Abstract
Purpose
Patient age is considered to play a unique prognostic role in papillary thyroid cancer (PTC), with a distinct staging dichotomization at 45 years of age. This is based on older, limited data demonstrating a marked rise in mortality around the ages of 40 to 50 years. We hypothesized that age is associated with compromised survival from cancer, with no cutoff denoting survival difference.
Patients and Methods
Patients with PTC who had surgery were identified from the SEER database (1998 to 2012). Multivariable proportional hazards modeling utilizing several flexible smoothing approaches were used to examine the association between age and cancer-specific survival (CSS) and to determine whether there is an age cut point that is associated with CSS decrement.
Results
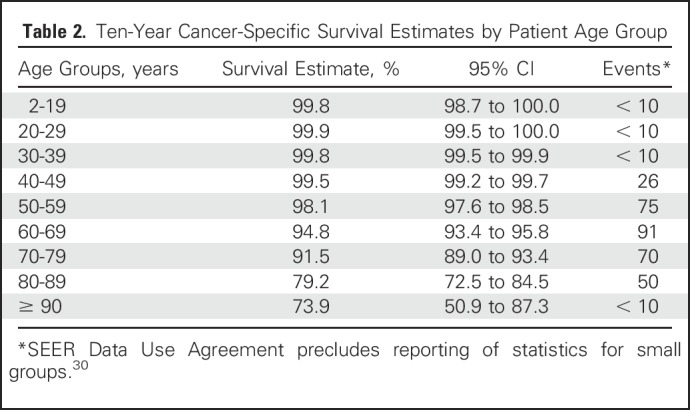
A total of 31,802 patients with PTC were included. Median age was 45 years (range, 2 to 105 years). Ten-year CSS according to age was as follows: 2 to 19 years, 99.8%; 20 to 29 years, 99.9%; 30 to 39 years, 99.8%; 40 to 49 years, 99.5%; 50 to 59 years, 98.1%; 60 to 69 years, 94.8%; 70 to 79 years, 91.5%; 80 to 89 years, 79.2%; and ≥ 90 years, 73.9%. After adjustment for patient demographic and clinicopathologic characteristics, increasing age was associated with increasing mortality from the disease in a dose-dependent fashion, without an apparent cut point. Each of the smoothing approaches demonstrated a similar linearity of risk over all ages and provided close measures of goodness of fit to the data.
Conclusion
Patient age is significantly associated with death from PTC in a linear fashion, without an apparent age cut point demarcating survival difference. These results challenge the appropriateness of a patient age cut point in current staging systems for PTC and argue for considering a revision in how we anticipate prognosis for patients with PTC.
INTRODUCTION
Thyroid cancer incidence is increasing faster than any other cancer in the United States, with 64,300 patients expected to be diagnosed in 2016.1 The incidence of thyroid cancer is expected to continue increasing to become the third most common cancer among women by 2019.2 Papillary thyroid cancer (PTC) is the predominant pathologic variant of thyroid cancer, and it almost entirely accounts for the increasing incidence of the disease.3
Although PTC is indolent overall, identification of patients with demographic, clinical, and pathologic features suggestive of compromised outcome is important. Older patient age was identified as a prognostic determinant of cancer-specific survival (CSS). In fact, differentiated thyroid cancer is the only human malignancy to include age as part of the American Joint Committee on Cancer (AJCC) staging system, with a distinct cut point at age 45 years. This dichotomization was based on data from the mid-1900s that demonstrated a sharp increase in thyroid cancer mortality starting around the ages of 40 to 50 years.4-6 Since then, the majority of thyroid cancer staging systems have incorporated patient age treated as a dichotomous variable into risk stratification as a formal consideration; these include the AJCC7; European Organization for Research and Treatment of Cancer8; National Thyroid Cancer Treatment Cooperative Study9; AGES (age, grade, extent, size)10; AMES (age, metastases, extent and size of tumor)11; and MACIS (metastases, age, completeness of resection, invasion, size).12,13
The current AJCC staging system for PTC includes a distinct patient age dichotomization at 45 years, on the basis of which tumor staging drastically changes. Patients < 45 years can only have two stages of disease on the basis of absence (stage I) or presence (stage II) of distant metastases, whereas patients ≥ 45 years can have stage I to V disease on the basis of tumor size, extent of thyroidal extension, status of cervical lymph nodes, and presence of distant metastases. Patients < 45 years of age with high-risk tumor features, such as presence of advanced locoregional disease and/or distant metastases, are still considered to have either stage I or II thyroid cancer, whereas they would be upstaged to stage III or IV disease if they were ≥ 45 years of age.
Despite the wide acceptance of treating patient age as a binary variable and an important prognostic factor for PTC, the concept of a patient age cut point recently has become more controversial, given recognition about the limitations of the initial supporting evidence, such as small sample size and lack of adjustment for potential clinical and pathologic confounders.14 Recent studies have re-examined the potential role of a patient age cut point in PTC, and their results have been contradictory.14-19 For instance, in a recent single-institution analysis of 1,807 patients with differentiated thyroid cancer, Nixon et al18 demonstrated age 55 years as a more appropriate cut point. However, in another recent study of 3,664 patients with differentiated thyroid cancer from the same institution, Ganly et al16 were unable to identify any age cut point that was associated with survival. Nationwide studies also have failed to establish consensus about the appropriate treatment of age in staging for PTC.17,19-24 A methodologic concern surrounding prior studies was their assumption that an age cut point actually exists; however, the resulting models were not compared with one without this assumption to demonstrate its superior fit to the data. In addition, in several studies, other concerns included a small number of events as well as a lack of appropriate control for multiplicity of hypotheses.17,18
We sought to explore the relationship between patient age at diagnosis and CSS in patients with PTC, without any preexisting assumption or bias. We used biostatistical methodologies to objectively test whether an age cut point exists, and our plan was to test for a cut point only if our initial analysis demonstrated the existence of such a cut point. We hypothesized that patient age would be linearly associated with compromised survival from PTC without an age cut point demarcating a survival differential.
PATIENTS AND METHODS
The study cohort was obtained from the SEER 9 database. SEER is a U.S. population-based cancer registry that is supported by the National Cancer Institute. This database represents approximately 28% of the population of the United States, capturing various demographic, clinical, tumor, and treatment data points from representative geographic regions.
The SEER database was queried for all patients diagnosed with PTC who underwent thyroidectomy between 1998 and 2012. Patients with PTC were identified using the following International Classification of Diseases for Oncology (3rd edition) codes: 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, and 8343/3. Patients who underwent removal of less than a thyroid lobe or had unknown extent of thyroid surgery were excluded. The study was granted exempt status by our institutional review board.
Demographic variables included patient age at diagnosis (hereafter denoted as age), gender, race, and year of diagnosis. Tumor characteristics included tumor size, presence of extrathyroidal extension, and status of lymph node metastases. Presence of distant metastases was determined from the variable historic SEER stage. Extent of thyroidectomy was documented as thyroid lobectomy and total thyroidectomy.25 Data on receipt of radioactive iodine (RAI) therapy were extracted from the data set, in addition to follow-up time and CSS.26
Statistical Analyses
Descriptive statistics were computed for patient characteristics. Unadjusted CSS proportions and 95% CIs were computed using the life-table method implemented in SAS Proc Lifetest.27,28 A multivariable Cox proportional hazards regression model with restricted cubic splines (RCS) was used to examine the functional relationship between patient age and CSS. RCS provides a flexible model to examine the relationship between age and the natural logarithm (log) of the hazard ratio (HR) without prior knowledge of the form of the association, while adjusting for the effects of covariables. RCS allows for visual determination of the nature of the relationship between a continuous predictor and the outcome, while also testing for significance of a nonlinear association. A bootstrap simulation incorporating the RCS function was conducted to internally validate the functional association between age and CSS in 1,000 simulation data sets.29 The following factors were accounted for in this multivariable model: patient gender, race, tumor size, extrathyroidal extension, lymph node metastases, distant metastases, extent of surgery, year of diagnosis, and receipt of RAI. The RCS model also was used to estimate the HRs and 95% CIs of different ages compared with age 45 years.
RCS applies some conditions to the functional relationship of the continuous covariable of interest and log HR, such as user-determined placement of knots and linearity at the tails. To verify that the association observed was not due to these assumptions, two additional types of smoothing functions were applied to the functional association of age with log HR, with adjustment for covariables in the generalized additive model setting. These approaches were selected to generalize our observations by removing the consideration of knots and also the assumption of linearity at the tails. Thin plate splines are knot free and therefore do not rely on knot numbers or placement, but instead apply a smoothing function on the basis of the second derivative across the distribution. Adaptive smoothers are also knot free, but instead allow the smoothing function to vary with the association. Thus, adaptive smoothers allow for a piece-wise smoothing process, allowing the smoother to vary over levels of age. Thin plate splines are low rank, meaning that they require estimation of only a few additional parameters, but adaptive smoothers require that there are enough data to approximate the variance. Plots were used to visualize the adjusted association of age and log HR after application of each of these smoothing techniques, and the Akaike information criterion (AIC) was used to compare model fit.
All P values reported are two-sided, with the significance level set to .05. Statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC) and the mgcv package in R (version 3.1.0; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
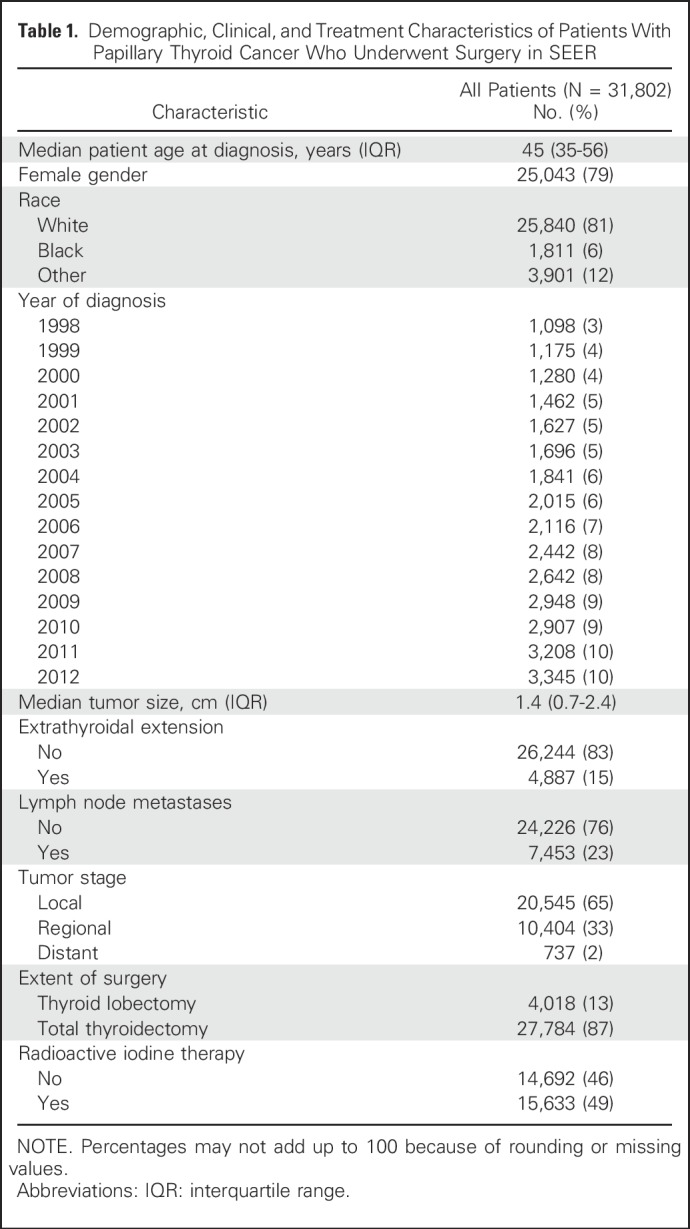
A total of 31,802 patients with PTC who underwent surgery were included. The majority of patients were female (79%), were white (81%), had intrathyroidal tumors (83%), and underwent total thyroidectomy (87%). Information about remaining demographic and clinical characteristics of the cohort is listed in Table 1. The median age was 45 years (range, 2 to 105 years).
Table 1.
Demographic, Clinical, and Treatment Characteristics of Patients With Papillary Thyroid Cancer Who Underwent Surgery in SEER
Median follow-up was 59 months (range, 0 to 179 months). There were 331 death events related to PTC. In unadjusted analysis, 10-year CSS estimates were inversely associated with increasing patient age: 99.8% for patients ages 2 to 19 years, 99.9% for patients 20 to 29 years, 99.8% for patients 30 to 39 years, 99.5% for patients 40 to 49 years, 98.1% for patients 50 to 59 years, 94.8% for patients 60 to 69 years, 91.5% for patients 70 to 79 years, 79.2% for patients 80 to 89 years, and 73.9% for patients ≥ 90 years (Table 2).
Table 2.
Ten-Year Cancer-Specific Survival Estimates by Patient Age Group
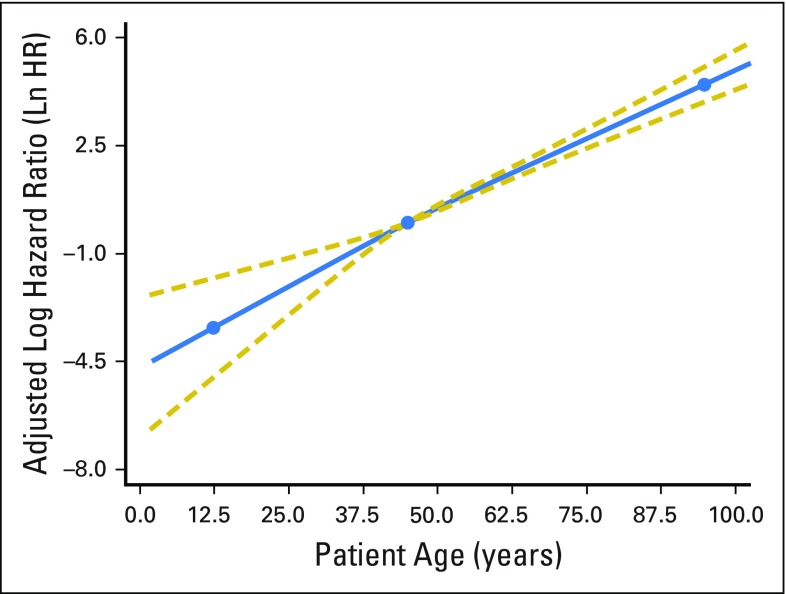
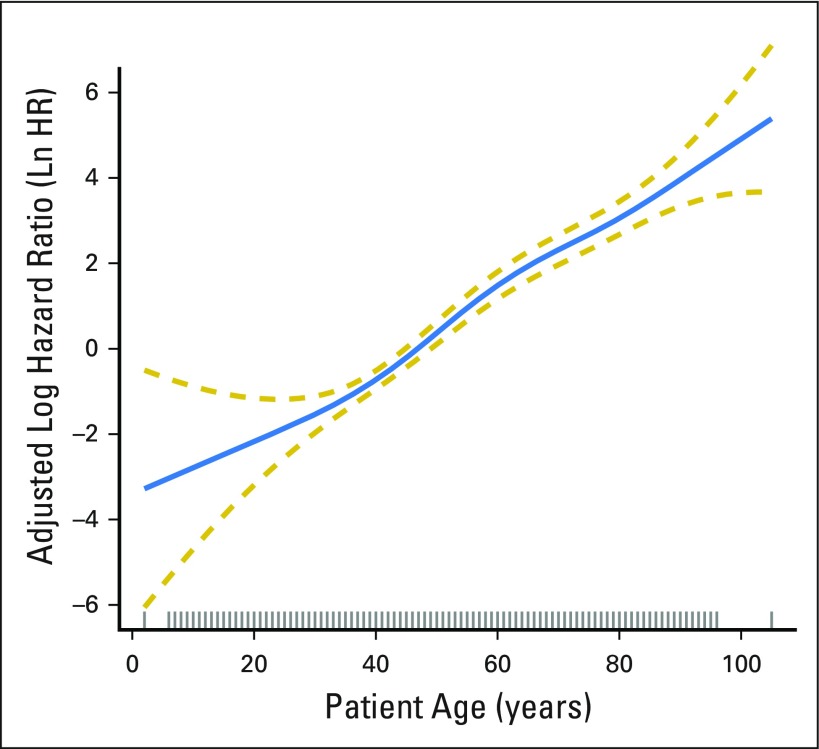
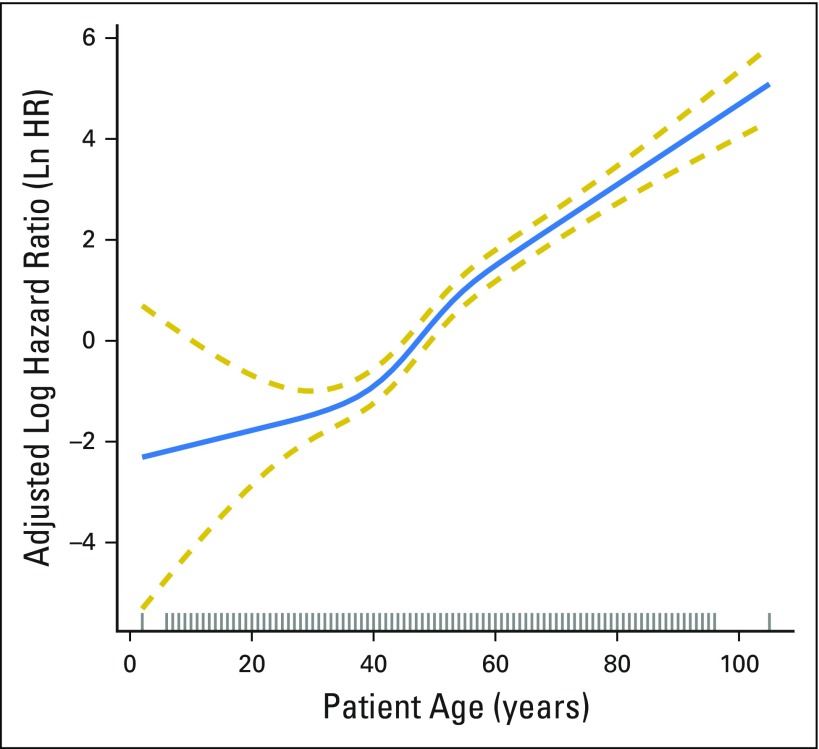
After adjustment for patient demographic, clinical, and treatment characteristics, increasing patient age was significantly associated with increasing mortality (P < .001). The RCS plot demonstrates a significant linear association between age and cancer-specific mortality, without an apparent cut point (Fig 1). The alternative models, using thin-plate splines (Fig 2) and the adaptive splines (Fig 3) demonstrated a similar linear association. A linear association between the predictor and outcome precludes the existence of a cut point in this relationship.1a
Fig 1.
Adjusted association between patient age at diagnosis and cancer-specific survival. The restricted cubic spline plot demonstrates the linear association between patient age (x-axis) and the adjusted log hazard ratio of death (y-axis). The blue solid line represents the fitted line of the association between age and the estimated hazard ratio of survival after adjustment; the two gold dashed lines represent the 95% CI. The three blue dots correspond to the location of three knots used in the model placed at the 10th, 50th, and 90th percentiles of patient age. The effects of the following were adjusted for in the model: patient gender, race, tumor size, extrathyroidal extension, lymph node involvement, distant metastases, extent of surgery, year of diagnosis, and radioactive iodine treatment. Ln HR, log hazard ratio.
Fig 2.
Adjusted association between patient age at diagnosis and cancer-specific survival with thin plate splines applied. The blue solid line represents the fitted line of the association between age and the estimated hazard ratio of survival after adjustment; the two gold dashed lines represent the 95% CI. The effects of the following were adjusted for in the model: patient gender, race, tumor size, extrathyroidal extension, lymph node involvement, distant metastases, extent of surgery, year of diagnosis, and radioactive iodine treatment. Ln HR, log hazard ratio.
Fig 3.
Adjusted association between patient age at diagnosis and cancer-specific survival with adaptive smoothers applied. The blue solid line represents the fitted line of the association between age and the estimated hazard ratio of survival after adjustment; the two gold dashed lines represent the 95% CI. The effects of the following were adjusted for in the model: patient gender, race, tumor size, extrathyroidal extension, lymph node involvement, distant metastases, extent of surgery, year of diagnosis, and radioactive iodine treatment. Ln HR, log hazard ratio.
We then compared the statistical fitness of different age-survival models with age treated as a continuous variable or a binary variable (with cut points at 45 years, 50 years, and 55 years). The model that included age as a continuous variable had the lowest AIC estimate (AIC = 2,953) compared with models that included age as a binary variable at 45 years (AIC = 3,108), 50 years (AIC = 3,071), or 55 years (AIC = 3,039). This indicates that treating age as a continuous versus a binary variable provides a better fit to the data.
DISCUSSION
This large study explored the relationship between patient age and CSS in patients with PTC. In unadjusted analysis, increasing patient age was associated with progressive compromise in CSS. After adjustment for patient clinical and treatment characteristics, patient age remained significantly associated with mortality from PTC in a linear dose-dependent fashion. There seems to be no patient age cut point corresponding to a marked decrement in survival, challenging the appropriateness of a patient age cut point in current staging systems for PTC.
Although emerging data recently have questioned the appropriateness of the concept of dichotomizing patient age in PTC,14,15,29,31 this issue continues to be controversial, given the inconsistency of published data on the subject.16,18-20 Nixon et al18 analyzed data for 1807 patients with differentiated thyroid cancer who underwent surgery at a single institution between 1986 and 2005. Their analysis focused on determining a patient age cut point associated with a significant change in CSS. With a median follow-up of 108 months, they reported 28 deaths from thyroid cancer. The analysis identified age 55 years as a more appropriate age cut point. The authors recommended that the age cut point used in the AJCC staging system be changed from 45 years to 55 years to provide more appropriate risk stratification and treatment.18 In another analysis published by the same research group using the same institutional database, the association between patient age and survival in differentiated thyroid cancer was examined using a different statistical methodology. The study included 3,664 patients with differentiated thyroid cancer who underwent surgery at the same institution. After a median follow-up of 54 months, there were 59 death events from thyroid cancer. The authors found that mortality from differentiated thyroid cancer increased progressively with advancing patient age, without a specific age cut point associated with a shift in survival. They concluded that it is more appropriate to treat patient age as a continuous variable in differentiated thyroid cancer.16
These two studies from the same institution reached two conflicting conclusions regarding the prognostic role of a patient age cut point in the arena of differentiated thyroid cancer. Differentiated thyroid cancer is an indolent disease, with death from the disease occurring only in a small minority of patients. Therefore, a large sample size is needed to adequately examine the relationship between patient age and survival. The numbers of thyroid cancer death events in both of these studies were small, challenging the sufficiency of statistical power. Our study included a cohort of 31,802 patients with PTC who had a significant long-term follow-up and an adequate number of death events (n = 331). This permitted us to adequately analyze this issue in a multivariable fashion, accounting for confounders.
A number of large studies examined the association between patient age and survival in differentiated thyroid cancer, but the conclusions reached in these studies also have been inconsistent. In a Canadian study of 2,115 patients with differentiated thyroid cancer, Mazurat et al20 demonstrated that patient age did not influence survival until the age of 55 years. In a multi-institution study of 9,484 patients with differentiated thyroid cancer, Nixon et al18 compared the appropriateness of an age cut point at 45 versus 55 years. They showed that modeling the cut point at 55 versus 45 years seems to better predict survival.24 In another study from SEER, Orosco et al19 examined the association between patient age and survival in 85,740 patients with differentiated thyroid cancer (1973 to 2009). They specified different possible age cut points between the ages of 25 and 55 years, all of which were significantly associated with survival. The authors concluded that patient age is associated with survival without an apparent cut point.19
The heterogeneity of published studies examining the prognostic role of a patient age cut point in differentiated thyroid cancer is likely related to how patient age was analyzed in each study. These studies have used different statistical methods for their analysis, with the majority using conventional multivariable regression analysis; others have used univariable or other cut point analyses.16-20 In one case, the conclusion was made based on 28 death events,18 and in the other case, the appropriate control for multiplicity of comparisons was not applied. Some of these studies were designed with the assumption that a binary age cut point exists,24 thereby forcing the model to identify a cut point value corresponding to a significant shift in survival between young and old patients, yet a comparison with the model without this assumption was not made.17,18,24 In our study, we examined the adjusted association between patient age and survival using a multivariable regression model incorporating RCS. The RCS function permitted us to explore the relationship between patient age and CSS without the need for any prespecified assumption about this relationship (ie, presence or absence of a cut point).1 Our analysis objectively demonstrated that the relationship between patient age and compromised survival is linear, without any apparent cut point. The results of this model were then internally validated using simulation analysis, which confirmed the linear form of this relationship. The linear relationship was also maintained after application of two alternative types of smoothing techniques, demonstrating robust results and conclusions.
Our results strongly challenge the appropriateness of the widely accepted treatment of age as a dichotomous factor in the staging for PTC. The concept of an age cut point has been propagated for decades on the basis of data from the mid-1900s, which demonstrated a sharp rise in mortality from thyroid cancer among patients older than 45 years.4-6 However, it is important to highlight that these studies are old, are based on a small number of patients, and lacked adjustment for important competing factors. Therefore, it would seem important to reassess the accuracy of current staging systems for PTC, most of which incorporate a patient age cut point of 45 years.7-13 In an analysis of nearly 70,000 patients with PTC, Adam et al29 examined the prognostic role of cervical lymph node metastases in patients < 45 years without distant metastases (stage I). After adjustment, lymph node metastases were significantly associated with a 32% increase in risk of death, which is comparable to the effect of lymph node metastases on survival among patients ≥ 45 years. This suggests that current staging schema might be understaging young patients with PTC, which in turn could result in their undertreatment.
Although we did not find a patient age cut point associated with a frameshift in the risk of mortality from PTC, our multivariable model demonstrated a significant association between patient age and CSS. The association between increasing patient age and mortality from thyroid cancer is not entirely clear, but several hypotheses have been put forth to explain this association, including several physiopathologic changes associated with older age, such as decreased avidity and response to RAI therapy, increasing levels of thyroid stimulating hormone, impaired immune response, and a higher frequency of the BRAF (V600E) mutation.32-35
There are several limitations to the current study, such as the potential for coding errors in SEER data. However, the SEER database uses standardized abstraction and coding methods. The study is retrospective in nature, with the possibility of selection bias. Information about tumor recurrence is not captured in SEER; therefore, it could not be analyzed. Although recurrence is an important outcome in PTC, CSS has traditionally been used to develop tumor staging systems. Despite these limitations, the strengths of our study include the large sample size and adequate number of cancer-specific death events that permitted us to conduct multivariable adjustment. Patient age was examined, using the RCS method, as a continuous variable without prespecification or assumption about a potential age cut point, allowing us to evaluate the nature of the association between patient age and CSS in an objective and transparent fashion, with successful validation by two other statistical techniques.
This large study provides valuable information regarding the relationship between patient age and CSS in PTC. Despite the widely accepted concept that dichotomizing patient age around a cut point of 45 years is an important prognostic indicator in PTC, the current study demonstrates that patient age is significantly associated with an increased risk of disease-specific mortality in a continuous, linear fashion, without an apparent age cut point demarcating survival difference. These results challenge the appropriateness of using a patient age cut point in current staging systems for PTC and suggest that current staging systems well might understage young patients (< 45 years) with intermediate- and high-risk tumors. The study findings are timely, because the AJCC is currently re-evaluating staging schemas for all cancer diagnoses, including differentiated thyroid cancer. Reconsideration of the current staging schema may be needed to inform a more appropriate system for risk stratification to better inform the management of young patients with PTC.
Footnotes
This work was supported in part by National Institute of Health grant number P30CA014236.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design Mohamed Abdelgadir Adam, Sanziana A. Roman, Julie A. Sosa
Provision of study materials or patients: Mohamed Abdelgadir Adam
Collection and assembly of data: Mohamed Abdelgadir Adam
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Exploring the Relationship Between Patient Age and Cancer-Specific Survival in Papillary Thyroid Cancer: Rethinking Current Staging Systems
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Mohamed Abdelgadir Adam
No relationship to disclose
Samantha Thomas
No relationship to disclose
Terry Hyslop
No relationship to disclose
Randall P. Scheri
No relationship to disclose
Sanziana A. Roman
No relationship to disclose
Julie A. Sosa
Consulting or Advisory Role: GlaxoSmithKline, Astra Zeneca, Novo Nordisk, Eli Lilly
Travel, Accommodations, Expenses: GlaxoSmithKline, Novo Nordisk, Astra Zeneca, Eli Lilly
REFERENCES
- 1. American Cancer Society: Cancer facts & figures 2015. Atlanta: American Cancer Society, 2015. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf.
- 1a.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 2.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, et al. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol. 2013;20:2746–2753. doi: 10.1245/s10434-013-2892-y. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Crile G, Jr, Hazard JB. Relationship of the age of the patient to the natural history and prognosis of carcinoma of the thyroid. Ann Surg. 1953;138:33–38. doi: 10.1097/00000658-195307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cady B, Sedgwick CE, Meissner WA, et al. Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg. 1976;184:541–553. doi: 10.1097/00000658-197611000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franssila KO. Prognosis in thyroid carcinoma. Cancer. 1975;36:1138–1146. doi: 10.1002/1097-0142(197509)36:3<1138::aid-cncr2820360346>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7. Edge SB, Byrd DR, Compton CC, et al (eds): AJCC Cancer Staging Manual (ed 7). New York, NY, Springer, 2010.
- 8.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–1041. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 9.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroiscarcinoma treatment: Initial analysis of staging and outcome. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: A retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 11.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 12.Dean DS, Hay ID. Prognostic indicators in differentiated thyroid carcinoma. Cancer Contr. 2000;7:229–239. doi: 10.1177/107327480000700302. [DOI] [PubMed] [Google Scholar]
- 13.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. [PubMed] [Google Scholar]
- 14.Adam MA, Reed SD, Roman SA, et al. Does current thyroid cancer staging accurately reflect the impact of lymph node metastases on survival in younger patients? Int J Endo Oncol. 2016;3:1–3. [Google Scholar]
- 15.Tran Cao HS, Johnston LE, Chang DC, et al. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery. 2012;152:145–151. doi: 10.1016/j.surg.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganly I, Nixon IJ, Wang LY, et al. Survival from differentiated thyroid cancer: What has age got to do with it? Thyroid. 2015;25:1106–1114. doi: 10.1089/thy.2015.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Myong JP, Suh H, et al. Optimal cutoff age for predicting mortality associated with differentiated thyroid cancer. PLoS One. 2015;10:e0130848. doi: 10.1371/journal.pone.0130848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nixon IJ, Kuk D, Wreesmann V, et al. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol. 2016;23:410–415. doi: 10.1245/s10434-015-4762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orosco RK, Hussain T, Brumund KT, et al. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid. 2015;25:125–132. doi: 10.1089/thy.2014.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazurat A, Torroni A, Hendrickson-Rebizant J, et al. The age factor in survival of a population cohort of well-differentiated thyroid cancer. Endocr Connect. 2013;2:154–160. doi: 10.1530/EC-13-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee M, Muenz DG, Chang JT, et al. Tree-based model for thyroid cancer prognostication. J Clin Endocrinol Metab. 2014;99:3737–3745. doi: 10.1210/jc.2014-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonklaas J, Nogueras-Gonzalez G, Munsell M, et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. 2012;97:E878–E887. doi: 10.1210/jc.2011-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLeod DS, Jonklaas J, Brierley JD, et al. Reassessing the NTCTCS Staging systems for differentiated thyroid cancer, including age at diagnosis. Thyroid. 2015;25:1097–1105. doi: 10.1089/thy.2015.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nixon IJ, Wang LY, Migliacci JC, et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016;26:373–380. doi: 10.1089/thy.2015.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam MA, Pura J, Gu L, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: An analysis of 61,775 patients. Ann Surg. 2014;260:601–605. doi: 10.1097/SLA.0000000000000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howlader N, Ries LA, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David DR. Regression models and life tables. J R Stat Soc (Ser A) 1972;34:187–220. [Google Scholar]
- 28.Cutler SJ, Ederer F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958;8:699–712. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- 29.Adam MA, Pura J, Goffredo P, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. 2015;33:2370–2375. doi: 10.1200/JCO.2014.59.8391. [DOI] [PubMed] [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results Program http://seer.cancer.gov/dataagreements/seer.pdf.
- 31.Ito Y, Miyauchi A, Kihara M, et al. Prognostic significance of young age in papillary thyroid carcinoma: Analysis of 5,733 patients with 150 months’ median follow-up. Endocr J. 2014;61:491–497. doi: 10.1507/endocrj.ej13-0529. [DOI] [PubMed] [Google Scholar]
- 32.Ronga G, Filesi M, Montesano T, et al. Lung metastases from differentiated thyroid carcinoma. A 40 years’ experience. Q J Nucl Med Mol Imaging. 2004;48:12–19. [PubMed] [Google Scholar]
- 33.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: Implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 34.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 35.Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009;14:216–221. doi: 10.1634/theoncologist.2008-0194. [DOI] [PubMed] [Google Scholar]