Abstract
Variation and cost in oncology care represent a large and growing burden for the US health care system, and acute hospital care is one of the single largest drivers. Reduction of unplanned acute care is a major priority for clinical transformation in oncology; proposed changes to Medicare reimbursement for patients with cancer who suffer unplanned admissions while receiving chemotherapy heighten the need. We conducted a review of best practices to reduce unplanned acute care for patients with cancer. We searched PubMed for articles published between 2000 and 2017 and reviewed guidelines published by professional organizations. We identified five strategies to reduce unplanned acute care for patients with cancer: (1) identify patients at high risk for unplanned acute care; (2) enhance access and care coordination; (3) standardize clinical pathways for symptom management; (4) develop new loci for urgent cancer care; and (5) use early palliative care. We assessed each strategy on the basis of specific outcomes: reduction in emergency department visits, reduction in hospitalizations, and reduction in rehospitalizations within 30 days. For each, we define gaps in knowledge and identify areas for future effort. These five strategies can be implemented separately or, with possibly more success, as an integrated program to reduce unplanned acute care for patients with cancer. Because of the large investment required and the limited data on effectiveness, there should be further research and evaluation to identify the optimal strategies to reduce emergency department visits, hospitalizations, and rehospitalizations. Proposed reimbursement changes amplify the need for cancer programs to focus on this issue.
INTRODUCTION
Variation and cost in oncology care represent a large and growing burden for the US health care system. In 2010, the cost of oncology care was estimated at nearly $125 billon; by 2020, this is projected to reach almost $160 billion.1 Significant regional variation in per capita spending on health care has been noted; higher spending is not associated with higher quality and has thus been identified as a marker of inefficient and low-quality care.2,3 Acute care utilization is the single largest driver of regional spending variation in oncology care, accounting for 48% of spending and 67% of variation.4 A proposed Centers for Medicare and Medicaid Service (CMS) rule seeks to reduce this variation. The rule, OP-35: Admissions and Emergency Department Visits for Patients Receiving Outpatient Chemotherapy, is meant to assess the quality of care for patients receiving chemotherapy and encourage performance improvement; if finalized, it will affect hospitals' outpatient Medicare payments beginning in 2020.5 Reducing preventable acute care utilization will be increasingly important for the financial well-being of cancer programs.
There are three primary measures of acute care for patients with cancer: emergency department (ED) visits, acute hospitalizations, and 30-day rehospitalizations. Patients with cancer visit the ED most often because of fever, pain, dehydration, abdominal complaints, and respiratory concerns.6-8 When patients do present to the ED, they are commonly admitted to the hospital. In one meta-analysis of 16 studies, the median rate of hospital admission from the ED was 58%; reported rates ranged from 31% to 100%.7 In a study completed for CMS, oncology patients had the highest 30-day readmission rate compared with other patient groups (such as surgery and cardiology), at 25%.9
An essential challenge in acute care for patients with cancer is identifying planned, unplanned, and preventable hospitalizations. The Agency for Healthcare Research and Quality has defined criteria for preventable hospitalizations in primary and preventive care.10 No such definition currently exists in oncology care; however, OP-35 would provide a definition for unplanned admissions.5 This rule focuses on patients with cancer (excluding patients with leukemia) who receive hospital-based outpatient chemotherapy and who have an inpatient admission or ED visit within 30 days for one of 10 conditions: anemia, nausea, dehydration, neutropenia, diarrhea, pain, emesis, pneumonia, fever, and sepsis.
We use the following taxonomy—all-cause, planned, unplanned, and preventable hospitalizations—in reviewing and reporting the effectiveness of various strategies to reduce acute care. All-cause hospitalizations include planned or unplanned hospitalizations. All-cause hospitalizations are the most commonly reported acute care event in the literature. Planned versus unplanned acute care differentiates between acute care events that are scheduled and those that occur in an unscheduled fashion. Examples of planned acute care include scheduled chemotherapy admissions, staged cancer surgeries, and scheduled stem cell transplantation. Unplanned acute care includes unscheduled treatments for neutropenic fever, chemotherapy-induced nausea and vomiting, and symptomatic cancer progression. Preventable acute care is a subset of unplanned acute care. Many episodes of unplanned acute care are likely preventable, with perceived rates of preventable hospitalization varying from 19% to 50%.7,11,12
STRATEGIES FOR REDUCING ACUTE CARE FOR PATIENTS WITH CANCER
We conducted a review of best practices to reduce acute care for patients with cancer. We searched PubMed for articles published between 2000 and 2017 and reviewed quality guidelines published by professional organizations. We also evaluated five care delivery models that have defined and developed systems for the delivery of high-quality oncology care. These models include the following: the National Committee for Quality Assurance patient-centered medical home, and patient-centered specialty practice; the Community Oncology Medical Home; the CMS Oncology Care Model; and the Commission on Cancer Oncology Medical Home.
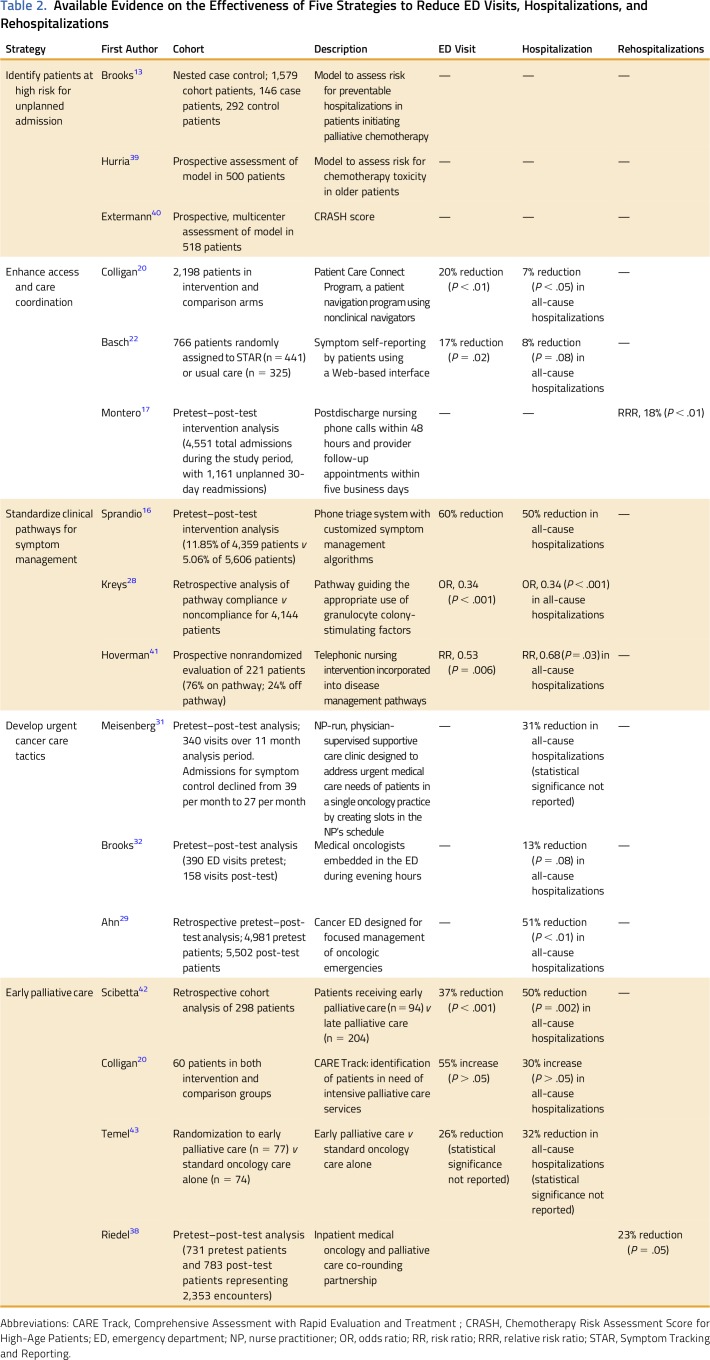
Table 1 lists five strategies—and specific interventions used to achieve each strategy—to reduce acute care for patients with cancer: (1) identify patients at high risk for unplanned acute care; (2) enhance access and care coordination; (3) standardize clinical pathways for symptom management; (4) develop urgent cancer care tactics; and (5) use early palliative care. Table 2 lists the available evidence on the effectiveness of each strategy to reduce ED visits, hospitalizations, and rehospitalizations. Findings are displayed in tabular format, using an approach to integrate multiple types of evidence.44 When the type of hospitalization event was not defined, we assumed all-cause hospitalization.
Table 1.
Five Strategies to Reduce Acute Care for Patients With Cancer and Example Interventions
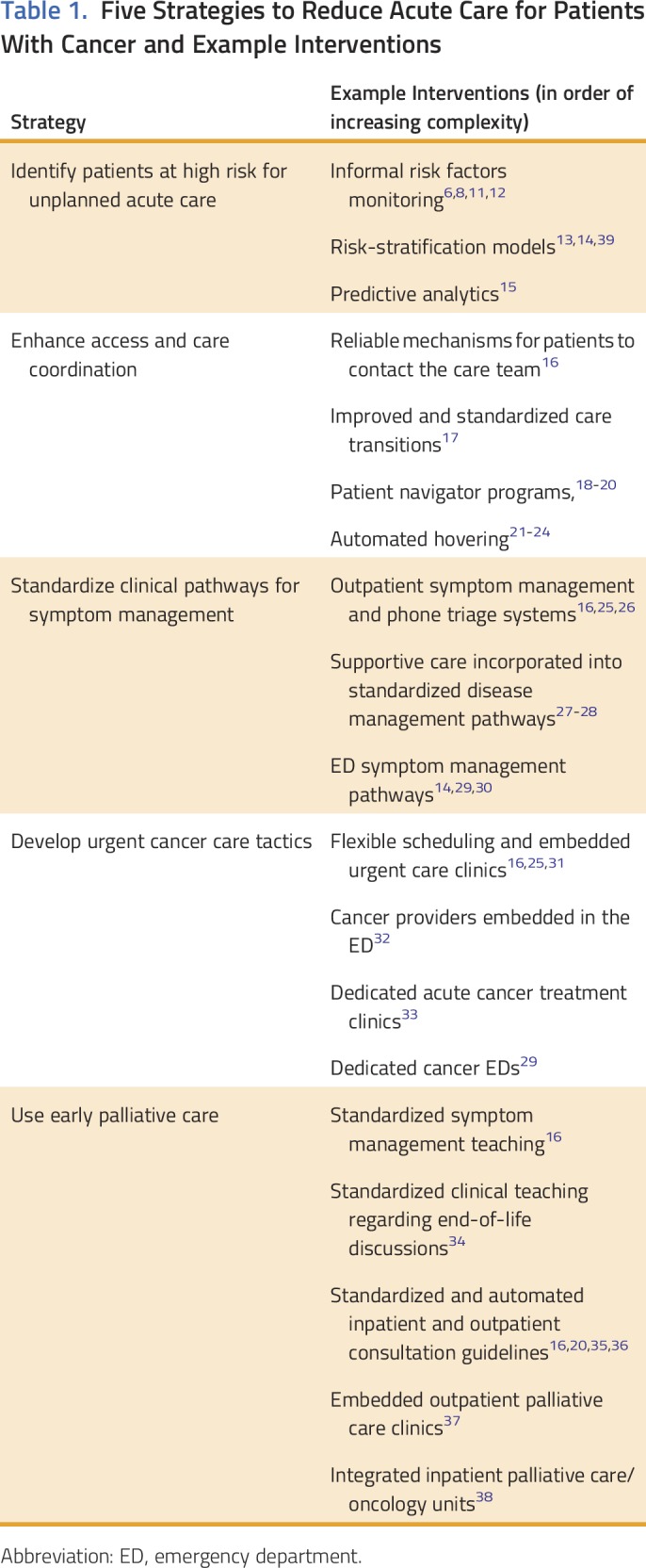
Table 2.
Available Evidence on the Effectiveness of Five Strategies to Reduce ED Visits, Hospitalizations, and Rehospitalizations
Identify Patients at High Risk for Unplanned Acute Care
Identifying patients at particularly high-risk for unplanned acute care enables oncology practices to target interventions and resources to specific populations who may most benefit from the additional support, ultimately saving the practices time, resources, and effort. Examples of this strategy include monitoring known risk factors, prospectively stratifying patients using published risk-stratification models, and prospectively stratifying patients using predictive analytics. Many such models exist in medicine45,46; however, few examples of these tools exist specific to patients with cancer. One group developed a clinical predictive model to assess risk of chemotherapy-related hospitalization in patients receiving palliative chemotherapy. In this model, seven variables were significantly associated with chemotherapy-related hospitalization: age, Charlson comorbidity score, creatinine clearance, calcium level, below-normal WBC and/or platelet count, polychemotherapy, and receipt of camptothecin chemotherapy.13
The model was moderately effective (concordance statistic, 0.71, with 49% sensitivity and 85% specificity at a risk threshold of 15%). However, this model was developed using a retrospective analysis of a clinical registry at a single community hospital and has not been subsequently validated, and its ability to affect unplanned acute care has not been studied.
Predictive analytic and risk stratification techniques for patients with cancer are in their infancy. Data suggest risk stratification models hold promise—for instance, models can predict which patients with neutropenic fever can be safely treated with oral antibiotics and early hospital discharge.14 These techniques should be more broadly developed, their effect on acute care should be studied, and electronic health record companies should help integrate these models into existing workflows. Interventions in other disease processes provide some insight into possible effect. For example, Parkland Health System instituted an algorithm to risk stratify patients with heart failure and introduced a discrete intervention to the high-risk group, reducing readmissions by 19% while conservatively allocating scarce care transition resources.46 Importantly, these models are designed to complement, rather than substitute for, physician involvement and discretion; it is unlikely that all important variables can be meaningfully incorporated into a predictive analytic process.
Enhance Access and Care Coordination
Enhanced access and care coordination consists of several elements, including providing clear and reliable mechanisms for patients to contact the care team, improving and standardizing care transitions, and developing patient navigator programs. Implementing a plan to ensure close communication with patients can reduce preventable admissions.16,25 In one study, over one of five unplanned presentations for oncology patients occurred because of a “need to talk with the treating physician.”6 In another study, 64% of oncology patients admitted to a hospital stated that the symptoms leading to their presentation had developed over several days; those symptoms could have been managed in a different setting had they been addressed in a timely fashion.47
Patient navigation provides a mechanism to enhance access and care continuity. Navigators may be clinical (nurse navigators) or nonclinical (patient navigators). The Academy of Oncology Nurse and Patient Navigators has developed a set of 35 metrics in eight domains that it recommends all oncology navigation programs evaluate and monitor.19
Data from the Patient Care Connect Program, a nonclinical patient navigation intervention for patients with cancer based at the University of Alabama at Birmingham, indicate that the effect of such programs may be substantial. The Patient Care Connect Program deployed nonclinical navigators to act as liaisons between patients and providers, clarify treatment plans, connect patients with resources, and encourage advance care planning. In the last 30 days of life, ED visits decreased by approximately 20% and all-cause hospitalizations decreased by approximately 7%.20
Future efforts to optimize access and care coordination should focus on patient-centric engagement facilitated by connected health efforts, a technique that has been described as automated hovering.21 Some data already exist in this arena. For example, eliciting patient-reported outcomes proactively using STAR (Symptom Tracking and Reporting) led to decreased rates of ED visits by 17% and all-cause hospitalizations by 8% for patients receiving chemotherapy.22 The effect of such remote monitoring may be generalizable beyond symptoms, to self-monitoring of biomarkers and vital signs.23 Telemonitoring may actually be more effective than office-based care: home blood pressure monitoring is useful for detection of early blood pressure changes in patients receiving sunitinib when office measurements do not show substantial changes.24 More studies should investigate the logistics of such automated hovering interventions and their effect on ED visits, hospitalizations, and rehospitalizations. Regardless of the methodology used, the importance of clear lines of communication cannot be overemphasized.
Standardize Clinical Pathways for Symptom Management
Standardized clinical pathways carry significant benefits with respect to reduction of unwanted variation, with some data suggesting a decrease in unplanned acute care.41,48 The specific ability of pathways to reduce unplanned acute care is probably best realized through integration of supportive care.27,48 Examples of such pathways include acute symptom management and phone triage systems, supportive care incorporated into disease management pathways, and ED symptom management pathways.
Pathways dedicated to acute symptom management and phone triage may have particular utility with respect to reducing acute care. For example, Consultants in Medical Oncology and Hematology has developed a set of customized symptom management protocols that are followed by both nurses (during business hours) and on-call physicians after hours. Protocols exist for dehydration, diarrhea, insomnia, and delayed chemotherapy-induced nausea and vomiting.
Consultants in Medical Oncology and Hematology has monitored the efficacy of these pathways since their implementation in 2004. Between 2005 and 2009, the percentage of patients directed to the ED as a result of a clinical call decreased by nearly 60% (11.85% to 5.06%). In addition, a standardized protocol for outpatient management of diarrhea decreased preventable hospitalizations for the treatment of Clostridium difficile infection by 50%. Patients are frequently managed in the home setting or given an appointment to be seen in the clinic.16
The ability of pathways to specifically affect readmissions in patients with cancer has not been well studied and merits further evaluation. A randomized trial demonstrated that integrating a discharge bundle into an inpatient pathway significantly reduced readmission (incidence rate ratio, 0.695) in medicine patients49; similar outcomes may be possible in patients with cancer.
Develop New Loci for Urgent Cancer Care
Patients with cancer who present to a general ED for urgent care are often subsequently admitted to the hospital. Many of these presentations occur during normal clinical hours.7 Thus, alternative sites of care might be appropriate for such patients. Example interventions to develop urgent cancer care tactics range in complexity and include the following: flexible scheduling and embedded urgent care clinics, cancer providers embedded in the ED, dedicated acute cancer treatment clinics and observation units, and dedicated cancer EDs.
By altering the scheduling system for providers, it is possible to see acute patients in a timely fashion in the outpatient clinic. For example, noting that patients could not be reliably seen for urgent needs, one group implemented an embedded supportive care clinic in a practice of nine oncologists and one advanced oncology-certified nurse practitioner by creating dedicated slots in the nurse practitioner’s daily schedule for acute patients.31 Using this strategy, the group was able to arrange same-day or next-day appointments for 87% of patients; unplanned hospitalizations for symptom-related care fell by 31%.
Data regarding the abilities of urgent cancer care management strategies to prevent hospitalization are plentiful; however, their effect on ED visits and rehospitalizations has been minimally studied and should be evaluated. Dedicated oncology EDs hold promise as a means to decrease unplanned hospitalizations, and a few such facilities exist in the United States, but published data regarding their efficacy are only available for international facilities and may not be generalizable.29
Use Early Palliative Care
Patients expect physicians to discuss treatment goals and end-of-life planning; physicians agree that this should be done. Frequently, such discussions occur late in a patient’s disease course (if at all), despite recommendations to initiate such conversations early, and such discussions often occur during an acute (and potentially terminal) hospitalization.50 Patients with advanced, metastatic disease often do not understand that their disease is uncurable.51Although oncologists often do refer patients for palliative care, such referrals often occur late in the disease in the setting of uncontrolled symptoms.52Examples of effective early palliative care strategies include standardized symptom management teaching, standardized clinical teaching regarding end-of-life discussions, standardized and automated inpatient and outpatient consultation guidelines, embedded outpatient palliative care clinics, and integrated inpatient palliative care/oncology units.
Standardized consultative triggers provide one mechanism to integrate palliative care and can reduce downstream acute needs. To increase the frequency of inpatient palliative care consultation, one group developed a set of such triggers on an inpatient solid oncology service. Consultation was requested if patients met at least one of the following eligibility criteria: an advanced solid tumor, prior hospitalization within 30 days, hospitalization > 7 days, or active symptoms. The group found that these triggers not only led to increased palliative care consultations, increased hospice referrals, and decreased chemotherapy use after discharge, but they also observed a nearly 50% decrease in 30-day readmission rates (17 of 48 [35%] to 13 of 65 [18%]; P = .04).35 In an ideal world, triggers for palliative care and hospice consultation would occur in the outpatient setting, before a potentially preventable symptom-driven admission. Standardized criteria for such outpatient consultation have also been developed.16,20
Data regarding the ability of early palliative care to reduce all types of unplanned acute care are robust. However, the appropriate balance between primary palliative care and specialty palliative care can be difficult to achieve. Several models by which to do so have been proposed but remain far from standardized.36
INTEGRATED PROGRAMS
Although these strategies have the potential to reduce unplanned acute care, implementation will require substantial resources and investment, and evidence regarding overall effectiveness is both limited and sobering. For example, an evaluation of the Community Oncology Medical Home model, which incorporates several of these strategies, showed a reduction in all-cause hospitalizations by 10% but an increase in ED visits by 5% (neither finding was statistically significant).20 Moreover, results of clinical transformation efforts outside of cancer care that use some of these strategies, such as the Comprehensive Primary Care Initiative and a variety of Patient-Centered Medical Home models, have demonstrated minimal improvements in unplanned acute care or reduction in cost of care (although it is important to note that the denominator of high-risk patients is typically lower in a general primary care population than in an oncology patient population).53 Targeting interventions to those patients most at risk rather than building capacity for all patients may prove particularly important in realizing the benefits of these programs most efficiently.53
VALUE-BASED PAYMENT MODELS
Many of the strategies described herein require up-front expenditures, returns from which may not be overtly apparent (or even realizable) in a fee-for-service system. If and when savings are realized in shared savings models, these financial benefits often occur several years in the future. Additional payments, such as Oncology Care Model’s per-beneficiary Monthly Enhanced Oncology Service payment, can facilitate implementation.
In conclusion, on the basis of a review of best practices and supporting evidence, we identify five strategies for reducing acute care for patients with cancer. Some of these strategies, such as palliative integration, have a robust evidence base; others, such as predictive analytics, remain in their nascency. These strategies could be implemented by oncology practices separately or, with perhaps more success, as a targeted, integrated program to reduce unplanned acute care for patients with cancer. Regardless, proposed reimbursement changes amplify the need for cancer programs to focus on this issue. Increased peer-reviewed evaluations of improvement and innovation efforts to reduce unplanned acute care for patients with cancer across the country could help move the field ahead by building a more robust body of evidence.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Provisions of study materials or patients: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Best Practices for Reducing Unplanned Acute Care for Patients With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Nathan R. Handley
No relationship to disclose
Lynn M. Schuchter
Consulting or Advisory Role: Incyte
Research Funding: GlaxoSmithKline (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
Justin E. Bekelman
No relationship to disclose
REFERENCES
- 1.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 1: The content, quality, and accessibility of care. Ann Intern Med. 2003;138:273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 3.Fisher ES, Wennberg DE, Stukel TA, et al. The implications of regional variations in Medicare spending. Part 2: Health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA, Li L, Uno H, et al. Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff (Millwood) 2014;33:1793–1800. doi: 10.1377/hlthaff.2014.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Advisory Board CMS proposes outcomes-based quality measure for outpatient cancer care. https://www.advisory.com/research/oncology-roundtable/oncology-rounds/2016/08/cms-outcomes-based-quality-measure.
- 6.Aprile G, Pisa FE, Follador A, et al. Unplanned presentations of cancer outpatients: A retrospective cohort study. Support Care Cancer. 2013;21:397–404. doi: 10.1007/s00520-012-1524-6. [DOI] [PubMed] [Google Scholar]
- 7.Adelson KB, Dest V, Velji S, et al. Emergency department (ED) utilization and hospital admission rates among oncology patients at a large academic center and the need for improved urgent care access. J Clin Oncol. 2014;32(suppl; abstr 19) [Google Scholar]
- 8.Manzano J-GM, Luo R, Elting LS, et al. Patterns and predictors of unplanned hospitalization in a population-based cohort of elderly patients with GI cancer. J Clin Oncol. 2014;32:3527–3533. doi: 10.1200/JCO.2014.55.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz L, Partovian C, Lin Z, et al. Hospital-wide (all-condition) 30-day risk standardization readmission measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf.
- 10. AHRQ: Preventable hospitalizations: A window into primary and preventive care. https://archive.ahrq.gov/data/hcup/factbk5/factbk5.pdf.
- 11.Brooks GA, Abrams TA, Meyerhardt JA, et al. Identification of potentially avoidable hospitalizations in patients with GI cancer. J Clin Oncol. 2014;32:496–503. doi: 10.1200/JCO.2013.52.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks GA, Jacobson JO, Schrag D. Clinician perspectives on potentially avoidable hospitalizations in patients with cancer. JAMA Oncol. 2015;1:109–110. doi: 10.1001/jamaoncol.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks GA, Kansagra AJ, Rao SR, et al. A clinical prediction model to assess risk for chemotherapy-related hospitalization in patients initiating palliative chemotherapy. JAMA Oncol. 2015;1:441–447. doi: 10.1001/jamaoncol.2015.0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klastersky J, Paesmans M, Georgala A, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006;24:4129–4134. doi: 10.1200/JCO.2005.03.9909. [DOI] [PubMed] [Google Scholar]
- 15.Furlow B. Predictive analytics reduces chemotherapy-associated hospitalizations. http://managedhealthcareexecutive.modernmedicine.com/managed-healthcare-executive/news/predictive-analytics-reduces-chemotherapy-associated-hospitalizations.
- 16.Sprandio JD. Oncology patient-centered medical home and accountable cancer care. Community Oncol. 2010;7:565–572. [Google Scholar]
- 17.Montero AJ, Stevenson J, Guthrie AE, et al. Reducing unplanned medical oncology readmissions by improving outpatient care transitions: A process improvement project at the Cleveland Clinic. J Oncol Pract. 2016;12:e594–e602. doi: 10.1200/JOP.2015.007880. [DOI] [PubMed] [Google Scholar]
- 18. AONN: Oncology Patient Navigator–Certified Generalist (OPN-CGTM) Certification. https://aonnonline.org/certification/patient-navigator-certification.
- 19.Strusowski T, Sein E, Johnston D, et al. Standardized evidence-based oncology navigation metrics for all models: A powerful tool in assessing the value and impact of navigation programs. http://www.jons-online.com/issue-archive/2017-issues/may-2017-vol-8-no-5/value-impact-of-navigation-programs/
- 20.Colligan EM, Ewald E, Ruiz S, et al. Innovative oncology care models improve end-of-life quality, reduce utilization and spending. Health Aff (Millwood) 2017;36:433–440. doi: 10.1377/hlthaff.2016.1303. [DOI] [PubMed] [Google Scholar]
- 21.Asch DA, Muller RW, Volpp KG. Automated hovering in health care--watching over the 5000 hours. N Engl J Med. 2012;367:1–3. doi: 10.1056/NEJMp1203869. [DOI] [PubMed] [Google Scholar]
- 22.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimako K, Lu S-K, Ayite B, et al. A pilot study of a novel home telemonitoring system for oncology patients receiving chemotherapy. J Telemed Telecare. 2013;19:148–152. doi: 10.1177/1357633X13483258. [DOI] [PubMed] [Google Scholar]
- 24.Azizi M, Chedid A, Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–97. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 25.McAneny BL. The future of oncology? COME HOME, the oncology medical home. http://www.ajmc.com/journals/evidence-based-oncology/2013/2013-1-vol19-sp1/the-future-of-oncology-come-home-the-oncology-medical-home/ [PubMed]
- 26.Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: Physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11:462–467. doi: 10.1200/JOP.2015.005256. [DOI] [PubMed] [Google Scholar]
- 27.Kreys ED, Koeller JM. Documenting the benefits and cost savings of a large multistate cancer pathway program from a payer’s perspective. J Oncol Pract. 2013;9:e241–e247. doi: 10.1200/JOP.2012.000871. [DOI] [PubMed] [Google Scholar]
- 28.Kreys ED, Kim TY, Delgado A, et al. Impact of cancer supportive care pathways compliance on emergency department visits and hospitalizations. J Oncol Pract. 2014;10:168–173. doi: 10.1200/JOP.2014.001376. [DOI] [PubMed] [Google Scholar]
- 29.Ahn S, Lee Y-S, Lim KS, et al. Emergency department cancer unit and management of oncologic emergencies: Experience in Asan Medical Center. Support Care Cancer. 2012;20:2205–2210. doi: 10.1007/s00520-012-1478-8. [DOI] [PubMed] [Google Scholar]
- 30.Carmona-Bayonas A, Jiménez-Fonseca P, Virizuela Echaburu J, et al. Prediction of serious complications in patients with seemingly stable febrile neutropenia: Validation of the Clinical Index of Stable Febrile Neutropenia in a prospective cohort of patients from the FINITE study. J Clin Oncol. 2015;33:465–471. doi: 10.1200/JCO.2014.57.2347. [DOI] [PubMed] [Google Scholar]
- 31.Meisenberg BR, Graze L, Brady-Copertino CJ. A supportive care clinic for cancer patients embedded within an oncology practice. J Community Support Oncol. 2014;12:205–208. doi: 10.12788/jcso.0049. [DOI] [PubMed] [Google Scholar]
- 32.Brooks GA, Chen EJ, Murakami MA, et al. An ED pilot intervention to facilitate outpatient acute care for cancer patients. Am J Emerg Med. 2016;34:1934–1938. doi: 10.1016/j.ajem.2016.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin J. Renovated Cancer Care Clinic provides 24/7 service. https://siteman.wustl.edu/renovated-cancer-care-clinic-provides-247-service/
- 34.Bernacki RE, Block SD. Communication about serious illness care goals: A review and synthesis of best practices. JAMA Intern Med. 2014;174:1994–2003. doi: 10.1001/jamainternmed.2014.5271. [DOI] [PubMed] [Google Scholar]
- 35.Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13:e431–e440. doi: 10.1200/JOP.2016.016808. [DOI] [PubMed] [Google Scholar]
- 36.Hui D, Meng Y-C, Bruera S, et al. Referral criteria for outpatient palliative cancer care: A systematic review. Oncologist. 2016;21:895–901. doi: 10.1634/theoncologist.2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muir JC, Daly F, Davis MS, et al. Integrating palliative care into the outpatient, private practice oncology setting. J Pain Symptom Manage. 2010;40:126–135. doi: 10.1016/j.jpainsymman.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Riedel RF, Slusser K, Power S, et al. Early palliative care on an inpatient oncology unit: Impact of a novel co-rounding partnership on patient and health system outcomes. J Clin Oncol. 2014;32(suppl, abstr 3) [Google Scholar]
- 39.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 41.Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: The impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10:63–67. doi: 10.1200/JOP.2013.001210. [DOI] [PubMed] [Google Scholar]
- 42.Scibetta C, Rabow MW, Kerr K. Care, quality, and cost implications of the timing of palliative care consultation among patients with advanced cancer. J Clin Oncol. 2014;32(suppl; abstr 8) [Google Scholar]
- 43.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 44.Eddy DM. Comparing benefits and harms: The balance sheet. JAMA. 1990;263:2493, 2498, 2501. doi: 10.1001/jama.263.18.2493. [DOI] [PubMed] [Google Scholar]
- 45.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: A systematic review. JAMA. 2011;306:1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amarasingham R, Patel PC, Toto K, et al. Allocating scarce resources in real-time to reduce heart failure readmissions: A prospective, controlled study. BMJ Qual Saf. 2013;22:998–1005. doi: 10.1136/bmjqs-2013-001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hjermstad MJ, Kolflaath J, Løkken AO, et al. Are emergency admissions in palliative cancer care always necessary? Results from a descriptive study. BMJ Open. 2013;3:e002515. doi: 10.1136/bmjopen-2012-002515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polite BN, Page RD, Nabhan C. Oncology pathways-preventing a good idea from going bad. JAMA Oncol. 2016;2:297–298. doi: 10.1001/jamaoncol.2015.5778. [DOI] [PubMed] [Google Scholar]
- 49.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: A randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mack JW, Cronin A, Taback N, et al. End-of-life care discussions among patients with advanced cancer: A cohort study. Ann Intern Med. 2012;156:204–210. doi: 10.1059/0003-4819-156-3-201202070-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wentlandt K, Krzyzanowska MK, Swami N, et al. Referral practices of oncologists to specialized palliative care. J Clin Oncol. 2012;30:4380–4386. doi: 10.1200/JCO.2012.44.0248. [DOI] [PubMed] [Google Scholar]
- 53.Nichols L, Cuellar AE, Helmchen L, et al. What should we conclude from “mixed” results in payment reform evaluations? http://healthaffairs.org/blog/2017/08/14/what-should-we-conclude-from-mixed-results-in-payment-reform-evaluations/