Abstract
Abemaciclib (Verzenio®) is approved as a tyrosine kinase inhibitor (TKI) for breast cancer treatment. In this study, in vitro phase I metabolic profiling of Abemaciclib (ABC) was done using rat liver microsomes (RLMs). We checked the formation of reactive intermediates in ABC metabolism using RLMs in the presence of potassium cyanide (KCN) that was used as a capturing agent for iminium reactive intermediates forming a stable complex that can be characterized by LC–MS/MS. Nine in vitro phase I metabolites and three cyano adducts were identified. The metabolic reactions involved in the formation of these metabolites and adducts are reduction, oxidation, hydroxylation and cyanide addition. The bioactivation pathway was also proposed. Knowing the electrodeficient bioactive centre in ABC structure helped in making targeted modifications to improve its safety and retain its efficacy. Blocking or isosteric replacement of α-carbon to the tertiary nitrogen atoms of piperazine ring can aid in reducing toxic side effects of ABC. No previous articles were found about in vitro metabolic profiling for ABC or structural identification of the formed reactive metabolites for ABC.
Keywords: Abemaciclib, reactive metabolites, iminium intermediates, side effects
1. Introduction
A malignant tumour is formed of a group of cancer cells that have the ability to grow and invade tissues either surrounding or at distant areas of the body [1]. Globally, breast cancer is the most frequently diagnosed cancer in women; it affects about 12% of women worldwide [2]. Saudi Arabia is no exception, where breast cancer is most commonly prevalent. It accounts for about 22% of all new cancers in women that are ranked first figures [3]. There is a group of novel drugs that specifically target gene changes in cancer cells that help the cells grow or spread. Tyrosine kinases (TKs) are important targets because of their important role in the modulation of growth factor signalling [4]. Controlling the activity of TK in the cell regulates many vital processes such as cell cycle, proliferation and cell death [5].
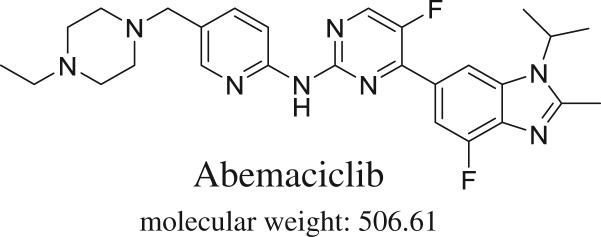
Cyclin-dependent kinases 4 and 6 (CDK4/6), as a class of tyrosine kinase inhibitor (TKI), play a crucial role in cell proliferation. When CDK4/6 pathway is dysregulated, it leads to an implication in breast cancer biology [6]. There are three highly selective CDK4/6 inhibitors that have been approved for breast cancer therapy: Palbociclib, Ribociclib and Abemaciclib (ABC) (Verzenio®) [7]. ABC (figure 1) is the third agent in this class, which was approved in September 2017 by the Food and Drug Administration (FDA), either in combination with fulvestrant for women or as monotherapy for women and men with HR-positive, HER2-negative advanced or metastatic breast cancer. Lately, in February 2018, FDA approved ABC with an aromatase inhibitor as the first-line endocrine-based therapy for metastatic breast cancer. The most frequent side effects of the drug were diarrhoea, which occurred in approximately 80% of patients, neutropenia, fatigue, infections, nausea, abdominal pain, anaemia, vomiting, alopecia, decreased appetite and leukopenia [8].
Figure 1.
ABC chemical structure.
Our group previously studied reactive metabolite formations of some TKI drugs. The current work proved that ABC formed three reactive intermediates through a specific bioactivation pathway. KCN was used as a trapping agent to capture the formed reactive metabolites and the bioactivation pathways were characterized [8–12]. The chemical structures of ABC contain N-ethyl piperazine ring. This group is expected to undergo metabolism generating reactive iminium intermediates that can be trapped by KCN forming cyano adducts. Oxidation at a carbon alpha to an N-substituted piperazine ring is known to result in iminium ions, which are considered hard electrophiles and can be trapped by cyanide anions to form cyano adducts. The N-substituted piperazine partial structures become bioactivated and subsequently trapped by the nucleophilic cyanide anion. The reactive intermediates were postulated as imine and imine-carbonyl conjugate (α,β-unsaturated) structures on the piperazine ring. Bioactivation of the N-substituted piperazine partial structure, which can be characterized and detected using tandem mass spectrometry (LC–MS/MS), is a possible explanation for reported idiosyncratic toxicity [13–16].
Iminium intermediates initiate several toxic side effects through previously reported mechanisms as in the case of brigatinib and ponatinib that bind covalently to a DNA base [9,17]. Knowing the electrodeficient bioactive centre in ABC structure helped in making targeted modifications to improve its safety and retain its efficacy. ABC rat liver microsome (RLM) incubation resulted in the identification of nine in vitro phase I metabolites and three cyano conjugates, and the proposed reactions involved include reduction, oxidation, hydroxylation and cyanide addition.
2. Chemicals and methods
2.1. Chemicals
All chemicals are described in table 1. RLMs were made in house using Sprague-Dawley rats following previously reported methods [9,17–19]. The experimental design for animal work was approved by the University's Ethics Review Committee.
Table 1.
List of chemicals and materials.
| name | source |
|---|---|
| Abemaciclib | Med. Chem. Express (NJ, USA) |
| acetonitrile (HPLC grade), ammonium formate, potassium cyanide and formic acid | Sigma-Aldrich (USA) |
| water (HPLC grade) | Milli-Q plus filtration system (USA) |
| rats (Sprague-Dawley) | King Saud University experimental animal care center (Saudi Arabia) |
2.2. Chromatographic conditions
An Agilent 6410 QQQ equipped with an ESI coupled to an Agilent 1200 HPLC was used. The liquid and mass chromatographic parameters were adjusted for each drug. ABC and its metabolites were produced in the collision cell by CID. The optimized conditions for chromatographic resolution of incubation mixture extract are given in table 2.
Table 2.
Chromatographic parameters of the proposed LC–MS/MS methodology.
| liquid chromatography | mass spectrometry | |||
|---|---|---|---|---|
| RRLC | Agilent 1200 series | mass spectrometer | Agilent 6410 QQQ | |
| mobile phase | A: 0.1% formic acid | ESI source | positive ESI | |
| B: ACN | N2 of low purity as drying gas: At flow rate of 12 l min−1 and 60 psi pressure | |||
| flow rate: 0.25 ml min−1 | ||||
| run time: 75 min | ||||
| Agilent Zorbax eclipse plus C18 column | L | 150 mm | ESI temperature: 350°C | |
| ID | 2.1 mm | capillary voltage: 4000 V | ||
| PS | 3.5 µm | gas inside collision cell | N2 of high purity | |
| T | 22 ± 1°C | detection modes | MS scan and PI | |
| elution system | time in minutes | % ACN | drug | ABC and its in vitro and reactive metabolites |
| 0 | 5 | |||
| 5 | 5 | fragmentation | fragmentor voltage (FV): 135 V | |
| 30 | 60 | collision energy (CE): 20 eV | ||
| 40 | 90 | |||
| 45 | 5 | |||
2.3. RLM incubations
ABC was incubated at 20 µM with 1 mg ml−1 RLMs, 1 mM NADPH, 1 mM KCN and 50 mM Na/K phosphate buffer (pH 7.4) containing 3.3 mM MgCl2. The mixtures were incubated at 37°C in a shaking water bath for 60 min before the metabolic reactions were terminated using protein precipitation by adding 2 ml of ice-cold ACN followed by centrifugation at 9000 g for 10 min at 4°C. The supernatants were removed to clean vials then evaporated to dryness, reconstituted in the mobile phase and analysed by the LC/MS system [8,12,17,20]. Two controls were done in the absence of NADPH or RLMs to confirm that ABC phase I metabolites were metabolically formed.
2.4. Characterization of ABC reactive intermediates in in vitro metabolic reactions
The same RLM metabolic incubation with ABC, previously described in §2.3, was repeated but in addition to 1.0 mM KCN to trap reactive iminium intermediates. This experiment was repeated three times to confirm the results and support our conclusions. Two controls were done in the absence of NADPH or KCN to confirm that cyano adducts are formed due to metabolic bioactivation.
2.5. Identification of ABC reactive metabolites
MS scan and extracted ion chromatogram (EIC) detection modes were used to characterize and locate metabolites in the incubation mixtures, while product ion (PI) was used to identify ABC in vitro metabolites and adducts of reactive intermediates formed in ABC metabolism. Locating metabolites in metabolic mixture extract chromatogram was performed by EIC of m/z of the supposed ABC metabolites.
3. Results and discussion
3.1. PI study of ABC
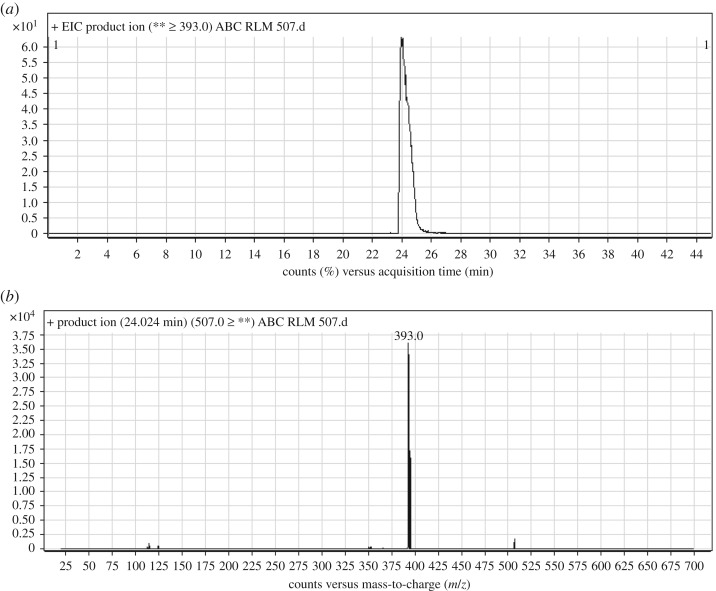
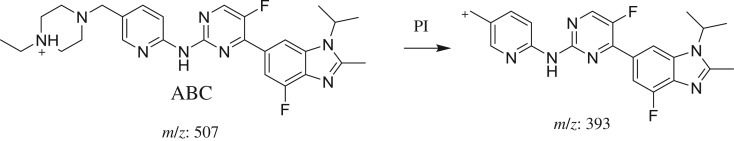
ABC chromatographic peak appears at 24.0 min in PI chromatogram (figure 2a). Collision-induced dissociation (CID) of ABC at m/z 507 generates one fragment ion (FI) at m/z 399 by the loss of ethyl piperazine moiety (figure 2b and scheme 1).
Figure 2.
ABC PI chromatogram (a) and ABC PI MS spectrum (b).
Scheme 1.
PIs of ABC.
3.2. Identification of in vitro and reactive ABC metabolites
After the purification and extraction of ABC RLM incubations, 15 µl was injected into LC–MS/MS. ABC incubation led to the identification of nine in vitro phase I metabolites and three cyano adducts, and the proposed reactions involved include reduction, oxidation, hydroxylation and cyanide addition (table 3). All these metabolites were formed in all incubations when repeated three times; this confirmed the validity of the used method. These results were confirmed by the absence of identified phase I metabolites and cyano adducts in all controls.
Table 3.
Identified in vitro and cyano adducts of ABC.
| MS scan | MS2 product ions | tR (min) | proposed phase 1 metabolic reaction | |
|---|---|---|---|---|
| ABC | 507 | 393 | 23.9 | |
| In vitro ABC metabolism | ||||
| ABC493a | 493 | 378.8 | 22.8 | demethylation |
| ABC493b | 493 | 378.9 | 25.8 | demethylation |
| ABC509 | 509 | 395.1, 509 | 24.3 | reduction |
| ABC521 | 521 | 365, 406.5, 521 | 28.8 | methyl oxidation |
| ABC523a | 523 | 393 | 23.2 | ethyl hydroxylation |
| ABC523b | 523 | 130, 393, 421, 495, 523 | 26.5 | piperazine hydroxylation |
| ABC525a | 525 | 395.1 | 23.32 | hydroxylation and reduction |
| ABC525b | 525 | 395.1 | 26.4 | hydroxylation and reduction |
| ABC537 | 537 | 392.5 | 30.74 | hydroxylation and oxidation |
| cyano adducts | ||||
| ABC532a | 532 | 392.9 | 26.5 | cyanide addition |
| ABC532b | 532 | 393 | 37.2 | cyanide addition |
| ABC548 | 548 | 393 | 29.36 | hydroxylation and cyanide addition |
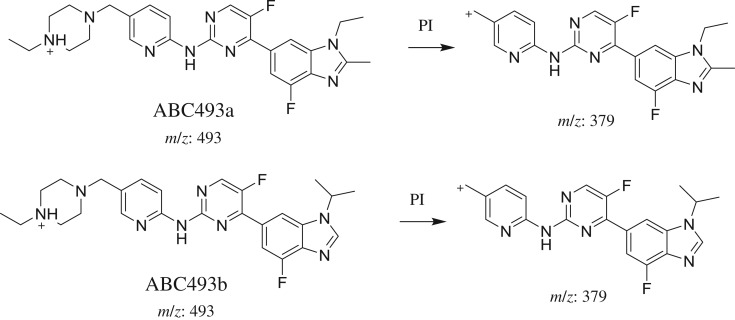
3.2.1. Identification of ABC493a and ABC493b phase I metabolites of ABC
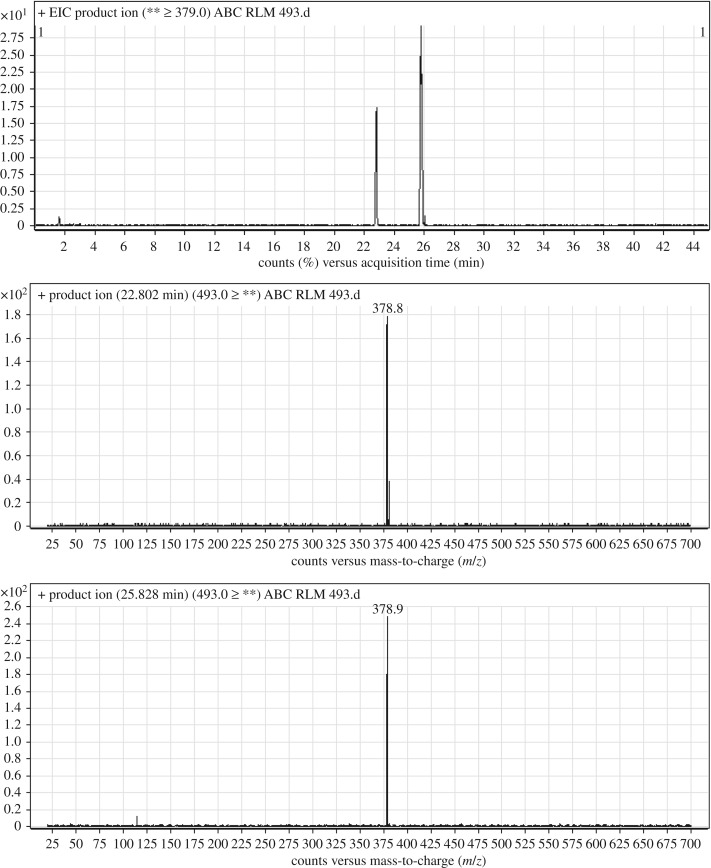
ABC493a and ABC493b chromatographic peaks appear at 22.8 and 25.8 min, respectively, in PI chromatogram (figure 3a). CID of AB493 generates one FI at m/z 379 (figure 3b,c). Comparing with PIs of ABC, a decrease of 14 m/z units was identified, which indicates that demethylation metabolic reaction occurred in benzimidazole ring (scheme 2).
Figure 3.
ABC493 PI chromatogram (a), ABC493a PI MS spectrum (b) and ABC493b PI MS spectrum (c).
Scheme 2.
PIs of ABC493a and ABC493b.
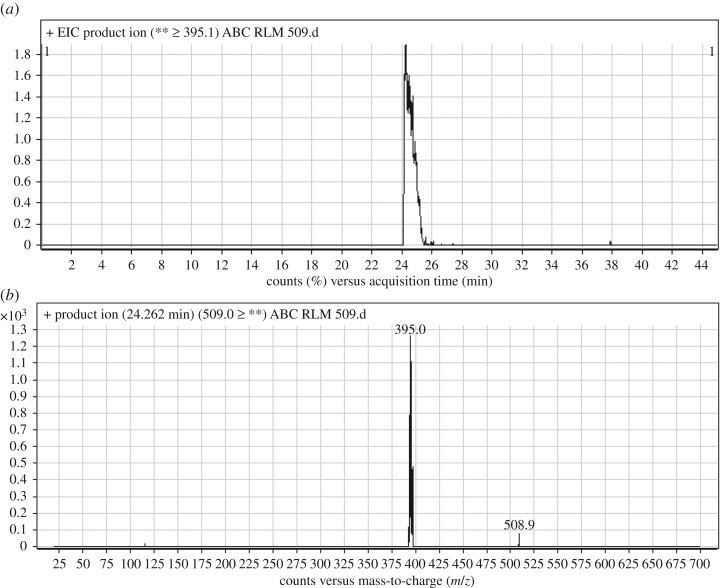
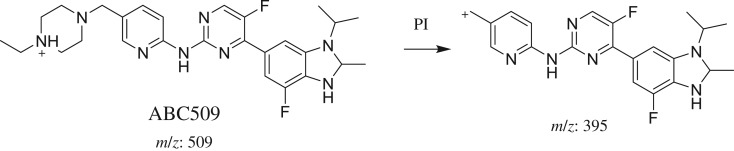
3.2.2. Identification of ABC509 phase I metabolite of ABC
ABC509 chromatographic peak appears at 24.3 min in PI chromatogram (figure 4a). CID of ABC509 generates one FI at m/z 395 (figure 4b). Compared with PIs of ABC, an increase of 2 m/z units was identified, which indicates that reduction metabolic reaction occurred in benzimidazole ring (scheme 3).
Figure 4.
ABC509 PI chromatogram (a) and ABC509 PI MS spectrum (b).
Scheme 3.
PIs of ABC509.
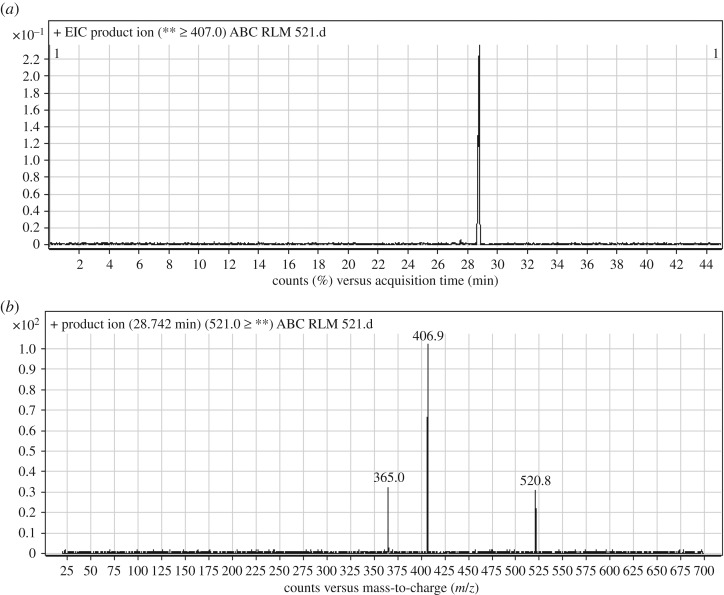
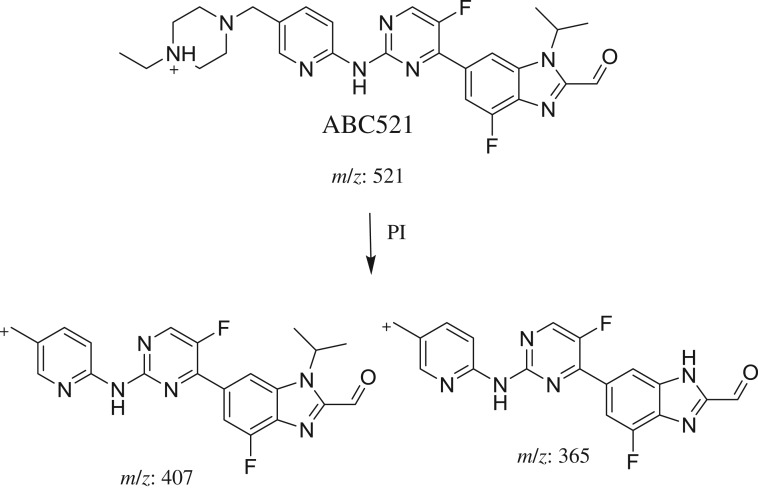
3.2.3. Identification of ABC521 phase I metabolite of ABC
ABC521 chromatographic peak appears at 28.7 min in PI chromatogram (figure 5a). CID of ABC521 generates two FIs at m/z 407 and m/z 365 (figure 5b). Compared with PIs of ABC, an increase of 14 m/z units was identified, which indicates that oxidation metabolic reaction occurred in benzimidazole ring (scheme 4).
Figure 5.
ABC521 PI chromatogram (a) and ABC521 PI MS spectrum (b).
Scheme 4.
PIs of ABC521.
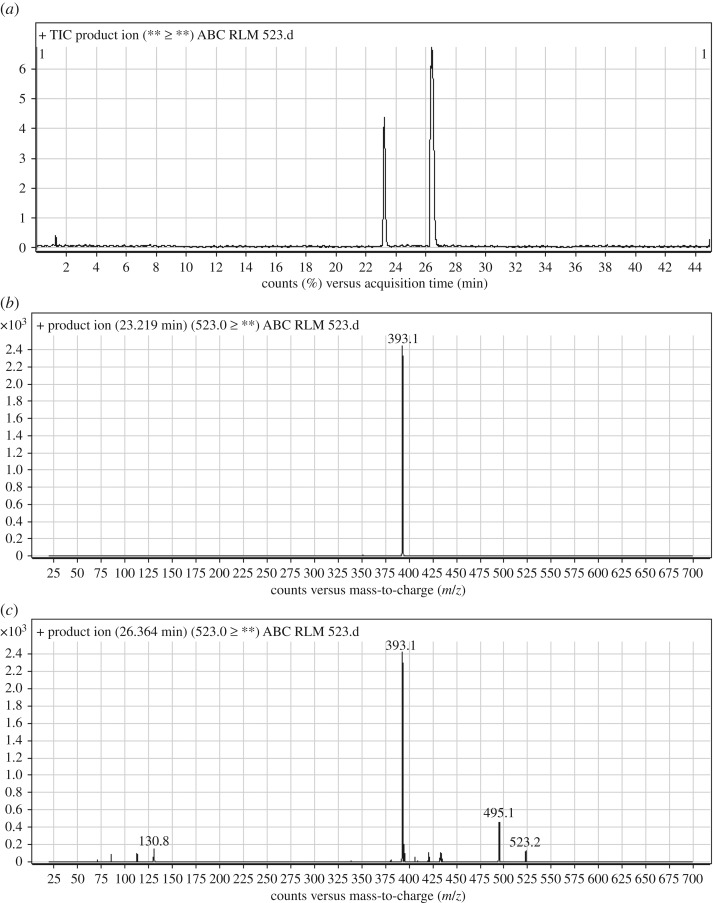
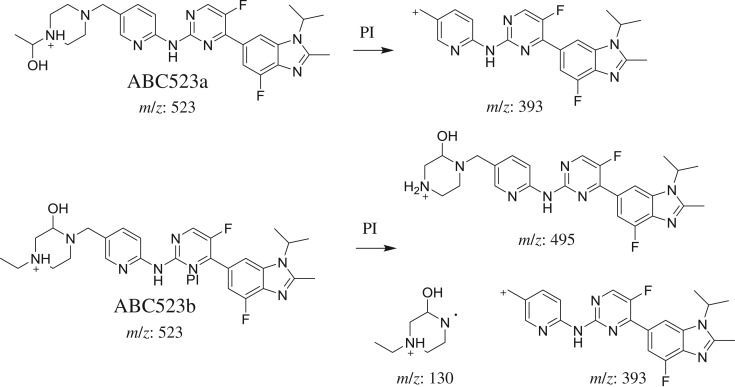
3.2.4. Identification of ABC523a and ABC523b phase I metabolites of ABC
ABC523a and ABC523b chromatographic peaks appeared at 23.2 and 26.4 min, respectively, in PI chromatogram (figure 6a). CID of ABC523a generates one FI at m/z 393 (figure 6b). CID of ABC523b generates three FIs at m/z 495, m/z 393 and m/z 130 (figure 6c). Compared with PIs of ABC, an increase of 16 m/z units was identified, which indicates that hydroxylation metabolic reaction occurred in N-ethyl piperazine ring (scheme 5).
Figure 6.
ABC523 PI chromatogram (a), ABC523a PI MS spectrum (b) and ABC523b PI MS spectrum (c).
Scheme 5.
PIs of ABC523a and ABC523b.
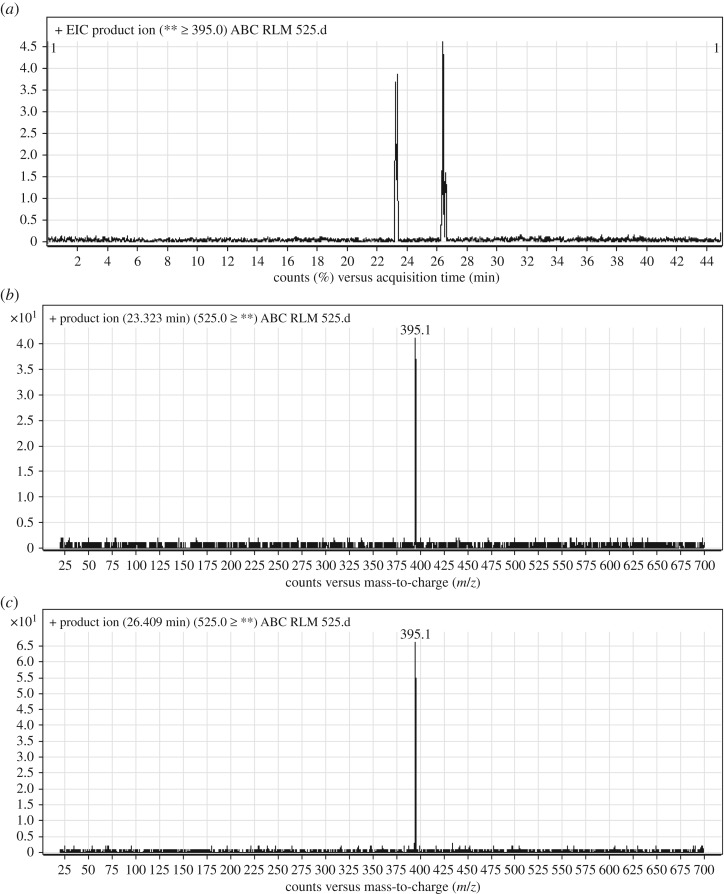
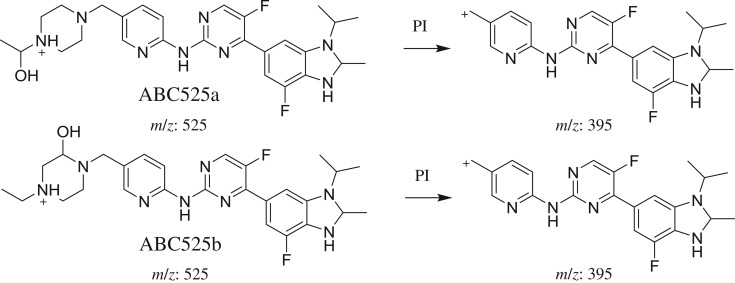
3.2.5. Identification of ABC525a and ABC525b phase I metabolites of ABC
ABC525a and ABC525b chromatographic peaks appeared at 23.3 and 26.4 min, respectively, in PI chromatogram (figure 7a). CID of ABC525 metabolites generates one FI at m/z 395 (figure 7b,c). Compared with PIs of ABC, an increase of 18 m/z units was identified, which indicates that reduction metabolic reaction occurred in benzimidazole ring in addition to hydroxylation metabolic reaction that occurred in N-ethyl piperazine ring (scheme 6).
Figure 7.
ABC525 PI chromatogram (a), ABC525a PI MS spectrum (b) and ABC525b PI MS spectrum (c).
Scheme 6.
PIs of ABC525a and ABC525b.
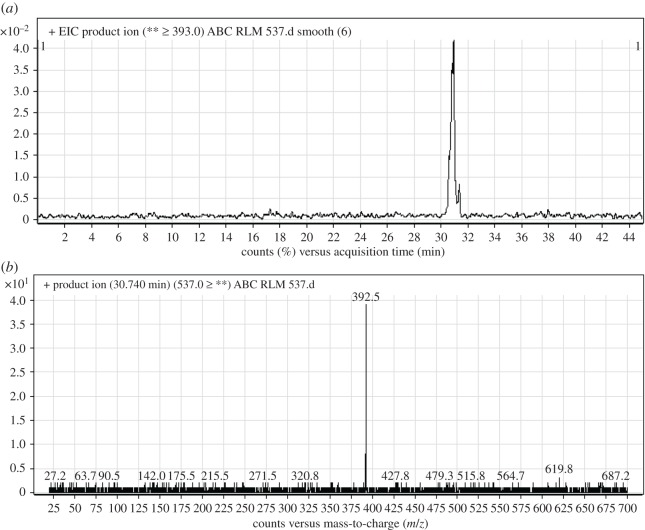
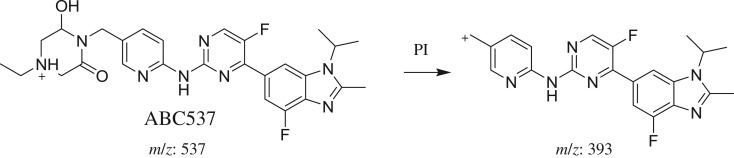
3.2.6. Identification of ABC537 phase I metabolite of ABC
ABC537 chromatographic peak appears at 30.7 min in PI chromatogram (figure 8a). CID of ABC537 generates one FI at m/z 393 (figure 8b). Compared with PIs of ABC, an increase of 30 m/z units was identified, which indicates hydroxylation and oxidation metabolic reactions occurred in N-ethyl piperazine ring (scheme 7).
Figure 8.
ABC537 PI chromatogram (a) and ABC537 PI MS spectrum (b).
Scheme 7.
PIs of ABC537.
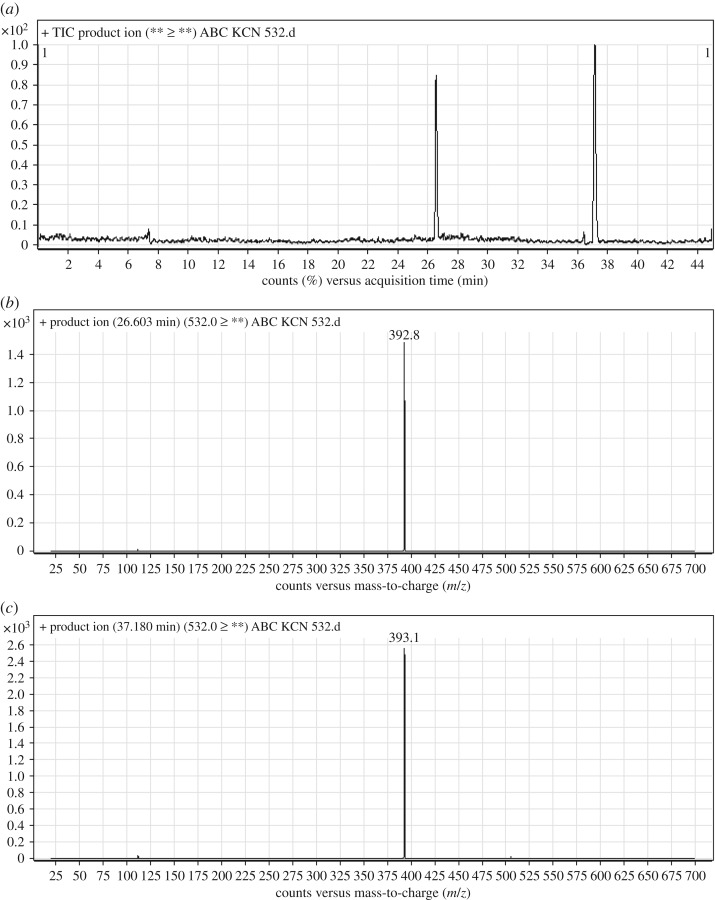
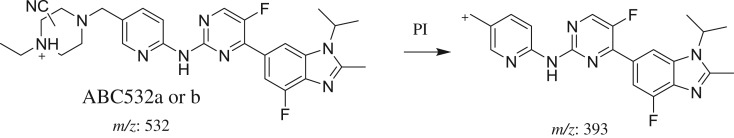
3.2.7. Identification of ABC532a and ABC532b cyano adducts of ABC
ABC532a and ABC532b chromatographic peaks appeared at 26.8 and 37.2 min, respectively, in PI chromatogram (figure 9a). CID of ABC532 metabolites generates one FI at m/z 393 (figure 9b,c). Compared with PIs of ABC, PI at m/z 393 indicates that all metabolic changes occurred in N-ethyl piperazine ring. An increase of 25 m/z units indicates that cyano addition occurred in N-ethyl piperazine ring (scheme 8).
Figure 9.
ABC532 PI chromatogram (a), ABC532a PI MS spectrum (b) and ABC532b PI MS spectrum (c).
Scheme 8.
PIs of ABC532a and ABC532b.
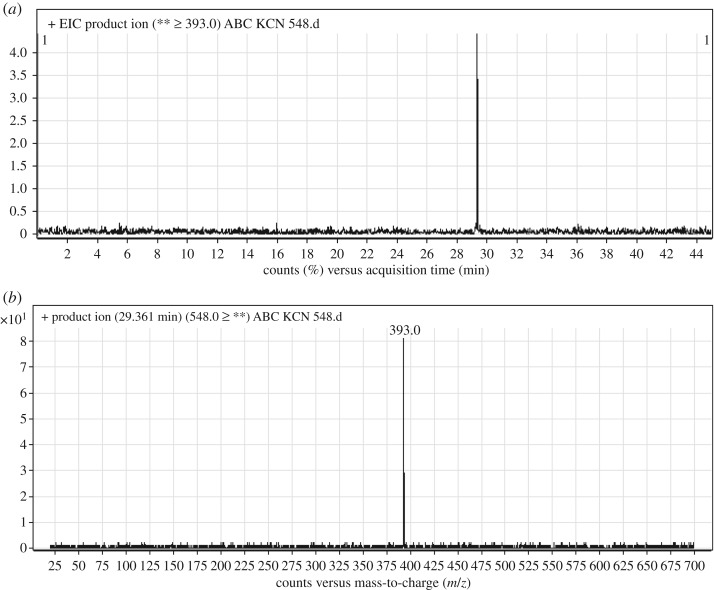
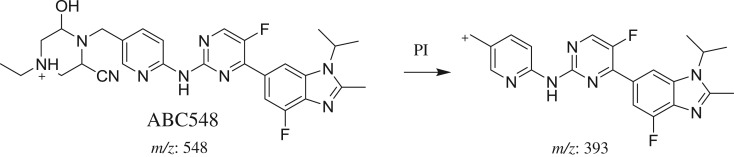
3.2.8. Identification of ABC548 cyano adduct of ABC
ABC548 chromatographic peak appears at 29.4 min in PI chromatogram (figure 10a). CID of ABC548 generates one FI at m/z 393 (figure 10b). Compared with PIs of ABC, PI at m/z 393 indicates that all metabolic changes occurred in N-ethyl piperazine ring. An increase of 41 m/z units indicates that hydroxylation and cyano addition occurred in N-ethyl piperazine ring (scheme 9).
Figure 10.
ABC548 PI chromatogram (a) and ABC548 PI MS spectrum (b).
Scheme 9.
PIs of ABC548.
3.3. Supposed pathways of bioactivation of ABC
Scheme 10 shows the different pathways for bioactivation of ABC. The formation of ABC532a, ABC532b and ABC548 cyanide adducts confirmed the formation of iminium intermediates in piperazine ring metabolism. Hydroxylation of piperazine ring in ABC followed by dehydration resulted in the generation of iminium ion intermediates that are unstable and reactive that can be trapped by cyanide-forming stable adduct which can be detected in LC–MS/MS. The formation pathway of iminium intermediate and bioactivation mechanism of ABC was previously described with cyclic tertiary amine-containing drugs [10,11,17,20].
Scheme 10.
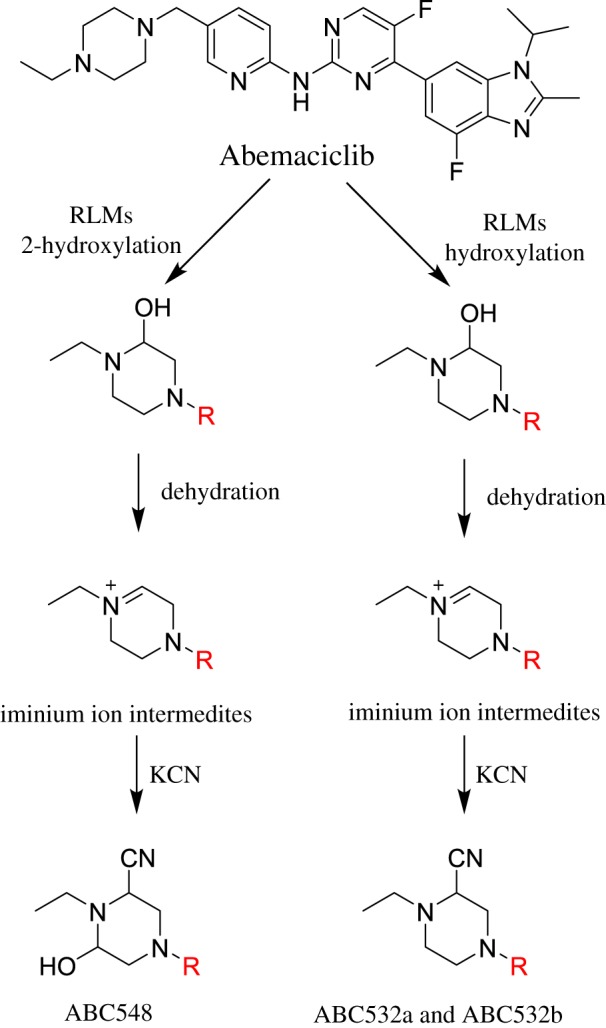
Proposed pathways for the iminium intermediate generation and trapping strategy.
4. Conclusion
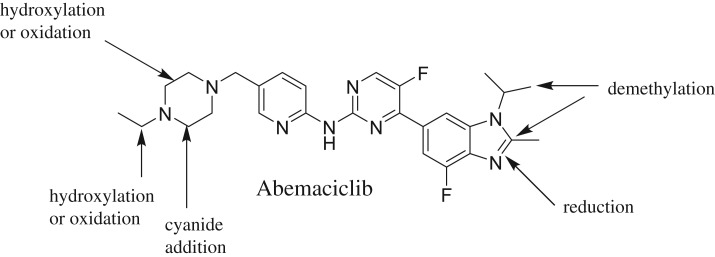
Three potential iminium reactive metabolites were detected and the bioactivation pathways were proposed (figure 11). Nine in vitro phase I metabolites were identified. The findings of potentially reactive intermediates of these drugs may give a deeper understanding of their adverse effects. Further drug discovery studies in ABC structure can shed more light on the possibility of blocking or reducing the formation of reactive intermediates by introducing alkyl substituents or isosteric replacement to the alpha position of the piperazine partial moiety which would probably block or interrupt enzymatic oxidation/hydroxylation on α-carbon atoms. This study opens the way for new drug development with more safety profile.
Figure 11.
In vitro metabolic pathways and bioactivation centres of ABC.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
Male Sprague-Dawley rats were maintained according to the Animal Care Center instructions at KSU that were accepted by the Local Animal Care and Use Committee of KSU. The animal experimental design used in the current work was approved by the University's Ethics Review Committee. Informed consent was taken from all participants.
Data accessibility
The data supporting the results in this article can be accessed through Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.71f1s18 [20].
Authors' contributions
T.A.A., H.W.D. and A.A.K. participated in study design. T.A.A. and M.W.A. carried out the experimental work and data analysis and helped draft the manuscript. A.A.K., H.W.D. and H.A.A guided the laboratory work. H.W.D. revised the final manuscript and is the corresponding author of this paper. All authors revised and approved the publication.
Competing interests
We declare that we have no competing interests.
Funding
The authors extend their sincere appreciation to the Deanship of Scientific Research at the King Saud University for funding this work through the Research Group Project no. RGP-322.
References
- 1.American Cancer Society. 2016. See http://www.cancer.org/acs/groups/cid/documents/webcontent/003090-pdf.pdf (accessed 31 October 2018).
- 2.McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. 2015. Effects of age on the detection and management of breast cancer. Cancers 7, 908–929. ( 10.3390/cancers7020815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Campaign for Breast Cancer Awareness. 2016. See http://www.moh.gov.sa/en/HealthAwareness/Campaigns/Breastcancer/Pages/stat.aspx (accessed 31 October 2018).
- 4.Martin TA, Ye L, Sanders AJ, Lane J, Jiang WG. 2013. Cancer invasion and metastasis: molecular and cellular perspective. In Madame Curie Bioscience Database [Internet] 2000–2013. Austin, TX: Landes Bioscience. Available from: https://www.ncbi.nlm.nih.gov/books/NBK164700/.
- 5.Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225. ( 10.1016/S0092-8674(00)00114-8) [DOI] [PubMed] [Google Scholar]
- 6.Kwapisz D. 2017. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 166, 41–54. ( 10.1007/s10549-017-4385-3) [DOI] [PubMed] [Google Scholar]
- 7.Bilgin B, Sendur MAN, Şener Dede D, Akıncı MB, Yalçın B. 2017. A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer. Curr. Med. Res. Opin. 33, 1559–1569. ( 10.1080/03007995.2017.1348344) [DOI] [PubMed] [Google Scholar]
- 8.Kadi AA, Amer SM, Darwish HW, Attwa MW. 2017. LC-MS/MS reveals the formation of aldehydes and iminium reactive intermediates in foretinib metabolism: phase I metabolic profiling. RSC Adv. 7, 36 279–36 287. ( 10.1039/C7RA06341E) [DOI] [Google Scholar]
- 9.Kadi AA, Attwa MW, Darwish HW. 2018. LC-ESI-MS/MS reveals the formation of reactive intermediates in brigatinib metabolism: elucidation of bioactivation pathways. RSC Adv. 8, 1182–1190. ( 10.1039/C7RA10533A) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attwa MW, Kadi AA, Alrabiah H, Darwish HW. 2018. LC–MS/MS reveals the formation of iminium and quinone methide reactive intermediates in entrectinib metabolism: in vivo and in vitro metabolic investigation. J. Pharm. Biomed. Anal. 160, 19–30. ( 10.1016/j.jpba.2018.07.032) [DOI] [PubMed] [Google Scholar]
- 11.Attwa MW, Kadi AA, Darwish HW, Alrabiah H. 2018. LC-MS/MS reveals the formation of reactive ortho-quinone and iminium intermediates in saracatinib metabolism: phase I metabolic profiling. Clin. Chim. Acta 482, 84–94. ( 10.1016/j.cca.2018.03.037) [DOI] [PubMed] [Google Scholar]
- 12.Attwa MW, Kadi AA, Darwish HW, Amer SM, Al-shakliah NS. 2018. Identification and characterization of in vivo, in vitro and reactive metabolites of vandetanib using LC–ESI–MS/MS. Chem. Cent. J. 12, 99 ( 10.1186/s13065-018-0467-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knowles SR, Uetrecht J, Shear NH. 2000. Idiosyncratic drug reactions: the reactive metabolite syndromes. The Lancet 356, 1587–1591. ( 10.1016/S0140-6736(00)03137-8) [DOI] [PubMed] [Google Scholar]
- 14.Ju C, Uetrecht J. 2002. Mechanism of idiosyncratic drug reactions: reactive metabolites formation, protein binding and the regulation of the immune system. Curr. Drug Metab. 3, 367–377. ( 10.2174/1389200023337333) [DOI] [PubMed] [Google Scholar]
- 15.Ma S, Zhu M. 2009. Recent advances in applications of liquid chromatography-tandem mass spectrometry to the analysis of reactive drug metabolites. Chem. Biol. Interact. 179, 25–37. ( 10.1016/j.cbi.2008.09.014) [DOI] [PubMed] [Google Scholar]
- 16.Stepan AF, Walker DP, Bauman J, Price DA, Baillie TA, Kalgutkar AS, Aleo MD. 2011. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: a perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem. Res. Toxicol. 24, 1345–1410. ( 10.1021/tx200168d) [DOI] [PubMed] [Google Scholar]
- 17.Kadi AA, Darwish HW, Attwa MW, Amer SM. 2016. Detection and characterization of ponatinib reactive metabolites by liquid chromatography tandem mass spectrometry and elucidation of bioactivation pathways. RSC Adv. 6, 72 575–72 585. ( 10.1039/C6RA09985H) [DOI] [Google Scholar]
- 18.von Jagow R, Kampffmeyer H, Kiese M. 1965. The preparation of microsomes. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 251, 73–87. ( 10.1007/BF00245731) [DOI] [PubMed] [Google Scholar]
- 19.Amer SM, Kadi AA, Darwish HW, Attwa MW. 2017. Identification and characterization of in vitro phase I and reactive metabolites of masitinib using a LC-MS/MS method: bioactivation pathway elucidation. RSC Adv. 7, 4479–4491. ( 10.1039/C6RA25767D) [DOI] [Google Scholar]
- 20.Kadi AA, Darwish HW, Abuelizz HA, Alsubi TA, Attwa MW. 2018. Data from: Identification of reactive intermediates formation and bioactivation pathways in Abemaciclib metabolism by LC-MS/MS: In vitro metabolic investigation Dryad Digital Repository. ( 10.5061/dryad.71f1s18) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Kadi AA, Darwish HW, Abuelizz HA, Alsubi TA, Attwa MW. 2018. Data from: Identification of reactive intermediates formation and bioactivation pathways in Abemaciclib metabolism by LC-MS/MS: In vitro metabolic investigation Dryad Digital Repository. ( 10.5061/dryad.71f1s18) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the results in this article can be accessed through Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.71f1s18 [20].