Abstract
Background
The diffusion gradient between ionized calcium (iCa) in the inlet dialysate and blood is considered to be the main driving force of calcium mass balance (CMB). The intradialytic change of parathyroid hormone (PTH) level corresponds to the change in plasma iCa. In contrast to the widely discussed calcium concentration of dialysis solution, the dialysate pH and bicarbonate concentration (DHCO3), important factors affecting the level of iCa, have not been studied with respect to the intradialytic change of plasma PTH level (ΔPTH) and CMB.
Methods
We measured ΔPTH and CMB (calcium flux from the dialysate to the patient) in 10 stable patients on haemodiafiltration. All patients underwent two treatments differing in DHCO3 (26 versus 32 mmol/L). The dialysate calcium concentration was 1.25 mmol/L for all treatments.
Results
We found significant difference in ΔPTH, which decreased with 26_DHCO3 and slightly increased with 32_DHCO3 (−110.5 versus +19.7 pg/mL, P < 0.01). CMB was negative for both DHCO3, but with higher DHCO3 there was a trend to minor intradialytic loss of calcium (−108 versus −309 mg).
Conclusions
DHCO3 increase at first glance leads to contrasting phenomena: the intradialytic rise of PTH and calcium gain. Both processes are caused by a pH-dependent decrease of plasma iCa, resulting in parathyroid stimulation and intradialytic increase of iCa diffusion gradient. We found no significant correlation between CMB and intradialytic change of plasma total Ca. With respect to plasma PTH level and CMB, the bicarbonate concentration should always be taken into account when selecting the optimal dialysis solution.
Keywords: calcium mass balance, dialysate bicarbonate, dialysate pH, haemodiafiltration, parathyroid hormone
INTRODUCTION
Abnormalities in plasma calcium and parathyroid hormone (PTH) concentrations are common in patients with end-stage renal disease and are associated with increased vascular calcification [1], bone disease [2], morbidity and mortality [3, 4]. Plasma PTH concentration is dependent upon the release of PTH stored in secretory granules within the parathyroid gland and by the synthesis of new PTH. Plasma ionized calcium (iCa) plays a major role in regulating PTH release and synthesis [5]. Altered mineral metabolism and PTH levels in dialysis-dependent patients can be modified by diet, pharmacologically and by selecting the appropriate dialysis solution. The optimal dialysate calcium concentration to maintain adequate calcium mass balance (CMB), normal mineralization and plasma PTH level in haemodialysis patients is still a matter of debate. Surprisingly, the optimal dialysate bicarbonate concentration in this context is not taken into account.
It is well known that solute iCa concentration is modified by pH. The acidaemia increases and alkalaemia decreases the level of plasma iCa. With respect to basic chemical principles, an intradialytic change in blood pH must affect the level of plasma iCa, and thereby the diffusion gradient of iCa between the blood and dialysate. These gradient changes may affect the CMB and the plasma iCa changes may also lead to shifts in PTH levels.
The aim of this study was to evaluate the effect of various dialysate bicarbonate concentrations on intradialytic changes of PTH level and CMB in patients on chronic haemodiafiltration (HDF).
MATERIALS AND METHODS
This study was approved by the B. Braun Ethics Committee and 10 patients gave informed consent to participate.
Study subjects
We enrolled 10 stable patients with end-stage renal disease on chronic post-dilution HDF dialysed three times a week. The characteristics of the patients are shown in Table 1. All of the patients were using calcium carbonate (1.5–4 g/day), one patient used calcitriol (0.25 µg three times a week after HDF) and seven patients received sodium bicarbonate (500–1500 mg/day). None of them used calcimimetics. The mean residual urine volume was 775 ± 301 mL/day.
Table 1.
Baseline characteristics of the patients
| Age (years) | 74 ± 5 |
| Gender (male/female) | 3/7 |
| Weight (kg) | 66 ± 14 |
| Height (cm) | 160 ± 7 |
| BMI (kg/m2) | 25.7 ± 0.2 |
| Aetiology of CKD | |
| Diabetic nephropathy | 4 |
| Vascular nephropathy | 2 |
| Combination of diabetic and vascular nephropathy | 3 |
| Chronic tubulointersticial nephritis | 1 |
Data are reported as means ± SD or as a number of all 10 patients.
BMI, body mass index; CKD, chronic kidney disease.
Design of the study
All of the patients underwent two HDF treatments in a random sequence (mid-week session) at least 2 weeks apart, using dialysate calcium (DCa) of 1.25 mmol/L. The difference in treatments was the dialysate concentration of bicarbonate (DHCO3) of 26 versus 32 mmol/L.
Blood was sampled for total calcium (tCa) and iCa, pH, pCO2, total protein (TP) and PTH from inserted fistula needle immediately before dialysis prior to heparin administration and using the standardized ‘slow-flow’ method after the end of dialysis.
Blood pH, pCO2 and plasma iCa were measured by an ion-selective electrode (Roche, Cobas B 221). Bicarbonate was calculated according to the Henderson–Hasselbalch equation. tCa was measured by o-cresolphthalein complexone (Roche, Integra 400; reference range, 2.17–2.51 mmol/L), plasma PTH by chemiluminescence immunoassay (Advia Centaur CP; reference range, 11.1–79.5 pg/mL) and plasma TP and albumin by routine automated methods.
The CMB (gradient from dialysate to patient) was determined from tCa levels in the dialyser inlet and from the continuous partial spent dialysate collection samples (rate 150 mL/h):
CMB = (tCa concentration in the inlet dialysate × QD × t) − [tCa concentration in spent dialysate × (QD × t + VUF)], where QD = dialysate flow (mL/min), t = session duration (min), VUF = ultrafiltration volume (L).
Positive CMB corresponds to calcium gain during dialysis. The inlet dialysate tCa concentration was measured during the first hour of all treatments; for calculation of CMB and diffusion gradient of tCa [Diff(tCa)], the means of 26_DHCO3 and 32_DHCO3 groups were used to eliminate the fluctuation in production of inlet dialysate concentration. All treatments were performed on the same dialysis machine.
The diffusion gradients were calculated as the differences between inflow dialysate and blood before the filter for pH [Diff(pH)], and between inflow dialysate and plasma water concentrations before the filter for ionized calcium [Diff(iCa)]: Diff(iCa) = 1.12 × inlet dialyser iCa concentration − plasma water iCa concentration, where 1.12 is the Gibbs–Donnan factor; and for HCO3 [Diff(HCO3)]: Diff(HCO3) = 0.96 × inlet dialyser HCO3 concentration − plasma water HCO3 concentration, where 0.96 is the Gibbs–Donnan factor. Plasma water concentrations were calculated as Cpw = Cp × [(100 – TP)/100)], where Cpw = plasma water solute concentration, Cp = plasma solute concentration and TP = total protein in g/dL.
We used the standard equation for tCa corrected for albumin [tCa(corr)]: tCa(corr) = [0.8 × (normal albumin − serum albumin)] + serum tCa, where normal albumin is 4.13 g/dL, serum albumin in g/dL and serum calcium in mg/dL. Furthermore, we calculated the differences between postdialysis and predialysis values [ΔtCa, ΔiCa, ΔtCa(corr), ΔTP, Δalbumin, ΔHCO3, ΔPTH, ΔpH, ΔDiff(iCa), ΔDiff(HCO3) and ΔDiff(pH)].
Other parameters were set as follows: session duration: 4 h, the dialysis machine: Dialog Plus B. Braun, the filter: high-permeability polysulfone membrane (Xevonta) 1.5–1.8 m2 according to body weight, the blood flow: 350 mL/min, the dialysate flow: 700 mL/min, the reinfusion flow 80 mL/min, postdilution mode, and the total exchange volume: 19.8 L. The composition of the dialysate was sodium, 140 mmol/L; potassium, 3.0 mmol/L; tCa, 1.25 mmol/L; magnesium, 0.5 mmol/L; chloride, 109.5 mmol/L; acetate, 3 mmol/L and glucose, 100 mg/dL. The mean ultrafiltration volume was 2360 ± 997 mL and 2330 ± 884 mL for 26_DHCO3 and 32_DHCO3, respectively. All patients had an arteriovenous fistula with no signs of recirculation measured by Crit-line®.
Statistical analysis
Numerical values are expressed as mean ± SD. Normality of the distribution of data was assessed by visual inspection of histogram and normal probability plot. Parametric continuous data were compared using Student t-tests and non-parametric using Mann–Whitney tests. Pearson’s or Spearman’s bivariate correlation analysis was used to examine the relationship between parametric and non-parametric numerical variables, respectively. Moreover, multiple regression models were considered to evaluate simultaneous effect of studied variables. P < 5% were considered as statistically significant. All statistical analyses were performed using Statistica 12.0 (StatSoft, Tulsa, OK, USA).
RESULTS
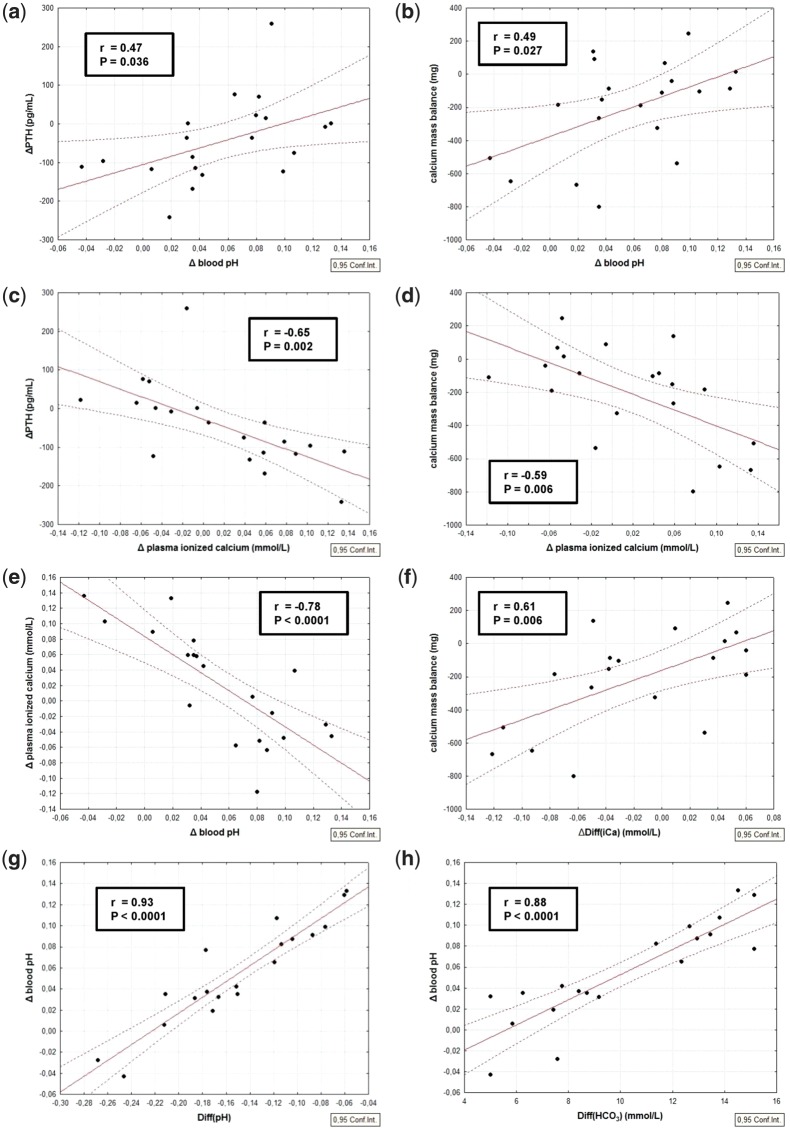
The main findings are shown in Tables 2 and 3. Predialysis plasma PTH levels were almost the same in both treatment groups. We found a significant difference in ΔPTH, which decreased when using 26_DHCO3 and slightly increased with 32_DHCO3 (−110.5 versus +19.7 pg/mL, P < 0.01). We found a significant intradialytic iCa increase with 26_DHCO3 (P < 0.001), whereas iCa significantly decreased in 32_DHCO3 (P < 0.01). With higher DHCO3, the intradialytic increase of blood pH together with a subsequent decrease of plasma iCa resulted in PTH increase (Figure 1a, c and d), which is expressed by correlations between ΔpH and ΔPTH (Figure 2a) as well as between ΔiCa and ΔPTH (Figure 2a and c). This fact is supported by the correlations between ΔPTH and predialysis diffusion gradient of pH (r = 0.512, P = 0.02) or bicarbonate (r = 0.521, P = 0.02). There was no significant correlation between ΔPTH and CMB.
Table 2.
The effect of different concentrations of dialysate bicarbonate on intradialytic change of plasma calcium, PTH, bicarbonate, albumin, TP, blood pH and CMB
| n = 10 (HDF) | 26_DHCO3 | 32_DHCO3 | Pa |
|---|---|---|---|
| Plasma PTH pre (pmol/L) | 291 ± 111 | 284.5 ± 125 | 0.9 |
| Plasma PTH post (pmol/L) | 180 ± 79*** | 304 ± 126 | 0.02 |
| CMB (mg) | −309 ± 329 | −108 ± 215 | 0.12 |
| Plasma tCa pre (mmol/L) | 2.18 ± 0.06 | 2.23 ± 0.10 | 0.17 |
| Plasma tCa post (mmol/L) | 2.27 ± 0.09* | 2.24 ± 0.13 | 0.55 |
| Plasma iCa pre (mmol/L) | 1.178 ± 0.062 | 1.189 ± 0.054 | 0.66 |
| Plasma iCa post (mmol/L) | 1.253 ± 0.05*** | 1.150 ± 0.026* | <0.001 |
| Plasma tCa(corr) pre (mmol/L) | 2.19 ± 0.09 | 2.23 ± 0.11 | 0.31 |
| Plasma tCa(corr) post (mmol/L) | 2.22 ± 0.09 | 2.19 ± 0.09 | 0.60 |
| Plasma TP pre (g/dL) | 6.71 ± 0.60 | 6.68 ± 0.53 | 0.91 |
| Plasma TP post (g/dL) | 7.28 ± 0.76*** | 7.10 ± 0.71** | 0.59 |
| Plasma albumin pre (g/dL) | 4.08 ± 0.21 | 4.11 ± 0.14 | 0.71 |
| Plasma albumin post (g/dL) | 4.38 ± 0.27** | 4.34 ± 0.32** | 0.77 |
| Blood pH pre | 7.364 ± 0.040 | 7.355 ± 0.035 | 0.61 |
| Blood pH post | 7.380 ± 0.021 | 7.450 ± 0.025*** | <0.001 |
| Blood HCO3 pre (mmol/L) | 21.2 ± 1.6 | 20.9 ± 1.4 | 0.62 |
| Blood HCO3 post (mmol/L) | 21.6 ± 0.74 | 26.7 ± 0.9*** | <0.001 |
| ΔPTH (pmol/L) | −110.5 ± 66.8 | 19.7 ± 103.4 | <0.01 |
| ΔtCa (mmol/L) | 0.08 ± 0.09 | 0.01 ± 0.13 | 0.12 |
| ΔiCa (mmol/L) | 0.075 ± 0.043 | −0.039 ± 0.042 | <0.001 |
| ΔtCa(corr) (mmol/L) | 0.03 ± 0.08 | −0.04 ± 0.11 | 0.11 |
| ΔTP (g/dl) | 0.57 ± 0.36 | 0.42 ± 0.38 | 0.38 |
| Δalbumin (g/dl) | 0.30 ± 0.22 | 0.23 ± 0.22 | 0.48 |
| Δ (mmol/L) | 0.32 ± 1.72 | 5.81 ± 1.57 | <0.001 |
| ΔpH | 0.017 ± 0.03 | 0.095 ± 0.022 | <0.001 |
| Ultrafiltration volume (mL) | 2360 ± 997 | 2330 ± 884 | 0.9 |
Data are shown as means ± SD.
post, postdialysis; pre, predialysis; Δ, intradialytic change.
P-value of unpaired test between groups using dialysate bicarbonate 26 and 32 mmol/L.
*P < 0.05 compared with pre-dialysis plasma concentration (paired test).
**P < 0.01 compared with pre-dialysis plasma concentration (paired test).
***P < 0.001 compared with pre-dialysis plasma concentration (paired test).
Table 3.
The effect of different concentrations of dialysate bicarbonate on dialysate composition and diffusion gradients
| n = 10 (HDF) | 26_DHCO3 | 32_DHCO3 | Pa |
|---|---|---|---|
| Dialysate tCa (mmol/L) | 1.36 ± 0.07 | 1.30 ± 0.04 | 0.03 |
| Dialysate iCa (mmol/L) | 1.260 ± 0.015 | 1.164 ± 0.01 | <0.001 |
| Plasma iCa pre (mmol/L) | 1.178 ± 0.062 | 1.189 ± 0.054 | 0.66 |
| Plasma iCa post (mmol/L) | 1.253 ± 0.05*** | 1.150 ± 0.026* | <0.001 |
| Dialysate pH | 7.18 ± 0.03 | 7.26 ± 0.03 | <0.001 |
| Dialysate HCO3 (mmol/L) | 28 ± 0.6 | 34.2 ± 1.0 | <0.001 |
| Diff(iCa) pre (mmol/L) | 0.312 ± 0.062 | 0.204 ± 0.045 | <0.001 |
| Diff(iCa) post (mmol/L) | 0.249 ± 0.05*** | 0.237 ± 0.027* | 0.42 |
| Diff(pH) pre | −0.194 ± 0.040 | −0.101 ± 0.035 | <0.001 |
| Diff(pH) post | −0.210 ± 0.021 | −0.196 ± 0.025*** | 0.18 |
| Diff(HCO3) pre (mmol/L) | 7.12 ± 1.51 | 13.49 ± 1.3 | <0.001 |
| Diff(HCO3) post (mmol/L) | 6.94 ± 0.82 | 8.13 ± 0.98*** | 0.02 |
| ΔDiff(iCa) (mmol/L) | −0.063 ± 0.040 | 0.033 ± 0.031 | <0.001 |
| ΔDiff(pH) | −0.017 ± 0.030 | −0.095 ± 0.022 | <0.001 |
| ΔDiff(HCO3) (mmol/L) | −0.18 ± 1.62 | −5.36 ± 1.58 | <0.001 |
Data are shown as means ± SD.
post, postdialysis; pre, predialysis; Δ, intradialytic change; Diff(iCa), diffusion gradient calculated as the differences between inflow dialysate and predialysis plasma water concentrations for iCa: Diff_iCa = 1.12 × inlet dialyser iCa concentration − plasma water iCa concentration, where 1.12 is the Gibbs–Donnan factor.
P-value of unpaired test between groups using dialysate bicarbonate 26 and 32 mmol/L.
*P < 0.05 compared with pre-dialysis plasma concentration (paired test).
***P< 0.001 compared with pre-dialysis plasma concentration (paired test).
FIGURE 1.
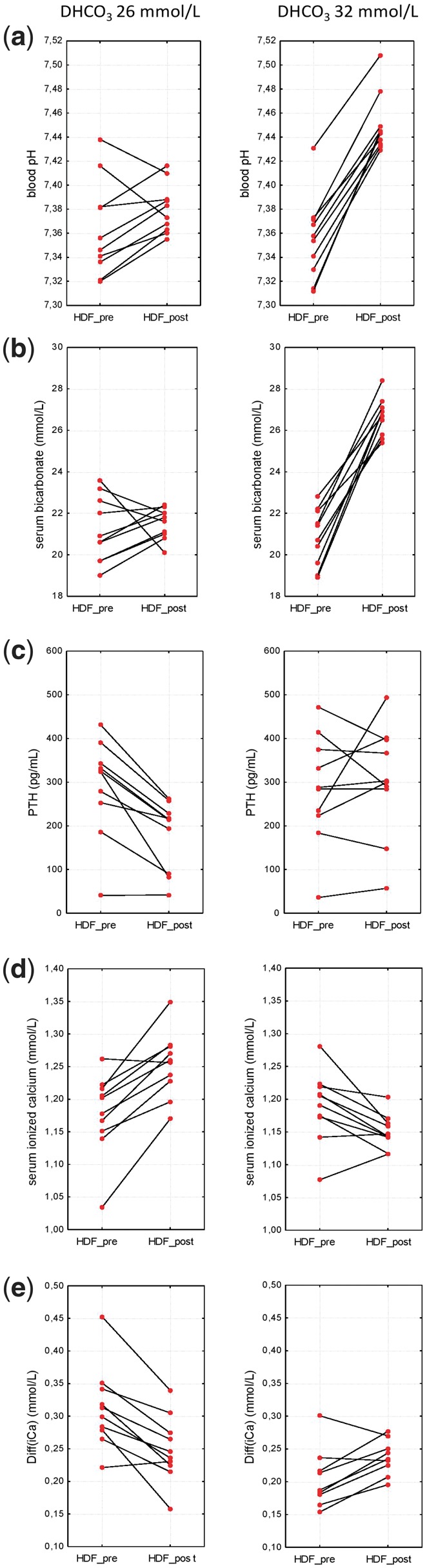
The intradialytic change before and after the dialysis procedure using dialysate bicarbonate 26 and 32 mmol/L.
FIGURE 2.
Linear regression and 95% confidence intervals, comparison between: (a) intradialytic change of blood pH and intradialytic change of plasma PTH; (b) intradialytic change of blood pH and CMB; (c) intradialytic change of plasma iCa and intradialytic change of plasma PTH; (d) intradialytic change of plasma iCa and CMB; (e) intradialytic change of blood pH and intradialytic change of plasma iCa; (f) intradialytic change of iCa diffusion gradient and CMB; (g) predialysis diffusion gradient of pH and intradialytic change of blood pH; (h) predialysis diffusion gradient of bicarbonate and intradialytic change of blood pH.
The mean CMB was negative for both dialysate bicarbonate concentrations since the calcium loss by ultrafiltration was included. Although the plasma tCa level increased significantly for 26_DHCO3 and remained stable for 32_DHCO3, we found an opposite trend of CMB with higher DHCO3 (−309 mg for 26_DHCO3 and −108 mg for 32_DHCO3).
The intradialytic increase of blood pH was associated with plasma iCa decline (Figure 2e) and with higher CMB (Figure 2b). We found a relationship between intradialytic rise of Diff(iCa) and CMB (Figure 2f). CMB correlated inversely with ΔiCa (Figure 2d). Furthermore, we found a correlation between the higher predialysis diffusion gradient of pH and increased CMB (r = 0.449, P = 0.05). The degree of intradialytic alkalinization was predominantly dependent on predialysis Diff(pH) and Diff(HCO3) (Figure 2g and h). We found no significant correlation between CMB and ΔtCa, ΔtCa(corr) or ultrafiltration volume, although the ultrafiltration has contributed to the loss of calcium.
Considering the simple difference between dialysate iCa and plasma iCa (e.g. for 32_DHCO3 1.16–1.19) it seems that iCa gradient is negative (−0.03 mmol/L). However, when calculating iCa gradient taking into account plasma water iCa concentration (obtained from plasma iCa using a coefficient of 0.93) and Gibbs–Donnan effect, the diffusion gradient for 32_DHCO3 is not negative (−0.03 mmol/L) but positive (0.204 mmol/L), resulting in a diffusive transfer of calcium from dialysate to the patient.
Multiple linear regression analysis was performed to evaluate the associations between CMB as a dependent variable and iCa, pH and bicarbonate diffusion gradients and their intradialytic changes. Two models were constructed (Table 4). Model 1 having R2 = 43.5%, describes the relation between CMB and the variables connected with iCa diffusion gradient associated parameters. In this model, there is a significant effect of ΔDiff(iCa) (P = 0.007). Model 2 (R2 = 70.0%) includes variables from Model 1 and, moreover, variables connected with acid–base parameters. In this model, there is still a significant effect of ΔDiff(iCa) (P = 0.003) and, further, a significant effect of Diff(iCa) (P = 0.017), ΔDiff(pH) (P = 0.048) and Diff(HCO3) (P = 0.019).
Table 4.
Multiple regression analysis results of CMB (mg) taken as dependent variable
| Model 1 (R2 = 43.5%, P = 0.008) |
Model 2 (R2 = 70.0%, P = 0.007) |
|||
|---|---|---|---|---|
| Predictor variable | Standard coefficient | P | Standard coefficient | P |
| Diff(iCa) | 0.645 | 0.098 | 0.96 | 0.017 |
| ΔDiff(iCa) | 1.14 | 0.007 | 1.35 | 0.003 |
| Diff(pH) | Not included in model | −0.53 | 0.291 | |
| ΔDiff(pH) | Not included in model | −1.33 | 0.047 | |
| Diff(HCO3) | Not included in model | −1.82 | 0.019 | |
| ΔDiff(HCO3) | Not included in model | 1.03 | 0.171 | |
P < 0.05 are given in bold.
DISCUSSION
In this study, the hypothesis that intradialytic alkalinization increases PTH level and CMB has been examined by assessing PTH level before and after HDF and assessing intradialytic CMB in 10 HDF patients when using different concentrations of dialysate bicarbonate.
The effect of dialysate calcium on intradialytic change of PTH is a well-known and long-debated topic. Basile et al. [6] theorized that the dialysate tCa concentration of 1.375 mmol/L might be preferable to 1.25 or 1.5 mmol/L because it is associated with mildly positive tCa mass balance values, plasma water iCa levels in the reference range and stable PTH levels during dialysis. They found that plasma PTH levels increased during dialysis using dialysate tCa concentration of 1.25 mmol/L and decreased with dialysate tCa concentrations of 1.375 and 1.5 mmol/L.
The concentration of plasma iCa is considered the main factor affecting PTH. In accordance with Argiles and Mion [7], we recorded a significant correlation between ΔiCa and ΔPTH. Although the effect of pH on iCa is well known, the above-mentioned studies did not took into account the effect of dialysate pH and bicarbonate concentration (DHCO3) on the level of PTH.
In our study, we confirmed the effect of higher DHCO3 on the intradialytic plasma iCa decrease together with PTH increase. The higher pH of dialysis solution led to more pronounced intradialytic alkalinization, which caused iCa decline and a subsequent increase of PTH. This was confirmed by the significant correlations between ΔpH and ΔPTH as well as between ΔiCa and ΔPTH.
Of course, it is necessary to distinguish between the short-term effect of intradialytic alkalinization on PTH level immediately after HDF and the long-term effect by evaluating PTH level during interdialytic period, which has not been studied in this work. Movilli et al. demonstrated that the correction of predialysis metabolic acidosis (MAC) in chronic HD patients by oral sodium bicarbonate supplementation during the interdialytic period reduces iPTH concentrations, possibly by a direct effect on iPTH secretion [8]. In our opinion, oral sodium bicarbonate supplementation is the optimal way to maintain acid–base balance and eliminate the effect of acidosis on PTH secretion within the interdialytic period. Furthermore, patients with higher predialysis blood pH and plasma bicarbonate do not require such large intradialytic loads of bicarbonate; thereby, there is a lower risk of intradialytic pH increase, iCa decrease and subsequent increase of PTH. On the contrary, the intradialytic loss of calcium can cause the increase of PTH level during the interdialytic period.
The intradialytic CMB is an important part of net calcium balance. The diffusion gradient between dialysate and plasma iCa is considered the main driving force of intradialytic CMB. However, the influence of pH and intradialytic alkalinization, important factors affecting the level of iCa, has not been systematically studied with respect to intradialytic calcium flux.
Malberti, in his review, mentioned the relationship between CMB and pH of dialysis solution [9]. He described a more positive CMB in acetate-free biofiltration (AFB) compared with standard bicarbonate HDF at similar dialysate tCa concentrations. This phenomenon was explained by the higher fraction of iCa in AFB and consequent higher diffusion iCa gradient. However, iCa gradient is a dynamic parameter, because blood pH is changing during the treatment (Figure 1a), whereas dialysate pH and iCa remain stable. Therefore, the intradialytic change of blood pH is the important factor determining iCa gradient during HDF (Figure 2e).
We compared two identical dialysis solutions differing only in the set of bicarbonate concentrations and recorded several interesting aspects related to CMB with higher DHCO3. As hypothesized, we confirmed a significant decrease of dialysate iCa concentration (Table 3). The reason for this is the decrease of dialysate tCa, which is more pronounced with 32_DHCO3. Taking into account the double-stage proportioning of concentrates in a dialysis machine, the higher setting of bicarbonate concentration leads to the decrease of all acid concentrate components as the result of increased base concentrate portion (NaHCO3) in order to maintain the preset sodium concentration and conductivity. Dialysate tCa was significantly decreased when using higher DHCO3 (1.30 versus 1.36 mmol/L, P = 0.03) due to the lower portion of acid concentrate in final inlet dialysate. Furthermore, the dialysate bicarbonate very likely creates complexes with calcium ions, thereby the higher DHCO3 enhances the decrease of dialysate iCa concentration. However, at the same time, the higher DHCO3 leads to the more pronounced intradialytic alkalinization (Figure 1a) and consequently more significant decrease of plasma iCa. Plasma iCa concentration decreases when it creates complexes with plasma anions (e.g. albumin and phosphate), whereas inlet dialysate tCa, iCa and pH remain constant. Therefore, the CMB is not only related to predialytic Diff(iCa) but also to the predialytic Diff(pH) and intradialytic change of blood pH and plasma iCa.
The observation that plasma iCa increased from 1.17 to 1.25 mmol/L with 26_DHCO3, whereas plasma iCa decreased from 1.19 to 1.15 mmol/L with 32_DHCO3 (average dialysate iCa 1.16 mmol/L) should apparently lead to the conclusion that there is a diffusive gain of calcium with 26_DHCO3 and a loss of calcium with 32_DHCO3. However, this statement can be applied only in the case of constant blood pH and plasma albumin/protein concentration. Paradoxically, in the context of this study it works inversely because of the different intradialytic change of blood pH with the two solutions. With 32_DHCO3, there is an intradialytic alkalinization that decreases plasma iCa and increases iCa diffusion gradient from the dialysate to the patient thus resulting in a more positive calcium mass transfer compared with 26_DHCO3 (Figure 2d)
This assumption was confirmed by the strong correlation between CMB and ΔDiff(iCa) (Figure 2f) as well as between CMB and ΔpH (Figure 2b). Although pre- and postdialytic Diff(iCa) were still lower when using 32_DHCO3 compared with Diff(iCa) using 26_DHCO3, the intradialytic change of Diff(iCa)—positive for 32_DHCO3 and negative for 26_DHCO3—seems to be the main driving force that is responsible for calcium gain or loss during HDF, as confirmed by multiple linear regression analysis (Table 4). Furthermore, the next reason for the higher calcium gain when using 32_DHCO3 could be the diffusability of calcium–bicarbonate complexes, which is very likely, due to their low molecular weight, nearly the same as the diffusability of iCa. This would explain the higher calcium gain despite the lower Diff(iCa) (Figure 3).
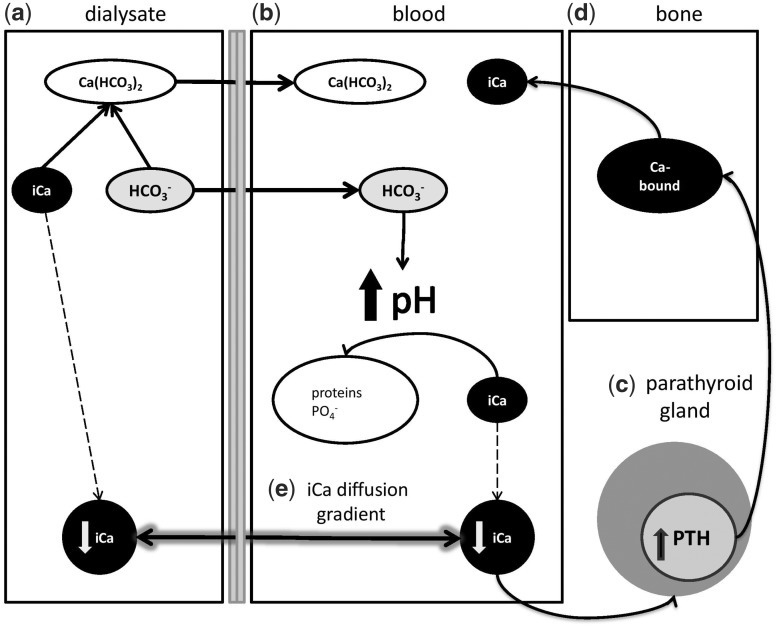
FIGURE 3.
Schematic illustration of intradialytic calcium flux. (a) The reaction of bicarbonate and iCa in dialysate leads to the formation of Ca(HCO3)2 and reduces dialysate iCa concentration. Ca(HCO3)2 diffuses probably through the membrane after the concentration gradient to the blood. (b) The bicarbonate load from dialysate alkalinized plasma to form water and CO2 (risk of hypercapnia). An increase of plasma pH facilitates iCa binding to plasma anions (protein, phosphate) and results in a decrease of plasma iCa (biologically active form of Ca) (c). This increases PTH secretion, which stimulates the release of iCa from bone (d). (e) The direction of iCa shift is determined by iCa gradient between dialysate and plasma, which depends on the degree of intradialytic plasma alkalization. The greater difference of plasma and dialysate pH leads to the greater plasma iCa decrease and greater iCa gain during dialysis.
The degree of intradialytic alkalinization was mostly determined by predialytic diffusion gradient of pH, which is supported by the strong correlation between ΔpH and predialytic Diff(pH) (Figure 2g). Therefore, in our opinion, the optimal DHCO3 should be individualized according to the predialytic diffusion gradient of pH with respect to desirable calcium gain.
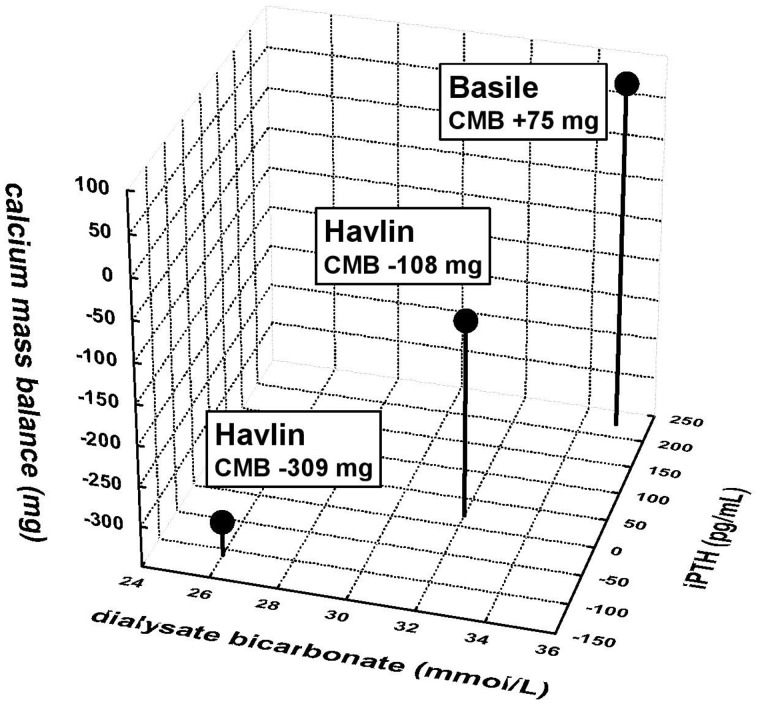
When we considered the factors affecting the CMB by a multiple regression analysis, we found that the predialysis iCa diffusion gradient and its intradialytic change plays a major role. Other important factors were the predialysis bicarbonate diffusion gradient and the intradialytic change of gradient for pH. We did not prove the significant effect of CMB on ΔPTH. On the contrary, we found a strong correlation between ΔDiff(iCa) and ΔPTH (r = 0.688, P = 0.001), which means that the intradialytic increase of iCa gradient—a consequence of plasma iCa decline—which is related to increased calcium gain, is also associated with a rise of PTH. When we compared our measurements using 26_DHCO3 and 32_DHCO3 with the study of Basile et al. [6], where 35_DHCO3 was used (DCa 1.25 mmol/L for all measurements), one can observe the relationship between higher DHCO3 and intradialytic increase of PTH together with the linear trend of higher CMB and with the increasing DHCO3 (Figure 4).
FIGURE 4.
The effect of different concentrations of dialysate bicarbonate (26, 32 and 35 mmol/L) on intradialytic change of PTH using dialysate calcium of 1.25 mmol/L in relationship with CMB. The higher DHCO3 is associated with a significant increase of intradialytic change of PTH and with a trend to higher CMB.
The Kidney Disease: Improving Global Outcomes guidelines [10] have offered an opinion-based recommendation of 1.25 mmol/L dialysate calcium concentration, weighing the long-term goals of maintaining bone and vascular health by neutral calcium balance with the effects of dialysate calcium on hyperparathyroidism and cardiac function. The Kidney Disease Outcome Quality Initiative—Nutrition and Bone disease guidelines recommend a predialysis plasma bicarbonate concentration >22 mmol/L [11].
Nevertheless, there are no guidelines for dialysate bicarbonate concentration and there is no effort to take into account the DHCO3 when considering the CMB.
Tentori et al. described the variation in DHCO3 in an international study including 17 031 patients from 11 countries (2002–11)—the mean DHCO3 was 35.5 mmol/L and ranged from 32.2 in Germany to 37 mmol/L in the USA [12]. The authors concluded that high DHCO3 may contribute to adverse outcomes, likely through the development of postdialysis metabolic alkalosis.
Due to the effect of alkalinization on iCa decrease, one can assume that high_DHCO3, frequently used in the USA and Canada, increases the risk of cardiac arrhythmias. The most threatened might be the patients with severe predialysis MAC, because the higher predialysis bicarbonate gradient increases the bicarbonate load and blood pH resulting in a higher risk of postdialysis hypokalaemia and hypocalcaemia. From a clinical point of view, the patients using low_DCa together with high_DHCO3 might be threatened by cardiac arrhythmias, whereas the combination of high_DCa and high_DHCO3 presents the highest risk of calcium gain and potential extraosseal calcifications. The high_DCa is a risk factor for inadequate calcium gain by itself, but the excessive intradialytic alkalinization, based on our data, enhances the CMB.
One of the most important findings of this work is that the intradialytic change of tCa, which is often used to assess the intradialytic calcium load, is not related to CMB. Furthermore, we found the inverse relationship between ΔiCa and CMB. Therefore, neither ΔtCa nor ΔiCa seem to be appropriate parameters for assessing CMB. The reason for this is very likely the large pool of rapidly diffusible Ca beyond the extracellular fluid distribution volume (e.g. connective tissue and bone surface), described by Gotch et al., where Ca could be mobilized or sequestered very rapidly at rate equal but opposite in sign to dialyser flux and thus effectively maintain near constant plasma Ca [13].
Finally, some limitations of this study must be considered. First, we used an unusually low concentration of dialysate bicarbonate. This was to provide a measurable difference between the two dialysates used. At the same time, we did not want to use DHCO3 >32 mmol/L in combination with DCa 1.25 mmol/L due to the increased risk of potential complications (cramps, hypotension, arrhythmias, etc.). Second, we measured only short-term intradialytic changes of PTH. From a clinical point of view, it might be appropriate to verify the long-term impact of different concentrations of bicarbonate dialysate on PTH. This must be assessed in the context of the overall calcium balance of the patient, which we did not measure in our study. The third limitation was the small number of the patients.
In conclusion, when using higher DHCO3 we observed the intradialytic increase of PTH even though the intradialytic loss of calcium was decreased. The higher PTH level after HDF is a result of blood pH increase and subsequent decrease of plasma iCa. The decline of intradialytic calcium loss, and respectively, the increase of CMB, is mainly caused by rise of iCa diffusion gradient during HDF, which is related to intradialytic alkalinization. Furthermore, there was no significant correlation between CMB and ΔtCa. With respect to intradialytic CMB and intradialytic PTH change, the dialysis concentration of bicarbonate should always be taken into account in addition to dialysate calcium concentration, when selecting the optimal dialysis solution.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Goodman WG, Goldin J, Kuizon BD. et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000; 342: 1478–1483 [DOI] [PubMed] [Google Scholar]
- 2. Torres A, Lorenzo V, Hernández D. et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int 1995; 47: 1434–1442 [DOI] [PubMed] [Google Scholar]
- 3. Tentori F, Blayney MJ, Albert JM. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530 [DOI] [PubMed] [Google Scholar]
- 4. Young EW, Akiba T, Albert JM. et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44 (Suppl 2): 34–38 [DOI] [PubMed] [Google Scholar]
- 5. Kumar R, Thompson JR.. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol 2010; 22: 216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basile C, Libutti P, Di Turo AL. et al. Effect of dialysate calcium concentrations on parathyroid hormone and calcium balance during a single dialysis session using bicarbonate hemodialysis: a crossover clinical trial. Am J Kidney Dis 2011; 59: 92–101 [DOI] [PubMed] [Google Scholar]
- 7. Argiles A, Mion CM.. Calcium balance and intact PTH variations during haemodiafiltration. Nephrol Dial Transplant 1995; 10: 2083–2089 [PubMed] [Google Scholar]
- 8. Movilli E, Zani R, Carli O. et al. Direct effect of the correction of acidosis on plasma parathyroid hormone concentrations, calcium and phosphate in hemodialysis patients: a prospective study. Nephron 2001; 87: 257–262 [DOI] [PubMed] [Google Scholar]
- 9. Malberti F. Non-pharmacological calcium metabolism control in patients undergoing hemodialysis. G Ital Nefrol 2009; 26: 670–678 [PubMed] [Google Scholar]
- 10. Moe SM, Drüeke TB, Block GA. et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009: (113): S1–S130. doi: 10.1038/ki.2009.188 [DOI] [PubMed] [Google Scholar]
- 11. Moe SM, Drüeke TB, Block GA. et al. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42 (Suppl 3): S1–S201 [PubMed] [Google Scholar]
- 12. National Kidney Foundation. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2013; 62: 738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gotch F, Kotanko P, Handelman G. et al. A kinetic model of calcium mass balance during dialysis therapy. Blood Purif 2007; 25: 139–149 [DOI] [PubMed] [Google Scholar]