Abstract
Adult T-cell lymphoma/leukemia (ATL) is a rare T-cell lymphoproliferative neoplasm caused by human T-lymphotrophic virus 1. In its more common, aggressive forms, ATL carries one of the poorest prognoses of the non-Hodgkin lymphomas. The disease has clinical subtypes (ie, acute, lymphoma, chronic, and smoldering forms) defined by the presenting features, and therefore, the clinical course can vary. For the smoldering and lower-risk chronic forms, combinations involving antiviral therapies have shown some success. However, in many patients, the more indolent forms will evolve into the more aggressive subtypes. In the more aggressive acute, lymphoma, and higher-risk chronic forms, the literature supports initial treatment with combination chemotherapy followed by allogeneic transplantation as a potentially curative approach. Recently, mogamulizumab and lenalidomide have shown promise in the treatment of ATL. With better understanding of the molecular drivers of this disease, we hope that the therapeutic landscape will continue to expand.
INTRODUCTION
Adult T-cell lymphoma/leukemia (ATL) is a rare lymphoproliferative neoplasm of mature CD4+ CD25+ T cells caused by infection with the retrovirus human T-lymphotropic virus type 1 (HTLV-1). The aggressive subtypes of ATL (ie, acute, lymphoma, and poor-risk chronic) carry some of the poorest prognoses of any of the non-Hodgkin lymphomas. In a large retrospective analysis, patients with ATL had 5-year failure-free and overall survival (OS) of only 12% and 14%, respectively.1 However, the clinical course can be quite varied with the chronic and smoldering variants of this disease.
EPIDEMIOLOGY AND PATHOGENESIS
In the United States, the incidence of ATL is approximately 0.05 per 100,000. However, in regions where HTLV-1 is endemic (eg, regions of Japan), the incidence has been reported to be as high as 27 per 100,000.2 Regions with the highest incidence of HTLV-1 include the southern and northern islands of Japan, the Caribbean, Central and South America, intertropical Africa, Romania, and northern Iran. The International Peripheral T-Cell Lymphoma Project has reported that ATL constitutes 25% of cases of T-cell lymphoma in Japan compared with 1% to 2% in Europe and North America.1,3-5 The mean age of patients with ATL is 62 years, without a sex predominance.
In the United States, approximately 0.4% of volunteer blood donors are infected with HTLV-1 or -2,6 which are retroviruses with 60% nucleotide similarity.7 HTLV-1 has infected approximately 10% to 15% of the population in many parts of Central and South America, southern Japan, Africa, and the Middle East.8,9 Unlike HTLV-1, HTLV-2 is not clearly associated with disease.10
The HTLV-1 genome includes Gag, Pol, and Env genes, as well as several regulatory genes, including those encoding the transcriptional transactivator protein Tax and the helix-basic-loop zipper protein HBZ.11 Tax is critical for immortalization of T cells but is highly immunogenic, and thus, its expression is repressed when ATL is clinically apparent.11 In cases of ATL, the HTLV-1 provirus has acquired mutations that prevent expression of viral proteins.12 Alternatively, the promoter regulating expression of Tax and viral proteins may be deleted or hypermethylated.13,14 In contrast, HBZ has weak tumor initiator activity; it is thought to function primarily in tumor maintenance, and it is continuously expressed in all ATL cases.15 The number of infected cells within an individual changes as a result of virus replication, release, and spread via cell-to-cell transmission16; infected cell division and resistance to apoptosis17; and cytotoxic T-lymphocyte (CTL) clearance of cells expressing viral proteins.11 Thus, therapies directed exclusively at steps in the virus replication cycle may have limited effects on the total number of infected cells. ATL patient cases carry a single provirus integrated almost randomly, but a majority of cases represent a clonal outgrowth of cells with a single integration site.18 The histone deacetylase inhibitor valproate activates Tax and Gag expression, enhances CTL activity, and results in reduced proviral load.19,20 Other recent data suggest that HTLV-1–specific CTLs can be induced in patients with ATL and that these CTLs can contribute to treatment and inhibit relapse. This provides important evidence supporting the concept that immunotherapy can be effective in this disorder.
Among approximately 10 to 20 million HTLV-1 carriers, the lifetime risk of developing ATL is approximately 2.5% to 4%, with a mean latency of more than 50 years.4,21 HTLV-1 is transmitted through breastfeeding, blood products, and unprotected sexual intercourse. The overwhelming majority of ATL cases occur in patients infected during the early years of life.4 Of note, HTLV-1 has also been associated with HTLV-1–associated myelopathy-tropical spastic paraparesis, which is a chronic inflammatory disease affecting the CNS. Patients present with progressive spastic paraparesis, lower-limb sensory disturbance, and bladder or bowel dysfunction.22
PATHOLOGY
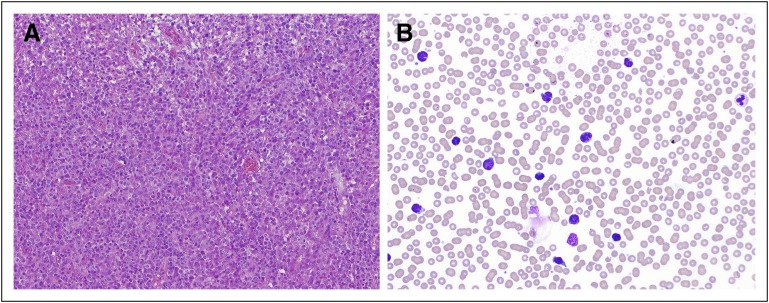
First described in 1977,23 the malignant cells carry a flower-like or clover-shaped appearance characterized by condensed nuclear chromatin and inconspicuous nucleoli. At times, these can be difficult to distinguish from Sezary cells (Fig 1). At least 5% of circulating abnormal T lymphocytes are required to diagnose ATL in patients without histologically proven tumor lesions.24 These cells express the surface T-cell lymphocytic markers CD2, CD4, CD5, CD45RO, CD29, and T-cell receptor (TCR) αβ and are usually negative for CD7, CD8, and CD26 and show reduced CD3 expression. The lymphocytic activation markers HLA-DP, DQ, DR, and interleukin-2Rα (CD25) are always present, whereas terminal deoxynucleotidyl transferase is typically absent.25 Rare immunophenotypic variants (CD4-negative, CD8-positive, and double-positive or double-negative variants) have been reported.26-28 The minimal flow cytometric analysis should include CD3, CD4, CD7, CD8, and CD25. Clonal rearrangements of the TCR genes are typically present.29-31 In addition, ATL can be associated with several histologic subtypes, including diffuse, poorly differentiated small-cell lymphoma; mixed large- and small-cell lymphoma; and large-cell immunoblastic lymphoma.32
Fig 1.
(A) Lymph node biopsy and (B) peripheral smear in adult T-cell lymphoma/leukemia (ATL). The neoplastic cells in ATL characteristically have a flower-like or clover-shaped appearance. By flow cytometry, they express CD2, CD4, CD5, CD45RO, CD29, and T-cell receptor αβ and do not express CD7, CD8, or CD26. CD3 expression is often reduced. Photographs courtesy of Ahmet Dogan.
The genomic landscape of ATL is being elucidated, with a vast majority of ATL patient cases carrying mutations in the TCR/nuclear factor kappa B activation pathway. Sequence analysis of ATL cells demonstrates a high rate of somatic nonsynonomous mutations, significantly higher than that in most other hematopoietic malignancies.12 The somatic mutations occur frequently in proteins that form the Tax interactome, thus providing a mechanism to substitute for Tax, which is repressed after tumor initiation. A high rate of mutations was found in genes encoding mediators of the TCR pathway and subsequent nuclear factor kappa B activation activation, analogous to that described in the B-cell receptor pathway in B-cell lymphomas. Thus, therapies targeting the TCR pathway have a role in ATL treatment. Also detected were frequent deletions, mutations, or hypermethylation of genes encoding components of the class I major histocompatibility complex, death receptors, and proteins involved in cell adhesion or immune checkpoints.12 In particular, one subgroup contains mutations in IRF4, which is implicated in the mechanism of action of lenalidomide.12 Whole-genome sequencing efforts have also demonstrated that ATL exhibits both gain-of-function and loss-of-function mutations in RHOA.33
CLINICAL FEATURES
The disease is typically subdivided into four clinical presentations: smoldering (generally indolent), chronic (variable course), lymphoma, and acute (both aggressive), with the poorest prognosis seen among those with lymphoma or acute as well as poor-risk or relapsed chronic subtypes.24 The acute variant of ATL represents 60% of cases and is characterized by a leukemic presentation with or without lymphadenopathy and/or visceral disease. An additional 20% of patients present with the lymphoma variant, which is characterized by lymphadenopathy and an absence of leukemic features (ie, < 1% of leukemic cells in the peripheral blood). A majority of patients present with hepatosplenomegaly (50% of cases), lymphadenopathy (almost all cases), elevated lactate dehydrogenase, hypercalcemia (50% of cases), and visceral and cutaneous lesions. The bone marrow is involved in approximately 35% of cases.32 Additional extranodal sites of disease include the lung, liver, skin, GI tract, and CNS, in which the disease can manifest itself as cord myelopathy or spastic paraparesis.
In contrast to the acute and lymphoma forms, the smoldering form of ATL typically presents with cutaneous or pulmonary lesions without visceral or bone marrow involvement and with a low level of peripheral blood (< 5% of lymphocytes) involvement. Chronic ATL presents with leukocytosis, lymphadenopathy, and organomegaly without elevated lactate dehydrogenase or visceral involvement.
Overall, prognosis in ATL remains poor. For those with the acute or lymphoma subtype, the median survival is less than 1 year. Although smoldering and chronic ATLs are characterized by indolent courses initially, the prognosis remains guarded, with 5-year survival of 40% and 50%, respectively, and approximately half of these patients experience progression to acute ATL.34
Opportunistic infections are common in patients with ATL, even indolent forms.35 Pneumocystis carinii infections, strongyloides, and cryptococcal meningitis are common, as are bacterial and other fungal infections.
TREATMENT
Despite limited long-term efficacy, cytotoxic combination chemotherapy remains the mainstay of therapy for ATL. Prospective clinical trials from Japan using regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or an aggressive combination chemotherapy, VCAP-AMP-VECP (vincristine, cyclophosphamide, doxorubicin and prednisone; doxorubicin, ranimustine, and prednisone; and vindesine, etoposide, carboplatin, and prednisone), for patients with the chronic, lymphoma, and acute subtypes showed median OS rates from 8.3 to 10.6 months.36,37 These initial therapies provide response rates of approximately 70%, with a complete response rate of approximately 30%.37 In a phase III trial comparing VCAP-AMP-VECP with CHOP-14, CHOP-14 carried a 25% complete response rate and a 13% 3-year OS, whereas VCAP-AMP-VECP was statistically superior, with a 40% complete response rate and 24% 3-year OS.38 Both arms in these studies included CNS prophylaxis with intrathecal therapy. Dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) has also been studied in ATL. Using dose-adjusted EPOCH with maintenance interferon and zidovudine, a study of 19 patients showed an overall response rate of 58%, with two complete remissions (CRs); however, the maintenance therapy did not seem to add to the efficacy.39 A more recent study of 18 patients treated with dose-adjusted EPOCH in combination with raltegravir and bortezomib showed an overall response rate of 67%, with three CRs and eight partial remissions.40
Unfortunately, despite aggressive first-line approaches, nearly 90% of patients experience relapse (often within months of completing therapy), and currently, consolidation of first remission with allogeneic stem-cell transplantation is strongly considered.32 In the largest retrospective series regarding the role of allogeneic transplantation in ATL, 586 Japanese transplant recipients had a 3-year OS of 36%, with similar outcomes between myeloablative approaches and reduced-intensity transplantation approaches.41 Retrospective series have shown that donor HTLV-I seropositivity adversely affected disease-associated mortality, and given the typical acquisition of the virus from the mother, it is often difficult to find matched siblings unaffected with the virus.42 Autologous stem-cell transplantation does not seem to provide benefit in managing ATL.43,44 For patients with relapsed or refractory ATL, the prognosis is even poorer.
In terms of alternate approaches, the role of antiviral therapy in ATL remains controversial. Although smaller initial studies regarding the use of antiviral therapy in combination with interferon were quite promising, studies in the United States have shown less promising results.45-47 Zidovudine remains the most studied antiviral used in this setting; however, other studies have incorporated raltegrevir and lamivudine.39,40 A meta-analysis of 254 patients with ATL demonstrated that for patients with acute, chronic, or smoldering ATL, there seems to be a benefit to first-line zidovudine including interferon, whereas patients with the lymphoma variant did not benefit.5 Patients with chronic or smoldering ATL treated with first-line antiviral therapy had a 100% 5-year OS. Patients received antiviral therapy in combination with either chemotherapy or an interferon. In this meta-analysis, patients with acute ATL treated with chemotherapy with or without antiviral therapy in the first-line setting had a 5-year OS of 28% compared with 10%, respectively. Additionally, maintenance antiviral therapy in patients treated with first-line chemotherapy also conferred an improved OS.5 However, when evaluated prospectively, the addition of antiviral therapy to initial therapy or as maintenance after combination chemotherapy has not yielded improved results.39,40
In 2015, mogamulizimab, an anti-CCR4 antibody, was approved in Japan for relapsed or refractory ATL. In a multicenter phase II study of 28 patients with relapsed or refractory ATL, treatment with mogamulizumab resulted in a 50% response rate and median OS of 13.7 months.48 A randomized phase II study of modified VCAP-AMP-VECP with or without mogmulizumab showed that the combination had a superior CR rate of 52%, compared with 33% with VCAP-AMP-VECP alone, at the cost of some increases in toxicity.49,50 A subsequent international study (in North America, Europe, the Caribbean, and South America) randomized phase II study of mogamulizumab in relapsed acute, lymphoma, or chronic ATL versus investigator’s choice of pralatrexate, gemcitabine plus oxaliplatin, or DHAP (dexamethasone, cisplatin, and cytarabine) showed an overall response rate of 28% with mogamulizumab compared with only 8% in the investigator’s choice arm.51
In addition to poor prognosis, patients with ATL have either been underrepresented or excluded from many trials of novel agents recently tested in T-cell lymphoma. Those with ATL were excluded from the registration studies of romidepsin, belinostat, and alisertib. In the phase II Southwest Oncology Group trial of alisertib, four patients with ATL were treated, and one of them responded.52 In the development of pralatrexate, there were a total of three patients included on the pivotal phase I and II trials. There has otherwise been only anecdotal use reported with brentuximab vedotin and romidepsin.53,54 Most recently, a phase II study from Japan of single-agent lenalidomide showed a 42% overall response rate in relapsed or refractory ATL. Interestingly, although the median progression-free survival with this approach was 4 months, the median OS was 20 months.55 Checkpoint inhibitors such as nivolumab are currently being studied in ATL as well in the United States (ClinicalTrials.gov identifier: NCT02631746).
OUR APPROACH
As reviewed in this article, our current results in treating ATL have been poor. We therefore strongly encourage participation in clinical trials investigating new approaches to this disease. In the absence of a clinical trial, for those with acute, lymphoma, or poor-risk chronic subtype, our preference is to use EPOCH-based regimens. Our results with EPOCH mirror those seen with VCAP-AMP-VECP in terms of response (unpublished data; Memorial Sloan Kettering Cancer Center). We try to consolidate remissions (when achieved) with allogeneic stem-cell transplantation and therefore refer for transplantation consults early in the treatment course. Because our goal of initial therapy is remission adequate to allow allogeneic stem-cell transplantation, we believe achieving a CR before a full course of EPOCH is adequate. For example, those in CR after four cycles who have ready donors may proceed to consolidation before completing a full six cycles. When the only available donors are also HTLV-1 positive, we will still proceed with transplantation, because despite the poorer results, this remains the only potentially curative strategy for these patients. In our experience, nearly 30% of our patients with ATL will have evidence of CNS involvement at diagnosis or relapse.56 Although it is not clear whether CNS prophylaxis with intrathecal therapy has reduced this rate, we routinely include it as part of initial therapy when administered with curative intent. For patients with relapsed or refractory disease, we recommend a clinical trial whenever possible. When a clinical trial is not available or patients decline, we use one of the agents we have described here with at least anecdotal evidence of response (mogamulizumab is not currently available outside of Japan) or extrapolate from other drugs or regimens shown to be active for relapsed peripheral T-cell lymphomas.
In conclusion, ATL remains a rare T-cell lymphoma caused by infection with HTLV-1. Despite efforts to intensify therapy with multiagent chemotherapeutic approaches, the prognosis remains poor, especially for those with the acute, lymphoma, or poor-risk chronic subtype of the disease. Recent studies have shown promising activity of nonchemotherapeutic agents such as mogamulizumab and lenalidomide, and it is hoped that these could form important parts of more-effective combination regimens.
ACKNOWLEDGMENT
Supported by the Paul Calabresi K12 Career Development Program (K12CA167540; N.M.-S.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adult T-Cell Leukemia/Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Neha Mehta-Shah
Research Funding: Bristol-Myers Squibb, Celgene, Verastem
Lee Ratner
No relationship to disclose
Steven M. Horwitz
Consulting or Advisory Role: Celgene, Millennium Pharmaceuticals, Kyoto-Hakka-Kirin, Seattle Genetics, Forty Seven, HUYA Bioscience International, Infinity Pharmaceuticals
Research Funding: Celgene, Infinity Pharmaceuticals, Seattle Genetics, Spectrum Pharmaceuticals, Takeda Pharmaceuticals, Kyowa Hakko Kirin, ADCT Therapeutics, Forty Seven, Aileron Therapeutics
REFERENCES
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Arisawa K, Soda M, Ono M, et al. Trends of incidence rate of adult T-cell leukemia/lymphoma in an HTLV-1 endemic area in Japan. Int J Cancer. 2009;125:737–738. doi: 10.1002/ijc.24420. [DOI] [PubMed] [Google Scholar]
- 3.Mahieux R, Gessain A. Adult T-cell leukemia/lymphoma and HTLV-1. Curr Hematol Malig Rep. 2007;2:257–264. doi: 10.1007/s11899-007-0035-x. [DOI] [PubMed] [Google Scholar]
- 4.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 6.Chang YB, Kaidarova Z, Hindes D, et al. Seroprevalence and demographic determinants of human T-lymphotropic virus type 1 and 2 infections among first-time blood donors: United States, 2000-2009. J Infect Dis. 2014;209:523–531. doi: 10.1093/infdis/jit497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimotohno K, Takahashi Y, Shimizu N, et al. Complete nucleotide seuqence of an infectious clone of human T-cell leukemia virus type II: An open reading frame for the protease gene. Proc Natl Acad Sci U S A. 82:3101–3105, 1985. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takatsuki K. Adult T-cell leukemia. Intern Med. 1995;34:947–952. doi: 10.2169/internalmedicine.34.947. [DOI] [PubMed] [Google Scholar]
- 9.Manns A, Hisada M, La Grenade L. Human T-lymphotropic virus type I infection. Lancet. 1999;353:1951–1958. doi: 10.1016/s0140-6736(98)09460-4. [DOI] [PubMed] [Google Scholar]
- 10.Feuer G, Green PL. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene. 2005;24:5996–6004. doi: 10.1038/sj.onc.1208971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangham CR, Ratner L. How does HTLV-1 cause adult T-cell leukaemia/lymphoma (ATL)? Curr Opin Virol. 2015;14:93–100. doi: 10.1016/j.coviro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kataoka K, Nagata Y, Kitanaka A, et al. Frequent activating somatic alterations in T-cell receptor / NF-κβ signaling in adult T-cell leukemia/lymphoma. Blood 126, 2015 (abstr 113) [Google Scholar]
- 13.Koiwa T, Hamano-Usami A, Ishida T, et al. 5′-long terminal repeat-selective CpG methylation of latent human T-cell leukemia virus type 1 provirus in vitro and in vivo. J Virol. 2002;76:9389–9397. doi: 10.1128/JVI.76.18.9389-9397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi Y, Nosaka K, Yasunaga J, et al. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology. 2005;2:64. doi: 10.1186/1742-4690-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satou Y, Yasunaga J, Zhao T, et al. HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 2011;7:e1001274. doi: 10.1371/journal.ppat.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dutartre H, Clavière M, Journo C, et al. Cell-free versus cell-to-cell infection by human immunodeficiency virus type 1 and human T-lymphotropic virus type 1: Exploring the link among viral source, viral trafficking, and viral replication. J Virol. 2016;90:7607–7617. doi: 10.1128/JVI.00407-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernal-Mizrachi L, Lovly CM, Ratner L. The role of NF-kappaB-1 and NF-kappaB-2-mediated resistance to apoptosis in lymphomas. Proc Natl Acad Sci USA. 2006;103:9220–9225. doi: 10.1073/pnas.0507809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook LB, Rowan AG, Melamed A, et al. HTLV-1-infected T cells contain a single integrated provirus in natural infection. Blood. 2012;120:3488–3490. doi: 10.1182/blood-2012-07-445593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook LB, Elemans M, Rowan AG, et al. HTLV-1: Persistence and pathogenesis. Virology. 2013;435:131–140. doi: 10.1016/j.virol.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Afonso PV, Mekaouche M, Mortreux F, et al. Highly active antiretroviral treatment against STLV-1 infection combining reverse transcriptase and HDAC inhibitors. Blood. 2010;116:3802–3808. doi: 10.1182/blood-2010-02-270751. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto JF, Goodman MT. Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002. Cancer Causes Control. 2008;19:379–390. doi: 10.1007/s10552-007-9097-2. [DOI] [PubMed] [Google Scholar]
- 22.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 24.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma: A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79:428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 25.Takatsuki K, Yamaguchi K, Hattori T. Adult T-cell leukemia/lymphoma. In: Gallo RC, Wong-Staal F, editors. Retrovirus Biology and Human Disease. New York, NY: Marcel Dekker; 1990. pp. 147–160. [Google Scholar]
- 26.Amagasaki T, Tomonaga Y, Yamada Y, et al. Adult T-cell leukemia with an unusual phenotype, Leu-2a positive and Leu-3a negative. Blut. 1985;50:209–211. doi: 10.1007/BF00320296. [DOI] [PubMed] [Google Scholar]
- 27.Takemoto S, Matsuoka M, Yamaguchi K, et al. A novel diagnostic method of adult T-cell leukemia: Monoclonal integration of human T-cell lymphotropic virus type I provirus DNA detected by inverse polymerase chain reaction. Blood. 1994;84:3080–3085. [PubMed] [Google Scholar]
- 28.Lorand-Metze I, Pombo-de-Oliveira MS. Adult T-cell leukemia (ATL) with an unusual immunophenotype and a high cellular proliferation rate. Leuk Lymphoma. 1996;22:523–526. doi: 10.3109/10428199609054793. [DOI] [PubMed] [Google Scholar]
- 29.Flug F, Pelicci PG, Bonetti F, et al. T-cell receptor gene rearrangements as markers of lineage and clonality in T-cell neoplasms. Proc Natl Acad Sci USA. 1985;82:3460–3464. doi: 10.1073/pnas.82.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertness V, Kirsch I, Hollis G, et al. T-cell receptor gene rearrangements as clinical markers of human T-cell lymphomas. N Engl J Med. 1985;313:534–538. doi: 10.1056/NEJM198508293130902. [DOI] [PubMed] [Google Scholar]
- 31.Waldmann TA, Davis MM, Bongiovanni KF, et al. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985;313:776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- 32.Bazarbachi A, Suarez F, Fields P, et al. How I treat adult T-cell leukemia/lymphoma. Blood. 2011;118:1736–1745. doi: 10.1182/blood-2011-03-345702. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 34.Takasaki Y, Iwanaga M, Imaizumi Y, et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood. 2010;115:4337–4343. doi: 10.1182/blood-2009-09-242347. [DOI] [PubMed] [Google Scholar]
- 35.Moriyama K, Muranishi H, Nishimura J, et al. Immunodeficiency in preclinical smoldering adult T-cell leukemia. Jpn J Clin Oncol. 1988;18:363–369. [PubMed] [Google Scholar]
- 36.Phillips AA, Shapira I, Willim RD, et al. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: A multicenter clinicopathologic experience and new prognostic score. Cancer. 2010;116:3438–3446. doi: 10.1002/cncr.25147. [DOI] [PubMed] [Google Scholar]
- 37.Suzumiya J, Ohshima K, Tamura K, et al. The International Prognostic Index predicts outcome in aggressive adult T-cell leukemia/lymphoma: Analysis of 126 patients from the International Peripheral T-Cell Lymphoma Project. Ann Oncol. 2009;20:715–721. doi: 10.1093/annonc/mdn696. [DOI] [PubMed] [Google Scholar]
- 38.Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25:5458–5464. doi: 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- 39.Ratner L, Harrington W, Feng X, et al. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PLoS One. 2009;4:e4420. doi: 10.1371/journal.pone.0004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratner L, Rauch D, Abel H, et al. Dose-adjusted EPOCH chemotherapy with bortezomib and raltegravir for human T-cell leukemia virus-associated adult T-cell leukemia lymphoma. Blood Cancer J. 2016;6:e408. doi: 10.1038/bcj.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishida T, Hishizawa M, Kato K, et al. Allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia-lymphoma with special emphasis on preconditioning regimen: A nationwide retrospective study. Blood. 2012;120:1734–1741. doi: 10.1182/blood-2012-03-414490. [DOI] [PubMed] [Google Scholar]
- 42.Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: A nationwide retrospective study. Blood. 2010;116:1369–1376. doi: 10.1182/blood-2009-10-247510. [DOI] [PubMed] [Google Scholar]
- 43.Phillips AA, Willim RD, Savage DG, et al. A multi-institutional experience of autologous stem cell transplantation in North American patients with human T-cell lymphotropic virus type-1 adult T-cell leukemia/lymphoma suggests ineffective salvage of relapsed patients. Leuk Lymphoma. 2009;50:1039–1042. doi: 10.1080/10428190902887571. [DOI] [PubMed] [Google Scholar]
- 44.Tsukasaki K, Maeda T, Arimura K, et al. Poor outcome of autologous stem cell transplantation for adult T cell leukemia/lymphoma: A case report and review of the literature. Bone Marrow Transplant. 1999;23:87–89. doi: 10.1038/sj.bmt.1701533. [DOI] [PubMed] [Google Scholar]
- 45.Gill PS, Harrington W, Jr, Kaplan MH, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995;332:1744–1748. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- 46.Hermine O, Bouscary D, Gessain A, et al. Brief report: Treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332:1749–1751. doi: 10.1056/NEJM199506293322604. [DOI] [PubMed] [Google Scholar]
- 47.White JD, Wharfe G, Stewart DM, et al. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leuk Lymphoma. 2001;40:287–294. doi: 10.3109/10428190109057927. [DOI] [PubMed] [Google Scholar]
- 48.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J Clin Oncol. 2012;30:837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 49.Jo T, Ishida T, Takemoto S, et al. Randomized phase II study of mogamulizumab (KW-0761) plus VCAP-AMP-VECP (mLSG15) versus mLSG15 alone for newly diagnosed aggressive adult T-cell leukemia-lymphoma (ATL) J Clin Oncol. 2013;31 (suppl; abstr 8506) [Google Scholar]
- 50.Ishida T, Jo T, Takemoto S, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: A randomized phase II study. Br J Haematol. 2015;169:672–682. doi: 10.1111/bjh.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips AA, Fields P, Hermine O, et al. A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL). J Clin Oncol 34, 2016 (suppl; abstr 7501) [Google Scholar]
- 52.Barr PM, Li H, Spier C, et al. Phase II intergroup trial of alisertib in relapsed and refractory peripheral T-cell lymphoma and transformed mycosis fungoides: SWOG 1108. J Clin Oncol. 2015;33:2399–2404. doi: 10.1200/JCO.2014.60.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez L. Complete response to single-agent brentuximab vedotin in relapsed adult T-cell leukemia lymphoma with high expression of CD30. Presented at the T-Cell Lymphoma Forum, San Francisco, CA, January 28-30, 2016. [Google Scholar]
- 54.Mukhi N, Verma V, Ahmed A, et al. Romidepsin in relapsed/refractory HTLV-1 associated adult T-cell lymphoma/leukemia: A case series. Blood 126, 2015 (abstr 5113) [Google Scholar]
- 55.Ishida T, Fujiwara H, Nosaka K, et al. Multicenter phase II study of lenalidomide in relapsed or recurrent adult T-cell leukemia/lymphoma: ATLL-002. J Clin Oncol. 2016;34:4086–4093. doi: 10.1200/JCO.2016.67.7732. [DOI] [PubMed] [Google Scholar]
- 56.Gurion R, Mehta N, Migliacci JC, et al. Central nervous system involvement in T-cell lymphoma: A single center experience. Acta Oncol. 2016;55:561–566. doi: 10.3109/0284186X.2015.1118656. [DOI] [PMC free article] [PubMed] [Google Scholar]