Abstract
Vascular endothelial growth factor (VEGF) receptor inhibition is a commonly used tool to prevent vascular proliferation in tumors and retinal diseases. The antiangiogenic effects of these drugs have made them potent adjunct therapies when given systemically for malignancies. They are also useful tools to ameliorate diminishing eyesight in retinopathy. Hypertension and proteinuria have been observed in systemic VEGF inhibitor therapy, with rarer presentations involving nephrotic-range proteinuria due to glomerulopathies. Pharmacokinetic studies have shown detectable blood levels of anti-VEGF inhibitors up to 30 days postintravitreal injection. Animal studies have also demonstrated binding of VEGF inhibitors in simian glomeruli 1 week after a single intravitreal injection. We report three patients who received intravitreal bevacizumab and/or aflibercept with worsening hypertension, proteinuria and renal injury. Data regarding emerging evidence of VEGF inhibitor nephrotoxicity after intravitreal injections are also presented. The clinical data and the existing literature are reviewed to support the hypothesis that intravitreal anti-VEGF agents may be unrecognized nephrotoxins. These agents are given to vulnerable patients with diabetes, hypertension and preexisting nephropathy and proteinuria. This case series is reported to spur further study of the systemic effects of intravitreal VEGF inhibitors.
Keywords: angiogenesis, diabetes mellitus, diabetic nephropathy, hypertension, proteinuria, VEGF
INTRODUCTION
Vascular endothelial growth factor (VEGF) inhibitors started to make an entrance in clinical use in the late 1990s as novel anti-neoplastic agents. Bevacizumab (Avastin®; Genentech, South San Francisco, CA, USA), the first agent of its class on the market, is a humanized anti-VEGF monoclonal antibody. It is currently indicated for non–small cell lung cancer, renal cell carcinoma, breast cancer, ovarian cancer, glioblastoma multiforme (GBM) and other malignancies [1–6]. Its side-effect profile as a systemic agent has been extensively studied and includes hypertension, proteinuria, thrombotic microangiopathy (TMA), renal injury and in some cases frank glomerular disease with nephrotic-range proteinuria [7–9].
Bevacizumab inhibits angiogenesis by direct binding of VEGF-A, which in turn disrupts signaling of VEGF receptors 1 and 2. VEGF receptors are predominately expressed on vascular endothelial cells. VEGF activity is also required in a paracrine manner as a trophic signal by the renal podocytes that line the glomerular basement membrane [10, 11]. Pathological VEGF signaling has been implicated in proteinuria due to preeclampsia and diabetic nephropathy (DN) and induced by mammalian target of rapamycin (mTOR) inhibitors like sirolimus and everolimus [12–14].
Because VEGF is a crucial regulator of angiogenesis, it has become an attractive target for treating ophthalmic diseases caused by inappropriate blood vessel proliferation. Bevacizumab is given through the intravitreal route as an ‘off-label’ treatment for the management of intraocular neovascular, edematous and proliferative disorders [15, 16]. Systemic administration of bevacizumab and related anti-VEGF agents is known to cause hypertension and proteinuria in a variable manner [17, 18]. The relationship between intravitreal anti-VEGF agents and systemic hypertension has not been well established due to conflicting study results [19–23]. Furthermore, the effects of intravitreal anti-VEGF injections on proteinuria and renal function have not been directly studied.
Given the extensive use of intravitreal anti-VEGF agents in diabetics with retinopathy and concomitant nephropathy, special attention needs to be focused on changes in proteinuria and blood pressure after these injections are administered. New studies show that anti-VEGF agents are absorbed systemically [24], actively suppressing VEGF activity in vivo [24]. There are also additional studies showing anti-VEGF agents binding to and disrupting the glomerular basement membranes of simian glomeruli 1 week after intravitreal injection [25]. A more positive effect is the ‘fellow eye effect’ that has been observed after intravitreal injection with anti-VEGF agents. The effect is that an injection of anti-VEGF agent in one eye ameliorates diabetic retinopathy in the contralateral eye [26].
An earlier study by Avery et al. [27] showed evidence of systemic absorption in patients with age-related macular degeneration (AMD). A more recent publication by the same group reproduced these results in patients with diabetic macular edema and central retinal vein occlusion [27]. The elegant pharmacodynamic studies showed serum concentrations as high as 0.1–0.2 nmol/L, which is comparable with data published by the US Food and Drug Administration (FDA). These levels were near or above the 50% inhibitory concentrations (IC50) of bevacizumab, aflibercept and ranibizumab as reported by Avery et al. [24]. Both studies noted that ranibizumab had the lowest level of drug absorption after intravitreal injection and caused the least amount of systemic VEGF inhibition [24, 26–28]. These same findings were validated by Rogers et al. [29], who analyzed the participants in the Inhibition of VEGF in Age-related choroidal Neovascularization (IVAN) trial. Rogers et al. also confirmed less systemic VEGF inhibition after intravitreal ranibizumab relative to intravitreal bevacizumab or intravitreal aflibercept.
Given the aforementioned evidence, we began inquiring about recent intravitreal exposures to anti-VEGF agents in our clinic visits with patients with chronic kidney disease (CKD). We looked for any changes in proteinuria, hypertension control or renal function in diabetic patients who were initiated on these agents. We quickly noted two diabetic patients who developed rapidly worsening proteinuria after initiation of bevacizumab injections. A third patient developed refractory hypertension after initiation of bevacizumab injections that persisted when she was switched to aflibercept. We present these three cases to examine the unexplored question of whether there are systemic effects of intravitreal injections of VEGF inhibitors.
CASE REPORTS
Patient 1
The first patient is a 54-year-old Hispanic female with poorly controlled diabetes mellitus with a hemoglobin A1C of >10 since diagnosis in 2011. She was treated with oral agents and insulin shortly after diagnosis, given her poor glycemic control. She initially did not have overt DN, but gradually started developing albuminuria and renal function decline. Her serum creatinine first started rising to 123.79 μmol/L (1.4–1.6 mg/dL) over 2014–15 and her proteinuria rose from 1000 μg albumin/mg creatinine (1000 μg albumin/mg creatinine = 1 g albumin/g creatinine) to 2000–3000 μg albumin/mg creatinine. Her hyperglycemia remained persistent in spite of her medication regimen. She began to take nonsteroidal anti-inflammatory drugs (NSAIDs) a few times a month, but as her renal function declined, she was instructed to stop NSAID use in 2016–17. Urine protein:creatinine ratios were not obtained in the Kaiser Permanente (KP) system, so trends of albumin:creatinine ratio (ACR) were followed at the University of California Los Angeles Health System.
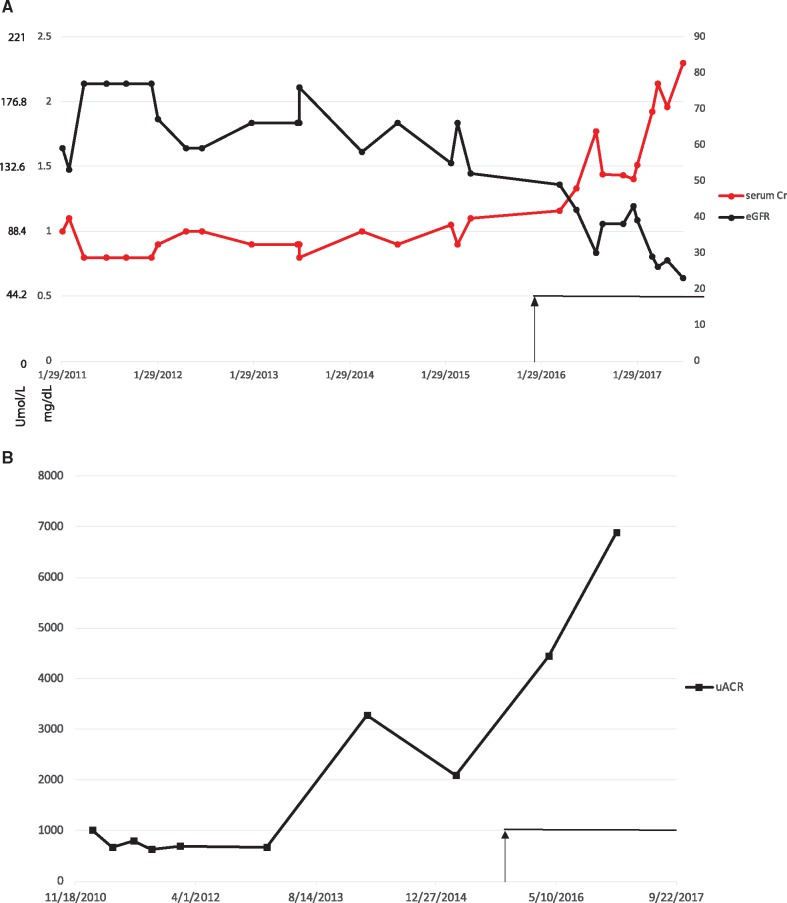
She was examined in KP’s ophthalmology clinic and was noted to have worsening glaucoma and proliferative DN. She started treatment with bevacizumab every 4 weeks as needed in January 2016. She required a monthly dose of 1.25 mg of bevacizumab over the next 13 months in either one or both eyes over a total of 10 separate visits, with the indication of proliferative diabetic retinopathy (DR). Thus the full dose administered was 12.5 mg of bevacizumab over 10 injections during this period. A notable increase in the slope of proteinuria was noted, with an increase in ACR to 4000 μg/mg and ultimately to 7000 μg/mg (see Figure 1 for trends of renal function decline and worsening proteinuria in this patient). The patient’s blood pressure did not rise beyond her usual range of 140–150 mmHg and, as such, it was not plotted.
FIGURE 1.
Changes in proteinuria and renal function in a diabetic (Patient 1) treated with bevacizumab. (A) Serum creatinine (μmol/L and mg/dL) and eGFR (by Cockroft–Gault, non-African American, in mL/min versus date). (B) ACR (μg albumin/mg creatinine) (equivalent to 0.001 g/g creatinine) versus date. Black arrow and line shows the period of bevacizumab injections (10 injections of 1.25 mg; total = 12.5 mg).
It was noted that her renal function seemed to worsen more rapidly in the year after bevacizumab was started. A full serological workup was performed, but was unrevealing. Due to the more rapid than expected decline in renal function, a renal biopsy was performed. The biopsy showed nodular diabetic glomerulosclerosis and hypertensive nephrosclerosis without any histological features suggestive of other glomerular disease. The timing of proteinuria exacerbation corresponded with the first few months after starting intravitreal bevacizumab injections. The mechanism of renal function decline may be due to worsening proteinuria and acute tubular necrosis. Given the severity of the patient’s DR, a change to a less potent agent, like ranibizumab, was not immediately feasible. The patient was managed with renin–angiotensin–aldosterone system (RAAS) blockade with plans to add a non-dihydropyridine calcium channel blocker (i.e. verapamil or diltiazem) for proteinuria control, with continuing attempts to optimize hypertensive control.
Patient 2
The second patient is a 53-year-old Asian male with a history of type 2 diabetes mellitus that was diagnosed in 2010. His medical history also includes hyperlipidemia, severe uncontrolled hypertension and advanced renal disease with Stage 4 CKD just before the need for intravitreal anti-VEGF therapy was noted. He had poor compliance with his prescribed diabetic diet. His hemoglobin A1C ranged between 10% and 11% at the time of initial diagnosis and remained consistency high for years afterwards. He was on oral agents for his diabetes mellitus since he declined insulin therapy despite elevated blood sugar levels and had a history of NSAID use earlier in his history that likely contributed to his CKD progression. As his renal function declined, he reduced his NSAID usage significantly and would only use NSAIDs during infrequent gout flares. His metformin was stopped in March 2017 and changed to a sulfonylurea due to his declining renal function.
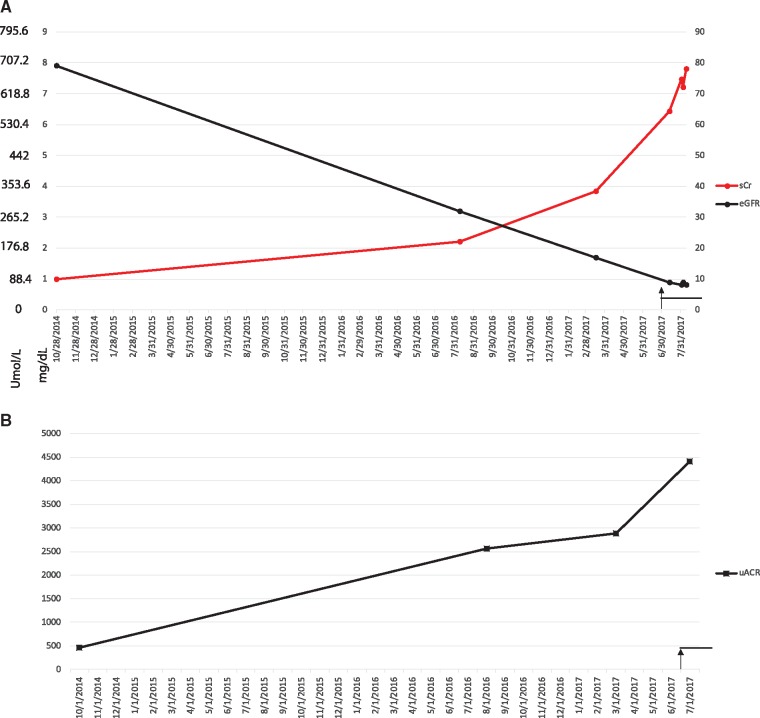
He had an estimated glomerular filtration rate (eGFR) of 17 mL/min at the initiation of bevacizumab injections on 1 July 2017. He had a nearly linear decline in eGFR (mL/min) as measured by the Cockroft–Gault equation. His proteinuria had been stable at 2800–3000 μg albuminuria/mg creatinine (= 2.8 g albumin/g creatinine), but after starting bevacizumab injections for proliferative DR his ACR increased to 4500 μg albumin/mg creatinine.
Within the first 2 months of initiating therapy he received four injections of 1.25 mg of bevacizumab at an interval of every 2 weeks alternating between each eye. His injections were planned so that he waited at least 1 month between injections in the same eye. The total dose delivered over these four injections was 5 mg, and within that short interval, his renal function deteriorated to the extent of needing renal replacement therapy (hemodialysis).
His renal function had already been declining prior to anti-VEGF therapy, but after starting therapy his creatinine rose from a baseline of 338 μmol/L (3.83 mg/dL) to 688.8 μmol/L (7.79 mg/dL). His blood pressure at baseline was 180–200 mmHg systolic (SBP) and 100–120 mmHg diastolic (DBP). There was no discernible change in the SBP or DBP, though his blood pressure was already severely elevated. The increase in urine protein from baseline to higher levels after the anti-VEGF injections and renal function trends are depicted in Figure 2.
FIGURE 2.
Changes in proteinuria and renal function in a diabetic (Patient 2) treated with bevacizumab. (A) Serum creatinine (μmol/L and mg/dL) and eGFR (by Cockroft–Gault, non–African American, in mL/min versus date). (B) ACR (μg albumin/mg creatinine) (equivalent to 0.001 g/g creatinine) versus date. Black arrow and line show the period of bevacizumab injections (four injections of 1.25 mg; total = 6.25mg).
Patient 3
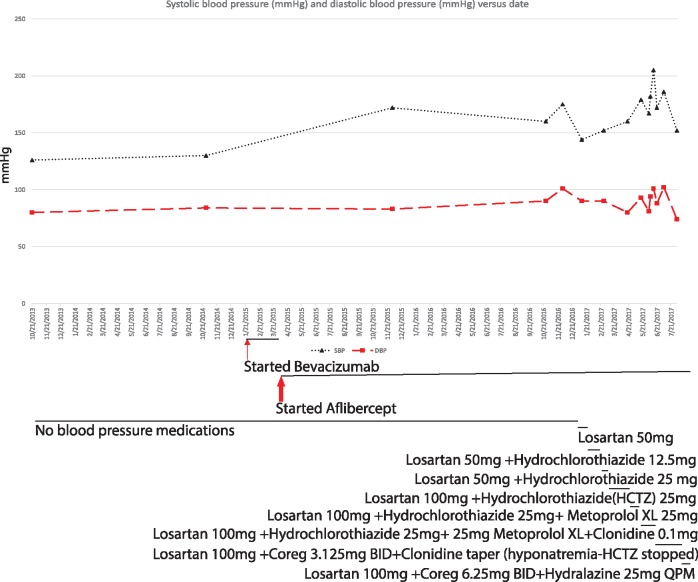
The third patient presented is a nondiabetic, 65-year-old female who was diagnosed with AMD. She had normal renal function at 56.59 μmol/L (0.64 mg/dL) with no measurable proteinuria. She started bevacizumab in January 2015 and continued receiving anti-VEGF injections every 4–6 weeks. After one dose of bevacizumab (1.25 mg) she was switched to aflibercept because of the need for a stronger antiproliferative agent. Initially the patient only met the criteria for prehypertension, with an SBP within the 130–140 mmHg range and normal DBP. However, we noticed her blood pressure began to rise from her recorded baseline after initiating injections. She was monitored until July 2016, when her SBP was routinely 170–200 mmHg and antihypertensive therapy was initiated. Thus she received 1.25 mg of bevacizumab and 16 injections of 2 mg aflibercept in the period between January 2015 and July 2016, for a total dose of 32 mg of aflibercept.
Despite multidrug regimens, her SBP rarely dropped below 150 mmHg. Her secondary hypertension workup for renal artery stenosis and hyperaldosteronism was negative. She had a borderline elevated urine cortisol test, but it was too low to be diagnostic of hypercortisolemia, and the lack of weight loss, potassium wasting or finding of an abdominal mass did not suggest another endocrine cause for the observed hypertension. (Refer to Figure 3 for blood pressure trends and antihypertensive regimens needed to control increasingly resistant hypertension in this patient.) A recommendation was made to change the patient to ranibizumab if possible. Some difficulties arose from the ophthalmology perspective, however, given the severity of her macular degeneration (MD) and the lower potency of ranibizumab. The plan of care was to work to optimize this patient’s blood pressure management with the goal of minimizing anti-VEGF intravitreal injections as able and to switch to a lower potency agent, like ranibizumab, when clinically feasible.
FIGURE 3.
Blood pressure changes in Patient 3 with AMD treated with bevacizumab followed by aflibercept. BID, twice a day; HCTZ, hydrochlorothiazide; QPM, every evening; XL, extended release. Black lines show the period of medication administration. Thin arrow = time of bevacizumab injection (one 1.25-mg injection of bevacizumab); thick arrow = period of aflibercept injections (16 injections of 2 mg aflibercept; total = 32 mg).
DISCUSSION
The observations presented above are not sufficient to establish a firm link between intravitreal anti-VEGF therapies and worsening of hypertension, proteinuria and/or renal function, although the timing observed suggests a correlation between beginning treatment with intravitreal injections of anti-VEGF agents and worsening of the aforementioned parameters. This is of interest given the established links between intravenous VEGF treatment and worsening of hypertension [12], proteinuria and glomerular disease [30].
Reports of glomerular disease [30] and worsening hypertension have also been noted in patients using intravitreal anti-VEGF injections [21, 22]. Pharmacokinetic and pharmacodynamic studies prove that intravitreal anti-VEGF drugs are absorbed and remain detectable in the blood for >1 month post-injection [24]. Other studies show that a week after intravitreal anti-VEGF agents are injected these agents can be detected binding to simian glomeruli, causing pathological changes in the glomerular basement membrane [25]. Thus it is not completely surprising that patients receiving intravitreal anti-VEGF injections display the same phenotypes of systemically treated patients. This is because intravitreal injections of bevacizumab, aflibercept and even ranibizumab are absorbed to varying degrees.
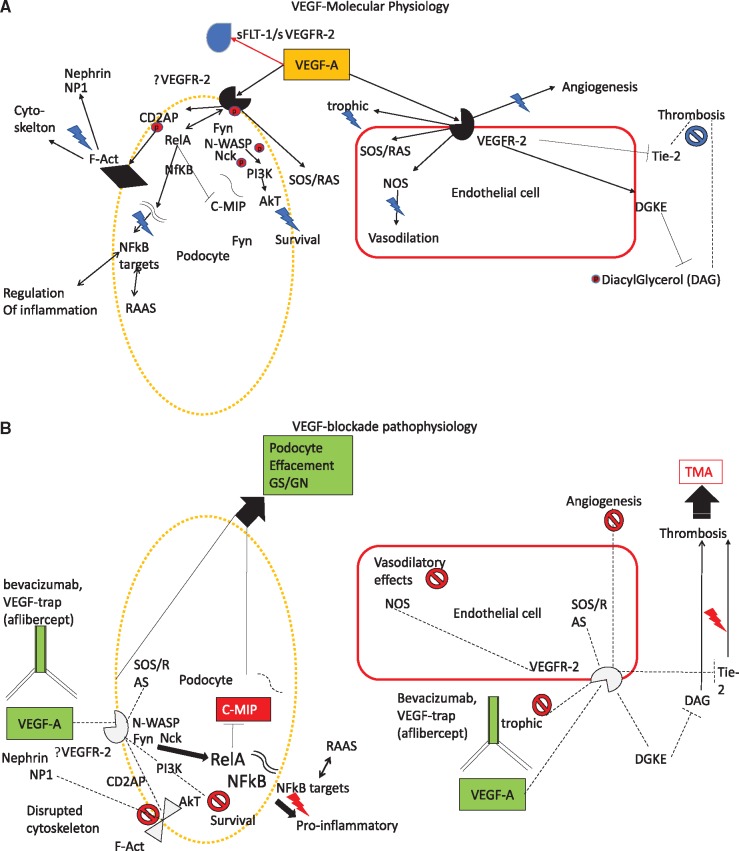
There have been nine other reports encompassing 13 patients who demonstrated renal failure, hypertension and glomerulopathies after intravitreal anti-VEGF injections [31–39]. This is postulated to occur through disruption of VEGF and downstream receptor tyrosine kinase signaling, leading to protein kinase C activation and dysregulation of nitric oxide (NO) generation [40, 41]. Refer to Table 1 for the 17 cases of hypertension and proteinuria, of which 13 are reported in the literature [31–39], 1 previously historic, unpublished report that we reference and the 3 cases reported in this series. Also see Figure 4 for the molecular physiology of VEGF signaling.
Table 1.
Cases of intravitreal injections of VEGF inhibitors resulting in glomerular disease or hypertension
| Reference | Anti-VEGF agent | Proteinuria | Renal biopsy | Treatment | Age (years) | Gender | DR | N | UC HTN |
|---|---|---|---|---|---|---|---|---|---|
| [31] | Bevacizumab, ranibizumab | 2.2–2.4 g/g Cr UPC | Case 1 MGN, Case 2 AKI, Inc Prot and TMA | Case 1: WD, Case 2: MMF, Px | 52, 67 | 2M | No (MD) | 2 | No |
| [32] | Bevacizumab | NR | No, decreased eGFR no biopsy | No immunosuppression | NR | NR | Yes | 3 | No |
| [33] | Ranibizumab, bevacizumab | Inc | No, decreased eGFR no biopsy | HD started | 51, 68 | 1F, 1M | Yes | 2 | No |
| [34] | Not specified | NR | No, decreased eGFR no biopsy | No immunosuppression | NR | NR | Yes | 1 | No |
| [35] | Bevacizumab | 8.6 g/g Cr UPC | MGN | No immunosupression, WD | 74 | M | Yes | 1 | No |
| [36] | Ranibizumab | 9.4 g/g Cr UPC | Class IV DN | No immunosuppresion | 56 | M | Yes | 1 | No |
| [37] | Ranibizumab | NR | TMA | WD | 77 | F | No (MD) | 1 | No |
| [38] | Bevacizumab | 11 g/g Cr UPC | MCD | High-dose prednisone | 54 | M | Yes | 1 | No |
| [39] | Bevacizumab | 4.2 g/g Cr UPC | MCD | High-dose prednisone, mizoribine | 16 | F | Yes | 1 | No |
| Unpub | Bevacizumab | 34 g/g Cr UPC | MCD | High-dose prednisone | 82 | F | Yes | 1 | No |
| Case 1 | Bevacizumab | 6890 μg/mg ACR | DN | No immunosupression | 54 | F | Yes | 1 | No |
| Case 2 | Bevacizumab | 4416 μg/mg ACR | No | No immunosupression, HD started | 53 | M | Yes | 1 | Yes, BL |
| Case 3 | Bevacizumab then aflibercept | None | No | No immunosupression, WD | 65 | F | No (MD) | 1 | Yes, new |
AKI, acute kidney injury; BL, high blood pressure at baseline; F, female; HTN, hypertension; Inc, increased; M, male; MCD, minimal change disease; MGN, membranous glomerulonephritis; MMF, mycophenolate mofetil; NR, not reported; Prot, protein; Px, plasma exchange; UC, uncontrolled; Unpub, unpublished; UPC, urine protein:creatinine ratio; WD, withdrawal of agent.
FIGURE 4.
Molecular physiology of VEGF signaling in podocytes and endothelial cells and renal pathophysiology that ensues with VEGF blockade. (A) Molecular physiology and (B) pathophysiology with VEGF blockade. VEGF-A signaling to renal podocytes maybe paracrine or mediated through VEGF-2 receptors. Akt, protein kinase B (PKB); CD2AP, CD2-associated protein; C-MIP, C-Maf-inducing protein; DAG, diacyl glycerol; DGKE, diglyceride kinase epsilon; F-Act, F-actin; Fyn, proto-oncogene tyrosine-protein kinase fyn; GN, glomerulonephritis; GS, glomerulosclerosis; Nck, NCK tyrosine kinase; NFkB, nuclear factor kappa light chain enhancer of activated B cells; NP1, neuronal pentraxin 1; N-WASP, Neural Wiskott–Aldrich syndrome protein; PI3K, phosphatidylinositol-4, 5-bisphosphate 3-kinase; RAS, rat sarcoma protein; Red P, phosphoryl group; RelA, v-rel avian reticuloendotheliosis viral oncogene homolog A; SOS, son of sevenless; sVEG2R, soluble VEGF receptor 2; VEGF-A, VEGF receptor A; VEGFR2, VEGF receptor 2; Tie2, tyrosine-protein kinase receptor TIE-2. Twin nucleic acid strands = messenger RNA.
The cases we present show a variety of phenotypes. Patient 1 showed worsening proteinuria and possible worsening of renal function with a biopsy showing only DN and hypertensive nephrosclerosis. Patient 2 showed only an increase in proteinuria at a data point observed days after bevacizumab injection, with worsening of severe Stage 4 CKD. Patient 3 showed only uncontrolled, resistant hypertension after starting ongoing bevacizumab and then aflibercept injections for MD. None of these phenotypes would surprise an experienced oncologist who has been treating patients with adjuvant anti-VEGF medications as systemic chemotherapy. The concern is that many patients who are receiving intravitreal anti-VEGF therapy also have diabetes, hypertension, proteinuria and CKD at baseline.
Avery et al. helped provide evidence of systemic absorption of anti-VEGF agents injected through the intravitreal route. This was demonstrated first in AMD patients in 2014 [24], then more recently in patients with central retinal vein occlusion and in patients with diabetic macular edema [27]. Avery et al. [26] also raised concerns about systemic side effects, and a meta-analysis in 2016 reported a possibly increased risk of cerebrovascular accidents in patients exposed to high levels of intravitreal VEGF inhibitors [28].
The recommendations on management of worsening proteinuria observed after intravitreal anti-VEGF injections remain limited given the very limited data on this subject. It is reasonable to consider decreasing the dose or switching to a less potent agent such as ranibizumab. This is due to the decreased systemic absorption and less severe systemic VEGF inhibition seen with ranibizumab [24, 26, 27]. Withdrawal of therapy may not be a suitable option if a patient’s eyesight is rapidly deteriorating, but it is an option in the most extreme cases of worsening proteinuria or renal function decline. Improving hypertensive control and RAAS blockade are reasonable adjunctive steps.
It is important to note that ranibizumab was linked to one case of TMA [37], as well as several other cases of renal function decline and proteinuria [31, 33, 36]. While ranibizumab represents a compromise as a less potent and less systemically absorbed anti-VEGF agent, it is not free from potential side effects [42]. Other less potent agents, such as pegaptanib, may offer better safety and fewer systemic side effects than the aforementioned agents, but are yet to be widely tested [43, 44]. Future investigations include prospectively confirming and quantifying the changes in proteinuria after intravitreal injections of anti-VEGF inhibitors. Given the different pharmacodynamic profiles of the various anti-VEGF agents, it is expected that each drug may have different effects on hypertension, proteinuria and CKD progression after intravitreal administration.
ACKNOWLEDGEMENTS
The authors thank Dr Ira Kurtz, Dr Shih-Fan Sun, Dr Christine Barhoma and Marina Barsoum for reviewing the text.
AUTHORS’ CONTRIBUTIONS
R.M.H. wrote the manuscript. E.A.L., H.H. and U.S. cowrote the manuscript. P.N.Y., J.W. and S.B. edited the manuscript. N.A. produced the figures and was a research volunteer. M.G. is the senior author for this case series.
CONFLICT OF INTEREST STATEMENT
None declared. This work does not contain human subject research material.
REFERENCES
- 1. Harshman LC, Xie W, Bjarnason GA. et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol 2012; 13: 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyake TM, Sood AK, Coleman RL.. Contemporary use of bevacizumab in ovarian cancer. Expert Opin Biol Ther 2013; 13: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mrugala MM, Crew LK, Fink JR. et al. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett 2012; 4: 1082–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossari JR, Metzger-Filho O, Paesmans M. et al. Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol 2012; 2012: 417673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soria JC, Mauguen A, Reck M. et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013; 24: 20–30 [DOI] [PubMed] [Google Scholar]
- 6. Sweet JA, Feinberg ML, Sherman JH.. The role of avastin in the management of recurrent glioblastoma. Neurosurg Clin N Am 2012; 23: 331–341 [DOI] [PubMed] [Google Scholar]
- 7. Bollee G, Patey N, Cazajous G. et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant 2009; 24: 682–685 [DOI] [PubMed] [Google Scholar]
- 8. Evans T. Utility of hypertension as a surrogate marker for efficacy of antiangiogenic therapy in NSCLC. Anticancer Res 2012; 32: 4629–4638 [PubMed] [Google Scholar]
- 9. Rosati G, Avallone A, Aprile G. et al. XELOX and bevacizumab followed by single-agent bevacizumab as maintenance therapy as first-line treatment in elderly patients with advanced colorectal cancer: the boxe study. Cancer Chemother Pharmacol 2013; 71: 257–264 [DOI] [PubMed] [Google Scholar]
- 10. Henao DE, Cadavid AP, Saleem MA.. Exogenous vascular endothelial growth factor supplementation can restore the podocyte barrier-forming capacity disrupted by sera of preeclamptic women. J Obstet Gynaecol Res 2013; 39: 46–52 [DOI] [PubMed] [Google Scholar]
- 11. Jin J, Sison K, Li C. et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell 2012; 151: 384–399 [DOI] [PubMed] [Google Scholar]
- 12. Hayman SR, Leung N, Grande JP. et al. VEGF inhibition, hypertension, and renal toxicity. Curr Oncol Rep 2012; 14: 285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kirsch AH, Riegelbauer V, Tagwerker A. et al. The mTOR-inhibitor rapamycin mediates proteinuria in nephrotoxic serum nephritis by activating the innate immune response. Am J Physiol Renal Physiol 2012; 303: F569–F575 [DOI] [PubMed] [Google Scholar]
- 14. Mima A, Kitada M, Geraldes P. et al. Glomerular VEGF resistance induced by PKCdelta/SHP-1 activation and contribution to diabetic nephropathy. FASEB J 2012; 26: 2963–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenfeld PJ, Heier JS, Hantsbarger G. et al. Tolerability and efficacy of multiple escalating doses of ranibizumab (Lucentis) for neovascular age-related macular degeneration. Ophthalmology 2006; 113: 623 e1 [DOI] [PubMed] [Google Scholar]
- 16. Rosenfeld PJ, Schwartz SD, Blumenkranz MS. et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 2005; 112: 1048–1053 [DOI] [PubMed] [Google Scholar]
- 17. Wu S, Kim C, Baer L. et al. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 2010; 21: 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu X, Wu S, Dahut WL. et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007; 49: 186–193 [DOI] [PubMed] [Google Scholar]
- 19. Lee K, Yang H, Lim H. et al. A prospective study of blood pressure and intraocular pressure changes in hypertensive and nonhypertensive patients after intravitreal bevacizumab injection. Retina 2009; 29: 1409–1417 [DOI] [PubMed] [Google Scholar]
- 20. Pande A, Lombardo J, Spangenthal E. et al. Hypertension secondary to anti-angiogenic therapy: experience with bevacizumab. Anticancer Res 2007; 27: 3465–3470 [PubMed] [Google Scholar]
- 21. Qi WX, Shen Z, Tang LN. et al. Risk of hypertension in cancer patients treated with aflibercept: a systematic review and meta-analysis. Clin Drug Investig 2014; 34: 231–240 [DOI] [PubMed] [Google Scholar]
- 22. Rasier R, Artunay O, Yuzbasioglu E. et al. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (Lond) 2009; 23: 1714–1718 [DOI] [PubMed] [Google Scholar]
- 23. Risimic D, Milenkovic S, Nikolic D. et al. Influence of intravitreal injection of bevacizumab on systemic blood pressure changes in patients with exudative form of age-related macular degeneration. Hellenic J Cardiol 2013; 54: 435–440 [PubMed] [Google Scholar]
- 24. Avery RL, Castellarin AA, Steinle NC. et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014; 98: 1636–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tschulakow A, Christner S, Julien S. et al. Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS One 2014; 9: e113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avery RL. What is the evidence for systemic effects of intravitreal anti-VEGF agents, and should we be concerned? Br J Ophthalmol 2014; 98(Suppl 1): i7–i10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Avery RL, Castellarin AA, Steinle NC. et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017; 37: 1847–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Avery RL, Gordon GM.. Systemic safety of prolonged monthly anti-vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA Ophthalmol 2016; 134: 21–29 [DOI] [PubMed] [Google Scholar]
- 29. Rogers CA, Scott LJ, Reeves BC. et al. Serum vascular endothelial growth factor levels in the IVAN trial; relationships with drug, dosing, and systemic serious adverse events. Ophthalmol Retina 2018; 2: 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanna RM, Lopez E, Wilson J. et al. Minimal change disease onset observed after bevacizumab administration. Clin Kidney J 2016; 9: 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheungpasitporn W, Chebib FT, Cornell LD. et al. Intravitreal antivascular endothelial growth factor therapy may induce proteinuria and antibody mediated injury in renal allografts. Transplantation 2015; 99: 2382–2386 [DOI] [PubMed] [Google Scholar]
- 32. Scott IU, Edwards AR, Beck RW. et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007; 114: 1860–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Georgalas I, Papaconstantinou D, Papadopoulos K. et al. Renal injury following intravitreal anti-VEGF administration in diabetic patients with proliferative diabetic retinopathy and chronic kidney disease–a possible side effect? Curr Drug Saf 2014; 9: 156–158 [DOI] [PubMed] [Google Scholar]
- 34. Jamrozy-Witkowska A, Kowalska K, Jankowska-Lech I. et al. [Complications of intravitreal injections–own experience]. Klin Oczna 2011; 113: 127–131 [PubMed] [Google Scholar]
- 35. Khneizer G, Al-Taee A, Bastani B.. Self limited membranous nephropathy after intravitreal nephropathy after intravitreal bevacizumab therapy for age related macular degeneration J Nephropathol 2017; 6: 134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morales E, Moliz C, Gutierrez E.. Renal damage associated to intravitreal administration of ranibizumab. Nefrologia 2017; 37: 653–655 [DOI] [PubMed] [Google Scholar]
- 37. Pellé G, Shweke N, Van Huyen J-PD. et al. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factor inhibitors. Am J Kidney Dis 2011; 57: 756–759 [DOI] [PubMed] [Google Scholar]
- 38. Perez-Valdivia MA, Lopez-Mendoza M, Toro-Prieto FJ.. Relapse of minimal change disease nephrotic syndrome after administering intravitreal bevacizumab. Nefrologia 2014; 34: 421–422 [DOI] [PubMed] [Google Scholar]
- 39. Sato T, Kawasaki Y, Waragai T. et al. Relapse of minimal change nephrotic syndrome after intravitreal bevacizumab. Pediatr Int 2013; 55: e46–e468 [DOI] [PubMed] [Google Scholar]
- 40. Singer RB, Milano AF.. Mortality in co-morbidity (I)–analysis of the results in the Multiple Medical impairment Study for impairments with elevated blood pressure as the second or co-morbid impairment. J Insur Med 2007; 39: 78–88 [PubMed] [Google Scholar]
- 41. Zeb A, Ali SR, Rohra DK.. Mechanism underlying hypertension and proteinuria caused by bevacizumab. J Coll Physicians Surg Pak 2007; 17: 448–449 [PubMed] [Google Scholar]
- 42. Zehetner C, Kralinger MT, Modi YS. et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol 2015; 93: e154–e159 [DOI] [PubMed] [Google Scholar]
- 43. Falavarjani KG, Nguyen QD.. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013; 27: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fogli S, Del Re M, Rofi E. et al. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye (Lond) 2018; 32: 1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]