Abstract
Background
This study assessed the short-term safety and efficacy of daprodustat (an oral hypoxia-inducible factor-prolyl hydroxylase inhibitor) to achieve a target hemoglobin in patients with anemia of chronic kidney disease (CKD).
Methods
Patients (n = 252) with Stages 3–5 CKD not receiving dialysis were enrolled in this 24-week, multicenter trial [hemoglobin entry criteria: 8–10 g/dL (Cohort 1) or 8–11 g/dL (Cohort 2) for recombinant human erythropoietin (rhEPO)-naïve participants; 9–10.5 g/dL (Cohort 1) or 9–11.5 g/dL (Cohort 2) for rhEPO users]. rhEPO-naïve participants were randomized 3:1 to daprodustat (1, 2 or 4 mg) or control (rhEPO per standard of care). rhEPO users were randomized 1:1 to daprodustat 2 mg or control. Study medication was titrated to maintain hemoglobin 9–10.5 g/dL (Cohort 1) or 10–11.5 g/dL (Cohort 2). Hemoglobin, iron metabolism markers and safety parameters were measured every 4 weeks.
Results
Mean hemoglobin levels at Week 24 were 10.2 g/dL (Cohort 1) and 10.9 g/dL (Cohort 2) in the daprodustat group and 10.7 g/dL (Cohort 1) and 11.0 g/dL (Cohort 2) in the control group. Participants had hemoglobin levels within the target range a median of 82% and 66% of the time between Weeks 12 and 24 in the daprodustat and control groups, respectively. The adverse event profile was consistent with clinical events in the CKD population.
Conclusions
Daprodustat effectively maintained target hemoglobin over 24 weeks in CKD patients with anemia who were rhEPO naïve or had switched from existing rhEPO therapy.
Keywords: chronic kidney disease, daprodustat, hypoxia-inducible factor, prolyl hydroxylase inhibitor, recombinant human erythropoietin
INTRODUCTION
In patients with chronic kidney disease (CKD) not on dialysis, therapy for anemia with recombinant human erythropoietin (rhEPO) and its analogs is often reserved for those with more advanced CKD [1]. Several large randomized trials [e.g. The Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial, [2] The Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) trial [3] and Trial to Reduce Cardiovascular Events with Aranesp Therapy (Treat) trial [4]] have reported adverse cardiovascular outcomes and death when using rhEPO and its analogs to target hemoglobin levels >13 g/dL, which led the US Food and Drug Administration to revise the safety language in labeling for rhEPO and its analogs in 2007 [5] and again in 2011 [6]. These changes included limiting treatment initiation in CKD patients not on dialysis to when the hemoglobin level is <10 g/dL, and reducing or interrupting the dose of rhEPO if the hemoglobin level is >10 g/dL [5–7].
To improve treatment options over existing marketed rhEPO and its analogs, new investigational agents including hypoxia-inducible factor (HIF)-prolyl hydroxylase inhibitors (PHIs), such as daprodustat, are being developed [8–13]. The mechanism of action of these agents is described in detail elsewhere [14]. Briefly, PHIs act by inhibiting HIF-prolyl hydroxylase enzymes (PHD1, PHD2 and PHD3), leading to activation of HIF-responsive genes that regulate the tissue response to hypoxia. One key activity is induction of erythropoiesis through direct activation of the EPO gene. In addition, HIF activation results in the induction of a number of genes directly or indirectly involved in iron uptake, mobilization and transport, resulting in decreased hepcidin production, as well as vascular endothelial growth factor (VEGF) [14]. Potential advantages of daprodustat and other PHIs over rhEPO and its analogs in the treatment of anemia of CKD include: (i) raising hemoglobin without exposing the patient to the supraphysiologic EPO levels and increases in blood pressure (BP) associated with intravenous (IV) rhEPO and its analogs; (ii) improving iron availability for erythropoiesis, which may reduce the need for iron replacement therapy; and (iii) avoiding the need for parenteral injection.
The dose response for daprodustat was previously defined in rhEPO-naïve patients with CKD [10]. Here, we report the results of a 24-week clinical trial that assessed the ability of daprodustat to achieve and maintain hemoglobin levels within a prespecified target range in patients with CKD Stages 3, 4 or 5, both as an initiation therapy in rhEPO-naïve participants and as a switch therapy for participants already receiving rhEPO.
MATERIALS AND METHODS
Study population
Informed consent was obtained from all participants. Eligible participants had CKD Stages 3, 4 or 5 as defined by the Japanese Society of Nephrology-Chronic Kidney Disease Initiatives equation [15] for Japanese participants residing in Japan and the CKD-Epidemiology Collaboration equation [16] for Caucasian and African-American populations. Participants who were rhEPO naïve were not to have used rhEPO within 8 weeks before screening; those who were rhEPO users were required to be on stable doses of the same rhEPO for the 4 weeks before entering the trial. Inclusion and exclusion criteria, and predefined study medication stopping criteria can be found in the Supplementary data, Item S1.
Study design
This global study (ClinicalTrials.gov identifier NCT01977573) was conducted from 17 December 2013 to 19 June 2015 at 84 sites in 15 countries in adherence with the Declaration of Helsinki, and was approved by the relevant institutional review boards or ethics committees. An internal GlaxoSmithKline Safety Review Team reviewed blinded safety data instream and an independent data monitoring committee periodically reviewed the same safety data, but it was unblinded.
The study consisted of a 4-week screening phase, a 24-week treatment phase and a follow-up visit ∼4 weeks after completing treatment. Participants who discontinued study medication or withdrew early from the study attended an early withdrawal (EW) visit, an EW follow-up visit 4 weeks later, phone assessments aligned with the remaining key study visits and an EW final visit at Week 24. Study investigators or staff were responsible for monitoring and recording adverse events (AEs). AEs were collected from the start of study treatment and until the follow-up contact. (Additional information on the study design and study assessments performed, including laboratory assessments, safety reporting, echocardiology and ophthalmology examinations, is included in the Supplementary data, Item S2; the study design is shown in Supplementary data, Figure S1.)
Participants were assigned to one of two groups based on previous exposure to rhEPO: those who were currently receiving rhEPO (rhEPO users) and those who were not (rhEPO naïve). Hemoglobin entry criteria and target values were modified during the study to more closely align with anemia guidelines and clinical practices outside the USA (Table 1). Separate randomization lists were generated for the two groups (rhEPO naïve and rhEPO users) by a GlaxoSmithKline statistician using the GlaxoSmithKline randomization system RandAll. The stratification variables (region and baseline hemoglobin range; Supplementary data, Table S1 in Item S2) were used as blocking factors. Block size of four was used. Participants were assigned a randomization number by an interactive voice/web response system. Participants who were rhEPO naïve were randomized 3:1 to once-daily daprodustat (1, 2 or 4 mg daily depending on baseline hemoglobin) or control, and rhEPO users were randomized 1:1 to daprodustat 2 mg daily or control. Starting doses of daprodustat were based on dose-response modeling from previous studies. Control participants received rhEPO (epoetins or their biosimilars or darbepoetin) as necessary per standard of care as determined by the investigator. Because of the different routes of administration, rhEPO dosing data were standardized in terms of epoetin IV dosing (reported in IU/kg/week) (see Supplementary data, Item S2).
Table 1.
Hemoglobin entry criteria
| Cohort 1a |
Cohort 2 (ex-USA) |
|||
|---|---|---|---|---|
| Hemoglobin entry | Hemoglobin target range | Hemoglobin entry | Hemoglobin target range | |
| rhEPO-naïve participants | 8–10 g/dL | 9–10.5 g/L | 8–11 g/dL | 10–11.5 g/dL |
| rhEPO users | 9–10.5 g/dL | 9–10.5 g/dL | 9–11.5 g/dL | 10–11.5 g/dL |
rhEPO, recombinant human erythropoietin.
Cohort included all countries until Cohort 2 was added; thereafter it applied to the USA only.
The dose of daprodustat was kept constant for the first 4 weeks, after which the dose could be adjusted based on a prespecified dose adjustment algorithm to achieve and/or maintain hemoglobin within the target range. Dose adjustments were made automatically by the interactive voice/web response system so the participant, investigator, site staff and study team were blinded to the dose being administered during the study. Those in the control group could receive rhEPO therapy, with doses adjusted as determined by the investigator with the intention of treating to the same hemoglobin target range as the daprodustat group. To ensure participants remained iron replete throughout the study, an iron protocol was instituted from Week 4 onwards (Supplementary data, Table S2 in Item S3). The complete study objectives and endpoints can be found in Table 2.
Table 2.
Study objectives and endpoints
| Objectives | Endpoints | |
|---|---|---|
| Primary | Characterize the ability of daprodustat to achieve mean hemoglobin response within the target range |
|
| Secondary | Characterize the ability of daprodustat to achieve hemoglobin within the target range |
|
| Characterize the effect of daprodustat on measures of iron metabolism and utilization, on indices of hematopoiesis, EPO and on VEGF |
|
|
| Characterize the steady-state population PK of daprodustat and metabolites |
|
|
| Evaluate the daprodustat dose adjustment scheme |
|
|
| Safety | Assess the safety and tolerability of daprodustat |
|
AE, adverse event; CHr, reticulocyte hemoglobin content; CV, cardiovascular; IV, intravenous; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; PK, pharmacokinetics; RBC, red blood cell; rhEPO, recombinant human erythropoietin; sPAP, systolic pulmonary artery pressure; TIBC, total iron-binding capacity; TSAT, transferrin saturation; VEGF, vascular endothelial growth factor.
Statistical analyses
The planned sample size for this study was driven by exposure requirements to achieve ∼100 evaluable participants treated with daprodustat, where a participant was considered evaluable if they had on-treatment data at Week 24. (See Supplementary data, Item S4 for additional justification.)
The intent-to-treat (ITT) population in the daprodustat groups comprised all randomized participants who received at least one dose of study treatment and had a baseline and at least one postbaseline on-therapy hemoglobin assessment. For the control groups, the ITT population comprised all randomized participants who had a baseline and at least one postbaseline on-therapy hemoglobin assessment. The safety population in the daprodustat groups comprised all participants who received at least one dose of study treatment, whereas it comprised all randomized participants in the control groups.
For the primary analysis, mean hemoglobin concentration at Week 24 was measured and corresponding 95% confidence intervals were calculated. As part of the secondary analysis, percentage of time hemoglobin values were within, above and below the target range between Weeks 12 and 24 (inclusive) was also calculated. Efficacy endpoints were summarized in the ITT population for each rhEPO group (naïve and user) and the two treatment groups (daprodustat and control) using summary statistics. In particular, hemoglobin was summarized by rhEPO group, the two cohorts (based on hemoglobin entry criteria) and treatment group. The data for the control groups served as a reference only; no formal comparisons with daprodustat were made.
RESULTS
Study population
Two hundred fifty-two participants were randomized to either daprodustat (n = 172) or control (n = 80) (Figure 1). Of those randomized, 250 (>99%) were included in the safety population and 235 (93%) in the ITT population, with 222 (88%) completing the study (i.e. participants who completed the Week 24 visit regardless of whether they remained on study treatment) [148 (86%) in the daprodustat group and 74 (93%) in the control group].
FIGURE 1:
Study flow diagram. aOne participant withdrew from the study because of an AE; however, this participant’s AE was actually a protocol-defined stopping criterion and is presented here in the stopping criteria category rather than the AE category. bCompleted all study visits or abbreviated schedule if study medication was discontinued. cFifteen participants discontinued study medication because of AEs; however, five of these participants had AEs that were actually protocol-defined stopping criteria and are presented here in the stopping criteria category rather than the AE category. dOne participant discontinued study medication because of an AE; however, this participant’s AE was actually a protocol-defined stopping criterion and is presented here in the stopping criteria category rather than the AE category. eProtocol-defined reasons for discontinuation of study medication included hemoglobin <7.5 g/dL, renal transplant, increased sPAP of ≥20 mmHg, drop of LVEF ≥10% from baseline and <50% and blood transfusion. The complete list of stopping criteria is included in Supplementary data, Item S1. IP: investigational product; PI: principal investigator.
The most common reasons for premature withdrawal were an AE in the daprodustat group (5%) and withdrawal by participant in the control group (3%).
Similar proportions of participants (20% randomized to daprodustat; 18% randomized to control) discontinued study treatment prematurely. The most common reasons for treatment discontinuation were an AE in the daprodustat group (10%) and reaching the protocol-defined stopping criteria in the control group (8%) [included renal transplant, increased systolic pulmonary artery pressure (sPAP) of ≥20 mmHg, drop of left ventricular ejection fraction (LVEF) ≥10% from baseline and <50% and blood transfusion]. Participants that finished the entire treatment as well as those with an abbreviated treatment schedule were included in the ITT population.
Overall, baseline characteristics were well balanced between the combined daprodustat and control groups. The majority of participants were female (59%), with a mean body mass index (BMI) of 27.8 kg/m2 and mean age of 66.1 years (Table 3). Mean baseline hemoglobin was 10.0 g/dL and most participants had Stage 4 (46%) or Stage 5 (36%) CKD.
Table 3.
Participant characteristics (ITT population)
| rhEPO naïve |
rhEPO user |
Total |
||||
|---|---|---|---|---|---|---|
| Daprodustat | Control | Daprodustat | Control | Daprodustat | Control | |
| (n = 123) | (n = 43) | (n = 33) | (n = 36) | (n = 156) | (n = 79) | |
| Age, years, mean (SD) | 67.6 (12.21) | 64.3 (14.22) | 62.0 (14.06) | 66.7 (12.89) | 66.5 (12.78) | 65.4 (13.6) |
| Sex, female, n (%) | 75 (61) | 23 (53) | 17 (52) | 23 (64) | 92 (59) | 46 (58) |
| Weight (kg), mean (SD) | 73.2 (19.64) | 77.1 (16.03) | 77.1 (23.49) | 71.7 (18.60) | 74.1 (20.49) | 74.6 (17.35) |
| BMI (kg/m2), mean (SD) | 27.7 (6.9) | 28.4 (5.4) | 28.1 (7.1) | 27.3 (6.4) | 27.8 (6.9) | 27.9 (5.9) |
| Hemoglobin criteriaan (%) | ||||||
| Cohort 1 | 52 (42) | 18 (42) | 21 (64) | 17 (47) | 73 (47) | 35 (44) |
| Cohort 2 | 77 (58) | 25 (58) | 12 (36) | 19 (53) | 83 (53) | 44 (56) |
| Baseline hemoglobin level (g/dL), mean (SD)b | 9.9 (0.80) | 9.9 (0.73) | 10.2 (0.62) | 10.2 (0.57) | 10.0 (0.77) | 10.1 (0.67) |
| Type of rhEPO, n (%) | ||||||
| Epoetin SC | 0 | 0 | 14 (42) | 5 (14) | 14 (42) | 5 (14) |
| Darbepoetin | 0 | 0 | 19 (58) | 30 (86) | 19 (58) | 30 (86) |
| Geographical region, n (%) | ||||||
| Japan | 17 (14) | 6 (14) | 9 (27) | 9 (25) | 26 (17) | 15 (19) |
| North America | 35 (28) | 6 (14) | 6 (18) | 7 (19) | 41 (26) | 13 (16) |
| Russia | 15 (12) | 10 (23) | 9 (27) | 2 (6) | 24 (15) | 12 (15) |
| Rest of world | 56 (46) | 21 (49) | 9 (27) | 18 (50) | 65 (42) | 39 (49) |
| Stage of CKD, n (%) | ||||||
| 2 | 0 | 0 | 0 | 1 (3) | 0 | 1 (1) |
| 3a | 4 (3) | 1 (2) | 1 (3) | 1 (3) | 5 (3) | 2 (3) |
| 3b | 23 (19) | 6 (14) | 3 (9) | 3 (8) | 26 (17) | 9 (11) |
| 4 | 58 (47) | 17 (40) | 17 (52) | 15 (42) | 75 (48) | 32 (41) |
| 5 | 38 (31) | 19 (44) | 12 (36) | 16 (44) | 50 (32) | 35 (44) |
| Baseline eGFR (mL/min/1.73 m2), mean (SD) | 21.3 (10.69) | 19.4 (10.9) | 17.9 (9.17) | 18.8 (11.97) | 20.6 (10. 45) | 19.2 (11.3) |
| Cardiovascular risk factors, n (%)c | n = 134 | n = 45 | n = 36 | n = 35 | n = 170 | n = 80 |
| Any | 29 (22) | 12 (27) | 9 (25) | 6 (17) | 38 (22) | 18 (23) |
| Angina pectoris | 7 (5) | 5 (11) | 0 | 3 (9) | 7 (4) | 8 (10) |
| Hyperlipidemia | 4 (3) | 2 (4) | 1 (3) | 0 | 5 (3) | 2 (3) |
| Hypertension | 2 (1) | 0 | 1 (3) | 0 | 3 (2) | 0 |
| Diabetes | 0 | 0 | 2 (6) | 0 | 2 (1) | 0 |
BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; rhEPO, recombinant human erythropoietin; SC, subcutaneous; SD, standard deviation.
Hemoglobin entry criteria for rhEPO-naïve participants were 8–10 g/dL in Cohort 1 and 8–11 g/dL in Cohort 2. Criteria in rhEPO users were 9.0–10.5 g/dL in Cohort 1 and 9.0–11.5 g/dL in Cohort 2.
Baseline hemoglobin levels for Cohort 1 and Cohort 2 combined.
Safety population.
Exposure to study medication
In the rhEPO-naïve group (n = 179), 134 participants received a median daprodustat dose of 1 mg at Week 20. Of the rhEPO users (n = 71), 36 received a median daprodustat dose of 2 mg at Week 20. Forty-five rhEPO-naïve participants in the control group (rhEPO per investigator discretion) received a median rhEPO dose, standardized to IV epoetin, of 16.5 IU/kg/week at Week 20. The remaining 35 rhEPO users in the control arm received 51.3 IU/kg/week at Week 20 (Supplementary data, Table S3 in Item S5). One study participant in the control arm was incorrectly randomized based on prior rhEPO exposure, which accounts for the difference in the safety/drug exposure populations as compared with the randomized population.
Hemoglobin response
Primary analysis
Mean hemoglobin levels at Week 24 were 10.2 g/dL (Cohort 1) and 10.9 g/dL (Cohort 2) in the daprodustat group versus 10.7 g/dL (Cohort 1) and 11.0 g/dL (Cohort 2) in the control group.
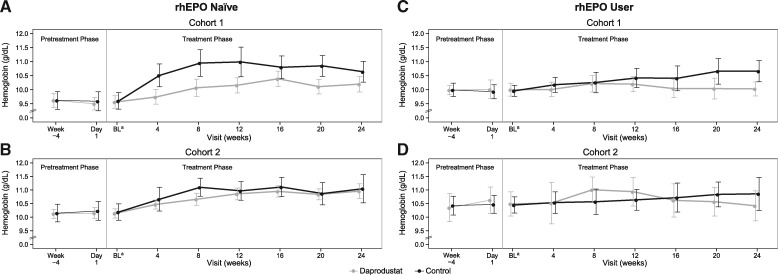
This represented an increase from baseline in rhEPO-naïve participants within the daprodustat and control groups for Cohorts 1 and 2 (Figure 2). In rhEPO users, hemoglobin at 24 weeks was similar to baseline levels in both daprodustat cohorts and increased in both control cohorts.
FIGURE 2:
Mean and 95% confidence interval for hemoglobin over time by original (A) and amended (B) hemoglobin criteria for the rhEPO-naïve group and original (C) and amended (D) hemoglobin criteria for the rhEPO user group. For Cohort 1, the hemoglobin entry criterion was 8–10 g/dL in the rhEPO-naïve group and 9–10.5 g/dL in rhEPO users, and the hemoglobin target range was 9–10.5 g/dL. For Cohort 2, the hemoglobin entry criterion was 8–11 g/dL in the rhEPO-naïve group and 9–11.5 g/dL in rhEPO users, and the hemoglobin target range was 10–11.5 g/dL. aBaseline values were calculated as the average of measurements at Week-4 and Day 1. BL, baseline; rhEPO, recombinant human erythropoietin.
Secondary analyses
Participants had hemoglobin levels within the target range a median of 82% and 66% of the time and above the target range a median of 0% and 18% of the time between Weeks 12 and 24 in the daprodustat and control groups, respectively (Table 4). Similar results were seen in a sensitivity analysis performed in all ITT participants where a participant on daprodustat was considered as having hemoglobin below target range once he/she discontinued randomized study medication. However, for participants in the control arm, the hemoglobin measurement was used. If the hemoglobin measurement was missing, we assumed it to be within the hemoglobin target range (Supplementary data, Table S4 in Item S6). Seventy-four percent and 59% of participants in the combined daprodustat and combined control groups, respectively, had hemoglobin levels within the target hemoglobin range at Week 24 (Table 4).
Table 4.
Hemoglobin time in or above target range (ITT population)a
| rhEPO naïve |
rhEPO user |
Total |
||||
|---|---|---|---|---|---|---|
| Daprodustat | Control | Daprodustat | Control | Daprodustat | Control | |
| (n = 112) | (n = 38) | (n = 30) | (n = 32) | (n = 142) | (n = 70) | |
| Percentage of time spent with hemoglobin in or above target range between Weeks 12 and 24 | ||||||
| Within the target range, medianb | 81 | 47 | 94 | 88 | 82 | 66 |
| Above the target range medianb | 0 | 33 | 0 | 5 | 0 | 18 |
| Participants with hemoglobin in or above target range at Week 24 | ||||||
| Within the target range, n (%) | 78 (74) | 20 (56) | 22 (73) | 19 (63) | 100 (74) | 39 (59) |
| Above the target range, n (%) | 23 (22) | 14 (39) | 5 (17) | 9 (30) | 28 (21) | 23 (35) |
rhEPO, recombinant human erythropoietin.
Target range was 9–10.5 g/dL in the original and 10–11.5 g/dL in the amended criteria.
The minimum and maximum for all values were 0 and 100, respectively.
The changes in hemoglobin were associated with expected changes in red blood cell (RBC) count, hematocrit and absolute reticulocyte count (Supplementary data, Table S5 in Item S7).
Three participants on daprodustat (two rhEPO naïve and one rhEPO user) discontinued treatment because of the protocol-defined hemoglobin stopping criteria (hemoglobin <7.5 g/dL). In the rhEPO-naïve group, six participants (4%) on daprodustat and four participants (5%) in the control group had hemoglobin ≥13 g/dL over the course of the 24-week treatment period. Among the rhEPO users, no participants on daprodustat and one participant (3%) in the control group had hemoglobin ≥13 g/dL.
For simplicity, data for parameters other than hemoglobin are presented for all participants on daprodustat (combined daprodustat group) and all control participants (combined control group).
Erythropoietin and vascular endothelial growth factor levels
Plasma EPO levels remained near baseline in the combined daprodustat group and the combined control group (single sample collected during the study visit, predose if scheduled to receive rhEPO) (Table 5).
Table 5.
Maximum observed plasma EPO levels (IU/L), plasma VEGF level (ng/L) and change from baseline (ITT population)
| Combined daprodustat | Combined control | |
|---|---|---|
| (n = 156) | (n = 78) | |
| Plasma EPO levels (IU/L) | ||
| Baseline, median (min, max) | 10.5 (2.5, 100.3) | 11.4 (2.5, 61.5) |
| Maximum observed EPO level,a median (min, max) | 16.1 (4.2, 109.3) | 19.2 (4.9, 620.6) |
| Plasma VEGF level (ng/L) | ||
| Baseline,b median (min, max) | 65.8 (17.9, 467.9) | 68.7 (22.9, 1118.5) |
| Maximum observed VEGF,c median (min, max) | 105.8 (33.1, 1205.3) | 94.5 (25.5, 472.7) |
rhEPO, recombinant human erythropoietin; VEGF, vascular endothelial growth factor.
Daprodustat measurements predose and postdose up to 15 h after dosing; control included a single sample during visit (predose if scheduled to receive rhEPO at visit).
Baseline is the last predose value.
Daprodustat measurements predose and postdose up to 15 h after dosing; control included a single sample during visit (predose if scheduled to receive rhEPO at visit).
Baseline plasma VEGF values were similar in the combined daprodustat and control groups (Table 5). VEGF levels were highly variable at all time points, with no clear signal for a change in any treatment group.
Iron use, metabolism and utilization
During the treatment period, ∼67% of participants received oral iron and ∼10% of participants received IV iron. Participants in the combined daprodustat group received a mean total of 38.1 g of oral elemental iron in the study (0.16 g/day) and participants in the control group received a mean total of 28.0 g (0.13 g/day).
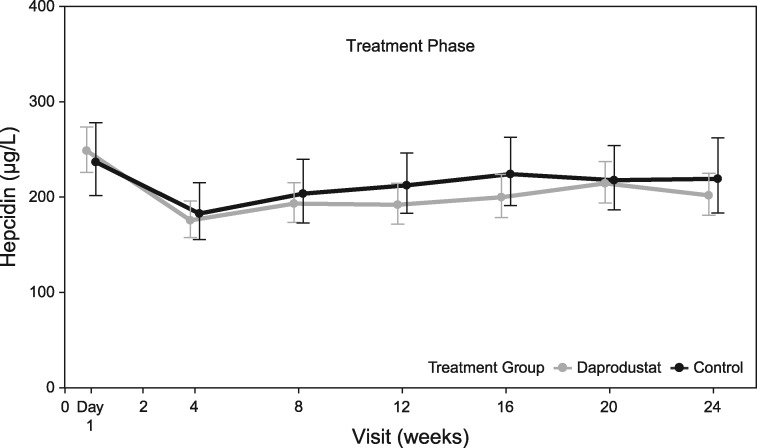
The time course of change in hepcidin is shown in Figure 3, and the baseline and change from baseline at Week 24 in measures of iron metabolism are shown in Supplementary data, Table S6 and Supplementary data, Figure S2 in Item S8.
FIGURE 3:
Geometric mean and 95% confidence interval for hepcidin (µg/L) by visit [intent-to-treat (ITT) population].
Safety
The incidence of any on-therapy AEs was 70% in the combined daprodustat and 68% in the combined control groups (Table 6). The most frequently reported AEs (≥5%) in the combined daprodustat group were nasopharyngitis, diarrhea and nausea. One participant (<1%) discontinued study treatment/withdrew from the study due to nausea.
Table 6.
AEs and frequency of MACE and component endpoints in the combined daprodustat or combined control group (safety population)
| Combined daprodustat | Combined control | |
|---|---|---|
| (n = 170) | (n = 80) | |
| AEs occurring in ≥5% of participants | ||
| Any event, n (%) | 119 (70) | 54 (68) |
| Nasopharyngitis | 20 (12) | 3 (4) |
| Diarrhea | 10 (6) | 6 (8) |
| Nausea | 8 (5) | 1 (1) |
| Arthralgia | 3 (2) | 4 (5) |
| Pruritus | 0 | 4 (5) |
| Frequency of MACE and component endpoints | ||
| Composite endpoints, n (%) | ||
| Any MACEa | 5 (3) | 1 (1) |
| Any MACE+b | 7 (4) | 4 (5) |
| Components of composite endpoints,cn (%) | ||
| All-cause mortality | 4 (2) | 1 (1) |
| MI (fatal or nonfatal) | 1 (<1) | 0 |
| Stroke (fatal or nonfatal) | 0 | 0 |
| Hospitalization due to heart failure | 4 (2) | 4 (5) |
MACE, major adverse cardiovascular event; MI, myocardial infarction.
MACE is defined as a first occurrence of all-cause mortality, a nonfatal MI or a nonfatal stroke.
MACE+ is defined as a first occurrence of MACE or hospitalization for heart failure.
Includes all events.
On-therapy serious AEs (SAEs) were reported in 26 participants (15%) in the combined daprodustat group and 13 participants (16%) in the combined control group. The only SAE reported in >1% of participants in either treatment group was worsening CKD [chronic renal failure-MedDRA (Medical Dictionary for Regulatory Activities Terminology)], which was reported in three participants (2%) in the combined daprodustat group and three participants (4%) in the combined control group. Cardiovascular events, including major adverse cardiovascular events (MACEs) and component endpoints, are summarized in Table 6. Other preidentified AEs of interest are described in the Supplementary data, in Item S9.
Mean (SD) estimated glomerular filtration rate (eGFR) at baseline was 20.6 (10.5) mL/min/1.73 m2 in the combined daprodustat group and 19.2 (11.3) mL/min/1.73 m2 in the combined control group and did not change by end of study [−0.2 mL/min/1.73 m2 (daprodustat) and −1.0 mL/min/1.73 m2 (control)]. Two participants (1%) on daprodustat and three (4%) on control started dialysis during the study.
Seven participants (4%) on daprodustat and one (1%) in the control group had a blood transfusion during the study. All transfusions occurred during hospitalization for various medical conditions (e.g. sepsis, congestive heart failure exacerbation, non-GI bleeding); in six of seven participants, these occurred at a hemoglobin level >7.5 g/dL.
LVEF, estimated sPAP and baseline values for other echocardiographic parameters were well balanced in all groups, as were the absolute changes from baseline in on-treatment assessments (Supplementary data, Table S7 in Item S9). Six (4%) participants in the combined daprodustat group and four (5%) in the combined control group had LVEF values <50% at any time during the study. Thirteen (9%) and five (7%) participants in the combined daprodustat and control groups, respectively, had an absolute decrease from baseline in LVEF of >10%. One (<1%) participant in the daprodustat group and one (2%) in the control group had an estimated sPAP value that increased >20 mmHg from baseline at any time during the study.
No adverse trends were noted for clinical laboratory values, electrocardiogram (ECG) values or BP; data for the latter are shown in Table 7. Furthermore, no changes were seen in visual acuity or intraocular pressure based on the protocol-specified ophthalmology examinations (data not shown).
Table 7.
Summary of SBP and DBP at Week 24 (safety population)
| Combined daprodustat | Control | |
|---|---|---|
| (n = 170) | (n = 80) | |
| SBP (mmHg) | ||
| Baselinea | ||
| n | 150 | 74 |
| Mean (SD) | 138.0 (18.5) | 136.4 (16.5) |
| Change from baseline at Week 24 | ||
| n | 137 | 66 |
| Mean (SD) | −0.4 (16.9) | −0.1 (15.3) |
| DBP (mmHg) | ||
| Baselinea | ||
| n | 150 | 74 |
| Mean (SD) | 73.0 (12.6) | 72.1 (10.6) |
| Change from baseline at Week 24 | ||
| n | 137 | 66 |
| Mean (SD) | 0.1 (10.5) | −0.3 (9.9) |
DBP, diastolic BP; SBP, systolic BP.
Baseline is the last predose value.
DISCUSSION
To our knowledge, this is the largest (>250 participants randomized) and longest (24-week treatment duration) study reported to date of a HIF-PHI in the anemic CKD population not yet on dialysis. Results demonstrate that daprodustat can increase and maintain hemoglobin over 24 weeks within a narrow prespecified target range, most notably evidenced by the percentage of time hemoglobin was in the target range between Weeks 12 and 24 for the rhEPO-naïve participants (81%) and rhEPO users (94%).
Consistent with its mechanism of action and similar to previous reports, [10] the effect of daprodustat on hemoglobin occurred with minimal changes in plasma EPO levels that were similar in magnitude to endogenous EPO levels seen during exposure to high altitudes in healthy individuals [17, 18]. If higher EPO levels contribute to the cardiovascular risk associated with rhEPO, [19] then daprodustat may provide a safer cardiovascular risk profile.
Daprodustat and control had minimal, if any, effect on VEGF, one of the target genes of HIF, consistent with prior observations at similar [10] and higher daprodustat doses (10–100 mg) [9] as well as with other HIF-PHIs [11]. This is also consistent with preclinical work demonstrating that VEGF expression is less sensitive to HIF activation than is EPO expression [20].
Measures of iron metabolism changed in a manner consistent with data from previous studies with several PHI agents [9–13]. Hepcidin levels, in general, are suppressed by erythropoiesis, due to increased iron utilization and decreased available stores [21–23]. In the combined daprodustat group, hepcidin levels decreased by 17% while hemoglobin increased, suggesting that this effect could be secondary to erythropoiesis. While the decrease in hepcidin is not as prominent as reported in the earlier dose-ranging studies with daprodustat [9, 10] in this study, lower doses of daprodustat were required to maintain hemoglobin in target ranges. It is conceivable that efficacy of distinct HIF-PHI investigational products differs and that higher daprodustat doses than needed to achieve target hemoglobin are required for increased hepcidin suppression.
Participants were considered iron replete at baseline based on the currently acceptable ferritin and transferrin saturation entry criteria and, although rates of IV iron supplementation in the study were very low (∼10%), 67% of participants received oral iron supplementation, albeit at low doses. Though HIF-PHIs are hypothesized to be iron sparing, this trial, as well as other published CKD trials [11–13], have failed to show a reduction in iron usage; it is likely that trials of longer duration are required to demonstrate a difference.
Treatment with daprodustat for up to 24 weeks demonstrated an AE profile consistent with the comorbidities typical of the CKD population [24]. No new safety signals based on the collective safety data were identified for daprodustat.
Seven (4%) participants in the daprodustat group and one (1%) in the control group had a blood transfusion, with the most frequent reason for transfusions cited by investigators being underlying chronic anemia followed by active bleeding (not gastrointestinal). It is possible that transfusions were infrequent in the control group because rhEPO in these participants could be titrated frequently at the investigator’s discretion. In the daprodustat group, adjustments were only allowed per the prespecified dose adjustment algorithm every 4 weeks.
Although the study was relatively small and too short in duration to determine MACE frequency and events were not formally adjudicated, the overall rate of MACE in the study was lower than anticipated based on the historical data [2, 4, 25]. A long-term cardiovascular outcome study is ongoing to conclusively assess these events.
There were no noteworthy changes in the measured echocardiographic parameters for either of the treatment groups. This is in contrast to a 24-week study of daprodustat in patients on hemodialysis where there was an imbalance in the number of participants with an increase from baseline in estimated sPAP of >20 mmHg in the daprodustat group relative to control due to a number of potential confounders [26]. Additionally, dedicated preclinical data (GlaxoSmithKline, unpublished data) and a clinical study in healthy volunteers [27] have demonstrated no effect of daprodustat on peak right ventricular pressure or sPAP, respectively, even in the setting of a hypoxic challenge.
No adverse trends were apparent in either treatment group following review of the laboratory data, vital signs, ECGs and ophthalmology examinations. This included no change in BP for the combined daprodustat and control groups. Contrary to the known risk of hypertension associated with rhEPOs and its analogs, [7, 28] the lack of effect on BP in the combined control group could be due to the overall smaller doses of rhEPO in this study and adequate BP control in the rhEPO user group.
There are several limitations in this study that need to be considered. Amending the hemoglobin entry criteria during study conduct necessitated analyzing changes in hemoglobin in the context of different entry criteria and target ranges. This, however, did not affect the interpretation of the hemoglobin time-in-range data where daprodustat was shown to achieve and maintain hemoglobin within a relatively narrow target range. Given the global nature of the study and differing practices in treating anemia, the control group received rhEPO per standard of care as necessary, with the added instruction to treat to the hemoglobin target range. This lack of standardization as to how and when rhEPO was utilized may have contributed to fewer participants with hemoglobin levels within the target range and more above the target range, which may have been consistent with regional standard of care. This was not the case with daprodustat, which was dosed based on a prespecified dose adjustment algorithm. Additionally, the tight eligibility criteria limit the generalizability of the findings, and the degree of noncompletion may complicate interpretation of the results.
Finally, the open-label design could potentially influence how AEs are reported by participants or recorded by study staff. For example, more AEs of nausea were reported in the daprodustat group, which could have been influenced by daprodustat being an investigational medication, or due to an oral route of daprodustat administration. Furthermore, cardiovascular events were not formally adjudicated, even though overall number of MACEs was low. Although this is the largest study to date, the sample size and 6-month duration is not long enough to draw conclusions regarding the long-term safety of daprodustat, particularly regarding cardiovascular risk and cancer.
In conclusion, in this 24-week study in anemic CKD patients, daprodustat effectively achieved target hemoglobin levels in rhEPO-naïve participants and maintained target hemoglobin levels in rhEPO users. Daprodustat was well tolerated with an AE profile consistent with the CKD population. These data support continued development of daprodustat to treat anemia of CKD, regardless of whether patients are rhEPO naïve or already taking rhEPO. This includes an ongoing large-scale cardiovascular outcomes trial (ASCEND-ND) in this population to assess the safety and efficacy of daprodustat (NCT02876835).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the study participants, investigators and site staff (see ClinicalTrials.gov NCT01977573 for site list). The authors acknowledge the following GlaxoSmithKline (GSK) employees for their assistance with study management and critical review: Deborah Kelly, MD, and Douglas Wicks, MPH, CMPP. Authors meet the authorship criteria set forth by the International Committee for Medical Journal Editors. Medical editorial support (Nancy Price, PhD, and Gautam Bijur, PhD) and graphic services were provided by AOI Communications, L.P., and were funded by GSK. B.M.J. was previously affiliated with Clinical Pharmacology Modeling and Simulation, GlaxoSmithKline, Research Triangle Park, NC, USA.
FUNDING
Funding for this study (clinicaltrials.gov: NCT01977573; EudraCT: 2013-002681-39; GSK registry: PHI113747) was provided by GlaxoSmithKline.
AUTHORS’ CONTRIBUTIONS
Concept and study was designed by L.H., B.C., A.M.M., N.B., B.M.J., D.J., J.J.L. and A.R.C.; S.G.K. and S.Z. were in charge of data acquisition; statistical analysis was conduted by N.B. and D.J. All authors reviewed the analysis and interpreted the data; L.H. wrote the initial draft and all authors contributed critically and approved of the final manuscript for submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
B.C., A.M.M., N.B., D.J., J.J.L. and A.R.C. are employees of, and hold stock in, GlaxoSmithKline (GSK). D.J., J.J.L. and A.R.C. also own stock options in GSK. L.H. and B.M.J. are former employees of GSK, and hold stock options in GSK. S.G.K. is an employee of Hallym University, and receives research funding from C.J. Healthcare and Fibrogen. S.Z. has no conflicts to declare. This manuscript is not under consideration for publication elsewhere. The results presented in this paper have not been published previously in whole or part, except in abstract form. Some of the data included in the present manuscript were presented at the American Society of Nephrology meeting in Chicago, IL, 15–20 November 2016.
REFERENCES
- 1. Bonomini M, Del Vecchio L, Sirolli V. et al. New treatment approaches for the anemia of CKD. Am J Kidney Dis 2016; 67: 133–142 [DOI] [PubMed] [Google Scholar]
- 2. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 3. Drüeke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 4. Pfeffer MA, Burdmann EA, Chen C-Y. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 5. Jenkins J. Statement regarding erythropoiesis-stimulating agents (ESA) before the Committee on Ways and Means Subcommittee on Health, US House of Respresentatives. Government Publishing Office https://www.gpo.gov/fdsys/pkg/CHRG-110hhrg49981/pdf/CHRG-110hhrg49981.pdf. Published 26 June 2007 (20 December 2017, date last accessed)
- 6.FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of erythropoiesis-stimulating agents (ESAs) in chronic kidney disease. US Food and Drug Administration https://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Published 24 June 2011 (20 December 2017, date last accessed)
- 7. Procrit [package insert]. Horsham, PA: Janssen Products, LP; 2013 [Google Scholar]
- 8. Johnson BM, Stier BA, Caltabiano S.. Effect of food and gemfibrozil on the pharmacokinetics of the novel prolyl hydroxylase inhibitor GSK1278863. Clin Pharmacol Drug Dev 2014; 3: 109–117 [DOI] [PubMed] [Google Scholar]
- 9. Brigandi RA, Johnson B, Oei C. et al. A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: a 28-day, phase 2A randomized trial. Am J Kidney Dis 2016; 67: 861–871 [DOI] [PubMed] [Google Scholar]
- 10. Holdstock L, Meadowcroft AM, Maier R. et al. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol 2016; 27: 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pergola PE, Spinowitz BS, Hartman CS. et al. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 2016; 90: 1115–1122 [DOI] [PubMed] [Google Scholar]
- 12. Besarab A, Provenzano R, Hertel J. et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 2015; 30: 1665–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Provenzano R, Besarab A, Sun CH. et al. Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol 2016; 11: 982–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lenihan CR, Winkelmayer WC.. The dawning of a new day in CKD anemia care? J Am Soc Nephrol 2016; 27: 968–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Imai E, Yasuda Y, Makino H.. Japan association of chronic kidney disease initiatives (J-CKDI). Jpn Med Assoc J 2011; 54: 403–405 [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milledge JS, Cotes PM.. Serum erythropoietin in humans at high altitude and its relation to plasma renin. J Appl Physiol 1985; 59: 360–364 [DOI] [PubMed] [Google Scholar]
- 18. Piperno A, Galimberti S, Mariani R. et al. Modulation of hepcidin production during hypoxia-induced erythropoiesis in humans in vivo: data from the HIGHCARE project. Blood 2011; 117: 2953–2959 [DOI] [PubMed] [Google Scholar]
- 19. McCullough PA, Barnhart HX, Inrig JK. et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 2013; 37: 549–558 [DOI] [PubMed] [Google Scholar]
- 20. Flamme I, Oehme F, Ellinghaus P. et al. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One 2014; 9: e111838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashby DR, Gale DP, Busbridge M. et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int 2009; 75: 976–981 [DOI] [PubMed] [Google Scholar]
- 22. Pinto JP, Ribeiro S, Pontes H. et al. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPalpha. Blood 2008; 111: 5727–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sasaki Y, Noguchi-Sasaki M, Yasuno H. et al. Erythropoietin stimulation decreases hepcidin expression through hematopoietic activity on bone marrow cells in mice. Int J Hematol 2012; 96: 692–700 [DOI] [PubMed] [Google Scholar]
- 24. Tonelli M, Wiebe N, Guthrie B. et al. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int 2015; 88: 859–866 [DOI] [PubMed] [Google Scholar]
- 25. United States Renal Data System. USRDS 2013 Annual Data Report. Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 26. Meadowcroft AM, Cizman B, Holdstock L. et al. Daprodustat for anemia: a 24-week, open-label, randomized, controlled trial in participants on hemodialysis. Clin Kidney J 2019; 12: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demopoulos L,, Haws T,, Mahar K. et al. Lack of correlation between PK exposure and changes in pulmonary artery systolic pressure in healthy volunteers on the prolyl hydroxylase inhibitor, GSK1278863. Pharmacotherapy 2014; 34: e222 [Google Scholar]
- 28. Aranesp [package insert]. Thousand Oaks, CA: Amgen Inc, 2017 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.