Abstract
Despite several attempts at setting up a standardized disease severity score for Fabry disease in the past, none have been established in routine clinical practice due to the multisystem nature and complexity of this inherited enzyme deficiency disorder. In this issue, Mignani et al. report a large multicentre application of the FASTEX, an online tool to assess disease progress over time that offers simple data inputting and graphic illustration of disease progression or stabilization. Mignani et al. succeeded in validating the tool in a large cohort of Fabry patients, including females and non-classical phenotypes, building on the first FASTEX introduction in 2016. We report on our own practical experience with the tool and comment on some limiting factors in its use as well as possible future prospects.
Keywords: activity score, disease progression, enzyme deficiency, Fabry disease, lysoGb3
Since the introduction of enzyme replacement therapy as a treatment for Fabry disease in 2001, physicians have struggled to answer one of the most frequent questions patients ask: is my therapy working? Indeed, monitoring therapy effects has proved difficult due to a multitude of issues.
First, Fabry disease is a multisystem disorder that can present with a very wide range of symptoms in various stages of the patients life, from mild phenotypes of isolated cardiac disease in the non-classical phenotype to the severely affected classical male phenotype with early organ failure and associated morbidity [1]. Thus extensive multidisciplinary investigations are needed at each patient visit [2]. Review and comparison of multiple reports from several medical specialties can be confusing for both the clinician and, more importantly, for the patient.
Second, until recently there were no reliable biomarkers to correlate disease severity and therapy response. Repeated biopsies to assess globotriaosylceramide (Gb3) accumulation in solid organs such as the heart or kidney are challenging [3]. A new, cheap and elegant approach to this problem is immunostaining for Gb3 deposits in peripheral blood mononuclear cells (PBMCs), which also seems to reflect therapy effectiveness [4]. The marker lysoGb3 is now a more widely accepted biomarker of disease activity in both classical and non-classical phenotypes [5] and is used for therapy monitoring. However, the long-term clinical effects of lysoGb3 reduction are still unknown.
Therefore several indices of disease activity have been developed over the years in an attempt to quantify disease activity and assess response to therapy. The first one was the Mainz Severity Score Index (MSSI) in 2004 [6], which attempted to quantify the phenotype as well as response to therapy. The index is cumbersome to use, with 4 domains and 24 items to be assessed, and it may be challenging to gather all relevant data. For example, one would have to assess for the presence cornea verticillata by slit lamp examination at each visit or perform 24-h urine collections, which patients often find difficult. What is more, in the initial study only the response to agalsidase alfa in 39 patients was assessed, with no follow-up validation in larger cohorts or other treatments.
Another attempt at disease score development was the Disease Severity Scoring System (DS3) in 2010, with 5 domains and 12 items [7], but this has not been validated in a patient population. To our knowledge, neither scoring system has proved itself in clinical practice.
In this issue, Mignani et al. [8] report a large multicentre application of the FASTEX, an online tool to assess disease progress over time that offers simple data inputting and graphic illustration of disease progression or stabilization. Mignani et al. [9] succeeded in validating the tool in a large cohort of Fabry patients, including females and non-classical phenotypes. The tool was developed in 2014 and reported in 2016 when applied to a small population of male classical phenotype patients in a 1-year follow-up. A consensus of experts have developed a raw and a weighted score, looking at the three main domains of system involvement—renal, cardiac and nervous. They then created FASTEX, a mathematical model to assess disease stability over time. The algorithm was applied and correlated with the DS3 and MSSI with very good results, even though it only involves three domains with easily elicited items. The tool is available online at www.fastex.online, making it particularly user friendly with visual graphics of disease stability over time.
The current study validates the FASTEX tool in a large multicentre study involving 132 male and female patients with both classical and non-classical phenotypes. Clinical assessment was standardized using recent guidance on the assessment and management of Fabry patients by Ortiz et al. [10]. The assessors were asked to classify their patients as ‘stable’ or ‘unstable’ blinded to FASTEX, which was calculated independently by an informatics company, achieving a good statistically significant correlation between the tool and specialist opinion. In addition, a 20% change cut-off point was validated to define disease stability.
In the absence of other standardized assessment tools, the simplicity of FASTEX and graphic animation of disease progression are certainly appealing to both patients and clinicians. Currently we rely on clinician expertise during yearly assessments, which can be a daunting prospect in such a rare disease where even hospitals with very large catchment areas might only see a few patients a year. Utilizing such a tool might support the physician in his/her decision making. It would be very interesting to see the relationship between the FASTEX tool and emerging biomarkers, in particular lysoGb3, as part of a comprehensive, well-rounded patient assessment.
Another advantage of this simple scoring system is direct comparison of study populations, thus facilitating research in the field. To date, assessments of therapy response have been non-standardized, with long observation periods required and different domains of organ response assessed. In the absence of prospective randomized controlled trials, assessing and comparing yearly FASTEX change might facilitate therapy comparison studies and provide valuable insights.
There are of course several limitations to this tool. The second study did not deliver a validation compared with existing DS3 and MSSI scores. Therefore it relied purely on the subjective clinical judgement of the physicians assessing their patients. The data were also collected retrospectively, therefore judging the prognostic value is difficult.
There are also limitations of using the tool in clinical practice, which we will illustrate with an example of two real-life patients, two brothers with a classical mutation, both on enzyme replacement therapy since 2001.
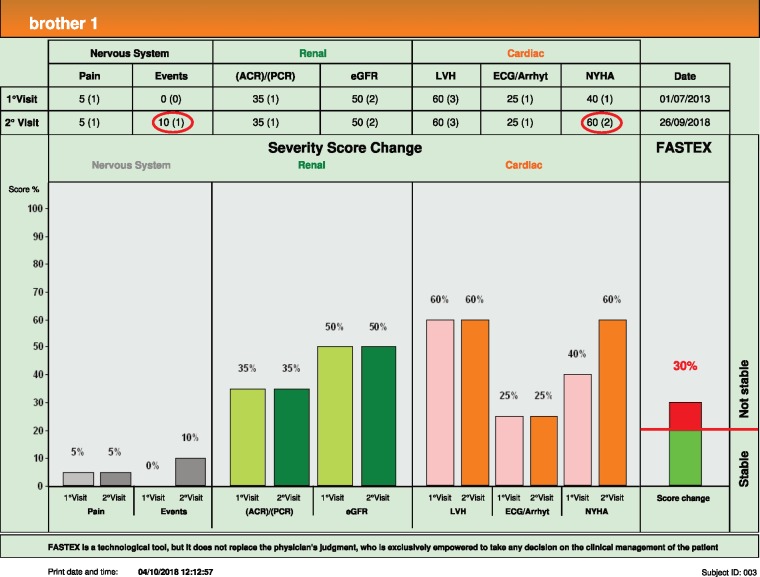
Brother 1 (age 48 years) (see Figure 1) presented to our centre after a 5-year gap, whereas Brother 2 (age 49 years) attends every 2 years. FASTEX was validated for a period of 6–12 months follow-up and the time interval implications have not been elaborated upon.
FIGURE 1.
FASTEX brother 1.
Looking at Brother 1, in 5 years he developed new changes in his brain magnetic resonance imaging (MRI) and subjectively feels more breathless on exertion. Therefore his FASTEX score change was 30% and his disease was deemed unstable under long-term enzyme replacement therapy.
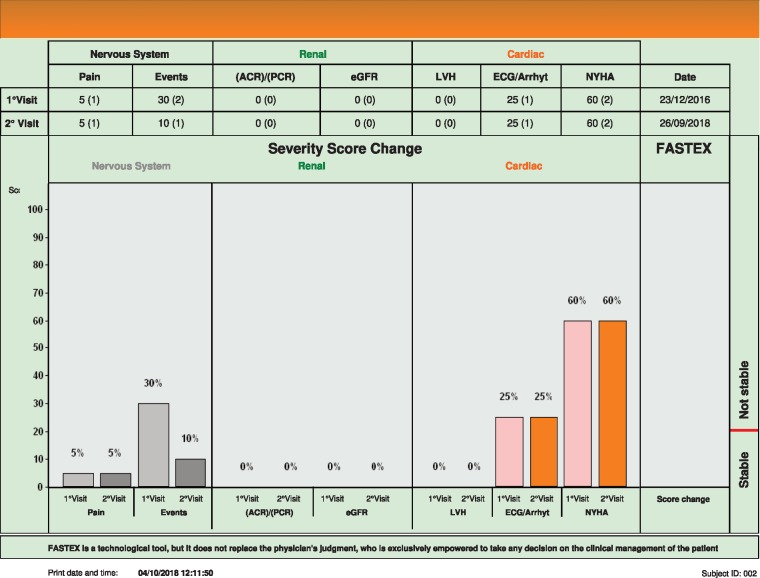
Brother 2 (see Figure 2), who 1 year older than Brother 1, is significantly less affected by the disease, with a much lower score. In the past he has suffered a transient ischaemic attack and on this occasion he had new T2 hyperintense lesions on his brain MRI. According to the score his disease is stable, even though arguably new lesions on his MRI might be assessed as unstable by some clinicians.
FIGURE 2.
FASTEX brother 2.
From experience using the FASTEX in our centre, some more practical issues have materialized. For example, the score is not easily applicable to kidney transplant patients. Also, the domains of pain and New York Heart Association score are very subjective and patients may have different scores under different circumstances; for instance, more pain during a hot summer, which may not necessarily indicate disease progression.
We would also welcome an automatic calculation of weighted scores, which may help the quantification of disease severity even on the first visit, without having to wait for interval change.
To conclude, FASTEX is a handy visual tool to use in patient consultations and has the potential to be utilized in larger comparative studies, despite the limitations outlined above. Prospective cohort studies are desirable and, in particular, correlation with lysoGb3 or Gb3 load in PBMCs and disease morbidity and mortality would aid in better understanding disease progression. The next key question—how to proceed in unstable disease—is a much trickier one to answer.
CONFLICT OF INTEREST STATEMENT
Dr Cairns and Dr Wanner report grants from Genzyme/Sanofi and Shire, personal fees and non-financial support from Genzyme/Sanofi, Actelion, Protalix and Shire, other from Fabry Registry European Board of Advisors, non-financial support from Genzyme/Sanofi, outside the submitted work.
REFERENCES
- 1. Arends M, Wanner C, Hughes D. et al. Characterization of classical and nonclassical Fabry Disease: a multicenter study. J Am Soc Nephrol 2017; 28: 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wanner C, Arad M, Baron R. et al. European expert consensus statement on therapeutic goals in Fabry disease. Mol Genet Metab 2018; 124: 189–203 [DOI] [PubMed] [Google Scholar]
- 3. Schiffmann R, Hughes DA, Linthorst GE. et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017; 91: 284–293 [DOI] [PubMed] [Google Scholar]
- 4. Uceyler N, Bottger J, Henkel L. et al. Detecton of blood Gb3 deposits as a new tool for diagnosis and therapy monitoring in patients with classic Fabry disease. J Intern Med 2018; 284: 427–438 [DOI] [PubMed] [Google Scholar]
- 5. Niemann M, Rolfs A, Störk S. et al. Gene mutations versus clinically relevant phenotypes: lyso-Gb3 defines Fabry disease. Circ Cardiovasc Genet 2014; 7: 8–16 [DOI] [PubMed] [Google Scholar]
- 6. Whybra C, Kampmann C, Krummenauer F. et al. The Mainz Severity Score Index: a new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 2004; 65: 299–307 [DOI] [PubMed] [Google Scholar]
- 7. Giannini EH, Mehta AB, Hilz MJ. et al. A validated disease severity scoring system for Fabry disease. Mol Genet Metab 2010; 99: 283–290 [DOI] [PubMed] [Google Scholar]
- 8. Mignani R, Pieroni M, Pisani A. et al. New insights from the application of FAbry STabilization indEX in a large population of Fabry cases. Clin Kidney J 2019; 12: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mignani R, Pieruzzi F, Berri F. et al. FAbry STabilization indEX (FASTEX): an innovative tool for the assessment of clinical stabilization in Fabry disease. Clin Kidney J 2016; 9: 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ortiz A, Germain DP, Desnick RJ. et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab 2018; 123: 416–427 [DOI] [PubMed] [Google Scholar]