Abstract
Along with amazing technological advances, the industrial revolution of the mid-19th century introduced new sources of pollution. By the mid-20th century, the effects of these changes were beginning to be felt around the world. Among these changes, health problems due to environmental air pollution are increasingly recognized. At the beginning, respiratory and cardiovascular diseases were emphasized. However, accumulated data indicate that every organ system in the body may be involved, and the kidney is no exception. Although research on air pollution and kidney damage is recent, there is now scientific evidence that air pollution harms the kidney. In this holistic review, we have summarized the epidemiology, disease states and mechanisms of air pollution and kidney damage.
Keywords: acute kidney injury, air pollution, chronic kidney disease, heavy metals, particulate matter
INTRODUCTION
The adverse health effects of ambient (outdoor) air pollution have been recognized since increased mortality due to smog was reported in London in 1952 [1, 2]. Since then, ambient air pollution is recognized as one of the leading causes of global disease burden [3, 4]. Air pollution is a complex mixture of gaseous components and solid and liquid particles suspended in the air and can vary substantially in chemical composition between different cities. There are various sources of these pollutants (Table 1).
Table 1.
Major sources of common air and other environmental pollutants
| Pollutant | Source | References |
|---|---|---|
| PM | Mostly traffic-related air pollution (mainly local emission) | [5, 6] |
| Other (domestic heating, industries, etc.) | ||
| NOX | Mainly derived from road traffic and the industrial burning of fuels | [7, 8] |
| Strongly related to diesel motor vehicles | ||
| SOX | Industrial production of sulphur-based products | [9] |
| O3 | Industrial combustion and processes | [10] |
| CO | Road traffic and industrial fuel burning | [7] |
| Cadmium | Diet in non-smokers | [11] |
| Tobacco in smokers | ||
| Lead | Gasoline, batteries, pipes and ammunition | [11] |
| In the past, paints and ceramic glazes | ||
| Occupational exposure to the inorganic form of Pb (Pb2+): welding manufacture of Pb-containing batteries, Pb melting and refining, and production of pottery | ||
| Children ingesting Pb2+ contaminated soil | ||
| Mercury | Occupational, environmental and dietary sources | [11] |
| Mainly through ingestion of food contaminated with CH3Hg+ | ||
| Arsenic | Primary route of human exposure is ingestion of contaminated drinking water | [11] |
| Methylated forms of As (MAs and DMAs) from pesticides used in cotton crops | ||
| Uranium | Mining and milling | [11] |
Particulate matter (PM), which primarily comprises solid particles derived from the combustion of coal, gasoline and diesel fuels, is the major element of air pollution that causes the most adverse health effects [12, 13]. Environmental air pollution may be composed of additional components (Figure 1), such as different sized PM (e.g. PM10 having an aerodynamic diameter ≤10 μm; PM2.5 which are ≤2.5 μm and PM2.5–10), gaseous pollutants [e.g. nitrogen dioxide (NO2), carbon monoxide (CO), sulphur dioxide (SO2) and ozone (O3)] and heavy metals [e.g. cadmium (Cd), lead (Pb) and mercury (Hg)] [14–16]. Over the past decade, a growing body of research has suggested a causal relationship between ambient air pollution exposure and adverse cardiovascular health [17].
FIGURE 1.
Components of environmental pollution.
Chronic kidney disease (CKD) is another worldwide public health problem with a variety of adverse outcomes, including premature death, and it is regarded as a cardiovascular disease (CVD) risk equivalent [18]. Apart from traditional risk factors, such as hypertension and diabetes, an increasing body of evidence demonstrates that air pollution may be a novel environmental risk factor for CKD [5, 19, 20]. In the present review, we systemically summarized the current data regarding air pollution and kidney disease.
METHODS
A literature search was performed using electronic databases MEDLINE, Ovid/MEDLINE (1988–2018), PubMed/MEDLINE, EMBASE and ISI Web/Web of Science for published studies from January 1988 to March 2018. We searched for relevant studies using the keywords ‘air pollution and chronic kidney disease’, ‘air pollution and end-stage renal disease’, ‘air pollution and proteinuria’, ‘environmental pollution and chronic kidney disease’, ‘air pollution and diabetes’, ‘air pollution and hypertension’, ‘toxic metals and chronic kidney disease’ and ‘heavy metals and chronic kidney disease’, limiting the search to research articles involving humans and published in English. Neither unpublished data nor abstracts were included.
NEPHROTOXIC METALS AND RENAL DISEASE
Heavy metals are among the best-known environmental pollutants causing kidney disease. It is less well known that they are also air pollutants, particularly Cd, Pb and Hg, but also arsenic (As) and uranium (http://www.euro.who.int/__data/assets/pdf_file/0007/78649/E91044.pdf).
Arsenic and CKD
Environmental, occupational and dietary exposure to As appear to contribute to the incidence of renal injury and the development of renal disease [11]. Contamination of drinking water with As has been linked to the development of hypertension and renal injury [21, 22]. Findings from a cross-sectional study of patients in Taiwan showed a positive correlation between urinary As and the incidence of CKD. It was concluded that high levels of urinary As may increase the risk of developing CKD by 4-fold [23]. Acute As-induced renal intoxication may lead to tubulointerstitial nephritis and acute tubular necrosis manifested by hypercalciuria, albuminuria, nephrocalcinosis and necrosis of the renal papillae [24, 25].
Cadmium and CKD
Cd is a prevalent nephrotoxic environmental pollutant. In non-smokers, diet is the primary source for Cd exposure. However, in smokers, the major source of exposure is related to tobacco products that contain high concentration of Cd. As paediatric age increases, Cd exposure is also increased [26]. Environmental tobacco smoke exposure was the most important determinant of Cd status in school-aged children. Moreover, serum Cd levels were higher in active smoker teenagers than in non-smokers. Interestingly, higher serum Cd concentration was associated with higher alpha-1-microglobulinuria in adolescents, suggesting subclinical renal toxicity after several years of cumulative exposure [27]. Cd is also present in air and drinking water, although the concentration of Cd in air is relatively low, and drinking water is normally not a major source of exposure for the general population. However, Cd contamination of drinking water and vegetables has been observed in some cities and regions [26]. Exposure to Cd is often assessed by measuring the concentration of Cd in urine and/or blood. In fact, urinary Cd excretion is considered as one of the most reliable indicators of renal and body burden of Cd [11].
Cd is directly nephrotoxic and can induce renal tubular damage (polyuria, generalized tubular dysfunction, i.e. Fanconi syndrome) and progressive loss of glomerular filtration rate (GFR) [28–30]. Long-term Cd exposure is thought to accelerate the CKD-related decline in GFR [31–33]. Cross-sectional studies have shown that Cd exposure is a risk factor for the development of CKD [34, 35]. Epidemiological studies have demonstrated a positive correlation between CKD and the renal accumulation of Cd in individuals exposed chronically to this metal [36, 37]. The most frequent long-term consequence of Cd exposure is proteinuria, and it may precede a slowly progressive and irreversible renal tubular dysfunction [38]. Proteinuria has been ascribed to the loss of megalin and cubilin, which mediate endocytosis of filtered proteins along the proximal tubule [39–41]. The incidence of kidney stones also increases in individuals exposed chronically to or to larger Cd dose, possibly due to the increased concentration of calcium in tubular fluid and urine [11]. Last but not least, Cd exposure has been associated with the severity of diabetes [42] and hypertension [43], which themselves are risk factors for CKD.
Lead and CKD
Together with bone, kidney is a primary site of Pb accumulation as Pb is excreted by kidneys [44]. The major kidney cellular effect of exposure to Pb is the induction of mitochondrial oxidative stress [45] and inflammation [11]. This results in lipid oxidation and DNA fragmentation [46]. Low-level Pb exposure early in life causes glomerular hypertrophy, which may disrupt glomerular development [11]. Acute Pb intoxication causes proximal tubular dysfunction (Fanconi syndrome) [47], and chronic intoxication leads to progressive tubulointerstitial nephritis [48]. There is convincing preclinical and clinical evidence supporting a direct relationship between Pb exposure and development of kidney disease [49, 50]. Additionally, cross-sectional general population studies in Mexico and Korea found a correlation between serum creatinine and blood Pb levels [51, 52].
Mercury and CKD
All forms of Hg are nephrotoxic through various mechanisms. Hg is well absorbed following inhalation, and air levels correlate with exposure estimated by urinary Hg excretion. Hg has been associated with CKD progression [11, 53]. The pars recta of the proximal tubule appears to be most sensitive to the Hg toxicity [54, 55]. Acute exposure causes altered mitochondrial structure, endoplasmic reticulum dilation and nuclear pyknosis [56]. After 12 h, microvilli are lost and cell death is associated with plasma membrane rupture and cell detachment from the basement membrane [57].
Chronic exposure to mercuric compounds can lead to glomerular injury [11]. In rats, chronic exposure to methyl Hg caused glomerulosclerosis and glomerular immunoglobulin deposition. In North West England, ambient background Hg concentration (3 ng/m3) is higher than the average background level in the UK (1.78 ng/m3). There were significant exposure–response relations between modelled estimates of Hg exposure (low: >3 to <4; medium: 4 to 10; high: >10; or very high exposure: >20 ng/m3) and risk of kidney disease mortality in both men and women after adjustment for age and socio-economic deprivation (test for trend: P = 0.02 for men and P = 0.03 for women) [58]. In another case–control study, occupational exposure in 272 men and women with CKD was compared with 272 controls matched for age, sex and region of residence. Hg exposure was independently associated with an increased risk of CKD [odds ratio (OR) = 5.13, 95% confidence interval (CI) 1.02–25.7] [59].
Uranium and CKD
Uranium toxicity, although relatively rare, may also cause renal damage and indeed, the kidney is the primary target for uranium toxicity following inhalation or ingestion. Approximately 40% of plasma uranium is complexed to transferrin and the remaining 60% is complexed with carbonate or bicarbonate, which are filtered by the glomerulus. In proximal tubules, the complexed uranium dissociates with decreasing pH, releasing the reactive uranyl ion, which can interact with the proximal tubule membrane. The most frequently used guideline for uranium kidney burdens is the International Commission on Radiological Protection value of 3 µg/g, a value that is based largely upon chronic animal studies [60]. A risk model equation to assess potential outcomes of acute uranium exposure was derived from 27 previously published case studies of acute exposure to soluble uranium compounds in workplace accidents. Kidney burdens of uranium for these individuals were determined from urine uranium, and correlated with health effects observed over a period of up to 38 years. Based upon the severity of health effects, each individual was assigned a score (− to 3+) and then placed into a Renal Effects Group (REG). A discriminate analysis was used to build a model equation to predict the REG based on the amount of kidney uranium [60] (Table 2).
Table 2.
Acute renal effects and predicted outcomes of kidney uranium accumulation
| REG | Kidney uranium concentration (µg/g) | Acute renal effect | Predicted outcome |
|---|---|---|---|
| 0 | ≤2.2 | None | No clinically detectable effects |
| 1 | >2.2 to ≤6.4 | Possible transient indicators of renal dysfunction | Not likely to become ill |
| 2 | >6.4 to ≤18 | Possible protracted indicators of renal dysfunction | May become ill |
| 3 | >18 | Possible severe clinical symptoms of renal dysfunction | Likely to become ill |
TOBACCO AND CKD
Smoking is also an air pollutant detrimental for kidney health [61]. A recent meta-analysis investigated the relationship between cigarette smoking and CKD in the general population. Summary relative risks (SRRs) and 95% CIs were calculated using a random effects model. A total of 15 prospective cohort studies, including 65 064 incident CKD cases, were included. Compared with never-smokers, the SRRs of incident CKD were 1.34 (95% CI 1.23–1.47) for current smokers and 1.15 (95% CI 1.08–1.23) for former smokers. The SRRs for end-stage renal disease (ESRD) development were 1.91 (95% CI 1.39–2.64) for current smokers and 1.44 (95% CI 1.00–2.09) for former smokers. However, the authors noted that there was considerable heterogeneity among these studies [62]. In African Americans from the Jackson Heart Study, after adjustment for various factors, the incidence of renal function decline was higher in current smokers than in never-smokers (incidence rate ratio 1.83, 95% CI 1.31–2.56). Current smokers of 1–19 and ≥20 cigarettes daily had an increased incidence rate ratio of residual renal function decline: 1.75 (95% CI 1.18–2.59) and 1.97 (95% CI 1.17–3.31), respectively. In addition, there was a significant progressive reduction in estimated GFR (eGFR) from Visits 1 to 3 in current and past smokers compared with never-smokers [63].
Apart from these findings, there is also evidence linking tobacco exposure to proteinuria in those with and without kidney disease [64–67]. In 990 middle-aged men recruited from a chemical plant, proteinuria was found in 4.6% of current smokers and 1.5% of never-smokers [68]. A meta-analysis of the relationship between tobacco smoking on the development of diabetic nephropathy (DN) in type 1 diabetes mellitus (T1DM) and T2DM, identified 19 observational studies (1 case–control, 8 cross-sectional and 10 prospective cohort studies), involving more than 78 000 participants and a total of 17 832 DN cases. The relative risk (RR) (95% CI) of DN was higher in ever-smokers with T1DM (1.31, 1.06–1.62; P = 0.006) or T2DM (1.44, 1.24–1.67; P < 0.001) than in never-smokers. In T1DM ever-smokers, the RR was 1.25 (95% CI 0.86–1.83) for microalbuminuria, 1.27 (95% CI 1.10–1.48) for macroalbuminuria and 1.06 (95% CI 0.97–1.15) for ESRD. In T2DM ever-smokers, the RR was 1.46 (95% CI 0.94–2.26) for microalbuminuria, 1.72 (95% CI 1.04–2.84) for macroalbuminuria and 1.10 (95% CI 0.36–3.33) for ESRD [69]. Thus, it appears that the highest risk associated with smoking is for macroalbuminuria, likely over a basis in incipient DN.
Passive smoking has also been associated with renal damage. Assessment of the nicotine metabolite cotinine in serum and in urine has been used to quantify smoking exposure. In a cohort of 366 children with CKD aged 1–16 years, secondhand smoke exposure obtained via questionnaire and urine cotinine was associated with the prevalence odds of nephrotic range proteinuria (OR = 2.64, 95% CI 1.08–6.42) [70]. In active (n = 24) and passive (n = 20) smokers, serum cotinine levels were higher than in controls (n = 20), as was the urinary albumin–creatinine ratio, whereas serum creatinine was higher in active smokers [71].
This evidence suggests that smoking harms the kidneys, but how? Several mechanisms may be at play. Cigarette smoking has been associated with idiopathic nodular glomerulosclerosis and microalbuminuria or overt proteinuria in healthy individuals, and with more severe proteinuria in individuals with pre-existing renal disease such as DN [72–74]. A correlation between smoking and nephrosclerosis and glomerulonephritis was found in a nationwide population-based case–control study that included Swedish subjects, 926 cases and 998 control [75]. Smoking promoted the progression of hypertensive [67, 76] and diabetic nephropathies [77]. Cigarette smoking has been directly associated with endothelial dysfunction, intimal hyperplasia and wall thickening of myocardial and renal arterioles and arteries.
Tobacco smoke caused mesangial proliferation, glomerulosclerosis and tubulointerstitial fibrosis in experimental studies [72, 78]. Specifically, nicotine activation of nicotine receptors promotes human mesangial cell extracellular matrix production [79]. Acrolein, an aldehyde from tobacco smoke, induces renal cell reactive oxygen species (ROS) production and apoptosis [80, 81]. Smoking also increases Cd levels and this could contribute to CKD progression. Smokers have 4- to 5-fold higher Cd levels in blood and 2- to 3-fold higher kidney Cd than non-smokers [82]. Indeed, NHANES 1999–2006 data for adults without CKD showed that urine Cd levels were highest for current smokers (3- to 13-fold higher), followed by former smokers (2- to 3-fold) compared with non-smokers. Cigarette smoking greatly increases RR of exceeding renal risk-associated urine Cd levels, particularly in former smokers [28].
Passive smoking has also being associated with renal fibrosis. Glomerulosclerosis comparable to the findings in idiopathic nodular glomerulosclerosis in human beings and upregulation of interstitial fibrosis-related genes has been observed in the absence of proteinuria, suggesting that histological changes precede biochemical changes [26]. Elevated sympathetic activity, blood pressure (BP), GFR and intraglomerular capillary pressure are candidate mechanisms for smoking-induced renal damage [72]. It was also suggested that chronic environmental tobacco smoke exposure induces systemic oxidative stress, which may subsequently trigger production of profibrotic factors [83].
However, there are also contradictory findings. In healthy rats exposed to smoke soon after birth for 4 months, as a model of passive smoking, the mean number of renal vessels, glomerulosclerosis and myointimal hyperplasia did not significantly differ from control rats [84]. However, it is unclear whether the timing was appropriate to observe any effect. No differences were found in the prevalence of glomerulosclerosis between non-smokers (51 patients, 47.7%) and ever-smokers (56 patients, 52.3%) in kidney biopsy. However, the number of glomeruli obtained at biopsy may have been insufficient for assessing glomerulosclerosis [85].
TRAFFIC AND NEARBY INDUSTRIAL POLLUTION AND CKD
Traffic air pollution is another kind of air pollution. Among 8497 Taipei city residents, regression models were used to estimate the participants’ 1-year exposures to PM of different sizes and traffic-related exhaust, PM2.5 absorbance, NO2 and nitrogen oxidases (NOx). Interquartile range (IQR) increments of PM2.5 absorbance (0.4 × 10−5/m) and NO2 (7.0 mg/m3) were associated with 1.07 and 0.84% lower eGFR, respectively. Similar associations were also observed for PM10 and PM2.5–10. Two-pollutant models showed that PM10 and PM2.5 absorbances were associated with lower eGFR. Authors conclude that 1-year exposures to traffic-related air pollution were associated with lower eGFR, higher CKD prevalence and increased risk of CKD progression among the elderly. Of note, there were no significant findings with regard to proteinuria, but albuminuria was not assessed [5]. Among 1103 consecutive Boston-area patients hospitalized with acute ischaemic stroke between 1999 and 2004, linear regression was used to evaluate the association between eGFR and categories of residential distance to major roadway (0 to ≤50, 50 to ≤100, 100 to ≤200, 200 to ≤400, 400 to ≤1000 and >1000 m) adjusting for age, sex, race, smoking, comorbid conditions, treatment with angiotensin-converting enzyme inhibitor and neighbourhood-level socioeconomic characteristics. Patients living closer to a major roadway had lower eGFR than patients living farther away (Ptrend = 0.01). Comparing patients living 50 m versus 1000 m from a major roadway, patients living within 50 m had 3.9 mL/min/1.73 m2 lower eGFR (95% CI 1.0–6.7; P = 0.007), a difference comparable in magnitude to the reduction in eGFR observed for a 4-year increase in age in population-based studies. The magnitude of this association did not differ significantly across categories of age, sex, race, history of hypertension, diabetes or socioeconomic status. However, as this study involved patients who were hospitalized with acute ischaemic stroke, the generalizability of the results to the general population is questionable. Proteinuria was not studied [86]. Excess kidney disease mortality was found in persons living within 2 km of industrial plants potentially releasing renal toxicants in Runcorn, UK: the standardized mortality ratio in males was 131 (95% CI 90–185) and in females 161 (95% CI 118–214) compared with a reference population living at 2.01–7.5 km [87].
PM AND CKD: EPIDEMIOLOGIC STUDIES
There are many studies showing association with various PM and CKD. A weak acceleration in the progression of albuminuria was observed during chronic exposure to PM10 in Multi-Ethnic Study of Atherosclerosis cohort [88]. In China, long-term exposure to PM2.5 was associated with an increased risk of membranous nephropathy in a nonlinear pattern in 71 151 native kidney biopsy samples from 938 hospitals in 282 cities. During the study period, PM2.5 exposure varied from 6 to 114 μg/m3 (mean 52.6 μg/m3) among the 282 cities. Each 10 μg/m3 increase in PM2.5 concentration was associated with 14% higher odds of a patient developing membranous nephropathy (OR = 1.14, 95% CI 1.10–1.18) in regions with PM2.5 levels >70 μg/m3, but the effect size in regions with PM2.5 <60 μg/m3 was greatly attenuated [89].
Another longitudinal observational cohort study that enrolled 2 482 487 US veterans demonstrated the association of PM2.5 concentrations with higher risk of incident CKD and progression to ESRD [90]. One-year PM2.5 exposure was associated with a lower eGFR among older men [91]. Among 21 656 Taiwanese adults, individual exposures to PM10, coarse particles (PMcoarse) or PM2.5 were related to renal function. An IQR increase in PM10 (5.83 μg/m3) was negatively associated with eGFR by –0.69 (95% CI –0.89 to –0.48) mL/min/1.73 m2 and positively associated with the prevalence of CKD with adjusted OR = 1.15 (95% CI 1.07–1.23). An IQR increase in PMcoarse (6.59 μg/m3) was significantly associated with lower eGFR by –1.07 (95% CI –1.32 to –0.81) mL/min/1.73 m2 and with CKD with OR = 1.26 (95% CI 1.15–1.38). In contrast, neither outcome was significantly associated with PM2.5. Stratified analyses indicated that associations of CKD with both PM10 and PMcoarse were limited to participants <65 years of age and were stronger in (for PM10) or limited to (PMcoarse) women. Associations also appeared to be stronger in those without hypertension than in those with hypertension, and in normal versus overweight participants [19].
In a recent cohort study, Bowe et al. [92] investigated the associations regarding coarse PM, NO2 and CO with the risk of kidney disease. The authors evaluated the association between PM10, NO2 and CO concentrations and risk of incident eGFR of <60 mL/min/1.73 m2, incident CKD, eGFR decline of ≥30% and ESRD. Participants were followed-up over a median of 8.52 years (IQR: 8.05–8.80). This study is one of the biggest, involving 2 010 398 participants. Among those individuals, the median concentration for PM10 was 20.45 μg/m³ (IQR: 14.64–24.81), NO2 was 14.54 parts per billion (9.69–17.91) and CO was 0.51 parts per million (0.40–0.64). An increased risk of eGFR <60 mL/min/1.73 m2 was associated with an IQR increase in concentrations of PM10 [hazard ratio (HR) = 1.07, 95% CI 1.06–1.08], NO2 (HR = 1.09, 95% CI 1.08–1.10) and CO (HR = 1.09, 95% CI 1.08–1.10). An increased risk of incident CKD was associated with an IQR increase in concentrations of PM10 (HR = 1.07, 95% CI 1.05–1.08), NO2 (HR = 1.09, 95% CI 1.08–1.11) and CO (HR = 1.10, 95% CI 1.08–1.11). An increased risk of an eGFR decline of ≥30% was associated with an IQR increase in concentrations of PM10 (1.08, 95% CI 1.07–1.09), NO2 (1.12, 95% CI 1.10–1.13) and CO (1.09, 95% CI 1.08–1.10). An increased risk of ESRD was associated with an IQR increase in concentrations of PM10 (1.09, 95% CI 1.06–1.12) and NO2 (1.09, 95% CI 1.06–1.12). This study, which has a high statistical power, showed that higher concentrations of PM10, NO2 and CO are associated with increased risk of incident CKD, eGFR decline and ESRD [92].
AIR POLLUTION AND BP
Air pollution has been associated with increased cardiovascular morbidity and mortality in numerous epidemiological studies. One of the proposed mechanisms involves increased BP, which is a risk factor for the development of CKD [93]. Large population studies showed that higher PM levels are associated with higher BP levels, even based on one BP measurement [94–99]. Data from the Nurses’ Health Study revealed that exposure to PM10 was associated with a slight increased risk of incident self-reported hypertension (PM10, HR 1.02, 95% CI 1.00–1.04) [100]. Similar findings were also observed in a Taiwanese study, which found that long-term exposure to PM2.5 was associated with increased risk of hypertension [101]. In another study, among 24 845 adults in Northeastern China, long-term exposure to air pollution was associated with increased odds of hypertension and more strongly with prehypertension [102]. Additionally, a recent systematic review and meta-analysis reported a positive association between ambient air pollution and elevated odds of hypertension [95, 103–108].
Apart from these studies, a very recent meta-analysis searched for the global association between ambient air pollution and BP. Seven international and Chinese databases encompassing 0.7 million participants from 16 countries were searched for studies examining the associations of PM2.5, PM2.5–10 or PM10 and gaseous (SO2, NO2, NOx, O3, CO) air pollutants with hypertension or BP. The overall meta-analysis showed significant associations of long-term exposures to PM2.5 with hypertension (OR = 1.05), and of PM10, PM2.5 and NO2 with diastolic BP (b-values: 0.47–0.86 mmHg). In addition, short-term exposures to four (PM10, PM2.5, SO2, NO2), two (PM2.5 and SO2) and four air pollutants (PM10, PM2.5, SO2 and NO2) were significantly associated with hypertension (ORs = 1.05–1.10), systolic BP (b-values: 0.53–0.75 mmHg) and diastolic BP (b-values: 0.15–0.64 mmHg), respectively. Stratified analyses showed a generally stronger relationship among men, Asians, North Americans and areas with higher air pollutant levels [103]. Additional studies have observed that not only single BP measurements but repeated BP measurements are also related to PM levels [106, 109–111].
An analysis of the association of exposure to PM10 (average concentration 23.5 ± 13.6µg/m3) with ambulatory BP and with sodium excretion in 359 adults disclosed that after controlling for potential confounders, a 10 µg/m3 increase in PM10 levels was positively associated with nighttime systolic BP, nighttime diastolic BP and negatively associated with nocturnal systolic BP dipping and daytime urinary sodium excretion, but not with nighttime sodium excretion. The effect sizes per 10 µg/m3 of PM10 were in the order of 1.0 mmHg for nighttime systolic BP and 0.5 mmHg for nighttime diastolic BP. The associations of short-term increase in PM10 with higher nighttime BP and blunted systolic BP dipping were preceded by associations with reduced ability of the kidney to excrete sodium during daytime. The authors suggested that the underlying mechanism linking air pollution to increased cardiovascular risk may include disturbed circadian rhythms of renal sodium handling and BP [112]. Smaller studies (48 healthy vehicular traffic controllers and 10 patients with impaired lung function) did not find any association between air pollution and ambulatory BP [113, 114].
Air pollution has also been associated with BP in children. A study of Chinese children aged 5–17 years found a positive association between short-term (2 years) exposure to ambient air pollution and elevated BP [115]. The interaction between exposure to pet ownership and air pollutants on hypertension was studied in 9354 Chinese children (aged 5–17 years) from 24 elementary and middle schools during 2012–13. Four-year average concentrations of PM10, SO2, NO2 and O3 were collected from 2009 to 2012. Hypertension was defined as average diastolic or systolic BP (three measurements) in the 95th percentile or higher based on height, age and sex. Children not exposed to pets exhibited consistently stronger effects of air pollution than those exposed to pets. The highest ORs per 30.6 mg/m3 increase in PM10 were 1.79 (95% CI 1.29–2.50) in children without current pet exposure compared with 1.24 (95% CI 0.85–1.82) in children with current pet exposure. As for BP, only O3 had an interaction for all exposure to pet ownership and showed lower BP in children exposed to pets. The increases in mean diastolic BP per 46.3 mg/m3 increase in O3 were 0.60 mmHg (95% CI 0.21–0.48) in children without pet exposure in utero compared with 0.34 mmHg (95% CI 0.21–0.48) in their counterparts. When stratified by age, pet exposure was more protective among younger children. This suggests that pet ownership reduces susceptibility to the health effects of pollutants [17].
However, there are also conflicting findings. For instance, a meta-analysis that examined the association between long-term exposure to air pollution and arterial BP in the European Study of Cohorts for Air Pollution Effects (n = 164 484) revealed that NO2 exposure was associated with a weak inverse relationship with systolic BP (0.29; 95% CI −0.70 to 0.12) among individuals not taking BP-lowering medication [116]. There are also some studies that found either no association [113, 117] or a negative association [118] of PM with BP. It should be noted that these negative studies are smaller in number and a large body of evidence still suggests a positive correlation between air pollution and hypertension.
AIR POLLUTION AND DIABETES
Diabetes is an ever-increasing worldwide health problem. Systematic reviews, meta-analyses and epidemiological studies confirm that exposure to air pollution may be associated with the incidence or prevalence of DM [119–124]. Several cohort studies also showed greater T2DM risk with exposure to higher levels of NO [125, 126] and PM2.5 [127, 128].
The effect of long-term exposure to ambient PM on the prevalence of T2DM and hypertension was explored in Iranian adults grouped by PM10 concentration <100 μg/m3 (5-year average 83.95 ± 7.81 μg/m3) or >100 μg/m3 (5-year average 120.15 ± 6.81 μg/m3). The prevalence of T2DM (13.8% versus 10.7%, P = 0.01; OR = 1.32, 95% CI 1.03–1.69) and hypertension (15.7 versus 11.9%, P = 0.005; OR = 1.55, 95% CI 1.20–1.99) was higher in the city with higher PM10 exposure [129]. The association between long-term exposure to PM, NOx and O3 and baseline prevalence and incidence of T2DM was also studied in a large administrative cohort in Rome, Italy. A total of 1 425 580 subjects aged 35+ years in January 2008 were assessed and followed for 6 years. A positive association was found between NOx exposures and prevalence of diabetes with ORs up to 1.010 (95% CI 1.002–1.017) and 1.015 (95% CI 1.009–1.021) for NO2 and NOx, respectively, per 10 μg/m3 and 20 μg/m3. They also found some evidence of an association between NOx and O3 and incidence of diabetes with HRs of 1.011 (95% CI 1.003–1.019) and 1.015 (95% CI 1.002–1.027) per 20 and 10 μg/m3 increases, respectively. The association with O3 with incident diabetes was stronger in women than in men and among those aged <50 years [6]. However, no association was found between PM10, PM2.5 or PM2.5–10, and diabetes. In the Netherlands, long-term exposure to NO2 was associated with prevalence of diabetes (OR = 1.07, 95% CI 1.05–1.09 per IQR increases) after adjustment for individual confounders such as body mass index (BMI) and physical activity [130].
In a very large-scale prospective study involving 1 729 108 participants who were followed-up for a median of 8.5 years, the relationship between PM2.5 and the risk of incident diabetes was investigated. All models were adjusted for age, race, sex, eGFR, systolic BP, hyperlipidaemia, chronic lung disease, CVD, cancer, BMI, smoking status, use of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and other covariates. In adjusted models, a 10 µg/m³ increase in PM2.5 was associated with increased risk of diabetes (HR = 1.15, 95% CI 1.08–1.22). The authors concluded that there is a significant association between increased PM2.5 exposure and the risk of diabetes with variable geographical distribution. More importantly, the risk is significant at concentrations below those recommended by regulatory agencies. This suggests that even a low concentration of air pollution might be unsafe, and adverse effects of air pollution become obvious at relatively low concentrations below those currently considered as safe by regulatory agencies [131]. In 429 overweight and obese African American and Latino Los Angeles children, both NO2 and PM2.5 were associated with higher fasting glycaemia and insulin, lower insulin sensitivity and higher insulin secretion. Specifically, each SD increase in NO2 and PM2.5 exposure was associated with 22.8% (P < 0.001) and 24.7% (P < 0.001) higher fasting insulin, respectively. At the same time, each SD increase in NO2 and PM2.5 exposure was associated with a 1.6% (P < 0.001) and 1.6% (P < 0.001) higher fasting glucose, respectively. HOMA-IR was 25.1% (P < 0.001) and 26.9% (P < 0.001) higher and insulin sensitivity 8.5% (P = 0.03) and 11.2% (P = 0.003) lower for each SD increase in NO2 and PM2.5 exposure. Regarding adiposity estimates, only elevated annual exposure to PM2.5 was associated with a higher BMI z-score (β = 0.05; P = 0.04). Each SD increase in non-freeway NOx exposure was related to a 12.1% higher fasting insulin (P < 0.001), 0.7% higher fasting glucose (P = 0.047), 12.9% higher HOMA-IR (P < 0.001) and 6.9% lower insulin sensitivity (P = 0.02) [132]. In a large American cohort (Cancer Prevention Study-II participants), a strong effect of ozone on diabetes-related mortality was found (HR = 1.16, 95% CI 1.07–1.26 per fixed increment of 10 parts per billion, equal to 20 μg/m3 O3) [133]. Rats exposed to realistic concentrations of ozone (0.8 parts per million = 1.69 μg/m3) developed insulin resistance induced by c-Jun N-terminal kinase activation that disrupted insulin signalling in skeletal muscles, suggesting a cause-and-effect relationship between ozone and insulin resistance [134]. The association of long-term residential exposure to PM2.5 with the prevalence and incidence of T2DM was studied in 61 447 elderly Hong Kong residents enrolled in 1998–2001, following participants without DM at baseline to 31 December 2010 to ascertain the first hospital admissions for T2DM. Over a mean follow-up of 9.8 years, 806 incident cases of T2DM occurred. After adjusting for potential confounders, the OR for every IQR (3.2 μg/m3) increase of PM2.5 concentration was 1.06 (95% CI 1.01–1.11) for prevalent DM, while the corresponding HR was 1.15 (95% CI 1.05–1.25) for incident T2DM [135]. These findings were in accordance with prior finding regarding PM2.5 and DM [136, 137] and with prospective cohort studies that examined the effect of long-term PM2.5 exposure on T2DM incidence [125, 127, 128].
Although all these findings suggest a strong relationship between air pollution and DM, some conflicting findings exist. Exposure to SO2 and PM10 was significantly related to the prevalence of T2DM in women, but not in men, suggesting gender differences [122]. Other prospective cohort studies also low in numbers failed to observe statistically significant associations between long-term PM2.5 exposure and incident DM [126, 138]. In the Framingham Offspring cohort, participants living 64 m (25th percentile) from a major roadway had 0.28% (95% CI 0.05–0.51%) higher fasting plasma glucose than participants living 413 m (75th percentile) away, and the association appeared to be driven by participants who lived within 50 m from a major roadway. Higher exposures to 3- to 7-day moving averages of black carbon and NOx were associated with higher plasma glucose, whereas the associations for ozone were negative, but there was no association with insulin resistance as evaluated by the HOMA-index [139].
Generally, in short-term exposure studies, exposure to air pollution was associated with higher levels of glucose, insulin or HOMA-IR [140, 141], whereas in longer term exposure, most of the positive associations were found with glucose but not with insulin or HOMA-IR [142, 143].
MESOAMERICAN NEPHROPATHY
MesoAmerican nephropathy (MeN) is another recently recognized endemic form of CKD. MeN can be considered as an environmental disease since it mostly affects people working in agricultural areas and with a tendency to be found in specific geographical locations, such as Nicaragua and El Salvador and other Central American countries. Additionally, global warming is thought to contribute to the increased incidence of MeN. Patients with MeN present with elevated creatinine levels, no hypertension and urine albumin levels are normal or of non-nephrotic range. The cause and pathogenesis of MeN are still not fully known; however, the repeated dehydration hypothesis is thought to play a role in the development of kidney disease. In this scenario, occupational heat exposure with repeated episodes of volume and salt depletion occurs during the disease process. Potential mechanisms include the development of hyperosmolarity with the activation of the aldose reductase–fructokinase pathway in the proximal tubule leading to local injury and inflammation, and the possibility that renal injury may be the consequence of repeated uricosuria and urate crystal formation as a consequence of both increased generation and urinary concentration, similar to a chronic tumour lysis syndrome. The epidemic is postulated to be increasing due to the effects of global warming. The renal morphology shows glomerulosclerosis of varying degrees, glomerular hypertrophy and signs of chronic glomerular ischaemia, together with mild to moderate chronic tubulointerstitial damage [131, 144, 145]. As MeN is considered as an environmental disease due to global warming, interventional studies to reduce heat stress may be of great importance. Indeed, a recent Phase 1 study showed that after an intervention with sugarcane workers, self-reported water consumption increased 25% and symptoms associated with heat stress and dehydration decreased [146]. It is now evident that new health problems with environmental and climate changes such as MeN will be of more interest in the near future, and preventive measures will be of the utmost importance for the prevention of a MeN epidemic.
BALKAN ENDEMIC NEPHROPATHY AND CHINESE HERB NEPHROPATHY
Balkan endemic nephropathy (BEN) is a kind of endemic nephropathy associated with upper urethelial cancer with specific geographical distribution along tributaries of the Danube River in Bosnia–Herzegovina, Croatia, Macedonia, Serbia, Bulgaria and Romania. Several hypotheses on the cause of BEN have been suggested such as mycotoxins, heavy metals, viruses and trace element insufficiencies. However, recent evidence suggests that chronic dietary exposure to aristolochic acid (AA)—a principal component of Aristolochia clematitis—which grows as a weed in the wheat fields of the endemic regions—is the cause of BEN [147].
BEN is described as a familial clustering and a slowly progressive kidney disease. The clinical signs and symptoms of BEN are non-specific and often remain latent for years and even decades. After an initial asymptomatic stage, patients suffer from weakness and lassitude, mild lumbar pain and pallor of the skin. At a later stage, anaemia is associated with a significant loss of renal function. Proteinuria of tubular type and specifically, β2-microglobulinuria is observed. Histologically, BEN is characterized by tubular atrophy with extensive hypocellular fibrosis decreasing from the outer to the inner cortex of the kidney [148, 149]. Nowadays, BEN is considered as an environmental disease since environmental exposure to AA by BEN patients has been confirmed [150, 151]. Other evidence of BEN being an environmental disease comes from the fact that in the last few decades, exposure to AA has decreased due to the significant improvement in farming and milling practices, disabling and preventing the contamination of flour [152].
Chinese herb nephropathy (CHN) is another nephropathy that is also related to AA. This nephropathy appeared to be the dramatic consequence of a substitution of Stephania tetrandra by Aristolochia fangchi, which is rich in AA, because both herbs share the same common name and one can be used instead of the other in traditional Chinese medicine irrespective of their botanical classification. The true incidence of aristolochic acid nephropathy (AAN) is largely unknown and probably underestimated, as numerous ingredients known or suspected to contain AA are used in traditional medicine in China, Japan and India. CHN is characterized by a rapidly progressive interstitial nephritis, leading to ESRD and urothelial malignancy [153]. In most of the cases, urinary sediment was unremarkable, and dipstick analysis for albuminuria was negative. Macroscopically, the kidneys were shrunk, asymmetric in about half of the cases with irregular outlines in one-third [154].
Microscopically, an extensive interstitial fibrosis with atrophy and loss of proximal tubules was the predominant lesion, which was mainly located in the superficial cortex and progressed towards the deep cortex. The glomeruli were relatively spared, although, in the later stage of the disease, they displayed a mild collapse of the capillaries and a wrinkling of the basement membrane [155]. Some suggest that BEN and CHN can be considered as two faces of Janus; however, recent evidence suggests that except in specific cases of acute tubular necrosis, no similarity could be found in the tubulointerstitial compartment with histological lesions observed in BEN and CHN [153]. However, as discussed above, both BEN and CHN can be considered as specific forms of nephropathy with specific geographical distribution.
PATHOGENESIS
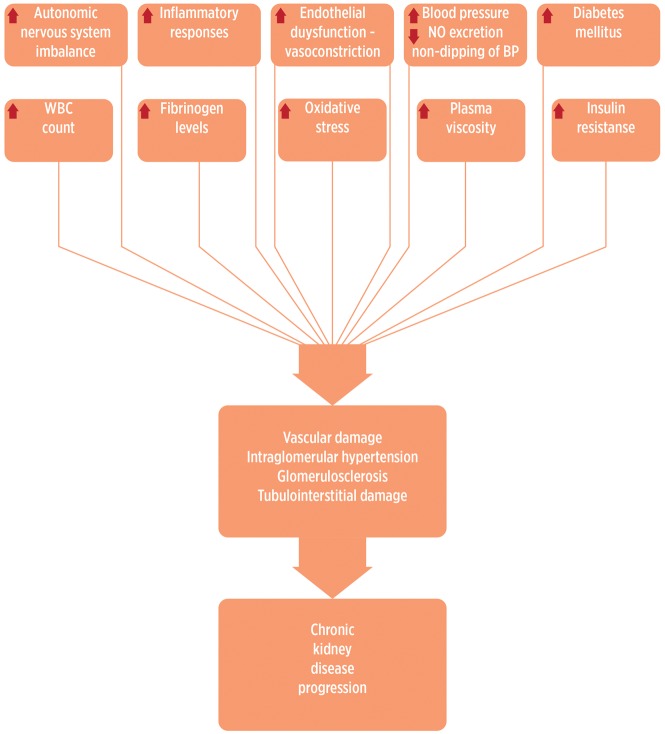
Several mechanisms have been proposed to link exposure to air pollution with BP and kidney damage (Figure 2). Inhaled PM can trigger acute autonomic nervous system imbalance and systemic proinflammatory responses and can activate vascular endothelial dysfunction and arterial vasoconstriction [156, 157]. Impaired sodium excretion associated with PM [112] may also increase BP since an impaired capacity to excrete sodium during daytime would lead to a non-dipping pattern [158]. Long-term exposure to traffic pollution leads to vascular endothelial injury, systemic inflammation, atherosclerosis and microvascular changes [13, 159]. Evidence from Apo-E knockout mice and hyperlipidaemic rabbits indicates that long-term exposure to urban air pollution causes increased atherosclerosis with inflammatory characteristics [160, 161]. Moreover, in community-dwelling adults, traffic-related pollution is positively associated with carotid intima–media thickness [162, 163]. Mice chronically and continuously exposed to ambient levels of air pollution for 4 months developed significant more thickening (decreased lumen/wall ratios) in pulmonary and coronary arteries than mice exposed to filtered air in which PM10 and NO2 were reduced by 50 and 75%, respectively. However, no evidence of influence of air pollution was detected in intra parenchymal renal arteries, which are well-known targets of systemic hypertension. One possibility is that pulmonary and cardiac effects were promoted by highly reactive substances present in the air, which may not reach sufficiently high levels in the renal circulation [164]. A recent experimental study reported that 16 weeks of exposure to concentrated ambient PM (average 13.3 μg/m3) versus filtered air was positively associated with glomerulosclerosis in rat T1DM [165]. Air pollution has been associated with objective markers of cardiovascular risk, such as circulating white cell counts, plasma fibrinogen levels and decreased heart rate variability [166, 167]. PM has been reported to be associated with increased plasma viscosity [168], changes in blood characteristics [169] and indicators of abnormal autonomic function of the heart, including increased heart rate, decreased heart rate variability and increased cardiac arrhythmias [170].
FIGURE 2.
Pathogenic mechanisms of environmental pollutants leading to kidney damage. WBC, white blood cell.
Smoking is an independent risk factor for CKD and might result in intraglomerular hypertension, vascular damage or glomerulosclerosis via multiple complex interactions of non-haemodynamic (angiotensin II, transforming growth factor-β1, endothelin-1) and haemodynamic factors [171], and occupational exposures to Cd and Pb may affect the magnitude of kidney damage conferred by smoking [172].
An interaction with obesity has also been described. Concentrated PM and ozone exposures induced inflammation and oxidative stress in peri-renal adipose tissue in rats [173]. Ambient fine PM matter may exaggerate adipose inflammation, induce insulin resistance and oxidative stress, mitochondrial functions and gene expression in adipose tissue [174–176]. Furthermore, diabetes and obesity may enhance the associations between PM2.5 and biomarkers of systemic inflammation [177], which in turn would lead to diminished insulin action [178]. Air pollution may also be associated with dysregulated release of peptides or proteins (adipokines) secreted by adipose tissue that regulates carbohydrate metabolism [174, 175, 179]. Mice exposed to PM2.5 developed a non-alcoholic steatohepatitis-like phenotype of impaired hepatic glycogen storage, glucose intolerance and insulin resistance. Moreover, exposure to PM2.5 activated inflammatory response pathways mediated through cJun N-terminal kinase, nuclear factor kappa B and toll-like receptor 4, and suppressed the insulin receptor substrate 1-mediated signalling [180].
RESEARCH NEEDS
The detrimental effects of air pollution on the kidney have just begun to be acknowledged. Various pollutants (toxic metals, PM, cigarette smoke and gases) may harm the kidney. However, it is not exactly known which pollutant by itself specifically causes damage or what type of damage.
Despite all accumulating data regarding pollution and its adverse health effects, there are still gaps that have to be recognized. This issue is very important since pollution is a global health threat that was responsible for an estimated 9 million premature deaths as well as for 268 million disability-adjusted life-years in 2015, which means great economic losses. The majority—71%—of the deaths attributed to pollution are caused by non-communicable diseases. To end neglect of pollution and advance prevention of pollution-related disease, commissions have been formed such as the Lancet Commission on Pollution and Health. The Commission identified substantial gaps in knowledge about pollution and noted that these gaps result in underestimation of pollution’s contribution to the global burden of disease. To close these gaps and guide prevention, the Commission made research recommendations and proposed creation of a Global Observatory on Pollution and Health. It is of no doubt that successful pollution research needs transdisciplinary collaborations among exposure science, epidemiology, data science, engineering, health policy and economics. Studies must be stimulated regarding the burden of disease due to pollution in cities and countries that include options for pollution control and disease prevention, source apportionment studies that analyse the amounts of pollution, country-level analyses of the burden of disease and loss of human capital attributable to various pollutants and all pollution in specific countries. These studies are essential for identifying the pollution sources with the most significant effects on human health and for prioritizing interventions [181].
Besides these issues, specific mechanisms of kidney injury are not known. PM is a complex mixture of chemical compounds, the behaviour of which strongly depends on the atmospheric conditions. Furthermore, it is not clear regarding the mechanisms that lead to development of CKD. For example, PM2.5 is associated both with DM and hypertension. However, it is not clear how much of the association of PM2.5 to CKD development is attributed to DM and hypertension separately. These issues are important to plan preferential preventive measures according to dominant-associated mechanisms. Last but not the least, more needs to be known about the causal relationship between exposure to levels of environmental pollution and ultimately cause-specific mortality. Further studies are needed to explore which compound(s) within PM might be responsible for the observed associations and what manoeuvres may prevent or treat these adverse effects.
Prospective studies should explore whether improving air pollution, setting preventive measures for clean air and using green energy have beneficial effects on hard endpoints, such as reduction in the incidence of diabetes, hypertension and CKD. Additionally, more longitudinal studies in different parts of the world with different pollution levels are needed to directly compare the effects of air pollution on human health. Recent initiatives to restrict the use of diesel fuels for urban transportation in Europe offer the opportunity to design and fund such studies.
CONCLUSION
The classic view that air pollution is a risk factor for upper and lower respiratory airways is now challenged by evidence that air pollution may also impact other organs such as heart, vessels and kidneys. The inflammatory mediators induced by PM and other pollutants in the lungs could spill over into the circulation, resulting in systemic inflammation, oxidative stress and damage to distant organs including kidneys [13]. However, there is also evidence of direct harm to the kidneys. The pathogenesis is still not fully understood. Studies are needed to characterize which type of air pollutant is primarily responsible for specific disease processes. It is necessary to raise awareness and recruit into action policy-makers, industry representatives establishing air quality standards, emissions controls and promoting the use of greener energy. More detailed longitudinal studies and also experimental designs are needed to demonstrate cause-and-effect relationships between specific air pollutants and kidney damage as well as the impact of manoeuvres that decrease air pollution.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bell ML, Davis DL.. Reassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollution. Environ Health Perspect 2001; 109 (Suppl 3): 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Park SK. Ambient air pollution and type 2 diabetes: do the metabolic effects of air pollution start early in life? Diabetes 2017; 66: 1755–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1659–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brook RD, Newby DE, Rajagopalan S.. The global threat of outdoor ambient air pollution to cardiovascular health: time for intervention. JAMA Cardiol 2017; 2: 353–354 [DOI] [PubMed] [Google Scholar]
- 5. Chen SY, Chu DC, Lee JH. et al. Traffic-related air pollution associated with chronic kidney disease among elderly residents in Taipei City. Environ Pollut 2018; 234: 838–845 [DOI] [PubMed] [Google Scholar]
- 6. Renzi M, Cerza F, Gariazzo C. et al. Air pollution and occurrence of type 2 diabetes in a large cohort study. Environ Int 2018; 112: 68–76 [DOI] [PubMed] [Google Scholar]
- 7. Kelly FJ, Fuller GW, Walton HA. et al. Monitoring air pollution: use of early warning systems for public health. Respirology 2012; 17: 7–19 [DOI] [PubMed] [Google Scholar]
- 8. Renzi M, Stafoggia M, Faustini A. et al. Analysis of temporal variability in the short-term effects of ambient air pollutants on nonaccidental mortality in Rome, Italy (1998-2014). Environ Health Perspect 2017; 125: 067019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kellogg WW, Cadle RD, Allen ER. et al. The sulfur cycle. Science 1972; 175: 587–596 [DOI] [PubMed] [Google Scholar]
- 10. Luecken DJ, Napelenok SL, Strum M. et al. Sensitivity of ambient atmospheric formaldehyde and ozone to precursor species and source types across the United States. Environ Sci Technol 2018; 52: 4668–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orr SE, Bridges CC.. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci 2017; 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X, Nie S, Ding H. et al. Environmental pollution and kidney diseases. Nat Rev Nephrol 2018; 14: 313–324 [DOI] [PubMed] [Google Scholar]
- 13. Brook RD, Rajagopalan S, Pope CA. et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010; 121: 2331–2378 [DOI] [PubMed] [Google Scholar]
- 14. Guo Y, Li S, Tian Z. et al. The burden of air pollution on years of life lost in Beijing, China, 2004-08: retrospective regression analysis of daily deaths. BMJ 2013; 347: f7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Liu Y, Cui LL. et al. Ambient air pollution, smog episodes and mortality in Jinan, China. Sci Rep 2017; 7: 11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milojevic A, Wilkinson P, Armstrong B. et al. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart 2014; 100: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawrence WR, Yang M, Lin S. et al. Pet exposure in utero and postnatal decreases the effects of air pollutants on hypertension in children: A large population based cohort study. Environ Pollut 2018; 238: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 19. Yang YR, Chen YM, Chen SY. et al. Associations between long-term particulate matter exposure and adult renal function in the Taipei Metropolis. Environ Health Perspect 2017; 125: 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim HJ, Min JY, Seo YS. et al. Association between exposure to ambient air pollution and renal function in Korean adults. Ann Occup Environ Med 2018; 30: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zalups RK. Enhanced renal outer medullary uptake of mercury associated with uninephrectomy: implication of a luminal mechanism. J Toxicol Environ Health 1997; 50: 173–194 [DOI] [PubMed] [Google Scholar]
- 22. Robles-Osorio ML, Sabath-Silva E, Sabath E.. Arsenic-mediated nephrotoxicity. Ren Fail 2015; 37: 542–547 [DOI] [PubMed] [Google Scholar]
- 23. Tsao DA, Tseng WC, Chang HR.. RKIP expression of liver and kidney after arsenic exposure. Environ Toxicol 2017; 32: 1079–1082 [DOI] [PubMed] [Google Scholar]
- 24. Aleksunes LM, Augustine LM, Scheffer GL. et al. Renal xenobiotic transporters are differentially expressed in mice following cisplatin treatment. Toxicology 2008; 250: 82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roggenbeck BA, Banerjee M, Leslie EM.. Cellular arsenic transport pathways in mammals. J Environ Sci (China) 2016; 49: 38–58 [DOI] [PubMed] [Google Scholar]
- 26. Beck KR, Bächler M, Vuorinen A. et al. Inhibition of 11beta-hydroxysteroid dehydrogenase 2 by the fungicides itraconazole and posaconazole. Biochem Pharmacol 2017; 130: 93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hossny E, Mokhtar G, El-Awady M. et al. Environmental exposure of the pediatric age groups in Cairo City and its suburbs to cadmium pollution. Sci Total Environ 2001; 273: 135–146 [DOI] [PubMed] [Google Scholar]
- 28. Mortensen ME, Wong LY, Osterloh JD.. Smoking status and urine cadmium above levels associated with subclinical renal effects in U.S. adults without chronic kidney disease. Int J Hyg Environ Health 2011; 214: 305–310 [DOI] [PubMed] [Google Scholar]
- 29. Roels HA, Hoet P, Lison D.. Usefulness of biomarkers of exposure to inorganic mercury, lead, or cadmium in controlling occupational and environmental risks of nephrotoxicity. Ren Fail 1999; 21: 251–262 [DOI] [PubMed] [Google Scholar]
- 30. Felley-Bosco E, Diezi J.. Fate of cadmium in rat renal tubules: a microinjection study. Toxicol Appl Pharmacol 1987; 91: 204–211 [DOI] [PubMed] [Google Scholar]
- 31. Gałażyn-Sidorczuk M, Brzóska MM, Jurczuk M. et al. Oxidative damage to proteins and DNA in rats exposed to cadmium and/or ethanol. Chem Biol Interact 2009; 180: 31–38 [DOI] [PubMed] [Google Scholar]
- 32. Matović V, Buha A, Ðukić-Ćosić D. et al. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 2015; 78: 130–140 [DOI] [PubMed] [Google Scholar]
- 33. So KY, Oh SH.. Cadmium-induced heme-oxygenase-1 expression plays dual roles in autophagy and apoptosis and is regulated by both PKC-delta and PKB/Akt activation in NRK52E kidney cells. Toxicology 2016; 370: 49–59 [DOI] [PubMed] [Google Scholar]
- 34. Liu J, Liu Y, Habeebu SS. et al. Metallothionein-null mice are highly susceptible to the hematotoxic and immunotoxic effects of chronic CdCl2 exposure. Toxicol Appl Pharmacol 1999; 159: 98–108 [DOI] [PubMed] [Google Scholar]
- 35. Brzóska MM, Roszczenko A, Galażyn-Sidorczuk M. et al. Zinc supplementation can protect from enhanced risk of femoral neck fracture in male rats chronically exposed to cadmium. Exp Toxicol Pathol 2011; 63: 491–498 [DOI] [PubMed] [Google Scholar]
- 36. Jarup L, Persson B, Elinder CG.. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup Environ Med 1995; 52: 818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jarup L, Elinder CG.. Incidence of renal stones among cadmium exposed battery workers. Br J Ind Med 1993; 50: 598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iwata K, Saito H, Moriyama M. et al. Renal tubular function after reduction of environmental cadmium exposure: a ten-year follow-up. Arch Environ Health 1993; 48: 157–163 [DOI] [PubMed] [Google Scholar]
- 39. Fujishiro H, Yano Y, Takada Y. et al. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012; 4: 700–708 [DOI] [PubMed] [Google Scholar]
- 40. He L, Wang B, Hay EB. et al. Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol Appl Pharmacol 2009; 238: 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jarup L, Berglund M, Elinder CG. et al. Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand J Work Environ Health 1998; 24 (Suppl 1): 1–51 [PubMed] [Google Scholar]
- 42. Edwards JR, Prozialeck WC.. Cadmium, diabetes and chronic kidney disease. Toxicol Appl Pharmacol 2009; 238: 289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Afridi HI, Kazi TG, Talpur FN. et al. Interaction between essential elements selenium and zinc with cadmium and mercury in samples from hypertensive patients. Biol Trace Elem Res 2014; 160: 185–196 [DOI] [PubMed] [Google Scholar]
- 44. Fowler BA, DuVal G.. Effects of lead on the kidney: roles of high-affinity lead-binding proteins. Environ Health Perspect 1991; 91: 77–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bogden JD, Gertner SB, Christakos S. et al. Dietary calcium modifies concentrations of lead and other metals and renal calbindin in rats. J Nutr 1992; 122: 1351–1360 [DOI] [PubMed] [Google Scholar]
- 46. Blake KC, Mann M.. Effect of calcium and phosphorus on the gastrointestinal absorption of 203Pb in man. Environ Res 1983; 30: 188–194 [DOI] [PubMed] [Google Scholar]
- 47. Goyer RA. Mechanisms of lead and cadmium nephrotoxicity. Toxicol Lett 1989; 46: 153–162 [DOI] [PubMed] [Google Scholar]
- 48. Evans M, Elinder CG.. Chronic renal failure from lead: myth or evidence-based fact? Kidney Int 2011; 79: 272–279 [DOI] [PubMed] [Google Scholar]
- 49. Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev 2006; 11: 114–127 [PubMed] [Google Scholar]
- 50. Basgen JM, Sobin C.. Early chronic low-level lead exposure produces glomerular hypertrophy in young C57BL/6J mice. Toxicol Lett 2014; 225: 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cárdenas A, Roels H, Bernard AM. et al. Markers of early renal changes induced by industrial pollutants. II. Application to workers exposed to lead. Br J Ind Med 1993; 50: 28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chung S, Chung JH, Kim SJ. et al. Blood lead and cadmium levels and renal function in Korean adults. Clin Exp Nephrol 2014; 18: 726–734 [DOI] [PubMed] [Google Scholar]
- 53. Bjorklund G, Dadar M, Mutter J. et al. The toxicology of mercury: current research and emerging trends. Environ Res 2017; 159: 545–554 [DOI] [PubMed] [Google Scholar]
- 54. Omata S, Sato M, Sakimura K. et al. Time-dependent accumulation of inorganic mercury in subcellular fractions of kidney, liver, and brain of rats exposed to methylmercury. Arch Toxicol 1980; 44: 231–241 [DOI] [PubMed] [Google Scholar]
- 55. Zalups RK. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology 1993; 79: 215–228 [DOI] [PubMed] [Google Scholar]
- 56. Zalups RK, Ahmad S.. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol 2004; 15: 2023–2031 [DOI] [PubMed] [Google Scholar]
- 57. Zalups RK, Koropatnick J.. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J Pharmacol Exp Ther 2000; 295: 74–82 [PubMed] [Google Scholar]
- 58. Hodgson S, Nieuwenhuijsen MJ, Elliott P. et al. Kidney disease mortality and environmental exposure to mercury. Am J Epidemiol 2007; 165: 72–77 [DOI] [PubMed] [Google Scholar]
- 59. Nuyts GD, Van Vlem E, Thys J. et al. New occupational risk factors for chronic renal failure. Lancet 1995; 346: 7–11 [DOI] [PubMed] [Google Scholar]
- 60. Roszell LE, Hahn FF, Lee RB. et al. Assessing the renal toxicity of Capstone depleted uranium oxides and other uranium compounds. Health Phys 2009; 96: 343–351 [DOI] [PubMed] [Google Scholar]
- 61. Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 2006; 164: 263–271 [DOI] [PubMed] [Google Scholar]
- 62. Xia J, Wang L, Ma Z. et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant 2017; 32: 475–487 [DOI] [PubMed] [Google Scholar]
- 63. Hall ME, Wang W, Okhomina V. et al. Cigarette smoking and chronic kidney disease in African Americans in the Jackson Heart Study. J Am Heart Assoc 2016; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hogan SL, Hogan SL, Vupputuri S. et al. Association of cigarette smoking with albuminuria in the United States: the third National Health and Nutrition Examination Survey. Ren Fail 2007; 29: 133–142 [DOI] [PubMed] [Google Scholar]
- 65. Pinto-Sietsma SJ, Mulder J, Janssen WM. et al. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med 2000; 133: 585–591 [DOI] [PubMed] [Google Scholar]
- 66. Chase HP, Garg SK, Marshall G. et al. Cigarette smoking increases the risk of albuminuria among subjects with type I diabetes. JAMA 1991; 265: 614–617 [PubMed] [Google Scholar]
- 67. Warmoth L, Regalado MM, Simoni J. et al. Cigarette smoking enhances increased urine albumin excretion as a risk factor for glomerular filtration rate decline in primary hypertension. Am J Med Sci 2005; 330: 111–119 [DOI] [PubMed] [Google Scholar]
- 68. Noborisaka Y, Ishizaki M, Nakata M. et al. Cigarette smoking, proteinuria, and renal function in middle-aged Japanese men from an occupational population. Environ Health Prev Med 2012; 17: 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang N, Huang F, Zhang X.. Smoking and the risk of diabetic nephropathy in patients with type 1 and type 2 diabetes: a meta-analysis of observational studies. Oncotarget 2017; 8: 93209–93218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Omoloja A, Jerry-Fluker J, Ng DK. et al. Secondhand smoke exposure is associated with proteinuria in children with chronic kidney disease. Pediatr Nephrol 2013; 28: 1243–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dulger H, Donder A, Sekeroglu MR. et al. Investigation of the relationship between serum levels of cotinine and the renal function in active and passive smokers. Ren Fail 2011; 33: 475–479 [DOI] [PubMed] [Google Scholar]
- 72. Boor P, Casper S, Celec P. et al. Renal, vascular and cardiac fibrosis in rats exposed to passive smoking and industrial dust fibre amosite. J Cell Mol Med 2009; 13: 4484–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Orth SR. Effects of smoking on systemic and intrarenal hemodynamics: influence on renal function. J Am Soc Nephrol 2004; 15 (Suppl 1): S58–S63 [DOI] [PubMed] [Google Scholar]
- 74. Markowitz GS, Lin J, Valeri AM. et al. Idiopathic nodular glomerulosclerosis is a distinct clinicopathologic entity linked to hypertension and smoking. Hum Pathol 2002; 33: 826–835 [DOI] [PubMed] [Google Scholar]
- 75. Ejerblad E, Fored CM, Lindblad P. et al. Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol 2004; 15: 2178–2185 [DOI] [PubMed] [Google Scholar]
- 76. Tylicki L, Puttinger H, Rutkowski P. et al. Smoking as a risk factor for renal injury in essential hypertension. Nephron Clin Pract 2006; 103: c121–c128 [DOI] [PubMed] [Google Scholar]
- 77. Orth SR. Smoking and the kidney. J Am Soc Nephrol 2002; 13: 1663–1672 [DOI] [PubMed] [Google Scholar]
- 78. Obert DM, Hua P, Pilkerton ME. et al. Environmental tobacco smoke furthers progression of diabetic nephropathy. Am J Med Sci 2011; 341: 126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jaimes EA, Tian RX, Raij L.. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 2007; 292: H76–H82 [DOI] [PubMed] [Google Scholar]
- 80. Schwerdt G, Gordjani N, Benesic A. et al. Chloroacetaldehyde- and acrolein-induced death of human proximal tubule cells. Pediatr Nephrol 2006; 21: 60–67 [DOI] [PubMed] [Google Scholar]
- 81. Jaimes EA, DeMaster EG, Tian RX. et al. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol 2004; 24: 1031–1036 [DOI] [PubMed] [Google Scholar]
- 82. Satarug S, Moore MR.. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect 2004; 112: 1099–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Visioli F, Galli C, Plasmati E. et al. Olive phenol hydroxytyrosol prevents passive smoking-induced oxidative stress. Circulation 2000; 102: 2169–2171 [DOI] [PubMed] [Google Scholar]
- 84. Culhaci N, Meteoglu I, Dundar M. et al. Histopathological evaluation of renal vascular changes in rats exposed to passive smoking. Pathol Oncol Res 2005; 11: 121–124 [DOI] [PubMed] [Google Scholar]
- 85. Lhotta K, Rumpelt HJ, Konig P. et al. Cigarette smoking and vascular pathology in renal biopsies. Kidney Int 2002; 61: 648–654 [DOI] [PubMed] [Google Scholar]
- 86. Lue SH, Wellenius GA, Wilker EH. et al. Residential proximity to major roadways and renal function. J Epidemiol Community Health 2013; 67: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hodgson S, Nieuwenhuijsen MJ, Hansell A. et al. Excess risk of kidney disease in a population living near industrial plants. Occup Environ Med 2004; 61: 717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O'Neill MS, Diez-Roux AV, Auchincloss AH. et al. Airborne particulate matter exposure and urinary albumin excretion: the Multi-Ethnic Study of Atherosclerosis. Occup Environ Med 2008; 65: 534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xu X, Wang G, Chen N. et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 2016; 27: 3739–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bowe B, Xie Y, Li T. et al. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2018; 29: 218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mehta AJ, Zanobetti A, Bind MA. et al. Long-term exposure to ambient fine particulate matter and renal function in older men: The Veterans Administration Normative Aging Study. Environ Health Perspect 2016; 124: 1353–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bowe B, Xie Y, Li T. et al. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. Lancet Planet Health 2017; 1: e267–e276 [DOI] [PubMed] [Google Scholar]
- 93. Brook RD. Why physicians who treat hypertension should know more about air pollution. J Clin Hypertens (Greenwich) 2007; 9: 629–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Choi J-H, Xu Q-S, Park S-Y. et al. Seasonal variation of effect of air pollution on blood pressure. J Epidemiol Community Health 2007; 61: 314–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Auchincloss AH, Diez Roux AV, Dvonch JT. et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 2008; 116: 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ibald-Mulli A, Stieber J, Wichmann HE. et al. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health 2001; 91: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dong GH, Qian Z, Xaverius PK. et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension 2013; 61: 578–584 [DOI] [PubMed] [Google Scholar]
- 98. Hoffmann B, Luttmann-Gibson H, Cohen A. et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect 2012; 120: 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. van Rossem L, Rifas-Shiman SL, Melly SJ. et al. Prenatal air pollution exposure and newborn blood pressure. Environ Health Perspect 2015; 123: 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Zhang Z, Laden F, Forman JP. et al. Long-term exposure to particulate matter and self-reported hypertension: a prospective analysis in the Nurses' Health Study. Environ Health Perspect 2016; 124: 1414–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang Z, Guo C, Lau AKH. et al. Long-term exposure to fine particulate matter, blood pressure, and incident hypertension in Taiwanese adults. Environ Health Perspect 2018; 126: 017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang B-Y, Qian Z, Vaughn MG. et al. Is prehypertension more strongly associated with long-term ambient air pollution exposure than hypertension? Findings from the 33 Communities Chinese Health Study. Environ Pollut 2017; 229: 696–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang B-Y, Qian Z, Howard SW. et al. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut 2018; 235: 576–588 [DOI] [PubMed] [Google Scholar]
- 104. Chuang KJ, Yan YH, Chiu SY. et al. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 2011; 68: 64–68 [DOI] [PubMed] [Google Scholar]
- 105. Giorgini P, Di Giosia P, Grassi D. et al. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des 2016; 22: 28–51 [DOI] [PubMed] [Google Scholar]
- 106. Dvonch JT, Kannan S, Schulz AJ. et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension 2009; 53: 853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baccarelli A, Barretta F, Dou C. et al. Effects of particulate air pollution on blood pressure in a highly exposed population in Beijing, China: a repeated-measure study. Environ Health 2011; 10: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen H, Burnett RT, Kwong JC. et al. Spatial association between ambient fine particulate matter and incident hypertension. Circulation 2014; 129: 562–569 [DOI] [PubMed] [Google Scholar]
- 109. Liu L, Ruddy TD, Dalipaj M. et al. Influence of personal exposure to particulate air pollution on cardiovascular physiology and biomarkers of inflammation and oxidative stress in subjects with diabetes. J Occup Environ Med 2007; 49: 258–265 [DOI] [PubMed] [Google Scholar]
- 110. Zanobetti A, Canner MJ, Stone PH. et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation 2004; 110: 2184–2189 [DOI] [PubMed] [Google Scholar]
- 111. Brook RD, Bard RL, Burnett RT. et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med 2011; 68: 224–230 [DOI] [PubMed] [Google Scholar]
- 112. Tsai D-H, Riediker M, Wuerzner G. et al. Short-term increase in particulate matter blunts nocturnal blood pressure dipping and daytime urinary sodium excretion. Hypertension 2012; 60: 1061–1069 [DOI] [PubMed] [Google Scholar]
- 113. de Paula Santos U, Braga ALF, Giorgi DMA. et al. Effects of air pollution on blood pressure and heart rate variability: a panel study of vehicular traffic controllers in the city of Sao Paulo, Brazil. Eur Heart J 2005; 26: 193–200 [DOI] [PubMed] [Google Scholar]
- 114. Chuang KJ, Chan CC, Shiao GM. et al. Associations between submicrometer particles exposures and blood pressure and heart rate in patients with lung function impairments. J Occup Environ Med 2005; 47: 1093–1098 [DOI] [PubMed] [Google Scholar]
- 115. Zeng X-W, Qian Z, Vaughn MG. et al. Positive association between short-term ambient air pollution exposure and children blood pressure in China-Result from the Seven Northeast Cities (SNEC) study. Environ Pollut 2017; 224: 698–705 [DOI] [PubMed] [Google Scholar]
- 116. Fuks KB, Weinmayr G, Foraster M. et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: an analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Environ Health Perspect 2014; 122: 896–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Madsen C, Nafstad P.. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO). Eur J Epidemiol 2006; 21: 485–491 [DOI] [PubMed] [Google Scholar]
- 118. Harrabi I, Rondeau V, Dartigues JF. et al. Effects of particulate air pollution on systolic blood pressure: a population-based approach. Environ Res 2006; 101: 89–93 [DOI] [PubMed] [Google Scholar]
- 119. Janghorbani M, Momeni F, Mansourian M.. Systematic review and metaanalysis of air pollution exposure and risk of diabetes. Eur J Epidemiol 2014; 29: 231–242 [DOI] [PubMed] [Google Scholar]
- 120. Wang B, Xu D, Jing Z. et al. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol 2014; 171: R173–R182 [DOI] [PubMed] [Google Scholar]
- 121. Balti EV, Echouffo-Tcheugui JB, Yako YY. et al. Air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014; 106: 161–172 [DOI] [PubMed] [Google Scholar]
- 122. Sohn D, Oh H.. Gender-dependent differences in the relationship between diabetes mellitus and ambient air pollution among adults in South Korean cities. Iran J Public Health 2017; 46: 293–300 [PMC free article] [PubMed] [Google Scholar]
- 123. Rajagopalan S, Brook RD.. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 2012; 61: 3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rao X, Patel P, Puett R. et al. Air pollution as a risk factor for type 2 diabetes. Toxicol Sci 2015; 143: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Krämer U, Herder C, Sugiri D. et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect 2010; 118: 1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Coogan PF, White LF, Jerrett M. et al. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation 2012; 125: 767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hansen AB, Ravnskjær L, Loft S. et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish Nurse Cohort. Environ Int 2016; 91: 243–250 [DOI] [PubMed] [Google Scholar]
- 128. Chen H, Burnett RT, Kwong JC. et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect 2013; 121: 804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hassanvand MS, Naddafi K, Malek M. et al. Effect of long-term exposure to ambient particulate matter on prevalence of type 2 diabetes and hypertension in Iranian adults: an ecologic study. Environ Sci Pollut Res Int 2018; 25: 1713–1718 [DOI] [PubMed] [Google Scholar]
- 130. Strak M, Janssen N, Beelen R. et al. Long-term exposure to particulate matter, NO2 and the oxidative potential of particulates and diabetes prevalence in a large national health survey. Environ Int 2017; 108: 228–236 [DOI] [PubMed] [Google Scholar]
- 131. Bowe B, Xie Y, Li T. et al. The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet Health 2018; 2: e301–e312 [DOI] [PubMed] [Google Scholar]
- 132. Toledo-Corral CM, Alderete TL, Habre R. et al. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes 2018; 13: 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Turner MC, Jerrett M, Pope CA. et al. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 2016; 193: 1134–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Vella RE, Pillon NJ, Zarrouki B. et al. Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes 2015; 64: 1011–1024 [DOI] [PubMed] [Google Scholar]
- 135. Qiu H, Schooling CM, Sun S. et al. Long-term exposure to fine particulate matter air pollution and type 2 diabetes mellitus in elderly: a cohort study in Hong Kong. Environ Int 2018; 113: 350–356 [DOI] [PubMed] [Google Scholar]
- 136. Liu C, Yang C, Zhao Y. et al. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int 2016; 92–93: 416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pearson JF, Bachireddy C, Shyamprasad S. et al. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care 2010; 33: 2196–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Puett RC, Hart JE, Schwartz J. et al. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect 2011; 119: 384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li W, Dorans KS, Wilker EH. et al. Ambient air pollution, adipokines, and glucose homeostasis: The Framingham Heart Study. Environ Int 2018; 111: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Brook RD, Xu X, Bard RL. et al. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ 2013; 448: 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chen L, Zhou Y, Li S. et al. Air pollution and fasting blood glucose: a longitudinal study in China. Sci Total Environ 2016; 541: 750–755 [DOI] [PubMed] [Google Scholar]
- 142. Ward-Caviness CK, Kraus WE, Blach C. et al. Association of roadway proximity with fasting plasma glucose and metabolic risk factors for cardiovascular disease in a cross-sectional study of cardiac catheterization patients. Environ Health Perspect 2015; 123: 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wolf K, Popp A, Schneider A. et al. Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes 2016; 65: 3314–3326 [DOI] [PubMed] [Google Scholar]
- 144. Correa-Rotter R, Wesseling C, Johnson RJ.. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis 2014; 63: 506–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Roncal-Jimenez CA, Garcia-Trabanino R, Wesseling C. et al. Mesoamerican nephropathy or global warming nephropathy? Blood Purif 2016; 41: 135–138 [DOI] [PubMed] [Google Scholar]
- 146. Bodin T, García-Trabanino R, Weiss I. et al. Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: Phase 1. Occup Environ Med 2016; 73: 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stiborova M, Arlt VM, Schmeiser HH.. Balkan endemic nephropathy: an update on its aetiology. Arch Toxicol 2016; 90: 2595–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Stefanovic V, Radovanovic Z.. Balkan endemic nephropathy and associated urothelial cancer. Nat Clin Pract Urol 2008; 5: 105–112 [DOI] [PubMed] [Google Scholar]
- 149. Jadot I, Declèves AE, Nortier J. et al. An integrated view of aristolochic acid nephropathy: update of the literature. Int J Mol Sci 2017; 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Grollman AP, Jelakovic B.. Role of environmental toxins in endemic (Balkan) nephropathy. October 2006, Zagreb, Croatia. J Am Soc Nephrol 2007; 18: 2817–2823 [DOI] [PubMed] [Google Scholar]
- 151. De Broe ME. Chinese herbs nephropathy and Balkan endemic nephropathy: toward a single entity, aristolochic acid nephropathy. Kidney Int 2012; 81: 513–515 [DOI] [PubMed] [Google Scholar]
- 152. Cvitković A, Vuković-Lela I, Edwards KL. et al. Could disappearance of endemic (Balkan) nephropathy be expected in forthcoming decades? Kidney Blood Press Res 2012; 35: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Debelle FD, Vanherweghem JL, Nortier JL.. Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008; 74: 158–169 [DOI] [PubMed] [Google Scholar]
- 154. Reginster F, Jadoul M, van Ypersele de Strihou C.. Chinese herbs nephropathy presentation, natural history and fate after transplantation. Nephrol Dial Transplant 1997; 12: 81–86 [DOI] [PubMed] [Google Scholar]
- 155. Depierreux M, Van Damme B, Vanden Houte K. et al. Pathologic aspects of a newly described nephropathy related to the prolonged use of Chinese herbs. Am J Kidney Dis 1994; 24: 172–180 [DOI] [PubMed] [Google Scholar]
- 156. Pope CA 3rd, Dockery DW.. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 2006; 56: 709–742 [DOI] [PubMed] [Google Scholar]
- 157. Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 2008; 115: 175–187 [DOI] [PubMed] [Google Scholar]
- 158. Fukuda M, Goto N, Kimura G.. Hypothesis on renal mechanism of non-dipper pattern of circadian blood pressure rhythm. Med Hypotheses 2006; 67: 802–806 [DOI] [PubMed] [Google Scholar]
- 159. Alexeeff SE, Coull BA, Gryparis A. et al. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect 2011; 119: 481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Suwa T, Hogg JC, Quinlan KB. et al. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol 2002; 39: 935–942 [DOI] [PubMed] [Google Scholar]
- 161. Sun Q, Wang A, Jin X. et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005; 294: 3003–3010 [DOI] [PubMed] [Google Scholar]
- 162. Bauer M, Moebus S, Möhlenkamp S. et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol 2010; 56: 1803–1808 [DOI] [PubMed] [Google Scholar]
- 163. Diez Roux AV, Auchincloss AH, Franklin TG. et al. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2008; 167: 667–675 [DOI] [PubMed] [Google Scholar]
- 164. Lemos M, Mohallen SV, Macchione M. et al. Chronic exposure to urban air pollution induces structural alterations in murine pulmonary and coronary arteries. Inhal Toxicol 2006; 18: 247–253 [DOI] [PubMed] [Google Scholar]
- 165. Yan Y-H, Chou CC-K, Wang J-S. et al. Subchronic effects of inhaled ambient particulate matter on glucose homeostasis and target organ damage in a type 1 diabetic rat model. Toxicol Appl Pharmacol 2014; 281: 211–220 [DOI] [PubMed] [Google Scholar]
- 166. Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect 2001; 109 (Suppl 3): 405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Perspect 2001; 109 (Suppl 4): 483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Peters A, Doring A, Wichmann HE. et al. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet 1997; 349: 1582–1587 [DOI] [PubMed] [Google Scholar]
- 169. Franchini M, Mannucci PM.. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 2011; 118: 2405–2412 [DOI] [PubMed] [Google Scholar]
- 170. Sun Q, Hong X, Wold LE.. Cardiovascular effects of ambient particulate air pollution exposure. Circulation 2010; 121: 2755–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Orth SR, Hallan SI.. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients–absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 172. EL-Safty IAM, Afifi AMH, Shouman AE. et al. Effects of smoking and lead exposure on proximal tubular integrity among Egyptian industrial workers. Arch Med Res 2004; 35: 59–65 [DOI] [PubMed] [Google Scholar]
- 173. Sun L, Liu C, Xu X. et al. Ambient fine particulate matter and ozone exposures induce inflammation in epicardial and perirenal adipose tissues in rats fed a high fructose diet. Part Fibre Toxicol 2013; 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Sun Q, Yue P, Deiuliis JA. et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009; 119: 538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Xu X, Liu C, Xu Z. et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci 2011; 124: 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Xu Z, Xu X, Zhong M. et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol 2011; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dubowsky SD, Suh H, Schwartz J. et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect 2006; 114: 992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Meigs JB, Hu FB, Rifai N. et al. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 2004; 291: 1978–1986 [DOI] [PubMed] [Google Scholar]
- 179. Piya MK, McTernan PG, Kumar S.. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol 2013; 216: T1–T15 [DOI] [PubMed] [Google Scholar]
- 180. Zheng Z, Xu X, Zhang X. et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol 2013; 58: 148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Landrigan PJ, Fuller R, Hu H.. Pollution and Global Health - An Agenda for Prevention. Environ Health Perspect 2018; 126: 084501. [DOI] [PMC free article] [PubMed] [Google Scholar]