, Carlin LM, Ameer-Beg S, Bowers K, Martin-Serrano J. Essential role of hIST1 in cytokinesis. Mol Biol Cell. 2009;20:1374–1387. doi: 10.1091/mbc.e08-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajorek M, Schubert HL, McCullough J, Langelier C, Eckert DM, Stubblefield WM, Uter NT, Myszka DG, Hill CP, Sundquist WI. Structural basis for ESCRT-III protein autoinhibition. Nat Struct Mol Biol. 2009;16:754–762. doi: 10.1038/nsmb.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajorek M, Morita E, Skalicky JJ, Morham SG, Babst M, Sundquist WI. Biochemical analyses of human IST1 and its function in cytokinesis. Mol Biol Cell. 2009;20:1360–1373. doi: 10.1091/mbc.e08-05-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Xiao T, Cheng S, Tong T, Gao Y. Cigarette smoke suppresses the ubiquitin-dependent degradation of OLC1. Biochem Biophys Res Commun. 2011;407:753–757. doi: 10.1016/j.bbrc.2011.03.095. [DOI] [PubMed] [Google Scholar]
- 9.Castro A, Bernis C, Vigneron S, Labbe JC, Lorca T. The anaphase-promoting complex: A key factor in the regulation of cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- 10.Manchado E, Eguren M, Malumbres M. The anaphase-promoting complex/cyclosome (APC/C): Cell-cycle-dependent and -independent functions. Biochem Soc Trans. 2010;38:65–71. doi: 10.1042/BST0380065. [DOI] [PubMed] [Google Scholar]
- 11.da Fonseca PC, Kong EH, Zhang Z, Schreiber A, Williams MA, Morris EP, Barford D. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature. 2011;470:274–278. doi: 10.1038/nature09625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins JA, Cross FR. Regulated degradation of the APC coactivator Cdc20. Cell Div. 2010;5:23. doi: 10.1186/1747-1028-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Chang L, Alfieri C, Zhang Z, Yang J, Maslen S, Skehel M, Barford D. Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature. 2016;533:260–264. doi: 10.1038/nature17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Entian KD, Schuster T, Hegemann JH, Becher D, Feldmann H, Güldener U, Götz R, Hansen M, Hollenberg CP, Jansen G, et al. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol Gen Genet. 1999;262:683–702. doi: 10.1007/PL00013817. [DOI] [PubMed] [Google Scholar]
- 15.Allison R, Lumb JH, Fassier C, Connell JW, Ten Martin D, Seaman MN, Hazan J, Reid E. An ESCRT-spastin interaction promotes fission of recycling tubules from the endosome. J Cell Biol. 2013;202:527–543. doi: 10.1083/jcb.201211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo EZ, Xu Z. Distinct mechanisms of recognizing endosomal sorting complex required for transport III (ESCRT-III) protein IST1 by different microtubule interacting and trafficking (MIT) domains. J Biol Chem. 2015;290:8396–8408. doi: 10.1074/jbc.M114.607903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Avino PP, Giansanti MG, Petronczki M. Cytokinesis in animal cells. Cold Spring Harb Perspect Biol. 2015;7:a015834. doi: 10.1101/cshperspect.a015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tormos AM, Taléns-Visconti R, Sastre J. Regulation of cytokinesis and its clinical significance. Crit Rev Clin Lab Sci. 2015;52:159–167. doi: 10.3109/10408363.2015.1012191. [DOI] [PubMed] [Google Scholar]
- 19.Jia C, Li X, Sun H, Sui L. Over-expression of the overexpressed in lung cancer 1 is associated with poor prognosis in epithelial ovarian cancer. J Surg Oncol. 2013;107:847–852. doi: 10.1002/jso.23336. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Song H, Yao L, Liu Y, Zhang Y, Zhao H, Ji H, Wang Y. Over-expression of the overexpressed in lung cancer-1 is associated with poor prognosis in colorectal cancer. Anticancer Res. 2014;34:367–372. [PubMed] [Google Scholar]
- 21.Ou-Yang QH, Duan ZX, Jin Z, Lei JX. OLC1 is overexpressed in breast cancer and its expression correlates with poor patient survival. Tumour Biol. 2014;35:8823–8827. doi: 10.1007/s13277-014-2130-7. [DOI] [PubMed] [Google Scholar]
- 22.Chondrogianni N, Petropoulos I, Grimm S, Georgila K, Catalgol B, Friguet B, Grune T, Gonos ES. Protein damage, repair and proteolysis. Mol Aspects Med. 2014;35:1–71. doi: 10.1016/j.mam.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Jain CK, Arora S, Khanna A, Gupta M, Wadhwa G, Sharma SK. The ubiquitin-proteasome pathway an emerging anticancer strategy for therapeutics: A patent analysis. Recent Pat Anticancer Drug Discov. 2015;10:201–213. doi: 10.2174/1574892810666150416111213. [DOI] [PubMed] [Google Scholar]
- 24.Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9:487–497. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao CC. Mechanisms of p53 degradation. Clin Chim Acta. 2015;438:139–147. doi: 10.1016/j.cca.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Lindon C. Control of mitotic exit and cytokinesis by the APC/C. Biochem Soc Trans. 2008;36:405–410. doi: 10.1042/BST0360405. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Barford D. Insights into the anaphase-promoting complex: A molecular machine that regulates mitosis. Curr Opin Struct Biol. 2014;29:1–9. doi: 10.1016/j.sbi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Barford D. Structure, function and mechanism of the anaphase promoting complex (APC/C) Q Rev Biophys. 2011;44:153–190. doi: 10.1017/S0033583510000259. [DOI] [PubMed] [Google Scholar]
- 29.Van Voorhis VA, Morgan DO. Activation of the APC/C ubiquitin ligase by enhanced E2 efficiency. Curr Biol. 2014;24:1556–1562. doi: 10.1016/j.cub.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho HJ, Lee EH, Han SH, Jeong JH, Kwon J, Kim H. Degradation of human RAP80 is cell cycle regulated by Cdc20 and Cdh1 ubiquitin ligases. Mol Cancer Res. 2012;10:615–625. doi: 10.1158/1541-7786.MCR-11-0481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.