Abstract
Background
Chronic kidney disease (CKD) patients experience a high symptom burden including fatigue, sleep difficulties, muscle weakness and pain. These symptoms reduce levels of physical function (PF) and activity, and contribute to poor health-related quality of life (HRQoL). Despite the gathering evidence of positive physiological changes following exercise in CKD, there is limited evidence on its effect on self-reported symptom burden, fatigue, HRQoL and physical activity.
Methods
Thirty-six patients [mean ± SD 61.6 ± 11.8 years, 22 (61%) females, estimated glomerular filtration rate: 25.5 ± 7.8 mL/min/1.73 m2] not requiring renal replacement therapy underwent 12 weeks (3 times/week) of supervised aerobic exercise (AE), or a combination (CE) of AE plus resistance training. Outcomes included self-reported symptom burden, fatigue, HRQoL and physical activity.
Results
Exercise reduced the total number of symptoms reported by 17% and had favourable effects on fatigue in both groups. AE reduced the frequency of ‘itching’, ‘impotence’ and ‘shortness of breath’ symptoms, and the intrusiveness for symptoms of ‘sleep disturbance’, ‘loss of muscular strength/power’, ‘muscle spasm/stiffness’ and ‘restless legs’. The addition of resistance exercise in the CE group saw a reduction in ‘loss of muscular strength/power’. No changes were seen in subjective PF or physical activity levels. AE increased self-efficacy for physical activity.
Conclusions
Supervised exercise had favourable effects on symptom frequency and intrusiveness, including substantial improvements in fatigue. Although the intervention did not improve self-reported physical activity levels, AE increased patients’ self-efficacy for physical activity. These favourable changes in self-reported outcomes support the important role of exercise in CKD.
Keywords: chronic kidney disease, exercise, fatigue symptoms, health-related quality of life
INTRODUCTION
Patients with chronic kidney disease (CKD) experience a high symptom burden [1]. The most commonly reported symptoms in non-dialysis-dependent (NDD)-CKD patients include fatigue, sleep difficulties, muscle weakness, restless legs, pruritus (i.e. itching) and bone/joint pain [1–8]. Our group found that 96% of patients not requiring dialysis reported experiencing at least one symptom [2], and the median number of symptoms experienced in those with CKD Stages 3–5 is between five and seven [2, 3, 7]. This elevated incidence of debilitating symptoms accentuates the reduced health-related quality of life (HRQoL) in this group [7–9], and high symptom burden is associated with increased hospitalization and mortality [10].
CKD patients have poor physical functioning [11, 12] and low habitual physical activity levels [13]. Both low physical activity [11] and poor physical function (PF) [12, 14] are independently associated with adverse outcomes and contribute to the reduction in activities of daily living and quality of life (QoL) in this group. Increased symptom burden [8, 15] may further reduce physical functioning, negatively impacting physical activity levels and intensifying the cycle of poor health [8, 9, 11]. Accordingly, efforts to alleviate symptom burden in CKD are likely to confer positive benefits on HRQoL and PF. While pharmacological intervention may help relieve some symptoms and consequently arrest HRQoL decline [6, 8], further interventions are necessary to restore and maximize it. The benefits of exercise in CKD are becoming increasingly established with improvements in exercise capacity, PF, strength and clinical outcomes observed [16–19].
Despite the evidence in regard to physiological changes following exercise in CKD, given the significance of self-reported HRQoL and symptom burden, there is a paucity of research on the effects of exercise on these outcomes [20]. We investigated the effects of 12 weeks of supervised exercise on self-reported symptom burden, fatigue, HRQoL and physical activity in CKD patients not requiring dialysis. We hypothesized that exercise would have favourable effects on symptom experience, fatigue, HRQoL and physical activity (as a result of improved symptoms and physiological factors).
MATERIALS AND METHODS
Participants
This was a secondary analysis of data from the ‘ExTra CKD’ study [17] (ISRCTN 36489137) conducted at the University Hospitals of Leicester National Health Service (NHS) Trust between December 2013 and October 2016. Patients gave written informed consent and National Ethical approval was obtained. The study was conducted in accordance with the Declaration of Helsinki. Eligible participants were: (i) diagnosed with moderately severe CKD (Stages 3b–5); (ii) aged ≥18 years; and (iii) free of physical impairment and comorbidities that were a contraindication to exercise [e.g. unstable hypertension, potentially lethal arrhythmia, uncontrolled diabetes mellitus (glycated hemoglobin (HbA1c) >9%)].
Exercise intervention
Full study methodology is described elsewhere [17]. In summary, patients attended the research gym three times a week for 12 weeks. Patients were randomized [stratified for estimated glomerular filtration rate (eGFR)] into one of the two supervised exercise groups:
aerobic exercise (AE): ∼70–80% of maximum heart rate, 30 min duration on each session, performed on standard cardiovascular equipment (e.g. treadmill);
a combination (CE) of AE (as above but for 20 min duration on two sessions, 30 min AE only for the remaining session), plus resistance training (∼70% one repetition maximum, three sets of 8–12 repetitions) on a leg extension and leg press machine, on two sessions where 20 min AE was completed.
Outcome measures
To negotiate the absence of a control group, prior to randomization, patients underwent a 6-week control period. All self-reported outcomes reported below were completed by patients in their own time prior to attendance at baseline or post-intervention physiological assessments. Questionnaires returned were screened for missing data and highlighted to the patient in person. Patients were reminded to return questionnaires if they had not done so already.
Symptom burden
Leicester Uraemic Symptom Scale
The 11-item Leicester Uraemic Symptom Scale (LUSS) [21, 22] evaluated the number, frequency (0 = ‘never’ to 4 = ‘every day’) and intrusiveness (symptom impact) (0 = ‘not at all intrusive’ to 4 = ‘extremely intrusive’) of uraemic symptoms. Each individual symptom was scored out of four, and summative scores were generated:
Total symptom number (LUSS-1): total number of symptoms experienced (out of 11).
Total symptom frequency score (LUSS-2): frequency of each 11 symptoms rated 0–4 (out of 44).
Total symptom intrusiveness score (LUSS-3): perceived intrusiveness of each 11 symptoms rated 0–4 (out of 44).
The LUSS has been widely used to determine symptom burden in renal populations [7, 12, 19, 21] and has recently been validated by our group [22].
Functional Assessment of Chronic Illness Therapy-Fatigue Scale
The validated Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F) assessed fatigue and its impact on daily activities. It uses a 5-point Likert scale (0 = ‘not at all’ to 4 = ‘very much’) based on a 7-day recall period. Different fatigue domains were measured (e.g. ‘physical’, ‘social/family’, ‘functional’ well-being, plus ‘additional concerns’). These were combined to form summative scores:
FACT-G: sum of subscale scores (out of 108);
Trial Outcome Index (TOI): sum of the ‘physical’ and ‘functional’ well-being and ‘additional concerns’ subscales. The TOI is regarded as an efficient index of physical/functional outcomes and used as a common endpoint in clinical trials (out of 108);
total FACIT-F score: sum of all domains (out of 160).
In all scales, a higher score denoted lower fatigue. Subscale scores were deemed acceptable when ≥50% of items were answered and summative scores when response rate was ≥80% [23].
Quality of life and PF
EuroQol Five Dimensions (five-level scale)
The EuroQol Five Dimensions (five-level scale) (EQ-5D-5L) is a well-established generic instrument for assessing HRQoL [24]. Patients classified their state of health by selecting one of the five different levels (1 = ‘none’, 2 = ‘slight’; 3 = ‘moderate’; 4 = ‘severe’; and 5 = ‘unable’) of problem severity within each health domain (mobility, capacity for self-care, conduct of usual activities, pain/discomfort, anxiety/depression). Scores were converted into a single index value (scored +1.0 to −0.594, where a higher score denoted better QoL). The EQ-5D-5L included a visual analogue scale (VAS) indicating current health status on a scale between 0 (worst) and 100 (best).
Medical outcomes 36-Item Short Form Survey
The 36-Item Short Form Survey (SF-36) is a measure of functioning and well-being [25] that is validated among the general population and numerous disease populations including kidney disease [5, 26, 27]. The SF-36 measures eight dimensions: PF, role limitations caused by physical problems (RP), pain (BP), general health (GH), vitality/energy (VT), social function (SF), mental health (MH)/emotional well-being and role limitations caused by emotional problems/MH (RE). For each parameter, scores were transformed from 0 to 100, with higher scores indicating better health. Summary physical and mental component scores were calculated [27]. The modified SF-36 (v 1.0) was used. Norm reference population (aged 60–64 years) scores are: PF = 69.6; RP = 70.1; BP = 62.3; GH = 60.9; VT = 55.6; SF= 76.9; RE = 84.9; MH = 74.1 [28].
Duke Activity Status Index
Validated in patients with CKD [29], the Duke Activity Status Index (DASI) was used to evaluate functional capacity. The DASI queried patients, ‘yes’ or ‘no’, whether they could complete a range of physical activities. Each question or activity was assigned a value based on the estimated peak oxygen uptake [metabolic equivalent (MET)]. ‘Yes’ responses were summed to give a raw DASI score (0–58.2). Higher scores indicated higher functional capacity.
Physical activity
Godin–Shephard Leisure Time Exercise Questionnaire
The Godin–Shephard Leisure Time Exercise Questionnaire (GSLTEQ) was used to determine physical activity levels [30]. Activities were classified into three subgroups [‘strenuous’ (nine METs), ‘moderate’ (five METs), ‘light’ (three METs)]. The frequency and type of activities or exercises performed weekly were used to calculate the total score (METs × times/week). A score of ≥24 U was regarded as active; 14–23 U regarded as moderately active; and ≤13 U regarded as inactive [30].
Self-efficacy for Physical Activity Scale
In order to assess whether exercise increased patients’ self-efficacy, we used the Self-efficacy for Physical Activity Scale (SEPA), a five-item scale with five-point Likert response levels (1 = ‘not at all confident’ to 5 = ‘extremely confident’). A summary score (1–5) was calculated by averaging responses. Higher scores reflect higher levels of self-efficacy [31]. The SEPA has shown high construct validity with numerous measures of physical activity and is used widely in clinical populations [32].
Statistical analysis
As a secondary per-protocol analysis of Watson et al. [17], no a priori sample size calculation is provided for the outcomes presented. Data are presented as mean (±SD). The 95% confidence intervals (CIs) were calculated for changes pre- and post-exercise. Significance was recognized as <0.05. Within-group differences were assessed using paired-samples t-tests. Between-group differences were assessed for changes pre- and post-exercise using linear regression modelling. Age, group, sex, eGFR and baseline values for that variable were used as covariants, and the presence of a significant group coefficient indicted a between-group difference. The expectation-maximization (EM) algorithm was used to impute missing questionnaire data (see Supplementary data, Table S1) and to restore adequate sample size for each outcome (n = 18 for both groups). EM is based on two iterating steps (50 iterations were used) that generate means and variances for missing data based on known values for that variable. Categorical data used in EM estimates were age, group, sex and eGFR, with values at pre- and post-exercise used as quantitative variables. Little’s missing completely at random (MCAR) test and separate variance t-tests confirmed the suitability of using EM. Raw data were evaluated to verify the imputed differences. Statistical guidance was provided by statisticians from the Leicester Biomedical Research Centre Clinical Trials Unit, and data were analysed using SPSS 24 software.
Results
Participants
Thirty-six patients completed the intervention (Table 1). For brevity, recruitment and retention rates to the study are reported elsewhere [17]. Out of 36 available exercise sessions, compliance was good and both groups completed 32 sessions (88%) on average.
Table 1.
Patient characteristics
| Variable | AE (n = 18) | CE (n = 18) |
|---|---|---|
| Age (years) | 63.7 (±8.5) | 59.6 (±14.4) |
| Sex, female, n (%) | 11 (61) | 11 (61) |
| BMI (kg/m2) | 30.2 (±5.8) | 29.4 (±5.8) |
| Ethnicity | ||
| White British, n (%) | 11 (61) | 9 (50) |
| White European, n (%) | 2 (11) | 1 (6) |
| Asian, n (%) | 4 (22) | 8 (44) |
| Black Caribbean, n (%) | 1 (6) | 0 (0) |
| Disease aetiology | ||
| Diabetic nephropathy, n (%) | 2 (11) | 1 (6) |
| Interstitial nephritis, n (%) | 2 (11) | 2 (11) |
| IgA nephropathy, n (%) | 2 (11) | 1 (6) |
| Polycystic kidney disease, n (%) | 0 (0) | 3 (17) |
| Other, n (%) | 0 (0) | 2 (11) |
| Unknown/aetiology uncertain, n (%) | 12 (67) | 9 (50) |
| Comorbidities | ||
| Diabetes mellitus type II, n (%) | 7 (39) | 2 (11) |
| Hypertension (essential or secondary), n (%) | 9 (50) | 7 (39) |
| Clinical parameters | ||
| eGFR (mL/min/1.73 m2) | 26.6 (±8.7) | (±6.9) |
| Stage 3b, n (%) | 6 (33) | 5 (27) |
| Stage 4, n (%) | 12 (67) | 12 (67) |
| Stage 5, n (%) | 0 (0) | 1 (6) |
| Haemoglobin (mg/dL) | 123.1 (±14.0) | 114.4 (±14.1) |
| Albumin (g/L) | 41.9 (±2.6) | 39.7 (±2.8) |
| Systolic blood pressure (mmHg) | 136.2 (±22.0) | 123.4 (±10.4) |
| Diastolic blood pressure (mmHg) | 73.7 (±12.5) | 68.2 (±9.4) |
Data are presented as mean (±SD), unless otherwise specified. BMI, body mass index; IgA, immunoglobulin A.
Outcome measures
No significant changes were seen in any questionnaire outcome over the 6-week control period (see Supplementary data, Table S2).
Symptom burden
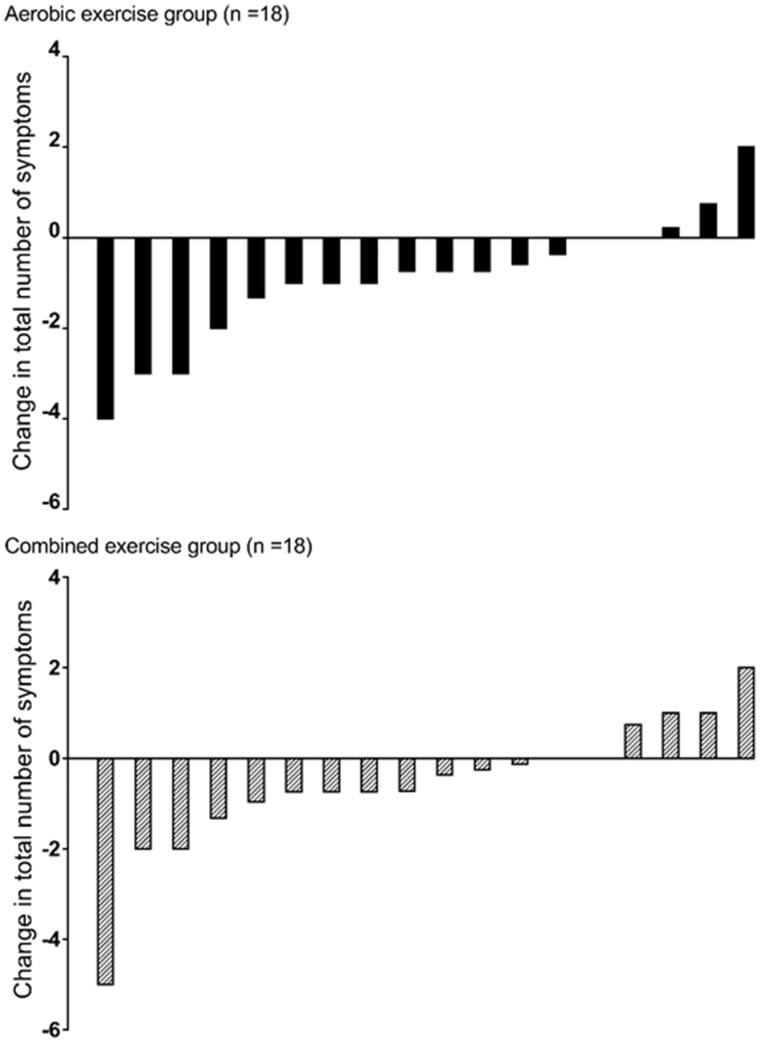
Changes in symptom burden (frequency and intrusiveness) following exercise can be found in Tables 2 and 3. In the AE group, the total number of symptoms were reduced by 1 (95% CI −2 to 0) from 6 (±3) to 5 (±2) (P = 0.01) (individual changes are shown in Figure 1). In regard to individual symptoms, reductions were seen in the frequency of ‘itching’ (P = 0.01), ‘impotence’ (P = 0.03) and ‘shortness of breath’ (P = 0.03), as well as a total symptom frequency score (P = 0.01). Following exercise, the AE group reported less intrusiveness for symptoms of ‘sleep disturbance’, ‘loss of muscular strength/power’, ‘muscle spasm/stiffness’ and ‘restless legs’ (P’s = 0.01–0.03), and as such, the total symptom intrusiveness score also reduced (P = 0.01).
Table 2.
Symptom burden (frequency) changes pre- and post-exercise
| Frequency of symptoms | AE (n = 18) |
CE (n = 18) |
P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change (95% CI) | P-value | Pre | Post | Change (95% CI) | P-value | ||
| Itching (0–4) | 2.0 (±1.3) | 1.2 (±1.1) | −0.7 (−1.2 to −0.3) | 0.01* | 1.6 (±1.0) | 1.6 (±1.2) | −0.0 (−0.7 to 0.6) | 0.88 | 0.04* |
| Sleep disturbance (0–4) | 2.0 (±0.9) | 2.0 (±0.7) | 0.0 (−0.3 to 2.2) | 0.73 | 1.8 (±1.4) | 1.8 (±1.4) | 0.0 (−0.2 to 0.2) | 0.9 | 0.68 |
| Loss of appetite (0–4) | 0.4 (±0.4) | 0.5 (±0.7) | +0.1 (−0.3 to 0.4) | 0.75 | 0.9 (±0.9) | 0.7 (±1.0) | −0.2 (−0.8 to 0.4) | 0.47 | 0.53 |
| Excessive tiredness (0–4) | 1.3 (±1.0) | 1.3 (±0.7) | 0.0 (−0.3 to 0.4) | 0.76 | 1.9 (±1.3) | 1.7 (±1.3) | −0.3 (−0.6 to 0.1) | 0.11 | 0.15 |
| Pain in bones/joints | 1.3 (±1.2) | 1.4 (±1.3) | +0.1 (−0.2 to 0.3) | 0.48 | 1.3 (±1.0) | 1.7 (±1.3) | +0.4 (0.0 to 0.9) | 0.05* | 0.19 |
| Poor concentration (0–4) | 0.5 (±0.6) | 0.8 (±0.9) | +0.3 (−0.1 to 0.7) | 0.09 | 0.6 (±0.6) | 1.1 (±1.1) | +0.5 (−0.2 to 1.1) | 0.15 | 0.48 |
| Impotence/lack of sex drive (0–4) | 1.3 (±1.2) | 1.0 (±1.3) | −0.3 (−0.6 to 0.0) | 0.03* | 1.6 (±1.4) | 1.6 (±1.7) | 0.0 (−0.2 to 0.2) | 0.83 | 0.03* |
| Loss of muscular strength/ power (0–4) | 1.0 (±0.9) | 0.8 (±1.1) | −0.2 (−0.6 to 0.2) | 0.31 | 1.7 (±1.0) | 0.9 (±1.1) | −0.7 (−1.1 to −0.4) | 0.01* | 0.07 |
| Shortness of breath (0–4) | 1.5 (±1.3) | 0.9 (±1.1) | −0.6 (−1.2 to −0.1) | 0.03* | 1.6 (±1.0) | 1.1 (±1.1) | −0.5 (−1.0 to 0.0) | 0.05 | 0.61 |
| Muscle spasm/stiffness (0–4) | 1.2 (±1.0) | 0.9 (±1.0) | −0.2 (−0.6 to 0.2) | 0.26 | 1.1 (±0.9) | 1.0 (±1.2) | −0.1 (−0.5 to 0.4) | 0.78 | 0.48 |
| Restless legs (0–4) | 0.8 (±1.1) | 0.5 (±0.7) | −0.3 (−0.8 to 0.2) | 0.27 | 0.9 (±0.9) | 0.6 (±1.1) | −0.3 (−0.7 to 0.1) | 0.14 | 0.88 |
| Total frequency (LUSS-2) (0–44) | 13.0 (±6.7) | 10.6 (±5.6) | −2.3 (−3.7 to −1.0) | 0.01* | 14.8 (±7.0) | 13.4 (±9.6) | −1.3 (−3.2 to 0.7) | 0.19 | 0.16 |
Data are presented as mean (±SD).
Between-group P-value with age, group, sex, eGFR and baseline values for that variable were used as covariants. Values are significant at P < 0.05 and denoted by *.
Table 3.
Symptom burden (intrusiveness) changes pre- and post-exercise
| Intrusiveness of symptoms | AE (n = 18) |
CE (n = 18) |
P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change (95% CI) | P-value | Pre | Post | Change (95% CI) | P-value | ||
| Itching (0–4) | 1.6 (±1.2) | 1.4 (±1.1) | −0.2 (−0.6 to 0.2) | 0.32 | 1.3 (±1.0) | 1.4 (±1.0) | +0.1 (−0.3 to 0.6) | 0.47 | 0.13 |
| Sleep disturbance (0–4) | 2.2 (±0.9) | 1.8 (±0.9) | −0.3 (−0.5 to 0.2) | 0.01* | 2.2 (±1.5) | 2.1 (±1.7) | −0.1 (−0.4 to 0.1) | 0.38 | 0.04* |
| Loss of appetite (0–4) | 0.6 (±0.5) | 0.6 (±0.5) | 0.0 (−0.2 to 0.1) | 0.76 | 0.9 (±0.9) | 0.9 (±0.9) | 0.0 (−0.3 to 0.4) | 0.82 | 0.55 |
| Excessive tiredness (0–4) | 1.8 (±1.1) | 1.8 (±0.8) | 0.0 (−0.3 to 0.4) | 0.71 | 2.4 (±1.6) | 2.1 (±1.4) | −0.3 (−0.7 to 0.2) | 0.21 | 0.29 |
| Pain in bones/joints (0–4) | 1.6 (±1.2) | 1.6 (±1.1) | 0.0 (−0.2 to 0.2) | 0.9 | 1.5 (±1.2) | 1.8 (±1.4) | +0.3 (−0.1 to 0.7) | 0.16 | 0.22 |
| Poor concentration (0–4) | 1.0 (±0.9) | 1.0 (±0.8) | 0.0 (−0.2 to 0.2) | 0.89 | 1.4 (±1.0) | 1.2 (±1.1) | −0.2 (−0.5 to 0.1) | 0.18 | 0.62 |
| Impotence/lack of sex drive (0–4) | 1.2 (±1.4) | 0.9 (±1.3) | −0.3 (−0.7 to 0.1) | 0.13 | 1.4 (±1.2) | 1.4 (±1.7) | +0.1 (−0.7 to 0.7) | 0.80 | 0.39 |
| Loss of muscular strength/ power (0–4) | 1.4 (±1.0) | 1.0 (±0.9) | −0.3 (−0.6 to 0.0) | 0.03* | 1.8 (±0.9) | 1.1 (±1.1) | −0.7 (−1.1 to −0.4) | <0.001* | 0.06 |
| Shortness of breath (0–4) | 1.2 (±1.0) | 1.0 (±0.9) | −0.2 (−0.6 to 0.2) | 0.32 | 1.7 (±1.3) | 1.1 (±1.2) | −0.6 (−1.0 to −0.2) | 0.01* | 0.27 |
| Muscle spasm/stiffness (0–4) | 1.7 (±1.2) | 1.2 (±1.1) | −0.5 (−0.8 to 0.1) | 0.02* | 1.3 (±0.9) | 1.1 (±1.1) | −0.2 (−0.5 to 0.0) | 0.04* | 0.17 |
| Restless legs (0–4) | 1.1 (±1.0) | 0.7 (±0.9) | −0.3 (−0.6 to −0.1) | 0.01* | 1.0 (±0.9) | 0.7 (±1.0) | −0.2 (−0.4 to 0.0) | 0.05* | 0.29 |
| Total intrusiveness (LUSS-3) (0–44) | 14.7 (±8.0) | 12.2 (±5.9) | −2.5 (−4.2 to −0.8) | 0.01* | 17.3 (±7.7) | 14.0 (±10.6) | −3.3 (−5.3 to − 1.3) | 0.01* | 0.9 |
Data are presented as mean (±SD).
Between-group P-value with age, group, sex, eGFR and baseline values for that variable were used as covariants. Values are significant at P < 0.05 and denoted by *.
FIGURE 1.
Individual changes in the number of symptoms reported pre- and post-exercise from the LUSS. Data show the reduction (or increase) in the number of symptoms for each of the 18 individual patients in each group.
In the CE group, a reduction in ‘loss of muscular strength/power’ (P = 0.01) was observed, as well as reports of less intrusiveness for ‘loss of muscular strength/power’, ‘shortness of breath’, ‘muscle spasm/stiffness’ and ‘restless legs’ (Ps < 0.001–0.05). Total symptom intrusiveness score was reduced (P = 0.01). Conversely, an increase in the frequency of ‘joint/bone pain’ symptoms was observed (P = 0.05). No significant change in the number of symptoms was seen [7 (±3) to 6 (±3), P = 0.1] although the number (–1) was comparable to the AE.
Fatigue changes are reported in Table 4. Exercise had favourable effects on the FACT-G (AE: P = 0.01, CE: P = 0.05), TOI (P = 0.01 in both groups) and total FACIT-F scores (P = 0.01 in both groups). No significant between-group differences were seen.
Table 4.
Changes pre- and post-exercise in the FACIT-F
| Component | AE (n = 18) |
CE (n = 18) |
Between-group |
||
|---|---|---|---|---|---|
| Mean (±SD) | P-value | Mean (±SD) | P-value | P-valuea | |
| FACT-G | |||||
| Pre | 84.3 (±15.0) | 75.9 (±14.8) | |||
| Post | 91.6 (±7.9) | 81.5 (±13.5) | |||
| Change (95% CI) | +7.3 (3.0 to 11.7) | 0.01* | +5.7 (−0.2 to 11.3) | 0.05 | 0.05 |
| TOI | |||||
| Pre | 82.0 (±17.7) | 65.9 (±24.7) | |||
| Post | 89.9 (±10.8) | 80.0 (±16.4) | |||
| Change (95% CI) | +7.9 (−2.9 to 12.9) | 0.01* | +14.0 (−6.5 to 21.4) | 0.01* | 0.81 |
| Total FACIT-F | |||||
| Pre | 122.9 (±21.1) | 103.2 (±27.9) | |||
| Post | 134.7 (±11.9) | 119.4 (±20.0) | |||
| Change (95% CI) | +11.8 (5.6 to 17.6) | 0.01* | +16.2 (7.3 to 24.0) | 0.01* | 0.26 |
Between-group P-value with age, group, sex, eGFR and baseline values for that variable were used as covariants. Values are significant at P < 0.05 and denoted by *.
HRQoL and PF
No changes were seen in the EQ-5D-5L index in either group [AE: 0.84 (±0.18) to 0.85 (±0.16), P = 0.7; CE: 0.87 (±0.12) to 0.87 (±0.15), P = 0.9; between-group difference P = 0.8]. An increase in the EQ-5D-5L VAS was seen in the AE group [70.2 (±17.2) to 75.2 (±14.9), P = 0.04] although no change was observed in the CE group [67.2 (±15.3) to 69.6 (±17.0), P = 0.40; between-group difference P = 0.34].
Changes in the SF-36 are shown in Table 5. Although no significant changes were observed, improvements in ‘Role Physical’ score [+12.4 (–1.8 to 26.6)] in the CE group, and the ‘Vitality’ score [+6.9 (–5.1 to 18.8)] in the AE group exceeded the minimal clinical important difference (MCID) (+5 points) [33]. No changes were seen in the DASI [AE: 40.8 (±13.8) to 39.9 (±17.5), P = 0.72; CE: 40.5 (±10.4) to 40.6 (±13.8), P = 0.9; between-group difference P = 0.9].
Table 5.
Changes pre- and post-exercise in the medical outcomes SF-36
| Component | AE (n = 18) |
CE (n = 18) |
Between-group |
||
|---|---|---|---|---|---|
| Mean (±SD) | P-value | Mean (±SD) | P-value | P-valuea | |
| PF | |||||
| Pre | 78.1 (±21.5) | 72.5 (±30.0) | |||
| Post | 80.2 (±20.8) | 70.3 (±30.0) | |||
| Change (95% CI) | +2.1 (−1.2 to 5.3) | 0.21 | −2.3 (−10.0 to 5.5) | 0.55 | 0.09 |
| RP | |||||
| Pre | 74.5 (±34.8) | 57.8 (±36.4) | |||
| Post | 78.2 (±37.6) | 70.2 (±31.1) | |||
| Change (95% CI) | +3.6 (−9.9 to 17.2) | 0.58 | +12.4 (−1.8 to 26.6) | 0.08 | 0.83 |
| BP | |||||
| Pre | 72.7 (±26.4) | 71.2 (±15.8) | |||
| Post | 75.2 (±22.4) | 68.0 (±17.7) | |||
| Change (95% CI) | +2.5 (−6.9 to 11.9) | 0.59 | −3.3 (−11.0 to 4.5) | 0.39 | 0.17 |
| GH | |||||
| Pre | 59.2 (±19.6) | 50.1 (±16.7) | |||
| Post | 62.7 (±15.9) | 53.7 (±17.9) | |||
| Change (95% CI) | +3.5 (−3.5 to 10.4) | 0.31 | +3.6 (−0.9 to 8.1) | 0.11 | 0.78 |
| VT | |||||
| Pre | 62.0 (±28.3) | 58.7 (±16.6) | |||
| Post | 68.9 (±19.4) | 62.4 (±15.3) | |||
| Change (95% CI) | +6.9 (−5.1 to 18.8) | 0.24 | +3.8 (−3.9 to 11.4) | 0.31 | 0.20 |
| SF | |||||
| Pre | 86.2 (±29.2) | 88.8 (±14.0) | |||
| Post | 88.6 (±17.9) | 89.3 (±11.1) | |||
| Change (95% CI) | +2.4 (−11.0 to 7.8) | 0.71 | 0.5 (−6.8 to 7.8) | 0.89 | 0.80 |
| RE | |||||
| Pre | 82.4 (±34.6) | 85.8 (±20.0) | |||
| Post | 85.3 (±33.0) | 85.9 (±25.0) | |||
| Change (95% CI) | +2.9 (−6.7 to 12.6) | 0.53 | +0.1 (−15.0 to 15.2) | 0.9 | 0.81 |
| MH | |||||
| Pre | 84.2 (±16.5) | 80.3 (±10.3) | |||
| Post | 87.6 (±7.9) | 79.4 (±18.8) | |||
| Change (95% CI) | +3.3 (−5.4 to 12.1) | 0.43 | −1.0 (−10.2 to 8.3) | 0.83 | 0.17 |
| PCS | |||||
| Pre | 47.0 (±8.8) | 43.0 (±7.9) | |||
| Post | 47.0 (±8.7) | 44.1 (±9.1) | |||
| Change (95% CI) | +0.8 (−0.9 to 2.5) | 0.35 | +1.1 (−0.2 to 2.4) | 0.09 | 0.84 |
| MCS | |||||
| Pre | 54.0 (±9.1) | 54.3 (±5.0) | |||
| Post | 55.7 (±6.2) | 54.5 (±8.5) | |||
| Change (95% CI) | +1.7 (−2.9 to 6.5) | 0.44 | +0.2 (−3.8 to 4.2) | 0.9 | 0.67 |
Between-group P-value with age, group, sex, eGFR and baseline values for that variable were used as covariants. Values are significant at P < 0.05.
Physical activity
Self-reported GSLTEQ physical activity scores were not changed [AE: 25.6 (±22.0) to 27.7 (±19.0), P = 0.45; CE: 26.2 (±20.5) to 22.2 (±13.3), P = 0.26, although the between-group difference was significant, P = 0.02]. Self-efficacy increased significantly in the AE group [2.5 (±1.4) to 3.1 (±0.9), P = 0.03] although no change was seen in the CE group [2.6 (±0.8) to 2.8 (±0.8), P = 0.57; between-group difference P = 0.17].
Discussion
In CKD patients, 12 weeks of supervised exercise had favourable effects on symptom frequency and intrusiveness, including improvements in fatigue-related outcomes. Although exercise did not improve self-reported physical activity levels, it did increase self-efficacy for physical activity. This secondary analysis supplements the improvements in physical performance and the strength reported in Watson et al. [17] and supports the necessary role of exercise in CKD.
Regardless of the exercise modality, we found beneficial effects on symptom burden with the number of symptoms falling from 6 to 5; a reduction of 17%. Kosmadakis et al. [19] also reported a decline in symptom frequency on the LUSS following 6 months walking (30 min, five times a week) in 40 CKD patients. In the AE group, reductions in the frequency of ‘itching’ (by 35%) and ‘shortness of breath’ symptoms (by 40%) were observed. Although these symptoms are frequently reported in CKD [2, 3, 8], there is a scarcity of research into the effect of exercise on them. Although preliminary data showed a reduction in itching after intradialytic cycling [34], further investigation is needed to elucidate the potential mechanisms behind this. Unsurprisingly, given the well-established benefits of exercise on aerobic capacity [16], we observed a reduction in dyspnoea (i.e. ‘shortness of breath’). In support of this, our original trial [17] reported an increase in peak oxygen uptake.
The AE group reported less intrusiveness for ‘sleep disturbance’ and a reduced total symptom intrusiveness score. Difficulties in sleep duration and quality are common in CKD and can have substantial impact on QoL. While research is limited in NDD-CKD, positive effects of exercise on sleep quality in renal transplant recipients [35] and older adults [36] have been shown. Possible mechanisms are complex and beyond the scope of this article; however, increased energy consumption, endorphin secretion and diurnal rhythm have been cited [35, 36].
The addition of resistance training (CE group) resulted a reduction in the number of ‘loss of muscular strength/power’ symptoms by 41%. Concurrently, we observed significant improvements in muscle mass and strength in Watson et al. [17], and this appears to have resulted in reductions in perceived weakness. This supports our recommendation that resistance training should form an integral part of exercise guidelines in CKD as skeletal muscle wasting and resultant loss of muscle function are common [15, 37]. It should be noted that the CE group reported an increase in the frequency of ‘joint/bone pain’ symptoms; whether this is a result of performing additional resistance-based training is unknown. Resistance training in CKD should be carefully progressed at a rate concomitant with patient ability and adequate recovery should be observed. As the CE group also completed an AE component, they too reported reductions in ‘shortness of breath’ symptom intrusiveness.
Both groups reported less intrusiveness for ‘loss of muscular strength/power’ and ‘muscle spasm/stiffness’ symptoms. This could be the result of the increased strength and PF observed [17]. Improvements in perceived muscle stiffness could be a consequence of general exercise-induced reductions in pain or inflammation, or improvements in range of motion and/or muscle architecture. Both groups reported less intrusiveness of ‘restless legs’ symptoms. Common in CKD, particularly in advanced disease stages, restless legs syndrome (RLS) is a sensorimotor disorder characterized by an uncontrolled urge to move the affected body parts [38]. Interestingly, exercise (both AE and CE) has been proved effective in improving RLS in both healthy middle-aged men [39] and in haemodialysis patients [38]. Symptoms are reportedly alleviated, at least temporarily, by physical activity [39], with a potential effect from endorphin release [40].
Exercise had large positive effects on fatigue symptoms in both groups. Symptoms of fatigue are consistently reported in CKD [1, 2, 8, 41]. With up to 80% of patients suffering from fatigue [4], it is a key barrier to regular participation in physical activity/exercise [8]. Although research is limited in renal populations, exercise is an effective intervention in reducing fatigue, especially in cancer patients [42]. Although the mechanisms are likely to be multi-factorial, it appears that improvements in aerobic/exercise/functional capacity and strength, as observed in our trial [17], resulted in reduced effort and improvement in the perception of fatigue during activities of daily living [42]. Fatigue in CKD has also been associated with poor sleep quality, RLS, excessive daytime sleepiness and low albumin levels [41]. Consequently, our improvements in sleep disturbance, restless legs, muscle weakness and stiffness, and shortness of breath symptoms may translate to reduced perceptions of fatigue.
Although a small change for the EQ-5D-5L VAS was seen in AE group, this did not reach the MCID [43], and no changes were seen in the EQ-5D-5L index in either group. Research by Mustata et al. [44], albeit in 10 CKD patients, found that AE (combined supervised and home-based) was able to elicit a clinically important improvement in the EQ-5D-5L index. However, in this trial, patients were older with greater disease progression and poorer exercise capacity; the baseline EQ-5D-5L index in that trial was 0.77 compared with 0.84–0.87 observed in our patients. Accordingly, our cohort may denote a relatively ‘healthy’ sample of patients, and our EQ-5D-5L index is highly comparable to the normative value of 0.86 for people aged 55–64 years in the UK [24].
The SF-36 values of our sample also suggest a somewhat ‘healthy’ cohort, and scores for ‘PF’, ‘BP’, ‘VT’, ‘SF’ and ‘MH’ were ∼11% above the mean scores for a norm reference population (60–64 years) [28]. Although no significant changes were seen as a result of exercise, the ‘RP’ score (+12.4) in the CE group, and ‘VT’ score (+6.9) in the AE group exceeded the MCID (+5 points) [33, 45]. Interestingly, Mustata et al. [44] also reported a clinically important change in the ‘RP’ domain (i.e. role limitations caused by physical problems [5]). The improvements in physical capacity observed in our trial could reduce limitations of daily activities. The ‘VT’ domain reflects ‘energy level’ and ‘fatigue’ [2, 45]. Therefore, improvements in other fatigue outcomes (as described above) are also reflected in increased ‘VT’.
Despite significant changes in objective functional capacity [17], we observed no changes in the DASI—a subjective measure of physical functioning. Despite its clinical utility, the DASI may not be suitable to differentiate individuals with high functional capacity (as apparent in our trial) due to a ‘ceiling effect’ [46]. This effect exists when ≥15% of the sample reaches the maximal score [47]. Indeed, 25% of our total sample reached this ‘ceiling’ post-exercise.
Self-reported physical activity scores from the GSLTEQ were not changed in both groups. A score of ≥24 U is regarded as ‘active’ [30]. As such, the mean baseline scores in patients recruited (∼26 U) indicate a relatively active cohort, and may explain why physical activity did not increase (i.e. patients reasoned themselves as sufficiently active already). Although physical activity levels did not change, self-efficacy for physical activity increased in the AE group. Self-efficacy, or confidence in one’s ability to perform a given behaviour despite obstacles, is a task-specific construct that influences the amount of time and effort individuals are willing to invest in order to overcome barriers [48]. Engaging in a supervised exercise intervention has been shown to have favourable effects on self-efficacy [49], including following pulmonary rehabilitation [50].
As stated elsewhere [17], the main limitation regards a lack of a non-exercising control group, which was excluded to promote recruitment to the study. However, we feel that this is somewhat negated by a 6-week control period prior to group allocation. Although we observed no changes in self-reported outcomes during this control period, suggesting the changes we observed are likely to be the result of the exercise intervention, the absence of a control group does not provide assessment on whether changes were driven by non-exercise psychosocial context variables including expectation, conditioning or social interactions. A lack of a ‘resistance training only’ group also means that we are unable to isolate the effects of this modality.
The questionnaire return rate in this study was poor (across all questionnaires ∼50% had completed pre- and post-intervention data). To that end, we used EM imputation to restore sample size and to provide a more accurate interpretation of any changes. Although the missingness of data was confirmed using Little’s MCAR test, we cannot exclude the possibility that patients with a more positive psychosocial response may have been more likely to return the questionnaires post-intervention. We used t-tests to investigate differences in age, sex, renal function and pre-intervention scores in patients who had complete and incomplete response rates for outcomes (i.e. the DASI and ‘number of symptoms’ question from the LUSS) identified as ‘missing at random’, but no explanatory variables could be identified. The number of missing data in our study highlights the importance by reducing patient outcome measure burden in study design, especially when the patient completes these in their own time. Efforts should be made to reduce the number of self-reported measures, but also ensure efficient management to achieve maximal return rates. Although getting patients to complete self-reported measures in the presence of a researcher may introduce observer bias, it would reduce missing data and improve response rate considerably. This was a secondary analysis of Watson et al. [17], which was powered to elicit physiological hypertrophic muscle responses. Consequently, the effects of exercise on self-reported outcomes reported here should be interpreted cautiously.
In conclusion, 12 weeks of exercise had favourable effects on symptom frequency and intrusiveness, including improvements in fatigue in CKD patients. These positive changes in self-reported outcomes support the important role of exercise in CKD management. Further research is needed to elucidate upon the physiological and psychological mechanisms of how exercise influences symptoms and other patient-reported outcomes.
FUNDING
This work was gratefully part-funded by the Stoneygate Trust. The research was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre (BRC). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR Leicester BRC or the Department of Health. At the time of writing this manuscript, E.L.W. was supported by a Kidney Research UK Post-Doctoral Fellowship. B.P.V. was funded by Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), an organization of the Brazilian federal government under the Ministry of Education.
AUTHORS’ CONTRIBUTIONS
T.J.W. was responsible for generation or collection of data, performed data analysis and data interpretation. He drafted and approved the final version of the manuscript to be submitted. E.L.W. was involved in conception and study design. He performed data interpretation. He revised and approved the final version of the manuscript to be submitted. D.W.G., S.X., A.L.C., and B.P.V. were responsible for generation/collection of data. They performed data interpretation and revised and approved the final version of the manuscript to be submitted. J.L.V. made contribution to conception and design of study. He performed data interpretation. He revised and approved the final version of the manuscript to be submitted. A.C.S. made contribution conception and design of study. He performed data interpretation. He revised and approved the final version of the manuscript to be submitted.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Almutary H, Bonner A, Douglas C.. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 2013; 39: 140–150 [DOI] [PubMed] [Google Scholar]
- 2. Brown SA, Tyrer FC, Clarke AL. et al. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J 2017; 10: 788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy EL, Murtagh FEM, Carey I. et al. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract 2009; 111: c74–c80 [DOI] [PubMed] [Google Scholar]
- 4. Abdel-Kader K, Unruh ML, Weisbord SD.. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pagels AA, Söderkvist BK, Medin C. et al. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 2012; 10: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senanayake S, Gunawardena N, Palihawadana P. et al. Symptom burden in chronic kidney disease; a population based cross sectional study. BMC Nephrol 2017; 18: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pugh‐Clarke K, Naish PF, Mercer TM.. Quality of life in chronic kidney disease. J Ren Care 2006; 32: 167–171 [DOI] [PubMed] [Google Scholar]
- 8. Clarke AL, Young HM, Hull KL. et al. Motivations and barriers to exercise in chronic kidney disease: a qualitative study. Nephrol Dial Transplant 2015; 30: 1885–1892 [DOI] [PubMed] [Google Scholar]
- 9. Soni RK, Weisbord SD, Unruh ML.. Health-related quality of life outcomes in chronic kidney disease. Curr Opin Nephrol Hypertens 2010; 19: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mapes DL, Lopes AA, Satayathum S. et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 2003; 64: 339–349 [DOI] [PubMed] [Google Scholar]
- 11. Zelle DM, Klaassen G, van Adrichem E. et al. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol 2017; 13: 152–168 [DOI] [PubMed] [Google Scholar]
- 12. Koufaki P, Mercer T.. Assessment and monitoring of physical function for people with CKD. Adv Chronic Kidney Dis 2009; 16: 410–419 [DOI] [PubMed] [Google Scholar]
- 13. West SL, Ma C, Chaudhry M. et al. The association of daily activity levels and estimated kidney function in men and women with predialysis chronic kidney disease. Kidney Int Rep 2017; 2: 874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roshanravan B, Robinson-Cohen C, Patel KV. et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roshanravan B, Gamboa J, Wilund K.. Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis 2017; 69: 837–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson T, Shur N, Smith A.. “Exercise as medicine” in chronic kidney disease. Scand J Med Sci Sports 2016; 26: 985–988 [DOI] [PubMed] [Google Scholar]
- 17. Watson EL, Gould DW, Wilkinson TJ. et al. 12-weeks combined resistance and aerobic training confers greater benefits than aerobic alone in non-dialysis CKD. Am J Physiol Renal Physiol 2018; 314: 1188–1196 [DOI] [PubMed] [Google Scholar]
- 18. Watson EL, Greening NJ, Viana JL, Aulakh J. et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis 2015; 66: 249–257 [DOI] [PubMed] [Google Scholar]
- 19. Kosmadakis GC, John SG, Clapp EL. et al. Benefits of regular walking exercise in advanced pre-dialysis chronic kidney disease. Nephrol Dial Transplant 2012; 27: 997–1004 [DOI] [PubMed] [Google Scholar]
- 20. Heiwe S, Jacobson SH.. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 64: 383–393 [DOI] [PubMed] [Google Scholar]
- 21. Gudex CM. Health-related quality of life in end-stage renal failure. Qual Life Res 1995; 4: 359–366 [DOI] [PubMed] [Google Scholar]
- 22. Brown SA, Tyrer FC, Clarke AL. et al. Kidney Symptom Questionnaire: Development, content validation and relationship with quality of life. J Ren Care 2018; doi: 10.1111/jorc.12247 [DOI] [PubMed] [Google Scholar]
- 23. Webster K, Cella D, Yost K.. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes 2003; 1: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Reenen M, Janssen B.. EQ-5D-5L User Guide: Basic Information on How to Use the EQ-5D-5L Instrument. Rotterdam: EuroQol Research Foundation; 2015 [Google Scholar]
- 25. Ware JE, Gandek B.. Overview of the SF-36 health survey and the international quality of life assessment (IQOLA) project. J Clin Epidemiol 1998; 51: 903–12 [DOI] [PubMed] [Google Scholar]
- 26. Liem YS, Bosch JL, Arends LR. et al. Quality of life assessed with the medical outcomes study Short Form 36‐Item Health Survey of patients on renal replacement therapy: a systematic review and meta‐analysis. Value Health 2007; 10: 390–397 [DOI] [PubMed] [Google Scholar]
- 27. Lowrie EG, Curtin RB, LePain N. et al. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 2003; 41: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 28. Burholt V, Nash P.. Short form 36 (SF-36) health survey questionnaire: normative data for Wales. J Public Health (Oxf) 2011; 33: 587–603 [DOI] [PubMed] [Google Scholar]
- 29. Ravani P, Kilb B, Bedi H. et al. The Duke Activity Status Index in patients with chronic kidney disease: a reliability study. Clin J Am Soc Nephrol 2012; 7: 573–580 [DOI] [PubMed] [Google Scholar]
- 30. Godin G. The Godin-Shephard leisure-time physical activity questionnaire. Health Fit J Canada 2011; 4: 18–22 [Google Scholar]
- 31. Marcus BH, Selby VC, Niaura RS. et al. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 1992; 63: 60–66 [DOI] [PubMed] [Google Scholar]
- 32. Mielenz TJ, Kubiak-Rizzone KL, Alvarez KJ. et al. Association of self-efficacy and outcome expectations with physical activity in adults with arthritis. Arthritis 2013; 621396: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hays RD, Woolley JM.. The concept of clinically meaningful difference in health-related quality-of-life research. Pharmacoeconomics 2000; 18: 419–423 [DOI] [PubMed] [Google Scholar]
- 34. Careless A, March D, Churchward D. et al. MP465 Intradialytic exercise: a non-pharmacological solution to a uraemic problem? [Abstract MP465]. Nephrol Dial Transplant 2017; 32: 599–600 [Google Scholar]
- 35. Pooranfar S, Shakoor E, Shafahi MJ. et al. The effect of exercise training on quality and quantity of sleep and lipid profile in renal transplant patients: a randomized clinical trial. Int J Organ Transplant Med 2014; 5: 157–165 [PMC free article] [PubMed] [Google Scholar]
- 36. Yang P-Y, Ho K-H, Chen H-C. et al. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother 2012; 58: 157–163 [DOI] [PubMed] [Google Scholar]
- 37. Workeneh BT, Mitch WE.. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010; 91: 1128S–1132S [DOI] [PubMed] [Google Scholar]
- 38. Sakkas GK, Hadjigeorgiou GM, Karatzaferi C. et al. Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis: a pilot study. ASAIO J 2008; 54: 185–190 [DOI] [PubMed] [Google Scholar]
- 39. Aukerman MM, Aukerman D, Bayard M. et al. Exercise and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med 2006; 19: 487–493 [DOI] [PubMed] [Google Scholar]
- 40. De Mello MT, Lauro FAA, Silva AC. et al. Incidence of periodic leg movements and of the restless legs syndrome during sleep following acute physical activity in spinal cord injury subjects. Spinal Cord 1996; 34: 294–296 [DOI] [PubMed] [Google Scholar]
- 41. Jhamb M, Liang K, Yabes J. et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol 2013; 38: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cramp F, Daniel J.. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 2012; 11: CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pickard AS, Neary MP, Cella D.. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mustata S, Groeneveld S, Davidson W. et al. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol 2011; 43: 1133–1141 [DOI] [PubMed] [Google Scholar]
- 45. Bjorner JB, Wallenstein GV, Martin MC. et al. Interpreting score differences in the SF-36 Vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin 2007; 23: 731–739 [DOI] [PubMed] [Google Scholar]
- 46. Alonso J, Permanyer-Miralda G, Cascant P. et al. Measuring functional status of chronic coronary patients: reliability, validity and responsiveness to clinical change of the reduced version of the Duke Activity Status Index (DASI). Eur Heart J 1997; 18: 414–419 [DOI] [PubMed] [Google Scholar]
- 47. Lim CR, Harris K, Dawson J. et al. Floor and ceiling effects in the OHS: an analysis of the NHS PROMs data set. BMJ Open 2015; 5: e007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977; 84: 191–215 [DOI] [PubMed] [Google Scholar]
- 49. Neupert SD, Lachman ME, Whitbourne SB.. Exercise self-efficacy and control beliefs: Effects on exercise behavior after an exercise intervention for older adults. J Aging Phys Act 2009; 17: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scherer YK, Schmieder LE.. The effect of a pulmonary rehabilitation program on self-efficacy, perception of dyspnea, and physical endurance. Heart Lung 1997; 26: 15–22 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.