Abstract
Purpose:
The US Food and Drug Administration (FDA) has approved epoetin and darbepoetin for chemotherapy-induced anemia (CIA). Approved epoetin and darbepoetin dosing schedules were three times per week and weekly, respectively, although off-label, less frequent scheduling was common. In 2004, 2007, and 2008, a US Food and Drug Administration Advisory Committees warned of risks associated with erythropoiesis-stimulating agents. During this period, lawsuits alleging illegal darbepoetin marketing practices have concluded, resulting in $1.1 billion in fines and settlements and one criminal conviction. No prior study, to our knowledge, has reported on the use of darbepoetin versus epoetin for CIA.
Methods:
We evaluated the dosing, utilization, and costs of erythropoiesis-stimulating agents among 3,761 South Carolina Medicaid patients with CIA.
Results:
Epoetin and darbepoetin utilization rates were 22% and 28% in 2003, 10% and 33% in 2007, and 3% and 7% in 2010, respectively. Mean per-patient per-administration epoetin and darbepoetin doses were 40,983 IU and 191 µg, respectively, in 2003 and 47,753 IU and 369 µg, respectively, in 2010. Mean monthly patient costs for epoetin and darbepoetin were $1,030 and $981, respectively, in 2003 and $932 and $1,352, respectively, in 2010. Epoetin use decreased steadily between 2002 and 2010; darbepoetin use increased steadily between 2003 and 2007 and then decreased steadily thereafter. Per-patient dosing of darbepoetin, but not epoetin, increased steadily between 2003 and 2010, and monthly per-patient epoetin costs decreased 3% while the per-patients costs of darbepoetin increased 30% between 2003 and 2010.
Conclusion:
To our knowledge, our findings are the first data reporting on epoetin versus darbepoetin use for CIA and support recently concluded lawsuits involving allegations of illegal marketing practices of the manufacturer of darbepoetin.
INTRODUCTION
The erythropoiesis-stimulating agents (ESAs) epoetin and darbepoetin treat chemotherapy-induced anemia (CIA) among patients with cancer.1 Epoetin has the same amino acid sequence as the endogenous RBC-stimulating protein erythropoietin.1 Darbepoetin has an additional oligosaccharide, resulting in a longer half-life.1 After epoetin and darbepoetin were approved by the US Food and Drug Administration (FDA) in 1993 and 2002, respectively, for CIA, their use expanded.
In 2003, ESA safety concerns in cancer were identified in two phase III clinical trials. In 2004, 2007, and 2008, these concerns were discussed at FDA Oncology Drug Advisory Committee (ODAC) meetings.1-4 Boxed warnings added in 2007 to product labels and revised guidelines from ASCO/American Society of Hematology and the National Comprehensive Cancer Network in 2008 warned against administering ESAs to patients with cancer with CIA receiving chemotherapy for curative intent. Risks of mortality, tumor progression, and venous thromboembolism (VTE) were identified in phase III trials, observational databases, meta-analyses; at FDA ODAC meetings; and in communications from ESA manufacturers and the FDA.1-6 Black Box Warnings in 2007 and clinical guidelines from ASCO/American Society of Hematology and the National Comprehensive Cancer Network in 2008 advised against administering high ESA doses and/or targeting high hemoglobin levels because of safety concerns.2,3,6,7 Two meta-analyses in 2008 and 2009 identified increased ESA-associated rates of VTE and mortality among patients with cancer.8,9 A National Coverage Determination from the Centers for Medicare and Medicaid Services in 2008 restricted ESA coverage to patients with cancer receiving chemotherapy only if hemoglobin levels were less than 10 g/dL.4
Despite ESA safety notifications, differential utilization of darbepoetin versus epoetin has not been evaluated previously. Initial dosing schedules for epoetin in 1993 and darbepoetin in 2002 were three times per week and weekly, respectively. Beginning with FDA approval of darbepoetin in 2002, off-label darbepoetin dosing at higher mean monthly doses every 2 weeks became common. Similarly, after FDA approval of epoetin in 1993 for CIA, off-label use of weekly epoetin was common until this schedule was formally approved by the FDA in 2006. In the same year, the FDA approved darbepoetin for use every 3 weeks (which resulted in higher mean monthly darbepoetin doses than the every-2-week schedule).
For the first time, to our knowledge, we report data on darbepoetin versus epoetin utilization, dosing, and costs for CIA. We evaluated whether ESA used decreased after ESA safety warnings for CIA were disseminated and, if so, when the decreases began. We also evaluated whether dosing and expenditures for CIA differed for darbepoetin versus epoetin, given off-label practices of administering higher doses every 2 weeks for darbepoetin between 2002 and 2006 and administering high doses of darbepoetin every 3 weeks between 2006 and 2010. Our findings also provide context for understanding settlements of three lawsuits involving allegations of illegal marketing and promoting unsafe dosing of darbepoetin as well as one criminal conviction for illegal marketing practices of darbepoetin between 2002 and 2007. Our data cover the years 2002 to 2010 and thus illustrate darbepoetin utilization for a 3-year period beyond the time period covered by the lawsuits.
METHODS
Empirical Data
A merged South Carolina Medicaid–Cancer Registry database for 2002 to 2010 contained information on cancer diagnosis and claims for pharmacy, physician, and hospital care for persons covered by Medicaid. Patients older than 18 years with breast cancer, non–small-cell lung cancer (NSCLC), or colorectal cancer (CRC) were identified. The first date of chemotherapy is the index date. Only individuals with first ESA claims after first chemotherapy claims were included; these patients are operationally designated as having CIA. ESA dosing from Healthcare Common Procedure Coding System (HCPCS) codes was determined using base units from HCPCS codes times the number of ESA services provided for an individual dose. For example, if the HCPCS code is for darbepoetin 1 µg and the number of services provided are 100, then the dose would be 100 µg. Likewise, if the HCPCS code is for epoetin 1,000 IU and the number of services is 20, then the dose would be 20,000 IU. For National Drug Codes, the individual dose was determined by the unit indicated in the drug name multiplied by quantity dispensed and then divided by day supply. For example, if the drug name indicated 100 µg of darbepoetin and the quantity dispensed was five with a day supply of two, then the dose would be 250 µg. Per-month doses were the sum of the individual doses for each month. Patients who received high ESA doses were those in the upper quartile of per-month dosing for the entire study period (160,000 IU for epoetin, 500 μg for darbepoetin) or per administration (45,000 IU for epoetin, 300 μg for darbepoetin). Data on use of packed RBC transfusions (yes or no) within 3 months of initiation of chemotherapy were also abstracted. The Charlson comorbidity index was used to measure comorbidity on the basis of diagnoses in claims files for the year before the index date.10 Costs for epoetin and darbepoetin were obtained from a publicly available presentation made by a health economist, whose research team included one economist used by the supplier of epoetin.11 A χ2 analysis was used to determine significance of time trends. Multivariable logistic regression evaluated outcomes (use of high ESA doses and administration of blood transfusion) after adjusting for demographic and clinical variables. SAS 9.4 (SAS Institutes, Cary, NC) was used.
RESULTS
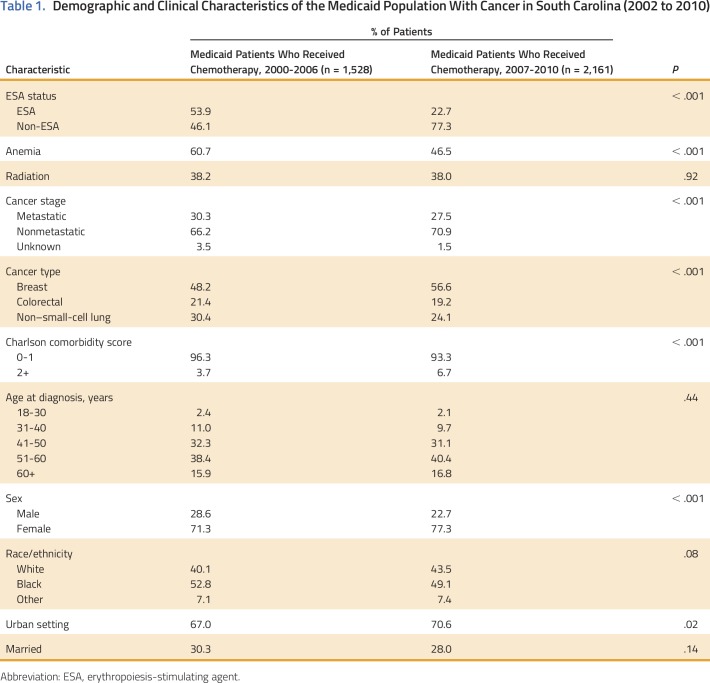
Overall, 3,689 patients with breast cancer, NSCLC, or CRC and CIA and covered by Medicaid between 2002 and 2010 in South Carolina were identified. Of those, 1,315 patients (35%) had at least one ESA claim after receiving chemotherapy (CIA). Of patients with CIA who received ESAs, 33% received epoetin and 67% received darbepoetin. Before 2007, 54% of patients with breast cancer, NSCLC, or CRC initiating chemotherapy received an ESA compared with 23% after 2007 (P < .001). Patients with one of these three cancers who received ESAs after 2007 had more comorbid conditions than those who received ESAs earlier (Table 1). ESA utilization rates for CIA were 51% in 2002, 56% in 2005, 43% in 2007, and 11% in 2010 (P < .001 for trend). In contrast, the chemotherapy population comprised 5% of all Medicaid enrollees in 2002, 12% in 2007, and 15% in 2010. Patients with CRC had a 48% reduction in the odds of receiving an ESA compared with patients with NSCLC (P < .001; Table 2). The odds of receiving an ESA were 78% lower after 2007 versus earlier (P < .001) after adjusting for cancer stage, listing of anemia as a medical diagnosis, comorbidity score, and demographics (Table 3).
Table 1.
Demographic and Clinical Characteristics of the Medicaid Population With Cancer in South Carolina (2002 to 2010)
Table 2.
Logistic Regression: Probability of Receiving an ESA
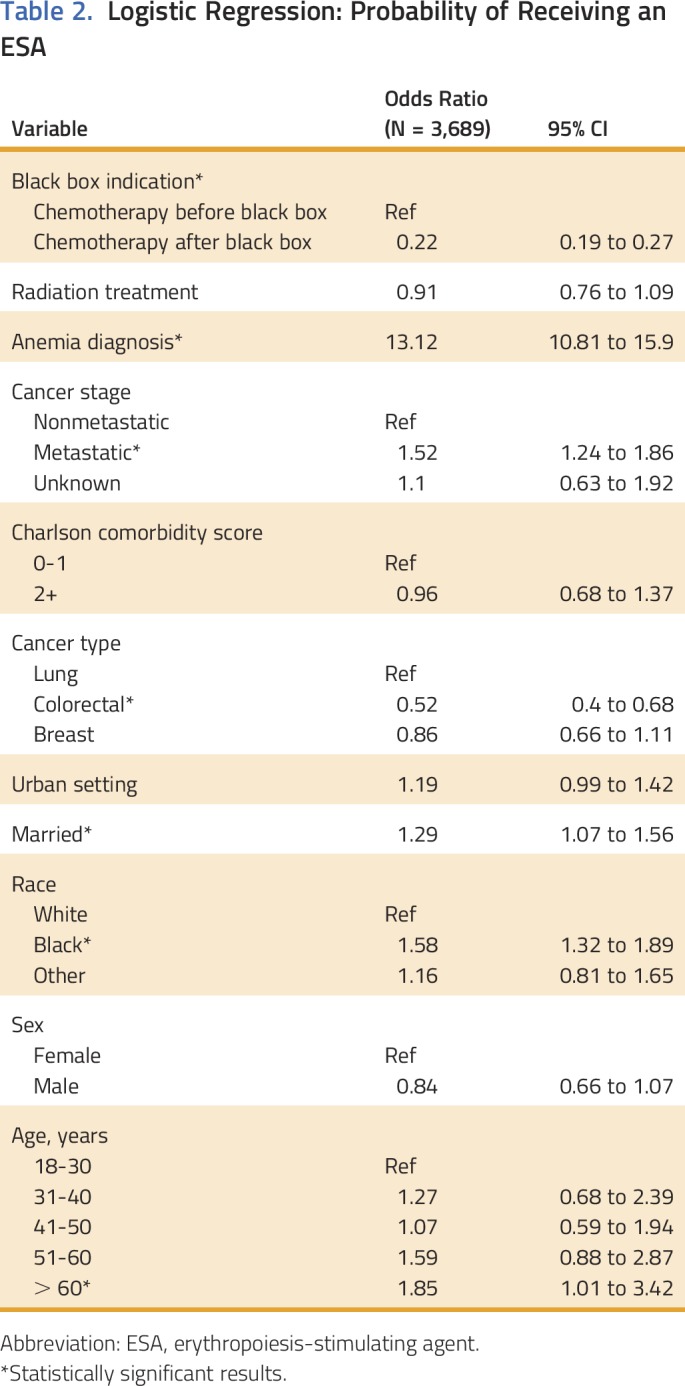
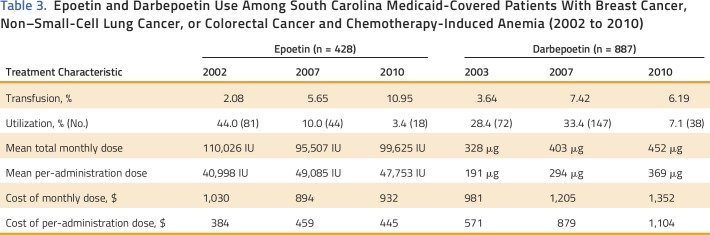
Table 3.
Epoetin and Darbepoetin Use Among South Carolina Medicaid-Covered Patients With Breast Cancer, Non–Small-Cell Lung Cancer, or Colorectal Cancer and Chemotherapy-Induced Anemia (2002 to 2010)
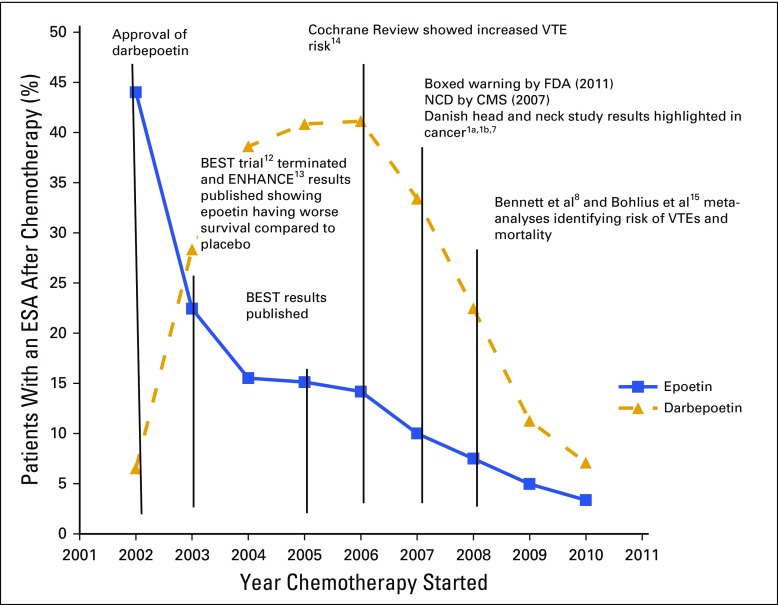
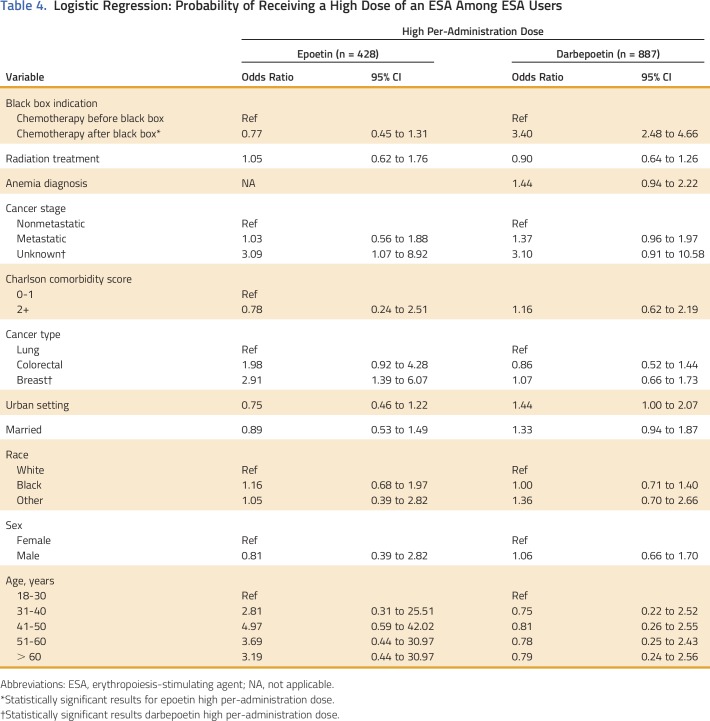
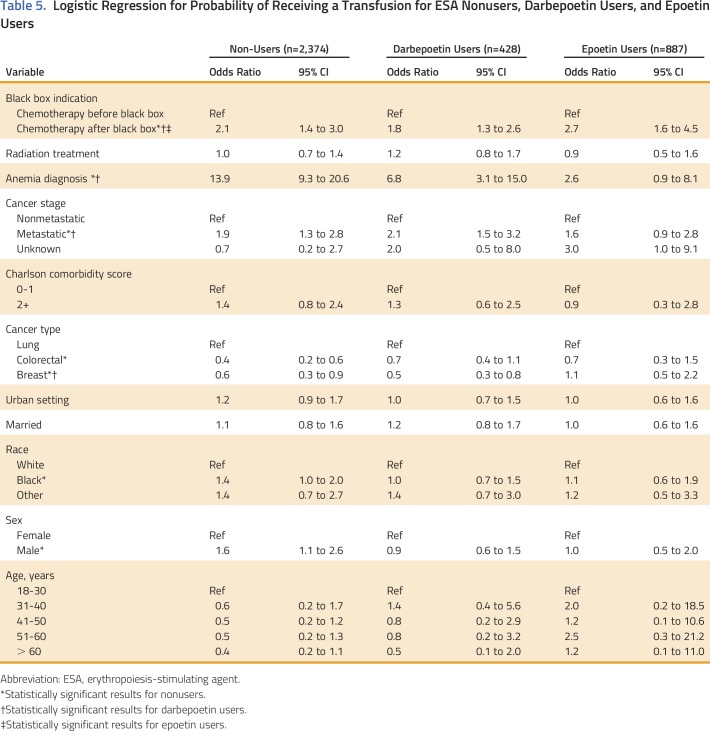
Epoetin and darbepoetin utilization rates were 22% and 28% in 2003, 10% and 33.4% in 2007, and 3.4% and 7.1% in 2010, respectively, among patients with breast cancer, NSCLC, or CRC and CIA (Fig 1). Mean per-patient administration of epoetin doses and per-administration epoetin costs were 40,983 IU and $384 in 2003, 49,084 IU and $459 in 2007, and 47,753 IU and $932 in 2010, respectively (Table 3). Mean per-patient monthly epoetin doses and epoetin costs were 110,665 IU and $1,030 in 2002, 95,507 IU and $894 in 2007, and 99,625 IU and $932 in 2010, respectively (Table 3). Darbepoetin dosing and darbepoetin costs show different trends (Figs 2A and 2B). Mean per-patient administration of darbepoetin doses and per-administration darbepoetin costs were were 191 μg and $571 in 2003, 294 μg and $879 in 2007, and 369 μg and $1,104 in 2010, respectively (Table 3). Mean per-patient monthly darbepoetin dose was 328 μg in 2003, 403 μg in 2007, and 452 μg in 2010 (Table 3). After adjustment for patient sociodemographic and clinical factors, the odds of receiving a high per-administration dose were 0.77 (95% CI, 0.45 to 1.31) for epoetin users compared with 3.35 (95% CI, 2.45 to 4.58) for darbepoetin users after versus before 2007 (Table 4). Transfusion rates among epoetin-treated patients with CIA were 0.15% in 2003, 0.14% in 2007, and 0.40% in 2010 (Table 2). Transfusion rates among darbepoetin-treated patients with CIA were 0.05% in 2003, 0.23% in 2007, and 0.22% in 2010. After adjustment for patient sociodemographic and clinical factors, the odds of receiving a transfusion were 2.7 (95% CI, 1.6 to 4.5) and 1.80 (95% CI, 1.3 to 2.6) for epoetin and darbepoetin users, respectively, after versus before 2007 (Table 5).
Fig 1.
Darbepoetin and epoetin use among South Carolina Medicaid-covered patients with chemotherapy-induced anemia and breast cancer, non–small-cell lung cancer, or colorectal cancer (2002 to 2010).12-15 BEST, Breast Cancer Erythropoietin Survival Trial; CMS, Centers for Medicare and Medicaid Services; ENHANCE, Erythropoietin in Head and Neck Cancer Study; ESA, erythropoiesis-stimulating agent; FDA, US Food and Drug Administration; NCD, National Coverage Determination; VTE, venous thromboembolism.
Fig 2.
Per-administration and per-month dosing among (A) epoetin and (B) darbepoetin patients covered by Medicaid in South Carolina who have chemotherapy-induced anemia and breast cancer, non–small-cell lung cancer, or colorectal cancer.
Table 4.
Logistic Regression: Probability of Receiving a High Dose of an ESA Among ESA Users
Table 5.
Logistic Regression for Probability of Receiving a Transfusion for ESA Nonusers, Darbepoetin Users, and Epoetin Users
DISCUSSION
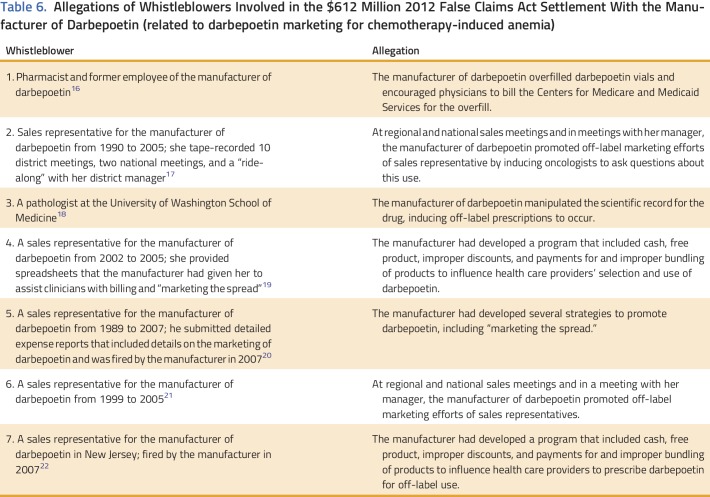
Among Medicaid-covered patients with cancer and CIA in South Carolina, marked variations in utilization, dosing, and costs for darbepoetin versus epoetin existed between 2002 and 2010. For epoetin, use decreased steadily during 2002 to 2010. For darbepoetin, use increased between 2003 and 2007 and then decreased between 2007 and 2010. Per-patient per-administration and per-patient per-month dosing and costs increased appreciably during the study years for darbepoetin but not epoetin. In interpreting our findings, several factors should be considered. Increases in darbepoetin administration and darbepoetin dosing in 2003 may be explained in part by corporate marketing practices. In addition, several concluded lawsuits against the manufacturer of darbepoetin provide context for interpreting these changes. In 2005, epoetin’s supplier (who had a licensing arrangement to purchase epoetin from darbepoetin’s manufacturer and then market it for cancer) filed an antitrust lawsuit alleging that between 2003 and 2005 darbepoetin’s manufacturer was illegally marketing the drug in the cancer setting. This case settled in 2008, with darbepoetin’s manufacturer paying $200 million.16 Additional allegations of illegal marketing were outlined in qui tam lawsuits (whistleblower lawsuits) joined by the Department of Justice (Table 6). In 2013, the manufacturer settled these cases for $612 million (the largest settlement ever paid by a biologic manufacturer) and paid a $136 million criminal fine.7,8,12,13,15-31 One whistleblower described a marketing program beginning in 2003 where the manufacturer provided rebates to hospitals on the price of two supportive care drugs marketed by the manufacturer of darbepoetin. In concluding one lawsuit, darbepoetin’s manufacturer settled, in 2015, a multistate Civil False Claims Act and a Washington DC Civil False Claims Act (for $72 million) alleging illegal promotion of darbepoetin doses higher than those approved by the FDA during 2002 to 2007. Settlements, fines, and repayments associated with these lawsuits accounted for $1.1 billion. These costs, as well as the total costs associated with the lawsuits themselves, should be included in any assessment of financial toxicity of ESAs because they represent part of the societal costs of cancer treatment.32
Table 6.
Allegations of Whistleblowers Involved in the $612 Million 2012 False Claims Act Settlement With the Manufacturer of Darbepoetin (related to darbepoetin marketing for chemotherapy-induced anemia)
ESA safety concerns were described at the 2004, 2007, and 2008 ODAC meetings. In 2004, ODAC sought advice regarding adverse effects on survival and shorter time to tumor progression observed with epoetin in the Erythropoietin in Head and Neck Cancer Study (ENHANCE) and Breast Cancer Erythropoietin Survival Trial (BEST) phase III clinical trials.12,13 FDA safety personnel presented data supporting marketing approval and labeling expansion of darbepoetin for treatment of anemia of cancer and results of the N93-004 study (conducted under a postmarketing commitment to assess tumor-stimulating potential of epoetin). The BEST and ENHANCE studies were designed to test whether use of ESAs at doses intended to maintain hemoglobin levels greater than 12 g/dL would improve tumor outcomes and survival. The studies identified detrimental effects on survival and tumor outcomes. In addition, dosing was inconsistent with FDA-approved labeling. The N93-004 study was a smaller phase III study designed to detect reduction in objective response rates. Published reports of three additional trials assessing ESAs in patients with homogeneous cancers and cancer treatment were reported. All trials were terminated prematurely because of evidence of unacceptable increases in risks of thrombotic and cardiovascular events in the ESA arms. An additional breast cancer trial was terminated early because of increased thrombotic events in the ESA arm.33 The 2007 ODAC meeting reviewed results from four phase III clinical trials reported since the 2004 ODAC meeting. These trials demonstrated shorter survival, shorter time to progression, and/or a lower rate of locoregional tumor control.5
The ODAC 2008 meeting reviewed two phase III studies that again identified harmful effects of ESAs (a total of eight phase III studies were reported at ODAC meetings).34 The FDA requested advice with regard to the following: removing FDA-approved indications for treatment of anemia as a result of cancer chemotherapy; restricting FDA-approved indications to ESA use only in patients with cancer who will not be cured by treatment and contraindicating ESA use in patients with cancers surgically resected for cure; restricting ESA use to specific cancer subtypes where safety was adequately assessed; contraindicating use in settings where harmful ESA effects were noted in phase III trials; or requiring risk management strategies. ODAC 2008 recommended restricting ESA use to CIA among patients with cancer receiving palliative chemotherapy and requiring risk mitigation programs.
Overall, several studies identified ESA risks, primarily VTE, tumor progression, and mortality, when patients with CIA received high ESA doses. In more recent years, compared with the years before boxed warnings, epoetin- and darbepoetin-treated patients with cancer and CIA were more likely to receive transfusions. An FDA-mandated postmarketing study reported in 2016 concluded that for safety reasons, transfusions are preferred over ESAs for anemia treatment care among women with metastatic breast cancer who receive chemotherapy.35 Decreased darbepoetin use beginning in 2007 is consistent with clinician responses to warnings, ODAC recommendations, and reimbursement changes occurring in 2007 and earlier.37-40
One study reported declining ESA use beginning in 2005, 2 years before the darbepoetin decline that we observed.36 The difference in the pivotal years may reflect that the earlier studies reported on overall ESA decline, whereas we reported epoetin decline in 2002 and darbepoetin decline in 2007. Clinicians who prescribed less ESAs before dissemination of boxed warnings in 2007 may have responded to reports of ESA-associated VTE risks as outlined at an ODAC meeting in 2004, in randomized clinical trials reported in 2003 and 2005, and in a meta-analysis in 2006 by the Cochrane Collaboration.5,8,12-15,24,33
Comparative epoetin and darbepoetin dosing data for CIA have not been reported before. Compendia supported weekly administration of epoetin before 2006, and the FDA approved this epoetin schedule in 2006. Empirical data indicated that per-patient epoetin dosing did not change over time, and the main schedule was weekly. However, 88% of community oncologists administered epoetin on a weekly schedule.27 In contrast, off-label practice commonly supported every-2-week darbepoetin dosing before 2006. In 2006, the FDA approved every-3-week dosing, with each dosing change of darbepoetin being accompanied by higher mean monthly doses. Our data analyses identified steadily increasing dosing of darbepoetin, but not epoetin, over time, consistent with these observations. In 2015, the manufacturer of darbepoetin settled litigation (for $72 million) with 48 state attorneys general alleging False Claims Act violations for marketing darbepoetin doses greater than those approved by the FDA between 2003 and 2007. ESA safety notification disseminated in 2007 and 2008 advised against administering high ESA doses to patients with cancer and CIA because cancer cells may have erythropoietin receptors.17 Consistent with these advisories, our data reveal the odds of receiving high epoetin doses decreased by 55% after dissemination in 2007 of warnings against high ESA dosing. In contrast, the odds of receiving high darbepoetin doses increased 3.8-fold after dissemination of these safety warnings.
Some limitations exist. The degree of anemia in epoetin- versus darbepoetin-treated patients with cancer may have differed, reflecting differences in chemotherapy administered or case mix. Medical record reviews and review of Medicaid policies over the study period are needed. Such information might provide insight into why Medicaid-covered patients with cancer receiving chemotherapy increased from 5% in 2002 to 15% in 2010, such as a change in the patient characteristics or available treatments for patients with cancer and CIA or a broadening of patient eligibility. In addition, payment and reimbursement programs, an important determinant of epoetin versus darbepoetin use, were not formally measured. For chronic kidney disease, lower ESA doses are administered at dialyses centers that are capitated or are at risk financially. Economic incentives can sometimes trump clinical acumen, particularly when incentives are misaligned. Alleged rebate programs outlined in federal False Claims lawsuits were reportedly offered to community- and academic-based oncology practices and provided rewards for bundling darbepoetin with pegfilgrastim (another product manufactured and marketed by the same company) when purchased in bulk and may partially explain persistence of darbepoetin use even after ESA toxicity warnings were disseminated. In addition, big data approaches are needed to evaluate ESA-associated adverse drug reactions, such as VTEs, and to see whether VTE risks differ between epoetin- and darbepoetin-treated patients with cancer. Clinicians are unlikely to independently report VTE occurrences to the FDA or to the manufacturer as a result of lack of economic incentives to do so and time constraints. Big data analysts may be reluctant to investigate adverse events unless protections such as those provided to qui tam relators (whistleblowers) are offered. Early warning approaches may have shortened the time to identification of ESA-associated toxicity from 10 years or more (as occurred in the CIA setting), mitigated legal costs, and improved cost-effectiveness of cancer treatments. Another source of adverse events, analyzing lawsuits invoking pharmaceutical safety, should also be considered in the future.
We conclude that among Medicaid-covered patients in South Carolina with breast cancer, NSCLC, or CRC and CIA, epoetin use for CIA decreased steadily between 2002 and 2010; for darbepoetin, use increased between 2003 and 2007 and then decreased thereafter. Per-patient dosing of epoetin did not change over time, whereas per-patient dosing of darbepoetin increased. Empirical data identifying high dosing of darbepoetin versus epoetin in the cancer setting between 2003 and 2010 have not been reported previously. Considering the context of darbepoetin use as outlined in four completed lawsuits focusing on allegations of illegal darbepoetin marketing and illegal marketing of high and unsafe darbepoetin doses in the CIA setting, our empirical data on darbepoetin use are in line with these lawsuits. To our knowledge, our findings are the first data reporting on epoetin versus darbepoetin use for CIA.
ACKNOWLEDGMENT
Supported in part by grants from the American Cancer Society (Grant No. IRG13-043-01), the National Institutes of Health (Grant No. R01CA165609), the University of South Carolina and the Centers for Economic Excellence Program of the state of South Carolina (Grant No. 11150-13-32600), and the Doris Levkoff Meddin Center for Medication Safety. We acknowledge the helpful comments and assistance with data analysis from Paul Ray, DO, Dennis Raisch, PhD, and Jun Wu, PhD. V.N. and K.B.K. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Virginia Noxon, LeAnn B. Norris, Brian Chen, Y. Tony Yang, Zaina P. Qureshi, William Hrushesky, Akida A. Lebby, Neset Hikmet, Mae Thamer, Dennis Cotter, Paul R. Yarnold, Charles L. Bennett
Administrative support: Akida A. Lebby, Benjamin Schooley, Neset Hikmet, Charles L. Bennett
Provision of study materials or patients: Neset Hikmet, Mae Thamer, Dennis Cotter, Charles L. Bennett
Collection and assembly of data: Virginia Noxon, Kevin B. Knopf, LeAnn B. Norris, Brian Chen, Y. Tony Yang, Zaina P. Qureshi, Neset Hikmet, Mae Thamer, Dennis Cotter, Paul R. Yarnold, Charles L. Bennett
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tale of Two Erythropoiesis-Stimulating Agents: Utilization, Dosing, Litigation, and Costs of Darbepoetin and Epoetin Among South Carolina Medicaid-Covered Patients With Cancer and Chemotherapy-Induced Anemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Virginia Noxon
No relationship to disclose
Kevin B. Knopf
Consulting or Advisory Role: TOMA Biosciences
Speakers' Bureau: Merck, Biocept
LeAnn B. Norris
Honoraria: Taiho Pharmaceutical, Bristol-Myers Squibb
Brian Chen
No relationship to disclose
Y. Tony Yang
No relationship to disclose
Zaina P. Qureshi
No relationship to disclose
William Hrushesky
No relationship to disclose
Akida A. Lebby
No relationship to disclose
Benjamin Schooley
No relationship to disclose
Neset Hikmet
No relationship to disclose
Michael Dickson
No relationship to disclose
Mae Thamer
Research Funding: Proteon Therapeutic (Inst)
Dennis Cotter
Employment: Results Physiotherapy (I)
Research Funding: Proteon Therapeutics (Inst)
Paul R. Yarnold
No relationship to disclose
Charles L. Bennett
No relationship to disclose
REFERENCES
- 1. US Food and Drug Administration: Information on Erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm109375.htm.
- 1a. US Food and Drug Administration: Revised FDA label for Aranesp, March 2007. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103951s5139lbl.pdf.
- 1b. US Food and Drug Administration: Revised FDA label for Procrit, March 2007. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103234s5122lbl.pdf.
- 2. US Food and Drug Administration: FDA strengthens safety information for erythropoiesis-stimulating agents (ESAs). http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108864.htm.
- 3. US Food and Drug Administration: FDA Briefing Document: Continuing reassessment of the risks of erythropoiesis stimulating agents for the treatment of anemia associated with cancer chemotherapy. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4301b2-02-FDA.pdf.
- 4. Centers for Medicare and Medicaid Services: National coverage determination (NCD) for erythropoiesis stimulating agents (ESAs) in cancer and related neoplastic conditions (110.21). https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=322&ncdver=1&bc=BAAAgAAAAAAA&.
- 5. US Food and Drug Administration: Continuing reassessment of the risks of erythropoiesis-stimulating agents (ESAs) administered for the treatment of anemia associated with cancer chemotherapy. https://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4301b2-02-FDA.pdf. [Google Scholar]
- 6.Rizzo JD, Somerfield MR, Hagerty KL, et al. : Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol 26:132-149, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Goldberg P: Danish researchers post long awaited Aranesp results: Ever so discreetly. Cancer Lett 33:1-6, 2007 [Google Scholar]
- 8.Bennett CL, Silver SM, Djulbegovic B, et al. : Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 299:914-924, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Hershman DL, Buono DL, Malin J, et al. : Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer. J Natl Cancer Inst 101:1633-1641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43:1130-1139, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Pashos CL, Bookhart B, MacKenzie RS, et al: Drug Cost Considerations for Erythropoietic Stimulating Agents (ESAs) in Patients Initiated at FDA-Approved Dosing: Results From Practice Patterns in a Prospective Observational Study, International Society for Pharmacoeconomic Research. Arlington, VA, Ortho Biotech, 2007. [Google Scholar]
- 12.Leyland-Jones B, Laszig R, Rube C, et al. : Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 4:459-460, 2003 [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1016/S0140-6736(03)14567-9. Henke M, Laszig R, Rube C, et al: Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet 362:1255-1260, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Bohlius J, Wilson J, Seidenfeld J, et al. : Recombinant human erythropoietins and cancer patients: Updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 98:708-714, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bohlius J, Schmidlin K, Brillant C, et al. : Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 373:1532-1542, 2009 [DOI] [PubMed] [Google Scholar]
- 16. United States ex rel. Westmoreland v. Amgen, Inc., 707 F. Supp. 2d 123 (D. Mass. 2010)
- 17. United States ex rel. Osiecki v. Amgen, Inc., Civil Action No. CV-05-5025 (E.D.N.Y.) [Google Scholar]
- 18. United States ex rel. Horwitz v. Amgen Inc., Civil Action No. C07-0248 (W.D. Wash.) [Google Scholar]
- 19. United States ex rel. Kelly v. Amgen Corporation, Civil Action No. CV-08-4157 (E.D.N.Y.) [Google Scholar]
- 20. United States ex rel. Hanks v. Amgen, Inc., Civil Action No. CV 08-3096 (E.D.N.Y.) [Google Scholar]
- 21. United States ex rel. Arriazola v. Amgen, Inc., Civil Action No. CV 06-3232 (E.D.N.Y.) [Google Scholar]
- 22. United States ex rel. Tucker v. Amgen, Inc., Civil Action No. CV-09-0887 (E.D.N.Y.) [Google Scholar]
- 23. US Department of Justice: Amgen to pay U.S. $24.9 million to resolve false claims act allegations. https://www.justice.gov/opa/pr/amgen-pay-us-249-million-resolve-false-claims-act-allegations.
- 24.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. : Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol 23:5960-5972, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Corporate Crime Reporter: AMGEN pleads guilty, to pay $762 million to settle false claims charge. http://www.corporatecrimereporter.com/news/200/amgenguilty12192012/
- 26. National Comprehensive Cancer Network: Practice Guidelines in Oncology: Cancer and Treatment Related Anemia. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 27. United States ex rel. Cantor v. Amgen, Inc., Civil Action No. CV-04-2511 (E.D.N.Y.)
- 28. United States ex rel. Ferrante v. Amgen, Inc., Civil Action No. CV-08-3931 (E.D.N.Y.) [Google Scholar]
- 29. United States ex rel. DJAE Partnership v. Amgen, Inc., Civil Action No. 11-CV- 11242 (D. Mass.)
- 30. Ortho Biotech Products, L.P. v. Amgen, Inc. (2:05-cv-04850), 2008.
- 31. Amgen Inc. v. Connecticut Retirement Plans and Trust Funds, 133 S.Ct. 1184 (2013)
- 32.Sorensen SV, Goh JW, Pan F, et al. : Incidence-based cost-of-illness model for metastatic breast cancer in the United States. Int J Technol Assess Health Care 28:12-21, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Luksenburg H, Wager R: Evolving safety issues associated with erythropoietic products: Assessment of the risks of erythropoiesis stimulating agents for the treatment of anemia associated with cancer chemotherapy. http://www.fda.gov/ohrms/dockets/ac/cder04.html#Oncologic.
- 34. US Food and Drug Administration: FDA Briefing Document. http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4345b2-01-FDA.pdf.
- 35.Leyland-Jones B, Bondarenko I, Nemsadze G, et al. : A randomized, open-label, multicenter, phase III study of epoetin alfa versus best standard of care in anemic patients with metastatic breast cancer receiving standard chemotherapy. J Clin Oncol 34:1197-1207, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Vadhan-Raj S, Zhou X, Sizer K, et al. : Impact of safety concerns and regulatory changes on the usage of erythropoiesis-stimulating agents and RBC transfusions. Oncologist 15:1359-1369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weir MA, Gomes T, Winquist E, et al. : Effects of funding policy changes and health warnings on the use of erythropoiesis-stimulating agents. J Oncol Pract 8:179-183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stafkey-Mailey D, Dickson M, Norris LB, et al: Changes in the use of erythropoietin-stimulating agents (ESAs) in cancer patients: Before and after FDA safety advisories. J Clin Oncol 30, 2012 (suppl; abstr 9143) [Google Scholar]
- 39. Reference deleted.
- 40.Tarlov E, Stroupe KT, Lee TA, et al. : Trends in anemia management in lung and colon cancer patients in the US Department of Veterans Affairs, 2002-2008. Support Care Cancer 20:1649-1657, 2012 [DOI] [PubMed] [Google Scholar]