Abstract
Tobacco smoking among those seeking treatment for cannabis use disorder (CUD) is common and is a negative predictor of cannabis outcomes. Quitting tobacco may be beneficial for those seeking to quit cannabis use. This initial proof of concept, controlled trial was designed to compare a simultaneous versus sequential tobacco intervention among those seeking treatment for CUD. Sixty-seven adults received either a simultaneous (SIM) or sequential (SEQ) approach to tobacco cessation in the context of outpatient treatment for CUD. A tobacco intervention (TI) that combined web-based counseling with nicotine replacement therapy (NRT) was provided during weeks 1–12 for SIM and was delayed until weeks 13–24 for SEQ. During weeks 1–12, no between-condition significant differences were observed on treatment participation or cannabis use outcomes. The majority of SIM participants initiated TI counseling (62%), 50% made at least one quit attempt and 41% initiated NRT. Interestingly, 39% in SEQ made tobacco quit attempts and 18% initiated NRT on their own before the TI was offered to them. However, only 30% of those in SEQ continued treatment during weeks 13–24, which compromised between-condition comparisons following the TI, but illustrated a potential clinical concern with delaying the TI. Tobacco cessation outcomes generally were poor and did not differ between conditions. This initial controlled trial suggests that addressing tobacco use during CUD treatment is acceptable and generates action towards tobacco cessation. Additional trials testing more intensive TI models may be necessary to identify more efficacious interventions for co-use of cannabis and tobacco.
1. Introduction
Co-occurring tobacco use is common among cannabis users in the US; over 90% of adult cannabis users have a lifetime history of tobacco use and over 60% report current (past month) tobacco use (SAMHSA, 2017) compared to 16% in the general population of US adults (Jamal et al., 2018). The prevalence of co-use appears to be on the rise in the United States, with the largest increases occurring in states where cannabis has been legalized for medical and/or recreational use (Schauer et al., 2015; Wang and Cataldo, 2016). Increasing trends in co-use rates have raised concerns that increased access to cannabis may reverse long-standing downward trends in tobacco use and increase negative consequences associated with use of each substance (e.g. United States Department of Health and Human Services, 2014; Volkow et al., 2014; Volkow et al., 2016).
Co-use of cannabis and tobacco is associated with elevated risks relative to use of each substance in isolation, particularly related to the onset and maintenance of problematic use. Tobacco use among cannabis users is associated with higher rates of daily cannabis use (Goodwin et al., 2018), greater severity of cannabis use disorder (CUD) (Agrawal and Lynskey, 2009; Hindocha et al., 2015; Peters et al., 2012), a higher probability of cannabis relapse in laboratory analog studies, and poorer outcome in CUD treatment studies than that for cannabis users who do not smoke tobacco (Haney et al., 2013; Moore and Budney, 2001; Peters et al., 2012). Moreover, cannabis use among tobacco smokers is associated with higher rates of nicotine dependence (Rubinstein et al., 2014) and poorer tobacco-cessation outcomes (Amos et al., 2004; Ford et al., 2002; Weinberger et al., 2018). Mechanisms underlying vulnerability to co-use remain unclear, however, genetic, neurobiological, and behavioral mechanisms likely contribute to establishing learned patterns of co-use, ultimately leading to increased rates of dependence or decreased ability to quit either or both substances (Agrawal et al., 2012; Castañé et al., 2005; Cohen et al., 2005; Cooper and Haney, 2009; Penetar et al., 2005; Rabin and George, 2015; Ream et al., 2008). These associations between cannabis and tobacco use pose difficult challenges to those who desire to quit using one or both substances, justifying the importance of developing cessation or reduction protocols that target both substances.
Results from controlled trials testing intervention models for targeting tobacco use during or following treatment for substance use disorders (SUD) have illustrated that offering cessation programs for tobacco does not adversely impact SUD outcomes, and can be efficacious for tobacco cessation (Kalman et al., 2001; Cooney et al., 2007, 2009, 2015, 2017; Dunn et al., 2010; Sigmon et al., 2016; Winhusen, et al., 2014). However, to date, only four uncontrolled trials have evaluated interventions for CUD that also target tobacco co-use (Hill et al., 2013; Becker et al., 2015; Lee et al., 2015; Adams et al., 2018). Each study examined an intervention combining behavioral treatment for CUD with behavioral therapy and/or pharmacotherapy for tobacco use (i.e. nicotine replacement therapy, varenicline). Across these uncontrolled studies, findings suggest that combined interventions are well-tolerated by CUD patients, have positive effects on tobacco use including prompting quit attempts and reducing tobacco use and dependence symptoms, while not adversely impacting cannabis outcomes.
Our pilot trial (Lee et al., 2015) compared cannabis abstinence rates achieved with an intervention that simultaneously addressed CUD and tobacco use to those observed in historical controls from prior CUD treatment studies that evaluated similar CUD interventions but did not address tobacco use (Budney et al., 2000; Budney et al., 2006; Budney et al., 2015). Cannabis abstinence rates achieved in this pilot trial were similar to those achieved in the historical control conditions. The majority (78%) also initiated the tobacco intervention and 56% made at least one tobacco quit attempt; however, sustained tobacco abstinence rates were minimal (12.5%).
The current proof-of-concept, randomized controlled trial sought to replicate and extend the findings from that pilot study by comparing our simultaneous intervention approach with a “sequential” approach that delayed delivery of the tobacco intervention. This experimental strategy was designed to (a) directly compare the effect of the simultaneous treatment approach on cannabis and tobacco use with the typical treatment approach that only targets cannabis, (b) obtain initial data on acceptability of a sequential intervention strategy, and (c) conduct an initial comparison of tobacco and cannabis outcomes achieved with these two alternative approaches.
2. Method
2.1. Participants.
Participants seeking treatment for CUD were enrolled between October 2014 to September 2016 at Dartmouth College and the University of Washington. The study was conducted in compliance with Internal Review Boards at Dartmouth College and the University of Washington and registered on clinicaltrials.gov (NCT02277665). Participants at both sites were recruited from advertisements posted online (Craigslist, Facebook), in newspapers, on radio, posters throughout the community, and notices to professionals and service agencies. Participants were included if they met the following criteria: 1) ≥ 18 years of age, 2) current diagnosis of cannabis abuse or dependence made using the Structured Clinical Interview for DSM-IV (SCID: First et al., 1995), 3) cannabis use on ≥45 of the past 90 days, 4) regular use of tobacco (i.e. ≥5 days per week) or report primary administration of cannabis via blunts/spliffs, and 5) self-reported at least minimal interest in quitting tobacco in the next six months during the pre-intake screening (i.e. rating of ≥ 2 on a 5-point scale). Exclusion criteria included: 1) current dependence (DSM-IV criteria) in the last 6 months on alcohol or any drug other than tobacco and cannabis with the exception of opiate dependence maintained with agonist replacement therapy, 2) use of non-tobacco nicotine (i.e. nicotine replacement therapy, NRT), or exclusive use of smokeless tobacco products, 3) currently in treatment for substance dependence, 4) severe psychological distress (e.g. active suicidal ideation, debilitating panic disorder), 5) medical condition that required physician approval before using NRT (e.g. pregnancy or myocardial infarction), 6) legal status that would interfere with participation, 7) living with someone enrolled in the study, 8) not fluent in English, or 9) living ≥45 miles from the study site.
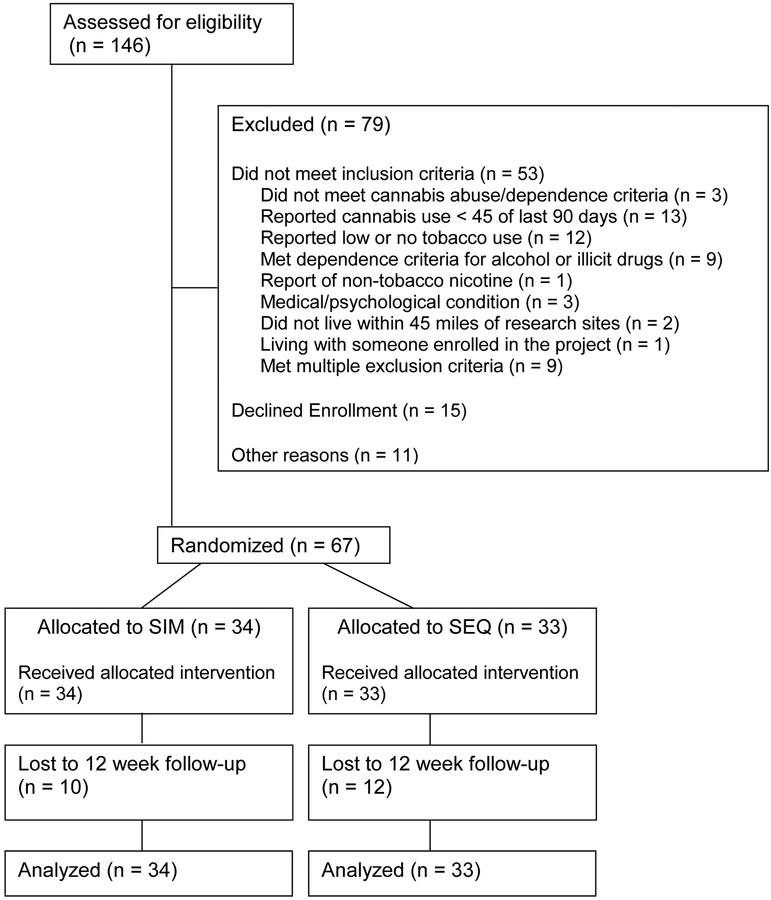
Of the 146 participants screened for the study, 53 did not meet inclusion / exclusion criteria, 15 were eligible but declined treatment, and 11 did not enroll for other reasons (e.g. lost contact after intake) (see Figure 1). Sixty-seven adults (45 male), age 18–60 years signed a study consent and were enrolled. Participant characteristics by treatment condition are presented in Table 1.
Figure 1.
Consort Diagram
Table 1.
Participant characteristics and substance use at intake
| Characteristic | SIM (n = 34) | SEQ (n = 33) | p-valuea |
|---|---|---|---|
| M (SD) or N (%) | M (SD) or N (%) | ||
| Age | 31.7 (11.2) | 36.3 (12.2) | 0.83 |
| Sex (male) | 23 (67.6%) | 25 (75.7%) | 0.46 |
| Race | 0.65 | ||
| Caucasian | 21 (61.7%) | 22 (66.7%) | |
| Other | 13 (38.2%) | 11 (33.3%) | |
| Cannabis Use | |||
| Age of Initiation | 14.9 (3.0) | 14.1 (2.5) | 0.21 |
| % days of Use (past 90) | 91.0 (14.6) | 92.3 (9.1) | 0.51 |
| Occasions per Day | 3.9 (2.5) | 4.3 (2.7) | 0.61 |
| Cannabis Dependence | 31 (91.2%) | 33 (100%) | 0.08 |
| Tobacco Use | |||
| Age of Initiation | 14.4 (4.4) | 14.9 (6.4) | 0.73 |
| Cigarette Usersb | 30 (88.2%) | 32 (96.9%) | 0.17 |
| % days of use (past 90) | 91.6 (20.5) | 93.8 (17.0) | 0.49 |
| Cigarettes per day | 10.7 (9.7) | 9.5 (5.5) | 0.54 |
| FTND | 3.4 (2.8) | 3.9 (2.4) | 0.44 |
| Use of Other Tobacco | 17 (50.0%) | 11 (33.3%) | 0.17 |
| Product | |||
| % days of use (past 90) | 59.4 (39.2) | 78.8 (31.1) | 0.18 |
| Occasions per Day | 2.4 (1.4) | 3.0 (2.3) | 0.40 |
| Motivation to Quit Tobacco | 8.8 (1.7) | 8.7 (1.9) | 0.78 |
| (0–10 pt scale) |
t-tests and chi square tests were used to assess group differences.
5 participants (4 SIM, 1 SEQ) reported exclusive use of other tobacco products (i.e. blunts, spliffs, cigarillos).
FTND: Fagerstrom Test of Nicotine Dependence; SEQ: sequential treatment condition; SIM: simultaneous treatment condition.
2.2. Interventions
2.2.1. CUD Intervention.
The CUD treatment approach has been described previously (Budney et al., 2011, 2015; Lee et al., 2014, 2015). Briefly, this treatment comprised a 9-session computer-assisted delivery of a motivational enhancement therapy (MET), cognitive behavioral therapy (CBT) and abstinence-based contingency management (CM) provided over 12 weeks. Participants attended the clinic twice weekly, where they received nine MET/CBT computer modules (weeks 1–8 and week 12), three 15 to 30-minute supportive counseling sessions (weeks 1, 4 and 12), and provided urine specimens. Computer modules were also available remotely with individualized passwords throughout the study. The CM program targeted cannabis abstinence by providing an escalating schedule of monetary incentives contingent on cannabis-negative urine toxicology derived from twice-weekly observed urine specimens. Participants could earn up to $435 for continuous abstinence throughout the intervention. Incentives were delivered after each visit via electronic deposits to a debit card.
2.2.2. Tobacco Intervention (TI).
The TI included NRT and seven computer-delivered psychoeducational and behavioral counseling modules developed specifically for this project. The first module was a translated and adapted version of a Swiss/French language, evidence-based, on-line assessment and feedback program (Stop Tabac: Etter, 2005, 2009; http://www.stop-tobacco.ch/en/), which focused on the pros and cons of smoking tobacco, encouraged motivated participants to set a tobacco quit date, and included a personalized tobacco use feedback report. The second module provided information about co-use of cannabis and tobacco, including potential additive health risks, difficulties with quitting one substance while continuing to smoke the other, planning to quit both substances, and roadblocks to quitting both. The third module provided NRT education and instruction. The fourth module focused on planning for change and setting a quit date. The fifth module provided reduction strategies for those interested in reducing rather than quitting. The sixth module included information and strategies for relapse prevention. The seventh module provided education on electronic cigarettes. The TI modules could be accessed remotely at any time with individualized passwords.
At each visit, participants were encouraged by staff, but not required, to complete at least one module and consider initiation of NRT. Participants were required to complete the NRT education module prior to receiving NRT. NRT was provided on a biweekly schedule free of charge and could be initiated anytime during the TI. NRT options included a combination of patch and gum or lozenges, following standard guidelines for dosing (Stead et al., 2012).
2.3. Trial Design, Treatment Conditions, Hypotheses
This two group, 24-week study randomized participants seeking treatment for CUD to either a simultaneous (SIM) or a sequential (SEQ) treatment condition (Figure 1). Urn randomization procedures balanced the conditions on the following baseline characteristics: cannabis abstinence prior to treatment initiation, tobacco dependence severity (low vs. high, based on the Fagerstrom), blunt/spliff smoker or not, ethnicity (African American or not), and gender. Assessments occurred at week 12 (end of Phase 1) and week 24 (end of Phase 2).
2.3.1. SIM Condition.
During weeks 1–12 (Phase 1), participants in the SIM condition received immediate access to the previously described CUD and tobacco interventions. The first five CUD MET/CBT modules were completed in a fixed order starting at the first visit. The TI modules and NRT were also available starting at the first visit, but for most participants, this did not occur until the second visit due to time constraints. TI modules could be completed in any order selected by participants, but as per above, NRT was not available until the NRT education module was completed. Both the CUD and TI modules viewed during clinic visits could then be accessed remotely with an ID and password. During weeks 13–24 (Phase 2) SIM participants could continue to access the cannabis and tobacco intervention modules ad libitum remotely but did not attend clinic visits or receive additional NRT. SIM participants that expressed an interest in continuing NRT after Phase 1 were provided with a one-week supply and instructed on how to purchase additional NRT on their own. For those not interested in continuing use, NRT dose was tapered during weeks 10–12 to reduce the probability of nicotine withdrawal symptoms from discontinuation.
2.3.2. SEQ Condition.
During Phase 1, SEQ participants received only the CUD MET/CBT/CM intervention. They could not access the TI modules, NRT was not provided by the clinic, and no counseling for tobacco cessation was offered. During their first visit, they were given a booklet on tobacco cessation, Clearing the Air (https://www.cancer.gov/publications/patient-education/clearing-the-air) that provided education and guidelines for quitting and a Quitline telephone number (SIM participants also received the booklet). This reflected “treatment as usual” for tobacco cessation in the context of outpatient treatment for CUD. SEQ participants interested in tobacco cessation during Phase 1 could make cessation attempts but had to obtain tobacco medications on their own. During Weeks 13–24, (Phase 2), SEQ participants were offered the TI as described above, and encouraged to attend weekly clinic visits to help facilitate engagement and adherence. Note that during Phase 2 clinic staff were highly flexible and encouraging of any attendance schedule with the primary goal of accommodating participants so that they could complete TI modules and obtain NRT.
2.3.3. Primary Hypotheses.
Based on prior pilot study and the literature on targeting tobacco use in the context of treatment for other SUDs, we hypothesized that: (1) the TI would be acceptable to participants in the SIM condition (i.e., Phase 1 retention and CUD engagement would not differ between conditions, and that the majority of SIM participants would engage with the TI components; (2) during Phase 1, the SIM condition would engender greater use of NRT, more tobacco quit attempts, greater sustained tobacco abstinence than SEQ, and cannabis abstinence would not differ between conditions; (3) at the end of Phase 2, after participants in both conditions were exposed to the TI, a greater percentage of SIM than SEQ participants would have initiated NRT and made a tobacco quit attempt.
2.4. Measures
2.4.1. Substance Use.
At intake, the Substance Use Disorders section of the SCID (First et al., 1995) was administered to determine SUD diagnoses. The timeline follow-back (TLFB) procedure (Sobell and Sobell, 1992) was used to obtain self-reported substance use information for the 90 days preceding intake, and then again at each clinic visit to assess substance use since the last clinic visit. TLFB assessments included cannabis, cigarettes, other tobacco and nicotine products (including smokeless tobacco and electronic cigarettes), alcohol, other drug use, and NRT use. Nicotine dependence was assessed at baseline using the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991).
Cannabis abstinence was verified via twice-weekly observed collection of urine specimens which were then tested immediately thereafter. Cannabis and adulterant testing were performed using dipstick tests with a minimum THC detection cutoff of 50 ng/ml (http://www.americanscreeningcorp.com). Failure to submit a specimen without an excused absence was treated as a cannabis-positive result.
Self-reported tobacco abstinence was verified using expired carbon monoxide (CO; coVita Micro + Smokerlyzer: http://covita.net/micro+.html). Participants with a CO level ≤ 5 were considered tobacco abstinent. In the event of self-reported tobacco abstinence, a breath CO >5, and self-reported cannabis use (which could increase CO level), tobacco abstinence was verified using urine cotinine (ONESCREEN Cotinine Test; http://www.americanscreeningcorp.com).
2.5. Outcomes.
Primary engagement outcomes included Phase 1 treatment retention defined as clinic attendance, and treatment engagement defined by completion of cannabis and tobacco computer modules, and initiation and duration of NRT. The primary cannabis outcome was the duration of continuous abstinence achieved during Phase 1, assessed using the twice-weekly observed urine specimens. Primary tobacco outcomes included the percent of participants who made at least one self-reported tobacco quit attempt during Phase 1, and percent of participants who achieved sustained tobacco abstinence over the final eight weeks of Phase 1. Secondary outcomes included: percent of participants who achieved any biologically-verified cannabis or tobacco abstinence (>1 negative urine THC/cotinine specimen or expired carbon monoxide <5 ppm), percent of participants who achieved cannabis abstinence throughout the final four weeks of treatment, reduction in percent days of cannabis and tobacco use during treatment relative to baseline, and point-prevalence cannabis and tobacco abstinence at the 12-week follow-up assessment.
2.6. Data Analysis
Sociodemographic and baseline drug use characteristics between treatment conditions were compared using t-tests for continuous measures and chi-square tests for nominal measures (Table 1). The primary cannabis and tobacco abstinence outcomes were analyzed using an intent-to-treat approach with missing biological verification data and dropouts treated as a positive indicator of cannabis or tobacco use. Longest duration of continuous abstinence from cannabis (primary), number of days abstinent from tobacco, and number of tobacco quit attempts (primary) were all zero-inflated and therefore could not be analyzed with models that assume continuous, normally distributed outcomes. We therefore analyzed each of these outcomes using a zero-inflated Poisson model which provides an odds ratio describing the relative likelihood of achieving a zero-response to the outcome in the two treatment arms and a ratio of means describing the relative mean of the outcome in the two treatment groups. Additionally, several binary outcomes were analyzed via chi-square tests: cannabis abstinence in the final 4 weeks, point prevalence abstinence at the end of treatment, achievement of at least 50% reduction in days of using cannabis from intake to the final 4 weeks, and achievement of continuous tobacco abstinence for ≥ 2 consecutive weeks. Phase 2 outcomes were analyzed using descriptive statistics. Statistical comparisons were not made between SEQ Phase 2 and SIM Phase 1 outcomes because of the lack of Phase 2 data due to only a 30% participation rate for SEQ during Phase 2. All analyses were performed using SAS version 9.4.
3. Results
3.1. Participant Characteristics.
No significant differences by treatment condition were found on participant socio-demographics or cannabis/tobacco use characteristics (Table 1). Across conditions, the sample was 67% male, 64% Caucasian, 18% African-American, and 18% other/multiple races, with a mean age of 34. Participants were experienced cannabis users (age of initiation: M = 14.5 ± 2.8 years), who generally reported near-daily cannabis use in the 90 days prior to intake (M = 82.4 ± 11.0 days) and used cannabis on multiple occasions each day (M = 4.1 ± 2.6). All participants met DSM-IV criteria for either cannabis abuse (4%) or dependence (96%). Most of the sample (81%) reported at least one previous cannabis quit attempt lasting ≥ 7 days.
Participants were experienced tobacco users (age of initiation: M = 14.7 ± 5.5 years). Nearly all participants (92%) smoked cigarettes regularly (used on M = 83.5 ± 16.8 of the 90 days prior to intake), smoked a mean of 10.0 ± 7.8 cigarettes per day, and had a mean FTND score of 3.7 ± 2.6. Forty-two percent reported using other types of tobacco, with the most common types being blunts (25%), followed by spliffs (15%) and e-cigarettes (4%). Five participants reported exclusive use of tobacco products other than cigarettes (4 used blunts/spliffs, and 1 used cigarillos). Motivation to quit tobacco appeared high, as participants reported a mean of 8.8 (SD= 1.8) on a 0–10 Likert scale assessing motivation to quit smoking in the next six months (0 = not at all, 10 = extremely), and 72% had made at least one previous tobacco quit attempt.
3.2. Retention and Participation.
Phase 1 participation and retention rates were similar between SIM and SEQ conditions (Table 2). No significant differences were observed for attendance at clinic visits, number of CUD modules completed, or treatment completion (defined as attending the final visit during Phase 1). Sixty-two percent of SIM participants completed at least one TI computer module, and 42% initiated NRT; unexpectedly, six SEQ participants (18%) initiated NRT on their own during Phase 1.
Table 2.
Treatment Retention and Participation
| SIM (n = 34) | SEQ (n=33) | Statistic | p-value | |
|---|---|---|---|---|
| Phase 1 | M (SD) or N (%) | M (SD) or N (%) | ||
| Treatment Attendance (# of specimens submitted out of 24 possible) | 9.7 (8.9) | 11.3 (9.0) | t = −0.74 | 0.46 |
| Completed Phase 1 TX (attended final visit) | 11 (32.3%) | 10 (30.3%) | χ2 = 0.03 | 0.86 |
| Attended Phase 1 Follow-up at Week 12 | 24 (70.5%) | 21 (63.6%) | χ2 = 0.37 | 0.55 |
| Number of CUD MET/CBT Modules Completed (of 9 possible) | 4.7 (3.3) | 5.3 (3.2) | t = −0.67 | 0.50 |
| Initiated TI (completed ≥ 1 TI module) | 21 (61.7)% | N/A | ||
| TI Modules Completed (of possible 6)a | 2.7 (1.8) | N/A | ||
| Initiated NRT | 14 (41.2%) | 6 (18.2%)b | χ2 = 4.23 | 0.04 |
| Days of NRT Use (of those that initiated) | 17.1 (19.2) | 19.0 (24.4)b | t = 0.18 | 0.86 |
| Phase 2 | ||||
| Initiated Phase 2 (submitted ≥1 specimen) | N/A | 10 (30.3%) | ||
| Treatment Attendance (# of specimens submitted of possible 24) | N/A | 2.3 (5.1) | ||
| Attended Phase 2 Follow-up at Week 24 | N/A | 20 (60.6%) | ||
| Completed ≥ 1 TI module | N/A | 8 (24.2)% | ||
| Number of TI Modules Completeda | N/A | 3.3 (2.3) | ||
| Initiated NRT | N/A | 3 (9.1%) | ||
| NRT Days of Use (of those that initiated NRT) | N/A | 47.3 (24.5) |
mean number of TI modules completed was calculated among participants that completed ≥ 1 TI module.
6 participants in SEQ TX purchased own NRT during Phase 1.
SEQ: sequential treatment condition; SIM: simultaneous treatment condition.
Direct comparison of TI participation (Phase 1 for SIM vs. Phase 2 for SEQ) indicated that a greater percentage of SIM than SEQ participants engaged with at least one TI computer module (62% vs. 24%; χ2(1) = 9.60, p=.002). Note that only 30% of SEQ participants initiated Phase 2 treatment, which is when the TI became available to them. Among those who engaged with the TI (i.e. completed at least one TI module), no significant differences emerged in total number of TI modules completed (M=3.3 (2.3) vs 2.7 (1.8), t(27)=−0.73, p=0.47). More SIM (41%) than SEQ (27%: total for Phase 1 and 2) participants initiated NRT, but this difference was not significant (χ2(1) = 1.44, p=0.23). Among those who initiated NRT, SEQ participants used it for a longer duration [M=33.7 (30.2) vs. 17.1 (19.1) days], but this difference was not significant (t(21)=1.61, p=0.12).
3.3. Phase 1 Cannabis Use Outcomes.
Table 3 presents primary and secondary cannabis use outcome data for Phase 1 treatment. As hypothesized, no statistically significant differences were observed between treatment conditions on any cannabis outcomes; however, the SEQ condition had numerically better outcomes on most cannabis outcomes, indicating a need for additional studies. Of note, across conditions, abstinence rates were poor compared to prior studies using similar CUD interventions.
Table 3:
Phase 1 Cannabis Outcomes
| SIM (n=34) | SEQ (n=33) | Summary Measure (SIM vs. SEQ) |
Test Statistic | |
|---|---|---|---|---|
| Primary abstinence outcome: weeks of continuous abstinence | ||||
| ≥ 1 week of documented abstinence, N (%) | 7 (20.6%) | 12 (36.4%) | OR (95% CI) 0.45 (0.15, 1.35) |
χ2(1) = 2.01, p = 0.16 |
| Weeks of continuous documented abstinence among those with ≥ 1 week, Mean (SD) | 5.9 (4.0) | 6.1 (3.7) | Means Ratio (95% CI) 0.97 (0.66,1.42) |
χ2(1) = 0.03, p = 0.87 |
| Secondary outcomes | ||||
| Point-prevalence documented abstinence (week 12) follow up assessment), N(%) | 7 (20.6%) | 6 (18.2%) | χ2(1) = 0.06, p = 0.80 |
|
| Self-report of abstinence (days of use = 0) during weeks 9–12a, N(%) | 6 (17.7%) | 8 (24.2%) | OR (95% CI) 0.67 (0.2, 2.2) |
χ2(1) = 0.44, p = 0.51 |
| ≥ 50% reduction in days of use (intake vs. final 4 weeks)a, N(%) | 9 (26.5%) | 15 (45.5%) | χ2(1) = 2.63, p = 0.11 |
self-report data available for n=22 per condition, missing data considered positive for cannabis use.
ETX: end of treatment; SEQ: sequential treatment condition; SIM: simultaneous treatment condition.
3.4. Phase 1 Tobacco Use Outcomes.
In contrast to our hypotheses, SIM did not clearly engender better tobacco use outcomes than SEQ during Phase 1 (Table 4). No significant differences were observed for percentage of participants who made a quit attempt, mean number of quit attempts made, continuous weeks abstinence from tobacco during weeks 5–12, or reduction in days of tobacco use.
Table 4:
Phase 1 Tobacco Outcomes
| SIM (n=34) | SEQ (n=33) | Summary Measure (SIM vs. SEQ) |
Test Statistic | |
|---|---|---|---|---|
| Primary outcomes | ||||
| ≥1 tobacco quit attempt, N(%) | 17 (50.0%) | 13(39.4)% | OR (95% CI) 0.56(0.17, 1.82) |
χ2(1) = 0.93, p = 0.33 |
| Number of quit attempts among participants with >1, Mean (SD) | 2.2 (1.4) | 2.5(1.9) | Means Ratio (95% CI) 0.87(0.50, 1.53) |
χ2(1) = 0.23, p = 0.63 |
| Continuous documented abstinence during weeks 5–12, N(%) | 2 (5.9%) | 1 (3.0%) | χ2(1) = 0.32, p = 0.57 |
|
| Secondary outcomes | ||||
| Achieved continuous documented abstinence for ≥ 2 consecutive weeks, N(%) | 5 (14.7%) | 5(15.2%) | χ2(1) = 0.00, p = 0.96 |
|
| ≥ 50% reduction in days of use (intake/final 4 weeks)a, N(%) | 8 (23.5%) | 7(21.2%) | χ2(1) = 0.31 p = 0.82 |
|
| Point prevalence abstinence (Week 12 Follow Up Visit), N(%) | 6 (17.6%) | 3(9.1%) | χ2(1)=1.05 p = 0.29 |
self-report data available for n=22 per condition, missing data considered positive for tobacco use.
ETX: end of treatment; SEQ: sequential treatment condition; SIM: simultaneous treatment condition.
3.5. Phase 2 SEQ Cannabis and Tobacco Outcomes.
Only 10 of the 33 participants randomized to SEQ initiated Phase 2, which compromised the validity of performing statistical comparisons between conditions. Among SEQ participants, 12% (n=4) provided at least one cannabis negative urine specimen and had a mean total of 2.4 (SD=4.0) cannabis negative urine specimens. Only one SEQ participant was cannabis abstinent at the 24-week follow-up visit. For tobacco, 18% (n=6) reported making one or more quit attempts, 6% (n=2) achieved documented tobacco abstinence for ≥ 24 hours (verified by CO/cotinine), and one participant was abstinent for at least 2 weeks. Only one SEQ participant reported tobacco abstinence at the 24-week follow-up.
4. Discussion
This study is the first to test the efficacy of a simultaneous treatment approach to CUD and tobacco use in a randomized controlled trial. Findings provide some support for the hypothesis that offering an intervention for tobacco use does not negatively impact cannabis outcomes during treatment for CUD. No robust differences in participation, retention or cannabis abstinence were observed between the group that received simultaneous treatment and the comparison condition that received the same treatment for CUD but no tobacco intervention during weeks 1–12. This observation is consistent with findings reported in alcohol literature (Cooney et al., 2015; 2017; Kalman et al., 2001, 2010; Nieva et al., 2011; Prochaska et al., 2004; Rohsenow et al., 2017a,b). However, a few non-significant differences in cannabis use outcomes during the initial 12 weeks of the study favored the SEQ condition, which raises some concern that simultaneous treatment may have undermined cannabis use outcomes in some participants. Replication with larger sample sizes is needed to ensure that this approach does not negatively impact CUD treatment outcome.
There was no indication that addressing tobacco use enhanced cannabis use outcomes during the 12-week CUD treatment in this study. Observations from previous studies targeting tobacco during treatment for other substance use disorders have produced equivocal findings, with most demonstrating either a null or weak effect of targeting tobacco on cessation or reduction of other substance use (Cooney et al., 2015; 2017; Prochaska, 2004; Rohsenow et al., 2017a,b; Winhusen et al., 2014). The potential for enhancing cannabis outcomes in the present study may have been limited by the failure to engender strong effects on tobacco cessation suggesting a need to explore more potent or tailored tobacco interventions.
Related to this, the majority of participants in the SIM condition engaged in the TI as hypothesized, yet, the lack of positive effects of the TI on tobacco quit attempts and cessation outcomes was unexpected. Several factors may have contributed to these negative findings. First, participants in both conditions were asked about tobacco use and quit attempts at intake and at weekly clinic visits, and all participants were provided with a tobacco cessation booklet at the initial study visit. Such attention to and monitoring of tobacco use may have prompted behavior aimed at reducing tobacco use in SEQ participants during Phase 1; indeed, six SEQ participants initiated NRT on their own. Second, CUD outpatients may require more potent, tailored or less burdensome tobacco interventions, such as varenicline or contingency management to engender enduring tobacco cessation outcomes. This would be consistent with literature on intervening with tobacco use among patients with other substance use disorders which have effectively used abstinence-contingent incentives and varenicline to boost rates of tobacco cessation in co-using clinical populations (Cooney et al., 2015, 2017; Dunn et al., 2010; Sigmon et al., 2016; Winhusen, et al., 2014; Rohsenow et al., 2017a,b).
Unfortunately, evaluation of the SEQ approach was challenging because only 30% of SEQ participants engaged in the TI offered during weeks 13–24, which inhibited a direct comparison of the impact of the SIM versus SEQ approach on outcomes after all participants had received the CUD and TI (week 12 for SIM vs. week 24 for SEQ). The observation of poor engagement with the delayed TI is consistent with an early study that compared simultaneous and sequential approaches to targeting tobacco use in alcohol use disorder patients (Kalman et al. 2001). Participants were not motivated to continue in treatment and complete the TI during Phase 2. Note that we did not provide any incentives for participation or for tobacco or cannabis abstinence during Phase 2. Participants did have remote access to the TI modules and could pick up a 2-week supply of NRT with each visit, however, these options were not accessed. Future tests of sequential approaches may need to consider motivational incentives and less burdensome approaches to keep participants engaged in the treatment process for an extended period of time.
Tobacco outcomes among the 30% of SEQ participants who initiated the TI in Phase 2 were similar to those in the SIM condition; the majority of SEQ participants made at least one quit attempt, and enduring tobacco abstinence was rare - only one SEQ participant was tobacco abstinent at the end of Phase 2. Note as mentioned above six SEQ participants started NRT on their own during Phase 1, and one of these continued into Phase 2 when the TI was delivered. Initiating NRT may have been prompted by calls to the Quitline number provided in the Clearing the Air booklet which assists with obtaining free NRT.
The general observation that most SIM participants were interested in and initiated the TI confirmed our previous observations and provides some reassurance and encouragement to clinicians to proactively discuss options for tobacco cessation with CUD patients. Offering a tobacco intervention to those interested, either concurrently with or immediately following an active CUD would seem to be reasonable and consistent with good SUD practice. Providing patients with a choice of when to initiate tobacco treatment would also seem prudent based on the limited clinical science in this area.
A few additional limitations of this study warrant mention. One might argue that our observations were gleaned from a group not representative of CUD patients who use tobacco because our inclusion criteria required indication of at least some motivation to quit (rating of ≥ 2 on a 5-point scale assessing interest in quitting in the next 6 months). However, only 6 of the 146 participants who were screened scored less than 2 on this scale, suggesting that the motivation level of the sample was representative of the great majority of those who seek CUD treatment and who use tobacco. Second, the inclusion of a wide range of tobacco users combined with a relatively small sample size severely limits the generalizability of our findings. Heavy and light tobacco users might respond quite differently to the TI that was provided, and the interaction between level of tobacco use and level of cannabis use also might influence response to the interventions provided in this study.
In summary, this study suggests that there does not appear to be a clear downside to offering tobacco interventions, either simultaneously or sequentially, to patients seeking treatment for CUD. Such approaches likely increase tobacco quit attempts and efforts to abstain. Larger sample sizes, better powered, and more intensive experimental tests of various approaches to tobacco cessation in this clinical population are needed to more clearly assess their impact on cannabis use outcomes, and to attempt to obtain more potent effects on tobacco reduction. Targeting multiple, related health behaviors adds complexity to the treatment process, but the potential for increased efficiency and clinical health benefits is likely high and warrants continued focus and investigation (Prochaska et al., 2008). That said, it is also clear that the CUD population poses a difficult challenge to treatment providers. Although we and others have demonstrated the efficacy of behavioral interventions for CUD (Sherman and McRae-Clark, 2016; Gates et al., 2016), the majority of CUD patients do not evidence a positive response to those interventions. Tobacco use has been one negative predictor of outcome in those studies (Moore and Budney, 2001; Peters et al., 2012), and the relatively poor cannabis outcomes observed in the present study strongly suggest that identifying interventions that can improve cannabis outcomes and engender tobacco cessation should continue to be a public health priority.
Highlights.
Cannabis users are receptive to tobacco interventions during treatment for CUD
Simultaneous treatment did not have robust effects on cannabis or tobacco outcomes
Sequential treatment had high dropout prior to receiving the tobacco intervention
More efficacious tobacco interventions are needed for CUD outpatients
Acknowledgements
We acknowledge Lauren Matthews, Alan Lun, and Stanley See, Shane Snyder, and Harold Yang for providing concerted research efforts during the conduct of the study.
Funding: This work was supported by the National Institutes of Health, National Institute on Drug Abuse, R01-DA032243, T32–037202, P30-DA29926
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest
Dr. Hughes has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction, and from several non-profit organizations that promote tobacco control. He consults with Swedish Match and Phillip Morris International on their efforts to develop less-risky tobacco products. No other authors have declarations of interest. Funding for this study was provided by NIH-NIDA.
REFERENCES
- Adams TR, Arnsten JH, Ning Y, Nahvi S, 2018. Feasibility and Preliminary Effectiveness of Varenicline for Treating Co-Occurring Cannabis and Tobacco Use. J. Psychoactive Drugs 50(1), 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Budney AJ, Lynskey MT, 2012. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction 107(7), 1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, 2009. Tobacco and cannabis co-occurrence: does route of administration matter? Drug Alcohol Depend. 99(1–3), 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A, 2004. ‘You can’t go without a fag…you need it for your hash’--a qualitative exploration of smoking, cannabis and young people. Addiction 99(1), 77–81. [DOI] [PubMed] [Google Scholar]
- Becker J, Haug S, Kraemer T, Schaub MP, 2015. Feasibility of a group cessation program for co-smokers of cannabis and tobacco. Drug and alcohol review 34(4), 418–426. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Fearer S, Walker DD, Stanger C, Thostenson J, Grabinski M, Bickel WK, 2011. An initial trial of a computerized behavioral intervention for cannabis use disorder. Drug Alcohol Depend. 115(1–2), 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL, 2000. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J. Consult. Clin. Psychol 68(6), 1051–1061. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Rocha HL, Higgins ST, 2006. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. J. Consult. Clin. Psychol 74(2), 307–316. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Stanger C, Tilford JM, Scherer EB, Brown PC, Li Z, Li Z, Walker DD, 2015. Computer-assisted behavioral therapy and contingency management for cannabis use disorder. Psychol. Addict. Behav 29(3), 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañé A, Berrendero F, Maldonado R, 2005. The role of the cannabinoid system in nicotine addiction. Pharmacology Biochemistry and Behavior 81(2), 381–386. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrié P, 2005. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: Reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30(1), 145–155. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Pilkey DT, Steinberg HR, & Oncken CA, 2007. Concurrent brief versus intensive smoking intervention during alcohol dependence treatment. Psychology of Addictive Behaviors, 21(4), 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney NL, Cooney JL, Perry BL, Carbone M, Cohen EH, Steinberg HR, Pilkey DT, Sevarino K, Oncken CA, & Litt MD, 2009. Smoking cessation during alcohol treatment: a randomized trial of combination nicotine patch plus nicotine gum. Addiction, 104(9), 1588–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney JL, Cooper S, Grant C, Sevarino K, Krishnan-Sarin S, Gutierrez IA, Cooney NL, 2017. A randomized trial of contingency management for smoking cessation during intensive outpatient alcohol treatment. J. Subst. Abuse Treat. 72, 89–96. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Sevarino KA, Levy L, Kranitz LS, Sackler H, Cooney JL, 2015. Concurrent alcohol and tobacco treatment: Effect on daily process measures of alcohol relapse risk. J. Consult. Clin. Psychol 83(2), 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2009. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 103(3), 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST, 2010. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Exp. Clin. Psychopharmacol 18(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F, 2005. Comparing the efficacy of two Internet-based, computer-tailored smoking cessation programs: a randomized trial. J. Med. Internet Res. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F, 2009. Comparing computer-tailored, internet-based smoking cessation counseling reports with generic, untailored reports: a randomized trial. Journal of health communication 14(7), 646–657. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB, 1995. Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute. [Google Scholar]
- Ford DE, Vu HT, Anthony JC, 2002. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 67(3), 243–248. [DOI] [PubMed] [Google Scholar]
- Gates PJ, Sabioni P, Copeland J, Le Foll B, Gowing L, 2016. Psychosocial interventions for cannabis use disorder. The Cochrane database of systematic reviews(5), Cd005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Pacek LR, Copeland J, Moeller SJ, Dierker L, Weinberger A, Gbedemah M, Zvolensky MJ, Wall MM, Hasin DS, 2018. Trends in daily cannabis use among cigarette smokers: United States, 2002–2014. Am. J. Public Health 108(1), 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Bedi G, Cooper ZD, Glass A, Vosburg SK, Comer SD, Foltin RW, 2013. Predictors of marijuana relapse in the human laboratory: robust impact of tobacco cigarette smoking status. Biol. Psychiatry 73(3), 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, 1991. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hill KP, Toto LH, Lukas SE, Weiss RD, Trksak GH, Rodolico JM, Greenfield SF, 2013. Cognitive behavioral therapy and the nicotine transdermal patch for dual nicotine and cannabis dependence: a pilot study. Am. J. Addict 22(3), 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindocha C, Shaban ND, Freeman TP, Das RK, Gale G, Schafer G, Falconer CJ, Morgan CJ, Curran HV, 2015. Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend. 148, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ, 2018. Current cigarette smoking among adults—United States, 2016. Morbidity and Mortality Weekly Report 67(2), 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, & Monti PM, 2001. Concurrent versus delayed smoking cessation treatment for persons in early alcohol recovery: a pilot study. Journal of Substance AbuseTreatment, 20(3), 233–238. [DOI] [PubMed] [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D, 2010. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin. Psychol. Rev 30(1), 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter JF, Stanger C, 2014. Treatment models for targeting tobacco use during treatment for cannabis use disorder: case series. Addict. Behav 39(8), 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Budney AJ, Brunette MF, Hughes JR, Etter JF, Stanger C, 2015. Outcomes from a computer-assisted intervention simultaneously targeting cannabis and tobacco use. Drug Alcohol Depend. 155, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BA, Budney AJ, 2001. Tobacco smoking in marijuana-dependent outpatients. J. Subst. Abuse 13(4), 583–596. [DOI] [PubMed] [Google Scholar]
- Nieva G, Ortega L, Mondon S, Ballbè M, Gual A, 2011. Simultaneous versus delayed treatment of tobacco dependence in alcohol-dependent outpatients. Eur. Addict. Res 17(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE, 2005. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 79(2), 211–223. [DOI] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM, 2012. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction 107(8), 1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Delucchi K, Hall SM, 2004. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J. Consult. Clin. Psychol 72(6), 1144. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Spring B, Nigg CR, 2008. Multiple health behavior change research: an introduction and overview. Prev. Med 46(3), 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, George TP, 2015. A review of co-morbid tobacco and cannabis use disorders: possible mechanisms to explain high rates of co-use. Am. J. Addict 24(2), 105–116. [DOI] [PubMed] [Google Scholar]
- Ream GL, Benoit E, Johnson BD, Dunlap E, 2008. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug Alcohol Depend. 95(3), 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Martin RA, Tidey JW, Colby SM, Monti PM, 2017a. Treating smokers in substance treatment with contingent vouchers, nicotine replacement and brief advice adapted for sobriety settings. J. Subst. Abuse Treat. 72, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Swift RM, Leggio L, & Monti PM, 2017b. Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms. Addiction, 112(10), 1808–1820. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Rait MA, Prochaska JJ, 2014. Frequent marijuana use is associated with greater nicotine addiction in adolescent smokers. Drug Alcohol Depend. 141, 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, Berg CJ, Kegler MC, Donovan DM, Windle M, 2015. Assessing the overlap between tobacco and marijuana: Trends in patterns of co-use of tobacco and marijuana in adults from 2003–2012. Addict. Behav 49, 26–32. [DOI] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, 2016. Treatment of Cannabis Use Disorder: Current Science and Future Outlook. Pharmacotherapy 36(5), 511–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Miller ME, Meyer AC, Saulsgiver K, Badger GJ, Heil SH, Higgins ST, 2016. Financial incentives to promote extended smoking abstinence in opioid-maintained patients: A randomized trial. Addiction 111(5), 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back, Measuring alcohol consumption. Springer, pp. 41–72. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T, 2012. Nicotine replacement therapy for smoking cessation. The Cochrane Library. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2017). Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- United States Department of Health and Human Services (2014). The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Printed with corrections, January 2014. [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR, 2014. Adverse health effects of marijuana use. N. Engl. J. Med 370(23), 2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Bloomfield MA, Curran HV, Baler R, 2016. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 73(3), 292–297. [DOI] [PubMed] [Google Scholar]
- Wang JB, Cataldo JK, 2016. Medical Marijuana Legalization and Co-use in Adult Cigarette Smokers. Am. J. Health Behav. 40(2), 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Platt J, Copeland J, Goodwin RD, 2018. Is Cannabis Use Associated With Increased Risk of Cigarette Smoking Initiation, Persistence, and Relapse? Longitudinal Data From a Representative Sample of US Adults. The Journal of Clinical Psychiatry 79(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG, 2014. A randomized trial of concurrent smoking-cessation and substance use disorder treatment in stimulant-dependent smokers. The Journal of Clinical Psychiatry 75(4), 336. [DOI] [PMC free article] [PubMed] [Google Scholar]