Abstract
Oncology guidelines clearly outline evidence-based recommendations for patients with newly diagnosed cancer to help oncologists determine which patients are appropriate for a genetic assessment. Ideally, patients with newly diagnosed cancer, who have personal or family histories suggestive of hereditary cancer predisposition, are referred for genetics work up in the nonurgent setting. However, in some cases, a genetics work up is delayed until the end of life. This is a time of heightened stress and additional obstacles, including discordance between family members regarding the obtainment of genetic information, paying for testing, selecting a surrogate to receive and disperse information in the case of a patient’s death, and the use of DNA banking for future evaluation. To meaningfully participate and support patients, family members, and our colleagues facing requests at the end of life for genetic testing, we provide a practical approach and highlight resources to effectively engage in this rising challenge.
INTRODUCTION
As patients with cancer approach the end of life (EOL), concerns often arise regarding the possibility of passing on a hereditary cancer predisposition to other family members. Ideally, a patient with newly diagnosed cancer, with a personal or family history suggestive of hereditary cancer predisposition, is referred for genetics work up in the nonurgent setting.1 Then, appropriate germline genetic testing can be considered and ordered.1-3 However, when patients with cancer are diagnosed with symptomatic, advanced-stage disease, new pragmatic and ethical challenges arise. This is a stressful time for both patients and family members, and there may be disagreement about genetic testing. Thoughts of a life-ending cancer may drive some patients away from completing a genetics work-up. Others may insist on knowing and sharing this information with surviving family members, even if testing is not clinically indicated. Sometimes a patient may have previously declined genetic testing, but when he or she loses decisional capacity, surrogates may request that the genetics work-up be completed. Trying to obtain genetic testing at the EOL may be further complicated by insurance issues. Payers are unlikely to cover genetic testing if it will not alter medical management of the patient with cancer, even if the information would benefit family members. Consequently, practicing oncologists increasingly may encounter urgent requests for genetic testing at the EOL, for which they need expertise and resources not widely available across all medical settings. To meaningfully participate and support patients, family members, and our colleagues facing urgent requests for genetic testing at the EOL, we provide a practical approach and highlight resources to effectively engage in this rising challenge.
Ideal Situation: Genetics Work Up in a Nonurgent Setting
Current oncology guidelines and practice indicators include clear referral recommendations for patients with newly diagnosed cancer to help oncologists determine which patients are appropriate for a genetic assessment, with a focus on germline mutations and familial patterns of cancer.1 In addition to germline genetic testing, molecular tumor profiling is increasingly used to identify treatment options or to determine eligibility for therapeutic trials. Tumor profiling may reveal possible (tumor-only) or definitive germline mutations (paired tumor-germline). It is important to distinguish between the types of testing with patients and families and to avoid confusion between tumor versus germline genetics when deciding if genetic testing is appropriate. Optimal genetic counseling engages the patient and family when both can engage in the following criteria:
Provide details about their personal and family medical history that may not be known to other relatives or their health care proxies
Provide informed consent for genetic testing
Consider that undergoing a genetic risk assessment (and possible genetic testing) can often provide reassurance for family members that there is not likely to be a strong genetic component to the patient’s illness
Consider that, alternatively, the genetic risk assessment may reveal a clear hereditary condition that is important for surviving family members. This allows the patient to actively participate in disclosing information to their relatives, providing a gift of health information as part of his/her legacy
Potentially review with family members any implications of previous genetic test results (particularly when extended or out-of-town family members have gathered and have been made aware of prior testing)
However, leaving this important discussion about the possible heritability of a condition until a patient’s last days can cause unnecessary distress for the patient and family. Given that there may not be enough time to address the logistical issues that are often needed when genetic testing is being considered, a sense of rushing to get testing completed is often created.
EOL: Genetics Work Up in an Urgent Setting
Too often, patients and families delay discussions regarding genetic counseling because they are overwhelmed by the diagnosis and treatment. Some patients are referred to genetics services, but decline for various reasons including anxiety, fear of results, objections from family members, and perceptions that genetic information is not relevant.4 Delaying genetic counseling discussions may create an urgent situation when a patient’s death is imminent and they can no longer fully engage in the process.5 In these cases, the responsibility may fall on the oncologist, who must determine if a genetics work-up was ever undertaken and, if not, how to initiate this work-up if it is consistent with the patient’s goals.
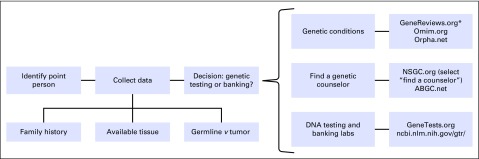
At the EOL, there are additional obstacles. For example, health insurers may not cover the cost of genetic tests because they will probably not alter medical management. Also, family members may not agree on whether they wish to pursue genetic testing. Lastly, the patient’s surrogate decision maker or health care power of attorney may be overwhelmed by the myriad of decisions that are being made at the EOL; thinking about a genetics work-up may seem especially burdensome or distressing. However, extended or out-of-town family members often gather around the patient, and this presence of additional family members may improve accuracy in collecting family medical history. In these urgent situations, we suggest the following approach to conduct genetic testing (Fig 1).
Fig 1.
Approach to conducting urgent genetic testing at the end of life. (*)GeneReviews.org: https://www.genetests.org/tests/mtb.php?mtb=DNA%20Banking
First, decide if the patient is an appropriate candidate for genetic testing. Potential indications for genetic testing include young age of onset, family history of the same or related cancers, or specific tumor types/histology types. Some patients with prior negative genetic testing may be appropriate for additional testing, especially if single-gene testing was performed but more extensive multigene panel testing might elucidate the cause of a particularly strong family history of cancer. At the EOL, it is critical to identify an appropriate family member to represent the patient, who may or may not be able to fully participate in informed decision making. This person will serve as the primary decision maker and contact to discuss any key genetic findings. Additionally, this point person will be responsible for communicating relevant information learned from genetic testing and its implications to surviving family members. It often takes several weeks for results of genetic testing to be reported, and results may not be available until after the patient has died. It is not necessary for the point person to also be the primary health decision maker for the patient. In many cases, it makes more sense for an alternative family member, ideally a biological relative, to assume this role.
In some cases, complete genetic testing is not possible at the EOL. One option for patients to consider is the banking of a DNA sample for future evaluation. This is an important middle-ground option to offer to select patients and families when agreement on genetic testing in the urgent setting cannot be achieved.6 The following list describes patients and families who frequently chose DNA banking:
Those who are suspected to have a hereditary condition, but who have had negative genetic testing results to date; genetic and genomic research is progressing at a rapid rate, and new genetic tests are being developed and made available as new disease-causing genes are discovered
Those who are suspected to have a hereditary condition, but who have not had any genetic testing
Those who have had a positive genetic test result for a genetic condition, but for which there is a limited understanding of the natural history of the syndrome or a great deal of clinical variability; DNA banking for future research may be desired by the patient and/or family as a way of contributing to research
DNA banking is relatively inexpensive (eg, 150 USD for indefinite banking), and several commercial banking options are available. An updated list of banking facilities can be found at GeneTests.org.7 In the unfortunate situation where the initial discussion about genetic testing occurs when the patient’s death is imminent, it may be prudent to consider drawing and refrigerating one or two tubes of whole blood in EDTA (lavender top) because this can afford the family a few days to discuss DNA banking, even after the patient has died.
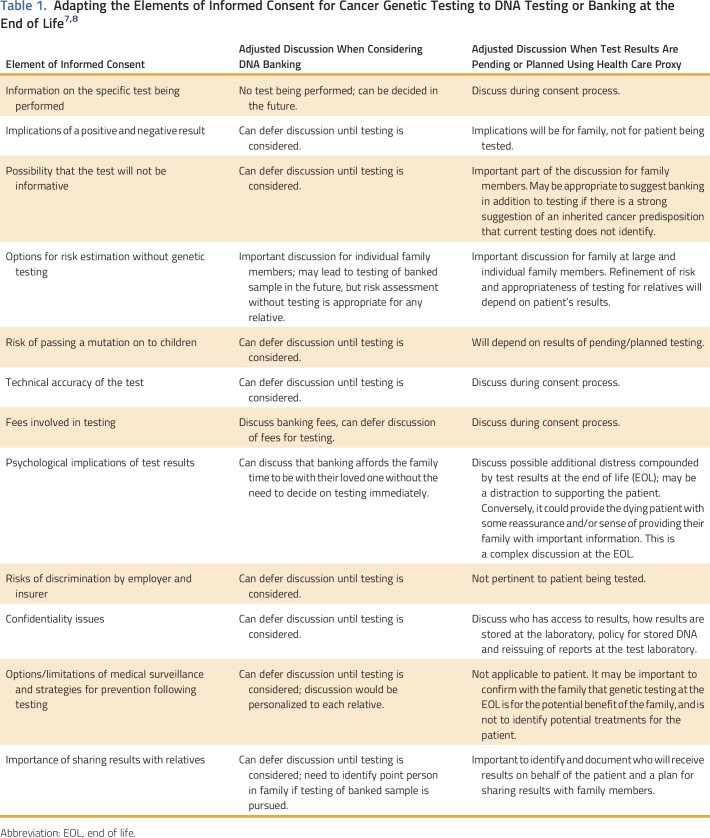
The informed consent process for genetic testing or DNA banking at the EOL has considerable overlap with the informed consent process that should occur with routine cancer genetic testing. In 2010, ASCO published an updated guideline summarizing the key elements of informed consent for cancer genetic testing,8 and again updated these elements in 2015 to adapt the recommendations to the increasing use of multigene panel testing and to tumor profiling that may include germline findings.9 We have adapted these recommendations further (Table 1) as a suggested framework for informed consent for testing/DNA banking at the EOL. For readers who are interested in further discussions of the ethical, legal, and social issues surrounding the disclosure of cancer genetic test results, duty to warn, and confidentiality, we suggest an article by Wouters et al10 and another by Cowley.11 It should also be noted that many of the issues regarding costs, availability of banking and testing, and designation of persons authorized to receive results will depend on local laws and health systems.
Table 1.
Ideally, patients with cancer who are interested and deemed appropriate for a genetics work-up will have the opportunity to pursue testing in a nonemergent setting. In some cases, however, consideration of genetic testing may not occur until near the EOL. If genetic testing is desired by patients and/or their families, key issues include identifying a family point person to receive any pending genetic test results (germline and/or tumor profiling), managing family dynamics and possible disagreements about testing, and helping families to decide between genetic testing and DNA banking. Family members can also be connected to cancer genetics specialty clinics for genetic counseling to review the family history and assist relatives with cancer screening recommendations if there are concerns about familial risk.
AUTHOR CONTRIBUTIONS
Conception and design: Eric J. Roeland, Lisa Madlensky
Collection and assembly of data: Alexandra D. Dullea, Chelsea H. Hagmann
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Addressing Hereditary Cancer Risk at the End of Life
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Eric J. Roeland
Honoraria: Pfizer
Consulting or Advisory Role: Eisai (Inst), Helsinn Healthcare (Inst), HERON
Speakers’ Bureau: Teva, Eisai, Depomed
Research Funding: XBiotech (Inst), AstraZeneca (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Eisai, Teva, Helsinn Healthcare
Alexandra D. Dullea
No relationship to disclose
Chelsea H. Hagmann
No relationship to disclose
Lisa Madlensky
Employment: Janssen (I)
REFERENCES
- 1.Lu KH, Wood ME, Daniels M, et al. : American Society of Clinical Oncology expert statement: Collection and use of a cancer family history for oncology providers. J Clin Oncol 32:833-840, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly MB, Pilarski R, Axilbund JE, et al. : Genetic/familial high-risk assessment: Breast and ovarian, version 1.2014. J Natl Compr Canc Netw 12:1326-1338, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Provenzale D, Gupta S, Ahnen DJ, et al. : Genetic/familial high-risk assessment: Colorectal version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14:1010-1030, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Schlich-Bakker KJ, ten Kroode HF, Wárlám-Rodenhuis CC, et al. : Barriers to participating in genetic counseling and BRCA testing during primary treatment for breast cancer. Genet Med 9:766-777, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Daniels MS, Burzawa JK, Brandt AC, et al. : A clinical perspective on genetic counseling and testing during end of life care for women with recurrent progressive ovarian cancer: Opportunities and challenges. Fam Cancer 10:193-197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quillin JM, Bodurtha JN, Siminoff LA, et al. : Physicians’ current practices and opportunities for DNA banking of dying patients with cancer. J Oncol Pract 7:183-187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GeneTests: Testing and banking services. https://www.genetests.org/tests/mtb.php?mtb=DNA%20Banking.
- 8.Robson ME, Storm CD, Weitzel J, et al. : American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol 28:893-901, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Robson ME, Bradbury AR, Arun B, et al. : American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol 33:3660-3667, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Wouters RH, Bijlsma RM, Ausems MG, et al. : Am I my family’s keeper? Disclosure dilemmas in next-generation sequencing. Hum Mutat 37:1257-1262, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Cowley L: What can we learn from patients’ ethical thinking about the right ‘not to know’ in genomics? Lessons from cancer genetic testing for genetic counselling. Bioethics 30:628-635, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]