Abstract
Purpose
Patients with double-hit lymphoma (DHL) rarely achieve long-term survival following disease relapse. Some patients with DHL undergo consolidative autologous stem-cell transplantation (autoSCT) to reduce the risk of relapse, although the benefit of this treatment strategy is unclear.
Methods
Patients with DHL who achieved first complete remission following completion of front-line therapy with either rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or intensive front-line therapy, and deemed fit for autoSCT, were included. A landmark analysis was performed, with time zero defined as 3 months after completion of front-line therapy. Patients who experienced relapse before or who were not followed until that time were excluded.
Results
Relapse-free survival (RFS) and overall survival (OS) rates at 3 years were 80% and 87%, respectively, for all patients (n = 159). Three-year RFS and OS rates did not differ significantly for autoSCT (n = 62) versus non-autoSCT patients (n = 97), but 3-year RFS was inferior in patients who received R-CHOP compared with intensive therapy (56% v 88%; P = .002). Three-year RFS and OS did not differ significantly for patients in the R-CHOP or intensive therapy cohorts when analyzed by receipt of autoSCT. The median OS following relapse was 8.6 months.
Conclusion
In the largest reported series, to our knowledge, of patients with DHL to achieve first complete remission, consolidative autoSCT was not associated with improved 3-year RFS or OS. In addition, patients treated with R-CHOP experienced inferior 3-year RFS compared with those who received intensive front-line therapy. When considered in conjunction with reports of patients with newly diagnosed DHL, which demonstrate lower rates of disease response to R-CHOP compared with intensive front-line therapy, our findings further support the use of intensive front-line therapy for this patient population.
INTRODUCTION
Double-hit lymphoma (DHL), defined as a B-cell non-Hodgkin lymphoma harboring rearrangement of MYC as well as BCL2 and/or BCL6,1 has been described as a high-risk variant of aggressive non-Hodgkin lymphoma in several retrospective series, with a reported median overall survival (OS) of < 2 years.2-7 Within the population of patients with DHL, a worse prognosis has been associated with multiple clinicopathologic features present at diagnosis, including B-cell lymphoma unclassifiable (BCLU) histologic classification,2 evidence of MYC-IG translocation,2,8,9 bone marrow involvement,2,4 elevated serum lactate dehydrogenase,4,6 and CNS involvement.4,6
With the recognition of the poor survival experienced by patients with DHL, chemotherapy intensification has been explored as one strategy for improving outcomes in this population. Although earlier single-institution reports did not reveal a benefit from intensified front-line chemotherapy,2,4 two large retrospective multicenter studies suggested prolongation of progression-free survival (PFS), but not OS, in patients with DHL treated with intensive front-line therapy compared with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).6,10
A second application of chemotherapy intensification for patients with DHL has been high-dose chemotherapy with autologous stem-cell transplantation (autoSCT) in patients achieving first complete remission (CR1). This treatment strategy may be particularly relevant for the population of patients with DHL, given the poor prognosis following relapse of this disease,5,6 and more specifically, the low likelihood of achieving long-term remission following salvage immunochemotherapy and autoSCT.11 Although receipt of autoSCT seemed to prolong PFS in patients with high-risk aggressive non-Hodgkin lymphoma achieving CR1,12 the benefit of autoSCT in CR1 could not be clearly determined in two retrospective series of patients with DHL,5,6 as a result of the small sample size of patients achieving CR1, as well as lack of analysis of patient-, disease-, and treatment-related factors that may be predictive of survival in this patient population.
Herein, we report outcomes for a large multicenter series of patients with DHL who achieved CR1, analyzing the impact of front-line therapy and autoSCT in CR1 on relapse and survival.
METHODS
Patients
DHL was defined as high-grade B-cell lymphoma with rearrangement of MYC/8q24 as well as BCL2/18q21 and/or BCL6/3q27 as assessed by fluorescence in situ hybridization (FISH) or conventional karyotype.1 Inclusion criteria included diagnosis between January 2006 and December 2015, age 18 to 72 years, an overall condition deemed fit for autoSCT by the local investigator, diffuse large B cell lymphoma (DLBCL) or BCLU histologic classifications and receipt of front-line treatment with either R-CHOP or intensive therapy, defined as either DA-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), R-hyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine), or R-CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate alternating with ifosfamide, etoposide, and cytarabine). Exclusion criteria included < 3 months in remission following completion of front-line therapy (equating to ≤ 7.5 months after diagnosis for patients receiving R-CHOP, DA-EPOCH-R, and R-CODOX-M/IVAC and ≤ 9.0 months after diagnosis for patients receiving R-hyperCVAD), the presence of HIV, receipt of allogeneic stem-cell transplantation, and a history of indolent lymphoma treated with cytotoxic chemotherapy. Cell of origin classification was defined per the Hans algorithm.13 When staging bone marrow aspiration and biopsy was not performed, patients with lymphomatous involvement of the peripheral blood by high-grade lymphoma were considered to have bone marrow involvement, whereas patients without abnormal [18F]fluorodeoxyglucose uptake in the bones were considered not to have bone marrow involvement by high-grade lymphoma.14 Cases were reviewed by hematopathologists at each academic medical center per routine clinical practice. Criteria and methods for performance of fluorescent in situ hybridization were per the policy of each center. Therapy was prescribed at the discretion of the treating physician. Data were censored in October 2016. This protocol was approved by the institutional review board of each participating center.
Statistical Analysis
As previously described,15 a landmark analysis was performed with time zero occurring at 3 months after completion of front-line therapy. Relapse-free survival (RFS) was defined as the interval from time zero to disease relapse or last follow-up in remission. OS was defined as the interval from time zero to death from any cause or last follow-up. Disease response by computed tomography with or without positron emission tomography was determined by the Revised Response Criteria for Malignant Lymphoma.16 Categorical data were analyzed by the χ2 test. RFS and OS curves were plotted using Kaplan-Meier estimates, and survival analysis was performed using the log-rank test. Univariable analysis was performed using Cox proportional-hazards regression. Statistical significance was defined as a two-tailed P value < .05. All statistical analyses were performed using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Patients
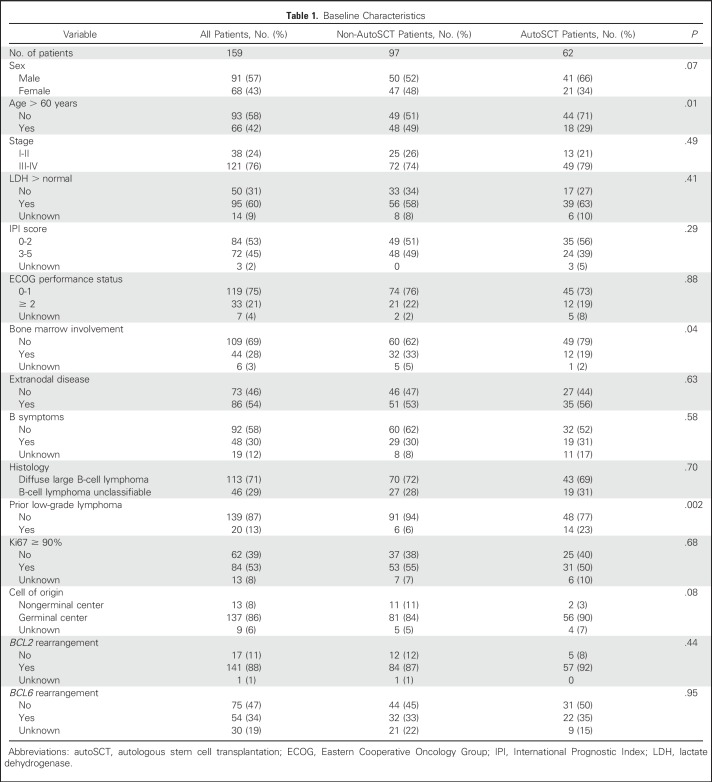
One hundred fifty-nine cases from 19 US academic medical centers were included in this analysis. Baseline clinicopathologic characteristics for all patients, as well as comparison by receipt of autoSCT in CR1, are listed in Table 1. The only statistically significant differences between the non-autoSCT (n = 97) and autoSCT (n = 62) patients were age > 60 years (49% v 29%; P = .01), bone marrow involvement (33% v 19%; P = .04), and prior indolent lymphoma (6% v 23%; P = .002). For those patients with prior indolent lymphoma (n = 20), management strategies for indolent lymphoma included no treatment (n = 17), rituximab monotherapy (n = 2), and radiation therapy (n = 1).
Table 1.
Baseline Characteristics
Outcomes
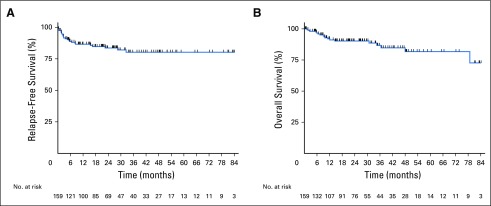
With a median follow-up of 26.5 months (range, 0.2 to 114.6 months) from time zero, the 3-year RFS and OS rates for all patients were 80% and 87%, respectively (Figs 1A and 1B). No significant differences in 3-year RFS and OS rates were seen between patients with de novo (n = 139) versus transformed indolent (n = 20) disease (78% v 94%, P = .18 and 86% v 93%, P = .54, respectively) nor International Prognostic Index score < 3 (n = 84) versus ≥ 3 (n = 71; 75% v 87%, P =.38 and 87% v 89%, P = .69, respectively). On the basis of gene rearrangement status, 3-year RFS and OS rates were 79% and 86% for patients whose tumors demonstrated BCL2 rearrangement, 77% and 80% for BCL6 rearrangement, and 70% and 73% for BCL2/BCL6 rearrangement (triple-hit lymphoma), respectively. For patients with lymphomas that were known to demonstrate MYC-IG translocation (n = 35), 3-year RFS and OS rates were 77% and 88%, respectively.
Fig 1.
(A) Relapse--free survival and (B) overall survival for all patients.
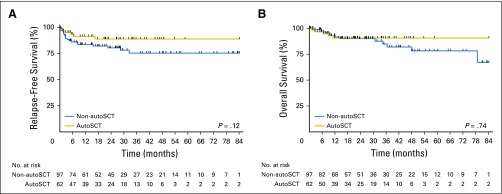
When analyzed by receipt of autoSCT in CR1, non-autoSCT and autoSCT patients experienced similar rates of 3-year RFS (75% v 89%; P = .12) and OS (85% v 91%; P = .74; Figs 2A and 2B). No significant differences in 3-year RFS and OS rates were observed when non-autoSCT (n = 91) and autoSCT (n = 48) patients with de novo disease were analyzed separately (74% v 88%, P = .15 and 84% v 91%, P = .68, respectively). Compared with patients in the autoSCT group, patients in the non-autoSCT group were less likely to be known to have received CNS prophylaxis with front-line therapy (57% v 75%; P = .02) and intensive front-line therapy (72% v 87%; P = .03).
Fig 2.
(A) Relapse-free survival and (B) overall survival for all patients by receipt of autologous stem cell transplantation (autoSCT).
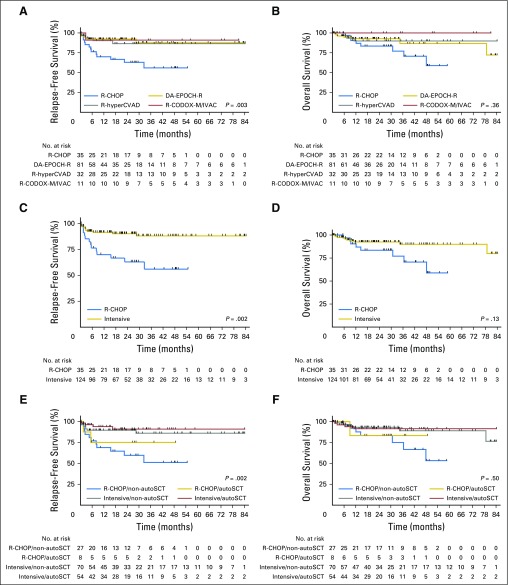
Additional analysis by front-line therapy received revealed that 3-year RFS rates differed significantly among patients treated with R-CHOP (n = 35), DA-EPOCH-R (n = 81), R-hyperCVAD (n = 32), and R-CODOX-M/IVAC (n =11; 56% v 88% v 87% v 91%, respectively; P = .003), but 3-year OS rates did not (77% v 87% v 90% v 100%, respectively; P = .36; Figs 3A and 3B). Given the similar 3-year RFS and OS for DA-EPOCH-R, R-hyperCVAD, and R-CODOX-M/IVAC patients (P = .90 and P = .57, respectively), these three regimens were combined into a single intensive front-line therapy cohort (n = 124), with a 3-year RFS rate of 88% (P = .002 compared with R-CHOP) and an OS rate of 90% (P = .13 compared with R-CHOP; Figs 3C and 3D). Patients in the R-CHOP group were less likely to be known to have received CNS prophylaxis with front-line therapy (28% v 72%; P = .001) and autoSCT in CR1 (23% v 44%; P = .03) compared with patients in the intensive group. Of note, comparison of baseline clinicopathologic characteristics listed in Table 1 between R-CHOP and intensive patients demonstrated a statistically significant difference only in age > 60 years (57% v 37%; P = .03).
Fig 3.
(A) Relapse-free survival and (B) overall survival by front-line regimen, (C) relapse-free survival and (D) overall survival by intensive front-line regimen versus rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and (E) relapse-free survival and (F) overall survival by front-line regimen and receipt of autologous stem-cell transplantation (autoSCT). DA-EPOCH-R, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; R-CODOX-M/IVAC, cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate alternating with ifosfamide, etoposide, and cytarabine; R-hyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine.
A final analysis of outcomes on the basis of both front-line therapy and receipt of autoSCT in CR1 was performed. For patients receiving R-CHOP front-line therapy without autoSCT in CR1 (R-CHOP/non-autoSCT, n = 27), 3-year RFS and OS rates were 51% and 75%; for patients receiving R-CHOP front-line therapy and autoSCT in CR1 (R-CHOP/autoSCT, n = 8), 3-year RFS and OS rates were 75% and 83%; for patients receiving intensive front-line therapy without SCT in CR1 (intensive/non-autoSCT, n = 70), 3-year RFS and OS rates were 86% and 89%, respectively; and for patients receiving intensive front-line therapy and autoSCT in CR1 (intensive/autoSCT, n = 54), 3-year RFS and OS rates were 91% and 92%, respectively. Intergroup comparison showed a statistically significant difference in 3-year RFS (P = .003), which was because of a significantly lower rate of 3-year RFS for R-CHOP/non-autoSCT compared with intensive/non-autoSCT (P = .003) and intensive/autoSCT (P = .001) patients, but similar 3-year OS (P = .50; Figs 3E and 3F).
None of the baseline clinicopathologic characteristics listed in Table 1 were significantly associated with 3-year RFS or OS in all patients by univariable analysis. In addition, univariable analysis of the baseline clinicopathologic features listed in Table 1 demonstrated that no factor was associated with either 3-year RFS or OS when analyzing patients receiving front-line R-CHOP and intensive front-line therapy separately. Receipt of autoSCT in CR1 was also not associated with 3-year RFS or OS for patients receiving front-line R-CHOP (P = .51 and P = .85, respectively) or front-line intensive therapy (P = .47 and P = .92, respectively) by univariable analysis.
Relapse and Death
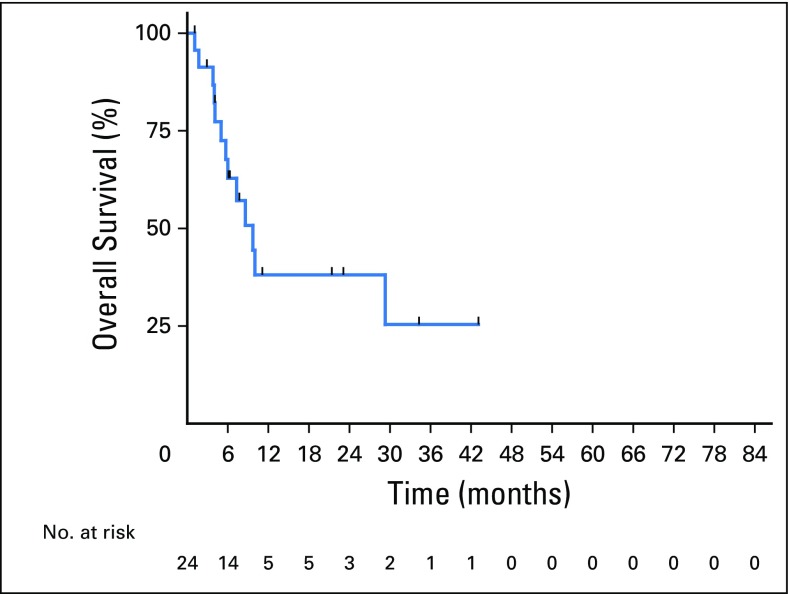
Twenty-five patients relapsed with a median OS of 8.6 months (range, 0 to 43.0 months) after relapse, with a 1-year postrelapse survival rate of 37% (Fig 4). Postrelapse survival did not differ by treatment with R-CHOP (n = 13) compared with intensive (n = 12) front-line therapy (10.0 v 6.0 months; P = .23) or receipt (n = 6) compared with no receipt (n = 19) of autoSCT in CR1 (9.7 v 3.8 months; P = .12). Only one patient with prior indolent lymphoma experienced disease relapse, which was of high-grade histologic classification. CNS relapse was diagnosed in five of these patients, three of whom received CNS prophylaxis with front-line therapy, with one each receiving intrathecal only, intravenous only, and combination intrathecal/intravenous therapy. Nineteen patients died, with five deaths during remission (one each from amyotrophic lateral sclerosis, lung cancer, and unknown causes in non-autoSCT patients and from infection and acute myeloid leukemia in autoSCT patients.
Fig 4.
Postrelapse overall survival.
DISCUSSION
DHL is an aggressive form of B-cell non-Hodgkin lymphoma that is associated with a poor prognosis. However, prior studies have suggested that outcome may be improved for DHL patients receiving intensive front-line immunochemotherapy. A series of 129 patients with DHL published by the MD Anderson Cancer Center reported a CR1 rate of 40% in patients treated with front-line R-CHOP, which was significantly lower than 68% for DA-EPOCH-R and R-hyperCVAD, and patients treated with DA-EPOCH-R experienced a significantly improved event-free survival rate compared with those who received R-CHOP.5 A US multicenter study of 311 patients with DHL reported a significantly lower CR1 rate for patients treated with R-CHOP compared with DA-EPOCH-R, and a shorter median PFS for patients receiving R-CHOP compared with intensive front-line therapy (7.8 v 21.6 months).6
What is apparent from these two studies is the difficulty in salvaging patients with DHL if they are experiencing relapse, with a 3-year OS rate of 7%5 and a median OS of 176 months in patients receiving therapy at relapse. Although it is possible for patients with DHL to achieve long-term survival if undergoing autoSCT after disease relapse, autoSCT is unlikely to provide durable disease control in this setting.11,17
Thus, despite the potential toxicity associated with autoSCT, the dismal outcome of relapsed DHL warrants consideration of consolidative autoSCT for patients achieving CR1 as a strategy to reduce the likelihood of disease relapse. Subgroup analyses of DHL patients achieving CR1 within the two aforementioned studies have demonstrated no significant difference in PFS or OS observed in 23 patients receiving SCT and 48 patients not receiving SCT in the MD Anderson Cancer Center series5 and 39 and 112 patients, respectively, in the US multicenter study.6 However, conclusions drawn about the benefit of SCT in CR1 within these studies are limited by multiple factors, including small cohorts of patients undergoing SCT, lack of analysis of baseline clinicopathologic characteristics and therapy received within the SCT and non-SCT cohorts, as well as inclusion of patients receiving allogenic SCT and cytotoxic therapy for prior indolent lymphoma.
Through a landmark analysis of 159 patients with DHL achieving CR1, we have demonstrated a 3-year RFS rate of 80% and a 3-year OS rate of 87% for all patients, which did not differ significantly on the basis of receipt of autoSCT in CR1. Comparison of baseline characteristics between autoSCT and non-autoSCT patients suggested that autoSCT patients were more likely to be ≤ 60 years of age, lack bone marrow involvement, and have a history of transformed indolent lymphoma; however, none of these characteristics were prognostic of 3-year RFS or 3-year OS on univariable analysis. Therefore, the apparent lack of benefit of autoSCT in CR1 is not confounded by a relevant difference in prognostic baseline clinicopathologic characteristics between patients receiving and not receiving autoSCT.
Interestingly, within this cohort, patients receiving R-CHOP front-line therapy experienced inferior rates of 3-year RFS compared with those receiving intensive front-line therapy. No significant differences in 3-year RFS and OS were noted in the R-CHOP or intensive therapy patient cohorts when analyzed by baseline clinicopathologic characteristics or receipt of autoSCT in CR1. Although patients treated with R-CHOP were more frequently older than 60 years compared with those receiving intensive induction, age older than 60 years was not associated with 3-year RFS or OS on univariable analysis of all patients. In addition, although patients treated with R-CHOP were less likely to receive CNS prophylaxis compared with those receiving intensive induction, the fact that CNS prophylaxis was received by three of five patients experiencing CNS relapse suggests that this statistical difference is of limited clinical significance. One explanation for improved outcomes for patients treated with intensive front-line therapy may be that although all patients in our cohort demonstrated radiographic complete response, those treated with intensive regimens may have been more likely to achieve a deeper disease response that is not distinguishable by standard postchemotherapy radiographic assessment. Techniques for detection of minimal residual disease following front-line therapy, such as circulating tumor DNA, should be investigated in future studies of patients with DHL.
Also of interest were the survival outcomes for patients in the R-CHOP/autoSCT cohort, which did not differ significantly from those for patients receiving intensive therapy. This former group of patients represented only 5% of all patients included in this analysis; thus the small sample size of this cohort limits the generalizability of these results. Also, given the aforementioned data, which demonstrate an inferior rate of CR1 achievement in patients with DHL treated with R-CHOP compared with intensive therapy, it cannot be safely assumed that patients with DHL treated with R-CHOP will necessarily achieve CR1 and subsequently be able to receive consolidative autoSCT. Nevertheless, if encountering a patient with DHL in clinical practice who achieved CR1 following R-CHOP, proceeding with consolidative autoSCT may be justifiable as the result of the relatively poor survival outcomes for R-CHOP/non-autoSCT patients demonstrated in this series.
The strengths of our study include a large sample size of patients with DHL collected from multiple academic medical centers (which may help to minimize biases regarding autoSCT referral patterns at individual centers), well-matched clinicopathologic characteristics between autoSCT and non-autoSCT patients despite the nonrandomized nature of this study, and the use of a landmark analysis to account for variation in duration of front-line therapy.
In addition to the retrospective nature of this study, we recognize potential limitations of our study. These include a lack of uniform testing for MYC, BCL2, and BCL6 rearrangements by FISH for all high-grade lymphoma cases at all participating centers. However, the lack of previously reported studies of unselected patients with DHL, as well as the fact that baseline clinicopathologic characteristics in our cohort are similar to those of other large retrospective series of patients with DHL identified by selective FISH testing,5,6 suggests that outcomes in our series can be extrapolated to the population of patients with DHL identified in routine clinical practice. Another limitation is the lack of central review of FISH results for MYC, BCL2, and BCL6 rearrangements, as was the case for prior multicenter studies of patients with MYC-rearranged lymphomas.6,18 Although we are not aware of studies reporting the interobserver reproducibility of FISH results for MYC, BCL2, and/or BCL6 probes, prior reports do suggest high interobserver reproducibility of FISH results for relevant genes in solid tumors.19,20
Finally, our series does not include information regarding prognostic pathologic data for all patients, such as MYC translocation partner2,8,9 or concurrent MYC and BCL2 expression by immunohistochemistry, the presence of which is associated with a poorer prognosis in patients with newly diagnosed DLBCL treated with R-CHOP.21,22 However, it is not clear that these high-risk pathologic features carry the same prognosis for patients with DHL who have achieved CR1, as evidenced by the relatively high rate of 3-year RFS and OS rates (77% and 88%, respectively) for patients in our cohort known to harbor the MYC-IG translocation. These pathologic features should be analyzed in subsequent studies of patients with DHL achieving CR1.
In conclusion, survival of patients with DHL achieving CR1 is not significantly prolonged by receipt of autoSCT, regardless of front-line therapy received. In addition, patients receiving R-CHOP experienced inferior 3-year RFS compared with those receiving intensive front-line therapy. There are no prospective randomized studies analyzing survival outcomes in patients with DHL on the basis of receipt of autoSCT in CR1, and although CALGB/Alliance 50303 was recently reported to show no significant difference in survival for patients with DLBCL receiving R-CHOP compared with DA-EPOCH-R, this study will be unable to determine a survival difference on the basis of front-line therapy received for patients with MYC-rearranged DLBCL.23 Therefore, we believe that our analysis provides the best available evidence to address the benefit of autoSCT in CR1 in patients with DHL, as well as the durability of CR1 on the basis of front-line therapy received. It also supports the management strategy of intensive front-line therapy without consolidative autoSCT in CR1 for fit patients with DHL.
Footnotes
Presented in part at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, December 3-6, 2016.
Listen to the podcast by Dr Dunleavy at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Daniel J. Landsburg
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Daniel J. Landsburg, Ryan D. Cassaday
Manuscript writing: Daniel J. Landsburg
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcomes of Patients With Double-Hit Lymphoma Who Achieve First Complete Remission
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Daniel J. Landsburg
Consulting or Advisory Role: Curis
Research Funding: Curis (Inst)
Marissa K. Falkiewicz
No relationship to disclose
Joseph Maly
No relationship to disclose
Kristie A. Blum
No relationship to disclose
Christina Howlett
Honoraria: Mallinckrodt
Speakers’ Bureau: Teva
Tatyana Feldman
Consulting or Advisory Role: Seattle Genetics, Celgene
Speakers’ Bureau: Seattle Genetics, Celgene, AbbVie, Pharmacyclics, Janssen
Research Funding: Eisai (Inst), Seattle Genetics (Inst), Pfizer (Inst), Millennium (Inst), cen rx (Inst), Cell Medica (Inst), Bristol-Myers Squibb (Inst)
Anthony R. Mato
Consulting or Advisory Role: AbbVie, Regeneron, Gilead Sciences, Pharmacyclics, Celgene
Speakers’ Bureau: Celgene
Research Funding: TG Therapeutics (Inst), AbbVie (Inst), Acerta Pharma (Inst), Regeneron (Inst), DTRM (Inst), Portola Pharmaceuticals (Inst), Pharmacyclics (Inst)
Brian T. Hill
Honoraria: Pharmacyclics, Gilead Sciences, Genentech
Consulting or Advisory Role: Seattle Genetics, Novartis, Genentech
Research Funding: AbbVie (Inst), Karyopharm Therapeutics (Inst), AbbVie (Inst)
Shaoying Li
No relationship to disclose
L. Jeffrey Medeiros
No relationship to disclose
Pallawi Torka
No relationship to disclose
Francisco Hernandez-Ilizaliturri
Speakers’ Bureau: Seattle Genetics
Nishitha M. Reddy
Consulting or Advisory Role: Celgene, Infinity Pharmaceuticals, AbbVie, Gilead Sciences
Speakers’ Bureau: Gilead
Arun Singavi
No relationship to disclose
Timothy S. Fenske
Honoraria: Celgene, Sanofi, Seattle Genetics, Pharmacyclics
Consulting or Advisory Role: Celgene, Seattle Genetics, Pharmacyclics, Sanofi
Speakers’ Bureau: Celgene, Sanofi
Julio C. Chavez
Consulting or Advisory Role: Incyte
Speakers’ Bureau: Janssen, AbbVie
Jason B. Kaplan
Research Funding: Janssen, Seattle Genetics
Travel, Accommodations, Expenses: Curis
Amir Behdad
No relationship to disclose
Adam M. Petrich
Employment: AbbVie
Stock or Other Ownership: AbbVie, Allergan
Martin A. Bast
No relationship to disclose
Julie M. Vose
Consulting or Advisory Role: Bio Connections
Research Funding: Celgene (Inst), Genentech (Inst), Incyte (Inst), Janssen Biotech (Inst), Acerta Pharma (Inst), Kite Pharma (Inst), Seattle Genetics (Inst), Novartis (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Allos Therapeutics (Inst)
Adam J. Olszewski
Research Funding: TG Therapeutics (Inst), Genentech (Inst), Incyte (Inst)
Cristiana Costa
No relationship to disclose
Frederick Lansigan
Consulting or Advisory Role: Celgene
Research Funding: Spectrum Pharmaceuticals (Inst)
James N. Gerson
No relationship to disclose
Stefan K. Barta
Consulting or Advisory Role: Janssen Oncology
Speakers’ Bureau: Janssen, Celgene
Research Funding: Seattle Genetics, Merck, Celgene
Oscar Calzada
Research Funding: Seattle Genetics
Travel, Accommodations, Expenses: Seattle Genetics
Jonathon B. Cohen
Consulting or Advisory Role: Pharmacyclics, Celgene, Novartis, Infinity Pharmaceuticals, AbbVie, Genentech
Research Funding: Bristol-Myers Squibb, Janssen, Novartis, Takeda
Jennifer K. Lue
No relationship to disclose
Jennifer E. Amengual
Research Funding: Acetylon Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst)
Xavier Rivera
Employment: Tenet Healthcare (I)
Daniel O. Persky
Consulting or Advisory Role: Verastem, Genentech, MorphoSys
Speakers’ Bureau: Gilead Sciences
Research Funding: Merck
David J. Peace
Stock or Other Ownership: Baxter, Alexion Pharmaceuticals, Celgene, Express Scripts, CVS
Patents, Royalties, Other Intellectual Property: US patent 8,557,777
Sunita Nathan
Consulting or Advisory Role: AstraZeneca (I), Janssen (I), Medtronic (I), Merit Medical Systems (I)
Ryan D. Cassaday
Consulting or Advisory Role: Pfizer, Amgen
Research Funding: Seattle Genetics (Inst), Gilead Sciences (Inst), Merck (Inst), Incyte (Inst), Pfizer (Inst)
REFERENCES
- 1.Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117:2319–2331. doi: 10.1182/blood-2010-09-297879. [DOI] [PubMed] [Google Scholar]
- 2.Johnson NA, Savage KJ, Ludkovski O, et al. Lymphomas with concurrent BCL2 and MYC translocations: The critical factors associated with survival. Blood. 2009;114:2273–2279. doi: 10.1182/blood-2009-03-212191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Desai P, Lin P, et al. MYC/BCL6 double-hit lymphoma (DHL): A tumour associated with an aggressive clinical course and poor prognosis. Histopathology. 2016;68:1090–1098. doi: 10.1111/his.12884. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Lin P, Fayad LE, et al. B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): An aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome. Mod Pathol. 2012;25:145–156. doi: 10.1038/modpathol.2011.147. [DOI] [PubMed] [Google Scholar]
- 5.Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: The MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 6.Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood. 2014;124:2354–2361. doi: 10.1182/blood-2014-05-578963. [DOI] [PubMed] [Google Scholar]
- 7.Wang W, Hu S, Lu X, et al. Triple-hit B-cell lymphoma with MYC, BCL2, and BCL6 translocations/rearrangements: Clinicopathologic features of 11 cases. Am J Surg Pathol. 2015;39:1132–1139. doi: 10.1097/PAS.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen MO, Gang AO, Poulsen TS, et al. Double-hit BCL2/MYC translocations in a consecutive cohort of patients with large B-cell lymphoma: A single centre’s experience. Eur J Haematol. 2012;89:63–71. doi: 10.1111/j.1600-0609.2012.01787.x. [DOI] [PubMed] [Google Scholar]
- 9.Copie-Bergman C, Cuillière-Dartigues P, Baia M, et al. MYC-IG rearrangements are negative predictors of survival in DLBCL patients treated with immunochemotherapy: A GELA/LYSA study. Blood. 2015;126:2466–2474. doi: 10.1182/blood-2015-05-647602. [DOI] [PubMed] [Google Scholar]
- 10.Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: A systematic review and meta-analysis. Br J Haematol. 2015;170:504–514. doi: 10.1111/bjh.13463. [DOI] [PubMed] [Google Scholar]
- 11.Cuccuini W, Briere J, Mounier N, et al. MYC+ diffuse large B-cell lymphoma is not salvaged by classical R-ICE or R-DHAP followed by BEAM plus autologous stem cell transplantation. Blood. 2012;119:4619–4624. doi: 10.1182/blood-2012-01-406033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369:1681–1690. doi: 10.1056/NEJMoa1301077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado J, Pereira A, Villamor N, et al. Survival analysis in hematologic malignancies: Recommendations for clinicians. Haematologica. 2014;99:1410–1420. doi: 10.3324/haematol.2013.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 17. doi: 10.1200/JCO.2016.68.2740. Herrera AF, Mei M, Low L, et al: Relapsed or refractory double-expressor and double-hit lymphomas have inferior progression-free survival after autologous stem-cell transplantation. J Clin Oncol 35:24-31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landsburg DJ, Falkiewicz MK, Petrich AM, et al. Sole rearrangement but not amplification of MYC is associated with a poor prognosis in patients with diffuse large B cell lymphoma and B cell lymphoma unclassifiable. Br J Haematol. 2016;175:631–640. doi: 10.1111/bjh.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brankley SM, Adams EJ, Christensen MR, et al. A study of the reproducibility of a fluorescence in situ hybridization bladder cancer detection assay. Anal Quant Cytol Histol. 2008;30:145–151. [PubMed] [Google Scholar]
- 20.Zhang G, Lanigan CP, Goldblum JR, et al. Automated bright-field dual-color in situ hybridization for MDM2: Interobserver reproducibility and correlation with fluorescence in situ hybridization in a series of soft tissue consults. Arch Pathol Lab Med. 2016;140:1111–1115. doi: 10.5858/arpa.2015-0249-OA. [DOI] [PubMed] [Google Scholar]
- 21.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 23.Wilson WH, Sin-Ho J, Pitcher BN, et al. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood. 2016;128:469. [Google Scholar]