Abstract
Background
In Fabry nephropathy, podocyturia is an early event that may lead to glomerulosclerosis and chronic kidney disease. The glycocalyx is a potential podocyte damaged compartment in glomerulopathies. We investigated glycocalyx podocalyxin in urinary detached podocytes compared with cytoplasmic synaptopodin.
Methods
This was a cross-sectional study including 68 individuals: Controls (n = 20) and Fabry patients (n = 48), 15 untreated and 33 treated. Variables included age, gender, urinary protein/creatinine ratio (UPCR), estimated glomerular filtration rate (eGFR), lyso-triasocylsphingosine (lyso-Gb3) levels and enzyme replacement therapy (ERT). Podocyturia was assessed by immunofluorescence and podocyte subpopulations were analyzed.
Results
Fabry patients displayed higher podocyturia than controls. Fabry treated subjects (n = 33) presented significantly higher UPCR compared with untreated ones (n = 15); podocyturia, eGFR and lyso-Gb3 levels were not different. All control podocytes colocalized synaptopodin and podocalyxin; 13 Fabry patients (27%) colocalized these proteins, while 35 (73%) were only synaptopodin positive. No podocalyxin-positive/synaptopodin-negative cells were encountered. In Fabry patients, podocyturia was significantly higher and proteinuria lower in those that colocalized.
Conclusion
Fabry patients present higher podocyturia and a presumably more damaged glycocalyx assessed by podocalyxin. Treated patients had significant higher proteinuria suggesting ERT is initiated late, at advanced stages. The degree of podocalyxin-negative podocytes was similar in both groups, but colocalization was associated with lower proteinuria. Podocyturia assessed by podocalyxin alone may be underestimated. The implications of podocyte glycocalyx damage deserve further investigations.
Keywords: Fabry nephropathy, podocalyxin, podocyte, podocyturia, synaptopodin
INTRODUCTION
Fabry disease is an X-linked lysosomal storage disorder due to deficient activity of the enzyme alpha-galactosidase A (α-GalA) caused by several mutations, resulting in the accumulation of glycosphingolipids intracellularly and in the circulation as well [1]. Clinically, there are two major subtypes: the early-onset Type 1 ‘classic’ and ‘late-onset’ Type 2 phenotypes. Affected males with the classic phenotype have low or no functional α-GalA enzymatic activity, marked microvascular endothelial glycosphingolipid accumulation, and childhood or adolescence onset of clinical manifestations [1]. Amongst the affected organs, kidneys are a major target, and particularly in men, Fabry nephropathy leads to end-stage kidney disease by approximately the fourth to fifth decade of life [2]. Affected males with the Type 2 late-onset phenotype have residual α-GalA activity, without relevant microvascular endothelial glycosphingolipid accumulation, and therefore lack the early manifestations of males with the Type 1 classic phenotype. Type 2 patients typically develop renal and/or cardiac disease in their fourth to seventh decades of life [1].
The prescription of enzyme replacement therapy (ERT) may delay this outcome but has been shown not to be sufficient to avoid it, probably due the fact that the specific treatment is on average started at advanced stages of Fabry disease, mainly secondary to late diagnosis [3–5]. The most common clinical aspects of Fabry nephropathy are microalbuminuria, proteinuria and/or elevated serum creatinine, with a progressive decline in the glomerular filtration rate (GFR) [6]. From a glomerular perspective, proteinuria due to glomerular origin can be due to glomerulosclerosis, hyperfiltration and/or podocytopathic abnormalities. In this respect, glomerular proteinuria is a hallmark of glomerular filtration barrier derangements, mainly due to the accumulation of the substrates of α-GalA as globotriasocylceramide (Gb3) and lyso-triasocylsphingosine (lyso-Gb3) in endothelial cells and podocytes [7]. Although some mechanisms of cellular dysfunction still remain elusive, it has been suggested that the accumulation of these enzyme substrates exerts many abnormal effects on affected cells beyond biomechanical strain, as oxidative stress, the triggering of inflammatory and fibrotic pathways, epithelial-to-mesenchymal transition and autophagy [2, 7–12]. We and others have demonstrated that in Fabry nephropathy, podocyte loss antedates proteinuria and is a marker of ongoing and progressive glomerular damage, being more specific than proteinuria [5, 13].
It has been demonstrated that the early initiation of high-dose ERT reduces Gb3 accumulation in cells, decreases lyso-Gb3 in plasma, and also improves symptoms, quality of life and prognosis, and delays the process of kidney dysfunction, but is unable to stop it [14, 15]. One of the most important causes of this appalling situation is the late diagnosis of Fabry disease and therefore the late initiation of ERT. Independently of the etiology, podocytes are a major target in Fabry nephropathy. These cells can be damaged at the basal membrane compartment, at the slit diaphragm zone, intracellularly and/or at the apical-lateral membrane [2, 16–18]. Glycolcalyx is essential for intercellular communication, molecule trafficking and intracellular signal transduction [19, 20]. Moreover, electron microscopy kidney biopsies of Fabry patients display gross abnormalities at the cellular membrane surface of podocytes, as foot process widening and effacements [2, 16, 17].
It has also been reported that podocalyxin, a glycocalyx sialoprotein located at the apical and lateral podocyte compartment, plays an important role in the attachment of cells to the contiguous extracellular matrix [21, 22]. Considering the fact that the product of α-GalA is a ceramide, a molecule with relevant roles at the cellular membrane and the transduction of signals intracellularly, we decided to investigate whether the glycocalyx of podocytes was affected in detached podocytes assessed by the identification of podocalyxin by immunofluorescent microscopy. We also investigated the presence or not of synaptopodin on the same assessed podocytes in order to identify potential different subpopulations of urinary detached podocytes. Moreover, we compared patients treated and untreated with ERT with respect to the identification of podocytes according to the presence or absence of these two molecules. Finally, as podocyturia may be questioned as a reliable biomarker of glomerular damage, in part due to its intermittent appearance in the urine, we collected three samples of urine form each patient every 7 days to assess this observation in our Fabry population.
MATERIALS AND METHODS
This is a cross-sectional, observational study that included 68 subjects. The control group consisted of 20 healthy individuals without known clinical morbidities or any pharmacological treatment with normal laboratory results. Forty-eight Fabry patients with the classic phenotype of Fabry disease were studied. Of them, 15 were not treated whereas 33 had received ERT for at least 24 months with agalsidase-β 1 mg/kg every fortnight (Fabrazyme, Genzyme Corp., Cambridge, MA, USA). All Fabry treated patients were on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at different doses. Podocyturia was analyzed by the identification of synaptopodin and podocalyxin by immunofluorescence.
Fabry disease was diagnosed in all cases by low enzymatic α-GalA activity in dried blood spots and peripheral blood leukocytes, later confirmed by the identification of an α-GalA gene mutation. Criteria for ERT therapy included symptoms related to Fabry disease (acroparesthesia, pain crisis or neuropathic pain, sensorineural loss, hypohydrosis, bowel abnormalities), cardiologic involvement as hyperthrophic myocardiopathy and/or arrhythmias and/or valve disease and/or myocardial fibrosis, cerebrovascular disease or renal compromise as proteinuria, decreased renal function and in some cases a kidney biopsy consistent with Fabry disease. Patient characteristics are outlined in Table 1. The following variables were studied: age, gender, estimated GFR (eGFR) by the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation, urinary protein/creatinine ratio (UPCR), podocyturia adjusted per gram of creatininuria and plasma lyso-Gb3 levels.
Table 1.
General clinical characteristics of controls and Fabry patients
| Variables | Control (n=20) | Fabry (n=48) | P-value control vs Fabry | Fabry untreated (n=15) | P-value control vs Fabry untreated | Fabry treated (n=33) | P-value control vs Fabry treated | P-value Fabry untreated vs treated |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 35 (26–53) | 36 (18–88) | NS | 24 (18–57) | NS | 38 (18–88) | NS | NS |
| Gender, males [n(%)] | 6 (30) | 15 (33) | NS | 3 (20) | NP | 12 (36) | NS | NS |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 111.45 (93.6–125.8) | 105.95 (40.7–143.3) | NS | 115 (48.8–137.8) | NS | 101.6 (18.2–156) | NS | NS |
| UPCR (g/g) | 0.04 (0.03–0.10) | 0.08 (0.3–2.45) | 0.002 | 0.07 (0.03–1.10) | 0.0093 | 0.08 (0.04–2.45) | <0.001 | NS |
| Podocyturia/creatininuria (cells/g) | 0 (0–0.14) | 0.15 (0–1.93) | <0.001 | 0.15 (0–0.36) | <0.001 | 0.14 (0–1.93) | <0.001 | NS |
| Colocalized podocytes synaptopodin+podocalyxin [n/N (%)] | 8/8 (100) | 13/48 (27) | <0.001 | 2 (13) | NP | 11 (33) | <0.001 | NP |
| Lyso-Gb3 (nmol/L) | 0.4 (0.3–0.5) | 5.2 (2.9–12) | <0.0001 | 5 (3.2–8) | NP | 6.4 (2.9–12) | <0.001 | NS |
Data are presented as median (range) unless otherwise indicated.
NS, non-significant; NP, not performed (due to low number of cases).
Podocyturia
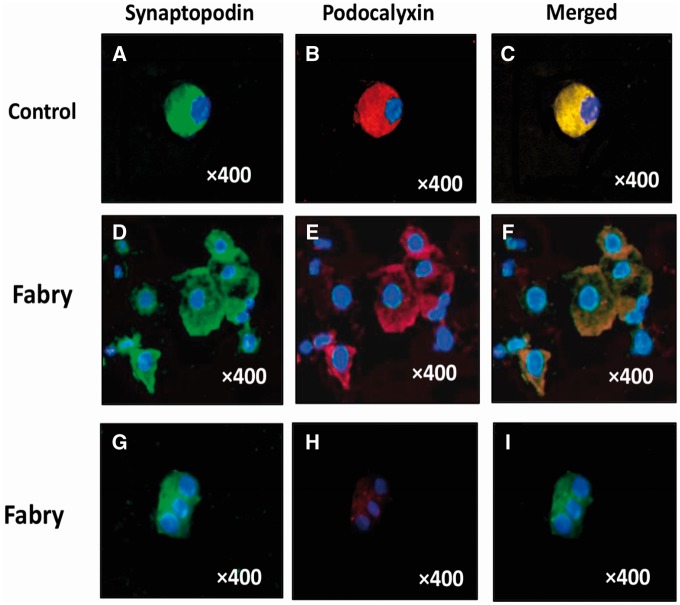
We have described the method to study podocyturia, although some modifications have been introduced compared with our previous work [5]. Briefly, a mid-stream freshly voided urine sample was collected on-site after a minimum of 3 h without voiding; 20 mL of urine were centrifuged at 700g for 5 min. The supernatant was discarded and the sediment was stored in 100 µL aliquots at room temperature mixed with an aliquot of 1.5 mL of formaldehyde buffer in Phosphate Buffered Saline (PBS) (pH 7.2–7.4). Then, we performed one centrifugation resuspending the pellet twice in 10% formaldehyde-buffer and once in 70% ethanol. The stretches were made in xilanized slides. Cells were preincubated with rabbit non-immunized serum (1:100) in a humid chamber at room temperature for 1 h. Thereafter podocytes were identified by indirect immunofluorescence using a mixture of antibodies formed by rabbit anti-synaptopodin as the primary antibody (1:100, Abcam, Cambridge, MA, USA) and mouse anti-podocalyxin antibody (1:100, Abcam) in a humid chamber at 4°C overnight. Three 5-min rinses with PBS were made and the samples were incubated with the secondary antibodies: anti-rabbit IgG ALEXA Fluor 488® (1:1000, Abcam) for synaptopodin and anti-mouse IgG ALEXA Fluor® 568 (1:100, Abcam) for podocalyxin in a humid chamber for 2 h at room temperature. Three 5-min rinses were followed by 40, 6-diamidino-2-phenylindole (DAPI) staining of nuclei. Samples were analyzed employing an epifluorescent Nikon Eclipse E200 microscope. Following our standardized technique, either synaptopodin-podocalyxin co-stained podocytes, synaptopodin-positive/podocalyxin-negative or synaptopodin-negative/podocalyxin-positive defined podocyturia and were counted in 10 randomly chosen 20× fields and the average of the counted podocytes in the microscopy fields was considered as the final count of podocyturia for each subject (Figure 1). The results were corrected based on the levels of urinary creatinine found in each sample. For that, the value of urinary creatinine was calculated for the initial urinary volume of 20 mL employed for podocyte counting. A total of three urine samples were collected from each subject every week. The podocyturia results of each of the three collections were compared amongst themselves to assess any significant difference along the 3-week analyzed period. During this time patients had to be in steady clinical conditions; otherwise, samples would be excluded until clinical stabilization.
FIGURE 1.
(A–C) Control podocyte. (A) Synaptopodin +; (B) podocalyxin +; (C) merged. (D–F) A cluster of Fabry podocytes (colocalization). (D) Synaptopodin +; (E) podocalyxin +; (F) merged. Fabry podocytes (no colocalization). (G–I) Fabry podocytes no colocalization: (G) Synaptopodin +; (H) podocalyxin. (I) Merging negative. Double indirect immunofluorescence. Magnification ×400.
Other laboratory determinations
Serum creatinine was assessed the same week that the urine was collected for podocyte counting employing an enzymatic method. UPCR was measured from the specimen employed for podocyte assessment. Plasma ethylendiaminotetraacetic acid samples were collected from Fabry patients to monitor. Lyso-Gb3 levels; these samples were collected prior to the scheduled ERT infusion. Lyso-Gb3 was extracted from plasma by solid phase (Waters Oasis McX, MA, USA). The separation and detection was done by ultra-performance liquid chromatography system coupled with a tandem mass spectrometer operated in multiple reaction monitoring (MRM) mode UPLC-MS/MS (Waters Corp., Milford, MA, USA) with a reverse phase column. The lyso-Gb3 calibration curve was linear between 0, 5 and 200 nmol/L. European research network for evaluation and improvement of screening (ERNDIM) samples (MCA Laboratories, Queen Beatrix Hospital, The Netherlands) were used as external quality control [23, 24].
Statistical analysis
Results are expressed as median and range. Variables were analyzed using the Wilcoxon Mann–Whitney test. Correlations between variables were obtained with the Spearman correlation coefficient. Results were considered significant when P < 0.05. The statistical program employed was InfoStat 2016, Córdoba, Argentina.
Ethical approval
The present protocol was approved by the Institutional Review Board of the Hospital Británico de Buenos Aires, Buenos Aires, Argentina. Informed consent was obtained from each study participant. All procedures performed in this work were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the 1975 Declaration of Helsinki and its later amendments, as revised in 2000.
RESULTS
Controls and Fabry patients did not differ with regard to age, gender and renal function (Table 1). However, patients had significantly higher UPCR, podocyturia and lyso-Gb3 levels than controls. In controls, 8/20 patients displayed podocyturia in the analyzed smears (Table 1). Podocyturia in control subjects consisted exclusively of synaptopodin-positive/podocalyxin-positive cells (Figure 1), whereas in Fabry patients synaptopodin-positive/podocalyxin-positive and synaptopodin-positive/podocalyxin-negative cells were encountered (Figure 1). No synaptopodin-negative/podocalyxin-positive podocytes were found in any Fabry patient. Therefore, for the purposes of this study due to the abovementioned results, we defined podocyturia as any cell that was synaptopodin positive. Moreover, to evaluate whether potential erratic urinary podocyte loss could lead to erroneous interpretation of podocyturia, three urinary samples were collected on a weekly basis from each patient and these three series were first analyzed separately. Thereafter, as changes in the count of cells were not different amongst the three urine collected series, four slides from each series were included in the analysis of podocyturia comprising a total of 12 slides per patient. Therefore, 576 slides belonging to Fabry patients were included in this study, plus the 60 from controls.
Untreated Fabry displayed significantly higher podocyturia and lyso-Gb3 levels than controls (Table 1). Fabry treated subjects were different with respect to UPCR, podocyturia and lyso-Gb3, albeit the significance of lyso-Gb3 levels versus controls was higher in Fabry untreated patients (Table 1). As mentioned, with respect to lyso-Gb3 levels, they were significantly elevated in Fabry subjects compared with controls, and this result was independent of ERT. However, the levels and statistical difference were more significant in the untreated group versus the treated group (Table 1). Mutations included the following both in the untreated and treated groups: L415P, D33G, D264Y, A292T, c.801-4A>G; c.640-1G>C, p.L180F, D155H, Y365X, p.D109G. When Fabry untreated and treated patients were compared, UPCR was the only variable that showed statistical significance (Table 1).
Analysis of podocyte subpopulations in Fabry patients
In Fabry patients, 13/48 colocalized synaptopodin and podocalyxin (27%) (Figure 1), whereas 35/48 did not (73%) (Figure 1). When the colocalization was analyzed according to therapy, untreated Fabry individuals showed synaptopodin-podocaylixin colocalization in 2/15 patients (13%) (Table 2), whereas in treated patients it was 33%, comprising 11/33 subjects (Table 3). This difference could not be ascribed to time on therapy (Table 3). In untreated patients, colocalized podocyturia was significantly higher than synaptopodin-positive/podocalyxin-negative podocytes (Table 2). In treated patients, colocalization was associated with significantly higher podocyte loss and lower proteinuria (Table 3).
Table 2.
Fabry untreated patients with and without colocalization
| Variables | Fabry untreated with colocalization | Fabry untreated without colocalization | P |
|---|---|---|---|
| (n=2) (13%) | (n=13) (87%) | ||
| Age (years) | 28 (18–38) | 24 (18–57) | NS |
| Gender, male [n(%)] | 1 (50) | 2 (15) | – |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 101 (84.7–131.6) | 114.2 (79.4–153.3) | NS |
| UPCR (g/g) | 0.04 (0.03–0.20) | 0.07 (0.03–0.88) | 0.03 |
| Podocyturia/creatininuria (cells/g) | 0.32 (0.28–0.36) | 0.11 (0–0.26) | 0.019 |
| Lyso-Gb3 (nmol/L) | 4.5 (3–7.5) | 5.0 (3.2–8) | NS |
Data are presented as median (range) unless otherwise indicated.
NS, non-significant.
Table 3.
Fabry treated patients with and without colocalization
| Variables | Fabry treated with colocalization | Fabry treated without colocalization | P |
|---|---|---|---|
| (n = 11) (33%) | (n = 22) (67%) | ||
| Age (years) | 36 (20–88) | 38.5 (18–76) | NS |
| Gender, male [n(%)] | 3 (27) | 9 (41) | – |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 101.6 (40.7–119) | 104.2 (18.2–156) | NS |
| UPCR (g/g) | 0.07 (0.04–0.34) | 0.10 (0.05–2.45) | 0.04 |
| Podocyturia/creatininuria (cells/g) | 0.35 (0.12–0.43) | 0.10 (0–0.43) | 0.0003 |
| Lyso-Gb3 (nmol/L) | 3.10 (2.9–6.4) | 7.25 (3.2–12.0) | NS |
| Time on therapy (months) | 96 (15–168) | 48 (13–180) | NS |
Data are presented as median (range) unless otherwise indicated.
NS, non-significant.
DISCUSSION
In the present 3-week observational cross-sectional study, we confirm other studies and ours which have shown that Fabry patients present higher levels of podocyturia and proteinuria (and in this work also lyso-Gb3 levels) compared with the general population. In this setting, within the Fabry population we found that detached podocytes displayed a decreased amount of podocalyxin-positive urinary detached podocytes when compared with synaptopodin-positive cells. Despite the fact that colocalized podocytes were higher in treated versus untreated subjects (33% versus 13%), the number of patients is not high enough to draw robust conclusions as to whether ERT may exert any protective effect on the glycocalyx. The decreased amount of the sialoprotein podocalyxin may be depicting a more damaged glycocalyx in Fabry patients. As this phenomenon occurred in all Fabry individuals but at a dissimilar non-significant extent, it may be commencing at early stages of the nephropathy.
Podocyturia assessed by podocalyxin alone may be underestimated, as no synaptopodin-negative/podocalyxin-positive podocytes were encountered in the 576 slides under observation. However, we believe that there may potentially also exist synaptopodin-negative podocytes that may have not been diagnosed as no additional podocyte proteins were tagged. The absence of podocalyxin-negative podocytes in our control group and the lower amount of podocalyxin-positive cells in both Fabry untreated and treated patients may indicate that the glycocalyx of Fabry patients is damaged.
It is known that podocalyxin is a sialomucin of ∼140 kDa that is localized predominantly at the apical compartment of the glycocalyx of podocytes and contributes to the negative charge of the glomerular filtration slits due to its high contents of sulfate and sialic acid [25, 26]. In the kidney, it is predominantly expressed by podocytes, albeit it can also be present in significantly lesser amounts in endothelial cells and also in other tissues [27, 28]. It has also been shown that podocalyxin is essential to maintain the structure of the glomerulus in order to prevent the occlusion of the slit diaphragms and urinary spaces [29–31]. In addition, podocalyxin interacts with intracellular proteins as integrins and also with extracellular ligands, and regulates cellular adhesion, migration and cell morphology as microvilli assemblance and patency [21, 22, 32]. Albeit not especifically addressed for podocalyxin, it has been shown that in Fabry nephropathy foot process widening and effacement are two ultrastructural findings commonly found in kidney biopsies [16, 17]. Particularly with respect to the increased width of foot processes, it has been shown to be an early event in Fabry nephropathy, and that it can antedate proteinuria [17].
Thus, the effects of podocalyxin in enhancing cell attachment, migration and organization of the cellular microfibrllar system implies the participation of integrins in the process of cell activation [22]. Therefore, a decrease in podocaylxin may be associated with less adhesiveness of cells to the extracellular matrix and enhance podocyturia. This statement may partially explain our findings that detached podocytes displayed lower podocalyxin-positive cells. Finally, podocalyxin interacts mainly with the Na+/H+ exchanger regulatory factor-1 (NHERF1) and NHERF2, two adaptor proteins involved in protein trafficking, ion transport and signaling, and associates with ezrin to cause actin stimulation, podocyte contraction and integrin β3 chain stimulation [33–37].
We have also shown that in human detached Fabry podocytes urokinase-type plasminogen activator receptor (uPAR) concentration is augmented and colocalizes with synaptopodin, whereas in in vitro experiments we have confirmed that lyso-Gb3 increases uPAR synthesis in human podocytes [38].
A number of studies reported that abnormalities in podocyte motility in vitro correlated with proteinuria in vivo [39]. In this regard, uPAR is required to activate the αvβ3 integrin in podocytes, which promotes cell motility, whereas activation of the small guanosine triphosphatases cell division control protein 42 (Cdc42) and ras-related C3 botulinum toxin substrate 1 (Rac1) [40]. Blockade of the αvβ3 integrin reduces podocyte motility in vitro and causes proteinuria in mice [40]. In fact, an alteration in podocyte motility has been postulated as the focus of specific proteinuric pathways involving enzymatic cleavage of essential regulators of podocyte actin dynamics by cytosolic cathepsin-L, resulting in a hypermotile podocyte phenotype [41]. A further important mechanism shown to regulate podocyte motility is via the highly expressed protein synaptopodin and its interaction with actin [39]. Synaptopodin is an upstream regulator of RhoA, inhibiting its degradation and thereby promoting podocyte motility [42]. These findings may also explain our findings that synaptopodin is elevated in detached podocytes: the podocyte contractile apparatus must be activated for a later detachment [38, 41].
Therefore, we propose that there may be at least two possible mechanisms by which podocyte detachment may occur in Fabry nephropathy: the stimulation by lyso-Gb3 of the αvβ3-uPAR coupling [38], and the decrease in podocalyxin concentration and distal interaction with the β3 subunit of integrins. Whether these two pathways are linked or not has not been yet investigated. Finally, the specific cause of podocalyxin decrease on podocytes in Fabry nephropathy is unknown. It has been reported that kidney disease is associated with glycocalyx degradation, and that sustained endothelial activation results in the stimulation of enzymes that eventually degrade the glycocalyx, as in diabetic nephropathy [43, 44].
In the kidney, under normal conditions both synaptopodin and podocalyxin are predominantly expressed in podocytes. Despite being morphologically different from podocytes, tubular cells can express low amounts of synaptopodin, whereas podocalyxin is normally present in certain subsets of neurons, endothelial cells and hemopoietic stems cells besides podocytes. In this respect, Fabry disease particularly affects neurons and the endothelium, the circulating, suggesting that filtered and/or intracellular enzyme substrate of α-GalA as lyso-Gb3 may be a potential cause of decreased podocalyxin concentration in podocytes. We have shown that lyso-Gb3 may not be a mere biomarker in Fabry disease; it also participates in the pathophysiology of Fabry nephropathy [38].
Many distinguished investigators have made significant contributions to unveil the pathophysiology of Fabry nephropathy and other glomerular diseases through the identification of detached podocytes employing podocalyxin as a target [13, 45]. Our findings may suggest that it is probable that higher amounts of urinary detached podocytes may be identified by employing synaptopodin. One hypothesis could be that as synaptopodin is located exclusively in the cytoplasm, this is a more protected compartment against extracellular insults, were this the case. In addition, as synaptopodin regulates actin contraction, and podocyte detachment requires the sustained contraction of the cell, larger amounts of synaptopodin may be present on detached podocytes with respect to podocalyxin. Albeit podocalyxin has also been identified in the cytoplasm, it is mainly located in the cellular membrane, being part of the glycocalyx of podocytes [22]. Finally, the podocalyxin kit employed in this study is different from the ones employed in other studies, and it could have also influenced the obtained results.
Finally, in this study, we have also shown that despite the fact that podocyturia may be potentially erratic on many occasions, it was not significantly different at least in the 3-week period taken into consideration. We therefore advocate for a single urinary sample to assess the degree of podocyturia amongst Fabry nephropathy patients. Whether this applies for other glomerulopathies deserves future investigations.
The employed method to identify podocytes was immunofluorescence, a colorimetric diagnostic tool that has not been validated yet. There are some limitations in this study that deserve to be commented on and the results should be interpreted with caution. The number of patients included may not be large, but considering Fabry disease as a rare disorder, we believe that the cohort is numerous enough so as to obtain relevant conclusions with respect to urinary detached podocytes. In this respect, the fact that three weekly urinary samples from each included patient were evaluated, provided this study with a large number of analyzed samples. We also lacked kidney biopsies against which our findings could be challenged, particularly with regard to podocalyxin distribution in tissular podocytes. We have calculated GFRs by eGFR employing the CKD-EPI equation. In this respect, Rombach et al. have compared these two common methods for creatinine-based eGFR against the gold standard measured GFR, and reported that regardless of the method employed, creatinine-based formulas tend to overestimate GFR in Fabry young male patients, where detection of glomerular hyperfiltration would be most likely expected [46]. To our knowledge, data about women with Fabry disease are not available.
In conclusion, we have again confirmed podocyturia as an early event in Fabry nephropathy, in which podocytes predominantly appear to display a damaged glycocalyx due to lower identification of podocalyxin-positive cells. Individuals treated with ERT had higher proteinuria and more diseased detached podocytes, suggesting that ERT may be started late despite similar GFRs between groups. This result may suggest that the current indications for the initiation of ERT in Fabry nephropathy, mainly based on the presence of proteinuria and/or deterioration in kidney function, ought to be revisited. Podocyturia assessed by podocalyxin alone may be underestimated, but it may also be the case if assessed by synaptopodin alone as well. The implications of decreased podocalyxin and/or podocyte glycocalyx damage in Fabry disease deserve further investigations, as it may be relevant to pharmacological interventions.
ACKNOWLEDGEMENTS
We wish to thank Ms Analía Pellegrini and Laura Ares for their professional assistance. The Collaborative Fabry Working Group wishes to thank the following colleagues for the referral of patients for this study: K. Aguilera, O. Amoreo, G.H. Cabrera, G. Compte, S.F. Di Pietrantonio, R. Migueles, F.J. Perretta and D. Ripeau.
FUNDING
Genzyme provided funding for the purchase of synaptopodin and podocalyxin kits. Genzyme had no participation in the design, elaboration, analysis or draft of the manuscript and did not interact with the investigators.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Brady RO, Gal AE, Bradley RM. et al. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med 1967; 276: 1163–1167 [DOI] [PubMed] [Google Scholar]
- 2. Fogo AB, Bostad L, Svarstad E. et al. Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 2010; 25: 2168–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortiz A, Abiose A, Bichet DG. et al. Time to treatment benefit for adult patients with Fabry disease receiving agalsidase beta: data from the Fabry Registry. J Med Genet 2016; 53: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weidemann F, Niemann M, Störk S. et al. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: evidence for disease progression towards serious complications. J Intern Med 2013; 274: 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trimarchi H, Canzonieri R, Schiel A. et al. Podocyturia in Fabry adult untreated and treated patients. A controlled study. J Nephrol 2016; 6: 791–797 [DOI] [PubMed] [Google Scholar]
- 6. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007; 49: S12–S154 [DOI] [PubMed] [Google Scholar]
- 7. Eikrem Ø, Skrunes R, Tøndel C. et al. Pathomechanisms of renal Fabry disease. Cell Tissue Res 2017; 369: 53–62 [DOI] [PubMed] [Google Scholar]
- 8. Das AM, Naim HY.. Biochemical basis of Fabry disease with emphasis on mitochondrial function and protein trafficking. Adv Clin Chem 2009; 49: 57–71 [DOI] [PubMed] [Google Scholar]
- 9. Lucke T, Hoppner W, Schmidt E. et al. Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab 2004; 82: 93–97 [DOI] [PubMed] [Google Scholar]
- 10. Weidemann F, Sanchez-Niño MD, Politei J. et al. Fibrosis: a key feature of Fabry disease with potential therapeutic implications. Orphanet J Rare Dis 2013; 8: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chévrier M, Brakch N, Céline L. et al. Autophagosome maturation is impaired in Fabry disease. Autophagy 2010; 6: 589–599 [DOI] [PubMed] [Google Scholar]
- 12. Liebau MC, Braun F, Hopker K. et al. Dysregulated autophagy contributes to podocyte damage in Fabry’s disease. PLoS One 2013; 8: e63506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fall B, Scott CR, Mauer M. et al. Urinary podocyte loss is increased in patients with Fabry disease and correlates with clinical severity of Fabry nephropathy. PLoS One 2016; 11: e0168346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schiffmann R, Hughes DA, Linthorst GE. et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017: 91; 284–293 [DOI] [PubMed] [Google Scholar]
- 15. Germain DP, Waldek S, Banikazemi M. et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 2007; 18: 1547–1557 [DOI] [PubMed] [Google Scholar]
- 16. Tøndel C, Bostad L, Hirth A. et al. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis 2008; 51: 767–776 [DOI] [PubMed] [Google Scholar]
- 17. Tøndel C, Kanai T, Larsen KK. et al. Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron 2015; 129: 16–21 [DOI] [PubMed] [Google Scholar]
- 18. Najafian B, Svarstad E, Bostad L. et al. Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int 2011; 79: 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lortat-Jacob H. The molecular basis and functional implications of chemokine interactions with heparan sulphate. Curr Opin Struct Biol 2009; 19: 543–548 [DOI] [PubMed] [Google Scholar]
- 20. Kosto KB, Deen WM.. Hindered convection of macromolecules in hydrogels. Biophys J 2005; 88: 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nielsen JS, McNagny KM.. The role of podocalyxin in health and disease. J Am Soc Nephrol 2009; 20: 1669–1676 [DOI] [PubMed] [Google Scholar]
- 22. Larrucea S, Butta N, Arias-Salgado EG. et al. Expression of podocalyxin enhances the adherence, migration, and intercellular communication of cells. Exp Cell Res 2008; 314: 2004–2015 [DOI] [PubMed] [Google Scholar]
- 23. Boutin M., Gagnon L, Lavoie P. et al. LC-MS/MS analysis of plasma Lyso-Gb3 in Fabry disease. Clin Chim Acta 2012; 414: 273–280 [DOI] [PubMed] [Google Scholar]
- 24. Boutin M, Auray-Blais C.. Multiplex tandem mass spectrometry analysis of novel plasma Lyso-Gb3-related analogues in Fabry disease. Anal Chem 2014; 86: 3476–3483 [DOI] [PubMed] [Google Scholar]
- 25. Andrews PM. Glomerular epithelial alterations resulting from sialic acid surface coat removal. Kidney Int 1979; 15: 376–385 [DOI] [PubMed] [Google Scholar]
- 26. Dekan G, Gabel C, Farquhar MG.. Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proc Natl Acad Sci USA 1991; 88: 5398–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sassetti AC, Van Zante A, Rose SD.. Identification of endoglycan, a member of the CD34/Podocalyxin family of sialomucins. J Biol Chem 2000; 275: 9001–9010 [DOI] [PubMed] [Google Scholar]
- 28. Horvat R, Hovorka A, Dekan G. et al. Endothelial cell membranes contain podocalyxin the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol 1986; 102: 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerjaschki D, Sharkey DJ, Farquhar MG.. Identification and characterization of podocalyxin the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 1984; 98: 1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kerjaschki D, Poczewski H, Dekan G. et al. Identification of a major sialoprotein in the glycocalyx of human visceral glomerular epithelial cells. J Clin Invest 1986; 78: 1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Charest PM, Roth J.. Localization of sialic acid in kidney glomeruli: regionalization in the podocyte plasma membrane and loss in experimental nephrosis. Proc Natl Acad Sci USA 1985; 82: 8509–8512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nielsen JS, Graves ML, Chelliah S. et al. The CD34-related molecule podocalyxin is a potent inducer of microvillus formation. PLoS One 2007; 2: e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan PC, Furness SG, Merkens H. et al. Na+/H+ exchanger regulatory factor-1 is a hematopoietic ligand for a subset of the CD34 family of stem cell surface proteins. Stem Cells 2006; 24: 1150–1161 [DOI] [PubMed] [Google Scholar]
- 34. Donowitz M, Cha B, Zachos NC. et al. NHERF family and NHE3 regulation. J Physiol 2005; 567: 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shenolikar S, Voltz JW, Cunningham R. et al. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology (Bethesda) 2004; 19: 362–369 [DOI] [PubMed] [Google Scholar]
- 36. Voltz JW, Weinman EJ, Shenolikar S.. Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 2001; 20: 6309–6314 [DOI] [PubMed] [Google Scholar]
- 37. Orlando RA, Takeda T, Zak B. et al. The glomerular epithelial cell anti-adhesin podocalyxin associates with the actin cytoskeleton through interactions with ezrin. J Am Soc Nephrol 2001; 12: 1589–1598 [DOI] [PubMed] [Google Scholar]
- 38. Trimarchi H, Canzonieri R, Schiel A. et al. The expression of uPAR in urinary podocytes in patients with Fabry disease. Int J Nephrol 2017; ID1287289: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Welsh GI, Saleem MA.. The podocyte cytoskeleton-key to a functioning glomerulus in health and disease. Nat Rev Nephrol 2012; 8: 14–21 [DOI] [PubMed] [Google Scholar]
- 40. Wei C, Möller CC, Altintas MM. et al. Modification of kidney barrier function by the urokinase receptor. Nat Med 2008; 14: 55–63 [DOI] [PubMed] [Google Scholar]
- 41. Mundel P, Reiser J.. Proteinuria: an enzymatic disease of the podocyte? Kidney Int 2010; 77: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Asanuma K, Yanagida-Asanuma E, Faul C. et al. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 2006; 8: 485–491 [DOI] [PubMed] [Google Scholar]
- 43. Nieuwdorp M, Mooij HL, Kroon J. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–1132 [DOI] [PubMed] [Google Scholar]
- 44. Nieuwdorp M, van Haeften TW, Gouverneur MCLG. et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006; 55: 480–486 [DOI] [PubMed] [Google Scholar]
- 45. Pereira EM, da Silva AS, Labilloy A. et al. Podocyturia in Fabry disease. J Bras Nefrol 2016; 38: 49–53 [DOI] [PubMed] [Google Scholar]
- 46. Rombach SM, Baas MC, ten Berge IJM. et al. The value of estimated GFR in comparison to measured GFR for the assessment of renal function in adult patients with Fabry disease. Nephrol Dial Transplant 2010; 25: 2549–2556 [DOI] [PubMed] [Google Scholar]