Abstract
Purpose
Patients who undergo surgery for papillary thyroid cancer with only a limited lymph node examination are thought to be at risk for potentially harboring occult disease. However, this risk has not been objectively quantified and may have implications for subsequent management and surveillance.
Methods
Data from the National Cancer Database (1998 to 2012) were used to characterize the distribution of nodal positivity of adult patients diagnosed with localized ≥ 1-cm papillary thyroid cancer who underwent thyroidectomy with one or more lymph nodes (LNs) examined. A β-binomial distribution was used to estimate the probability of occult nodal disease as a function of total number of LNs examined and pathologic tumor stage.
Results
A total of 78,724 patients met study criteria; 38,653 patients had node-positive disease. The probability of falsely identifying a patient as node negative was estimated to be 53% for patients with a single node examined and decreased to less than 10% when more than six LNs were examined. To rule out occult nodal disease with 90% confidence, six, nine, and 18 nodes would need to be examined for patients with T1b, T2, and T3 disease, respectively. Sensitivity analyses limited to patients likely undergoing prophylactic central neck dissection resulted in three, four, and eight nodes needed to provide comparable adequacy of LN evaluation.
Conclusion
To our knowledge, our study provides the first empirically based estimates of occult nodal disease risk in patients after surgery for papillary thyroid cancer as a function of primary tumor stage and number of LNs examined. Our estimates provide an objective guideline for evaluating adequacy of LN yield for surgeons and pathologists in the treatment of papillary thyroid cancer, and especially intermediate-risk disease, for which use of adjuvant radioactive iodine and surveillance intensity are not currently standardized.
INTRODUCTION
Thyroid cancer is the most rapidly increasing cancer in the United States, with an estimated 62,980 incident cases diagnosed in 2014.1 Papillary thyroid cancer predominates, and lymph nodes (LNs) represent the most common site of persistent, recurrent, and/or progressive disease.2 Surgical resection is the mainstay of treatment; however, the adequacy of the number of LNs resected and examined at the time of surgery has not been determined and remains a controversial issue. Adjuvant treatment using radioactive iodine (RAI) may be used to mitigate the risk of recurrence; however, its use is known to have adverse effects, including impaired fertility and secondary malignancies.3 It is not recommended for all patients and must be balanced with the benefit of reduced risk of recurrence or improved survival. The presence of nodal involvement impacts survival in patients older than age 45 years, and new data suggest that the same is true for younger patients.4 As a result, in part, of this concern, roughly half of all patients with differentiated thyroid cancer receive adjuvant RAI,5 and nearly 20% may be inappropriately treated,6 stressing the need for objective data to guide treatment decisions.
One approach to addressing the concern for occult LN disease is to use prophylactic central LN dissection (PCLND) in which LNs are harvested from the central neck in the absence of clinically apparent metastases. The benefit of this approach is to provide more adequate staging and, in some cases, pre-emptively obviate the need for adjuvant treatment.7 However, added risks of surgical complications, such as hypocalcemia and recurrent laryngeal nerve injury, may outweigh the benefits for many patients.7-11 Current guidelines from both the National Comprehensive Cancer Network12 and American Thyroid Association13 recommend consideration of LN dissection and RAI ablation for intermediate- and high-risk tumors, which are associated with an elevated risk of recurrence, progression, and mortality. Specifically, these guidelines recommend central LN compartment dissection for all patients with clinically involved central nodes (therapeutic) and consideration of PCLND for patients with advanced primary tumors or where such information might be used to plan further steps in therapy and indicate that PCLND may or may not be appropriate for small, clinically node-negative tumors. Despite these guidelines, significant variation in practice surrounding LN resection and RAI use exists at a national level,5,14 suggesting that additional objective criteria are needed to assess the risk of residual disease in these patients.
In this study, we present a statistical model to describe the risk of occult nodal disease in patients with papillary thyroid cancer as a function of primary tumor (T) stage and the number of nodes examined, using a large sample from a nationwide database. We use this model to provide objective, evidence-based estimates of the risk of residual occult nodal disease to help guide in the assessment of patients who have undergone thyroidectomy with limited nodal sampling.
METHODS
Data Source
Data from the National Cancer Database (1998 to 2012) were used to characterize the distribution of nodal positivity among adult patients diagnosed with ≥ 1-cm papillary thyroid cancers who underwent thyroidectomy with one or more LNs surgically examined and no evidence of distant metastatic disease at diagnosis. Patient characteristics were summarized and compared by nodal status using χ2 tests for categorical variables and t tests for continuous variables. The study was deemed exempt by the institutional review board.
Model of Adequacy of LN Yield
We adapted a previously described mathematical model15 using the β-binomial distribution to estimate the probability of occult nodal disease as a function of total number of LNs examined (equations 1 to 4 in Appendix, online only). In step 1, we used node-positive patients to estimate the distribution percentage of positive LNs in these patients. Analysis was limited to patients with at least two nodes examined because, by definition, patients with node-positive disease and only one node examined would be 100% positive. We then estimated the probability of a false-negative LN evaluation as a function of LNs examined (equation 5, Appendix). In step 2, we estimated the true prevalence of nodal disease within the overall study population, accounting for false-negative results using the estimates from step 1 (equation 6, Appendix). Finally, in step 3, we combined the probability of a false-negative dissection in a truly node-positive patient (derived in step 1) with the true prevalence of nodal positivity (step 2) to estimate the risk of occult nodal disease (equations 7 and 8, Appendix). The American Joint Committee on Cancer TNM staging16 was used to categorize patient tumor sizes and analyze the risk of nodal disease occurrence. Although patients with pT4 tumors were used to derive the β-binomial estimates of nodal positivity, the risk of occult disease in T4 patients was not reported because the risk of nodal recurrence was uniformly high and adjuvant RAI therapy already would be indicated in such patients. We examined the association of quartile occult nodal disease risk with overall survival (OS) in patients with pathologically node-negative disease. Given the strong dependence of our metric on T stage, we further examined OS within T stage by comparing quartiles of occult nodal risk.
Analyses were independently derived, programmed, and cross-validated by two authors (T.J.R. and S.T.) to ensure accurate derivation and reproducibility of the mathematical model using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). The VGAM package was used to fit α and β parameters of the β-binomial distribution using a maximum likelihood approach.17 Survival analysis was completed using the Kaplan-Meier method. The remaining analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Sensitivity Analyses Limited to Patients With Central Neck LNs
The intent of the main analysis was to assess the adequacy of LN evaluation for all patients who underwent neck dissection, not just patients undergoing PCLND for cN0 disease. To address this latter clinical scenario, we performed sensitivity analyses restricted to patients who were most likely to have only undergone central neck lymphadenectomy. A direct variable to indicate PCLND was not available; therefore, to select for these patients, we restricted our analyses to patients who only had positive central neck LNs (pathologic stage N1a) and then further limited analysis to patients who were clinically node-negative (cN0).
RESULTS
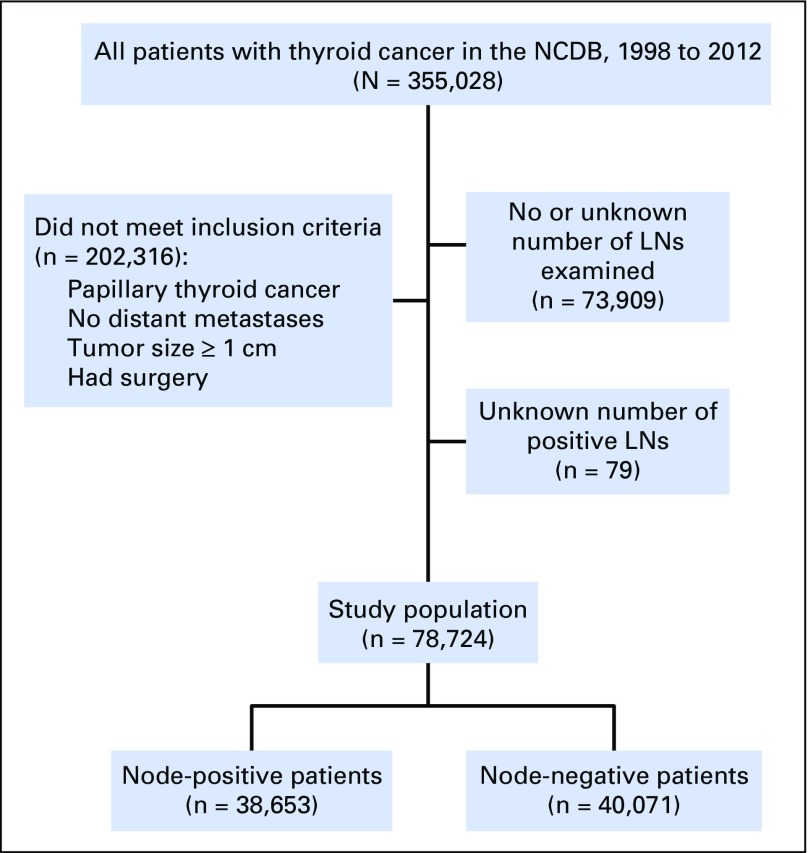
Patient Cohort
Overall, 78,724 patients met inclusion criteria for modeling (Fig 1). Of these, 38,653 patients (49.1%) were observed to have node-positive disease and were analyzed to derive estimates of false-negative disease as a function of the number of LNs examined. The remaining 40,071 patients with pN0 disease were incorporated into estimates of true LN-positive disease prevalence among the overall cohort. Patient characteristics were reflective of the general population of patients with papillary thyroid cancer (Table 1). Factors associated with node-positive versus node-negative disease included advanced T stage, extrathyroidal extension, positive surgical margins, and male sex. Node-positive disease prevalence did not vary substantially by geographic region, patient insurance, or income status. Patients with pathologic LN-positive disease were significantly more likely to undergo adjuvant RAI than patients with LN-negative disease (69.6% v 58.3%, respectively; P < .001).
Fig 1.
CONSORT diagram. LN, lymph node; NCDB, National Cancer Database.
Table 1.
Patient Demographic, Clinical, and Pathologic Characteristics on the Basis of Lymph Node Status
| Characteristic | No. of Patients (%) | P* | ||
|---|---|---|---|---|
| All Patients (N = 78,724) | Node-Negative (n = 40,071) | Node-Positive (n = 38,653) | ||
| Median age, years (IQR) | 44 (34-54) | 45 (36-55) | 41 (32-53) | < .001 |
| Median LNs examined, No. (IQR) | 4 (1-9) | 2 (1-5) | 7 (3-17) | < .001 |
| T stage | < .001 | |||
| 1 | 30,369 (38.6) | 19,184 (47.9) | 11,185 (28.9) | |
| 2 | 24,322 (30.9) | 13,253 (33.1) | 11,069 (28.6) | |
| 3 | 17,878 (22.7) | 6,319 (15.8) | 11,559 (29.9) | |
| 4 | 6,155 (7.8) | 1,315 (3.3) | 4,840 (12.5) | |
| Race | < .001 | |||
| White | 67,992 (86.4) | 34,754 (86.7) | 33,238 (86) | |
| Black | 3,379 (4.3) | 1,973 (4.9) | 1,406 (3.6) | |
| Other | 5,548 (7) | 2,411 (6) | 3,137 (8.1) | |
| Margins† | < .001 | |||
| Positive | 12,666 (16.1) | 3,810 (9.5) | 8,856 (22.9) | |
| Negative | 61,544 (78.2) | 34,583 (86.3) | 26,961 (69.8) | |
| RAI use | 50,264 (63.8) | 23,348 (58.3) | 26,916 (69.6) | < .001 |
| Facility type | < .001 | |||
| Academic | 36,215 (46) | 17,857 (44.6) | 18,358 (47.5) | |
| Community | 4,504 (5.7) | 2,295 (5.7) | 2,209 (5.7) | |
| Comprehensive | 37,974 (48.2) | 19,912 (49.7) | 18,062 (46.7) | |
| Extrathyroidal extension | 16,745 (21.3) | 4,497 (11.2) | 12,248 (31.7) | < .001 |
| Sex | < .001 | |||
| Female | 59,981 (76.2) | 32,820 (81.9) | 27,161 (70.3) | |
| Male | 18,743 (23.8) | 7,251 (18.1) | 11,492 (29.7) | |
| Facility location | < .001 | |||
| Midwest | 18,329 (23.3) | 9,220 (23) | 9,109 (23.6) | |
| Northeast | 19,648 (25) | 10,863 (27.1) | 8,785 (22.7) | |
| South | 24,319 (30.9) | 12,365 (30.9) | 11,954 (30.9) | |
| West | 16,428 (20.9) | 7,623 (19) | 8,805 (22.8) | |
| Insurance status | < .001 | |||
| Private | 60,643 (77) | 31,317 (78.2) | 29,326 (75.9) | |
| Government | 13,264 (16.8) | 6,719 (16.8) | 6,545 (16.9) | |
| Not insured | 2,490 (3.2) | 1,006 (2.5) | 1,484 (3.8) | |
| Income | < .001 | |||
| < $35,000 | 17,217 (21.9) | 8,508 (21.2) | 8,709 (22.5) | |
| ≥ $35,000 | 58,290 (74) | 29,912 (74.6) | 28,378 (73.4) | |
| Surgery | < .001 | |||
| Total thyroidectomy | 73,006 (92.7) | 36,384 (90.8) | 36,622 (94.7) | |
| Lobectomy | 3,999 (5.1) | 2,966 (7.4) | 1,033 (2.7) | |
| Other surgery | 1,719 (2.2) | 721 (1.8) | 998 (2.6) | |
NOTE. Percentages may not add up to 100% as a result of rounding or missing values.
Abbreviations: IQR, interquartile range; LN, lymph node; RAI, radioactive iodine.
χ2 test for categorical variables and t test for continuous variables.
Margin status was either missing or could not be assessed in 5.7% of patients.
Step 1: Estimate Risk of False-Negative LN Dissection as a Function of Number of LNs Examined
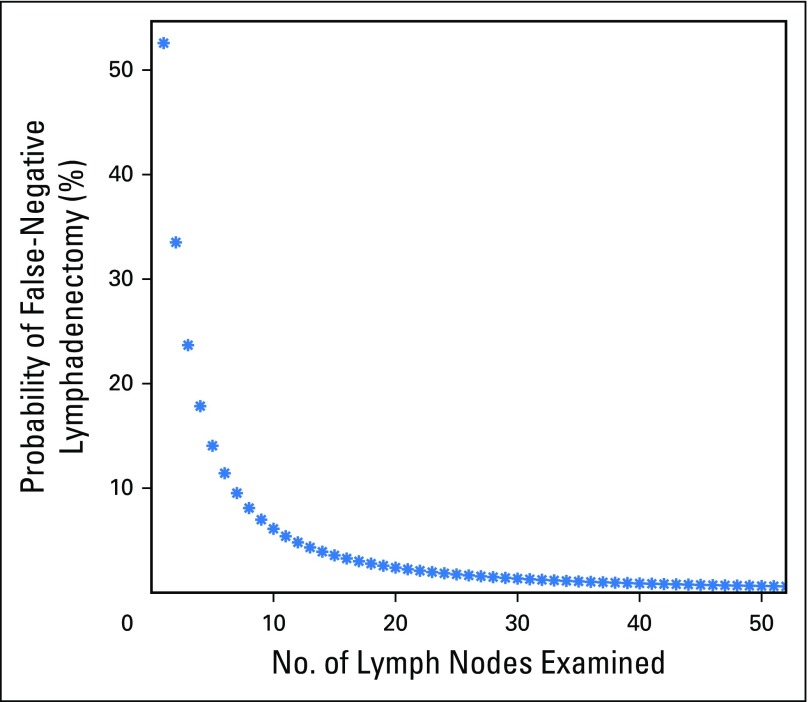
The distribution of the percentage of positive metastatic LNs among all patients with at least one positive LN (n = 38,653) was fit using a β-binomial distribution with resulting model parameter estimates of α = 1.54 (95% CI, 1.51 to 1.57) and β = 1.71 (95% CI, 1.67 to 1.75). This single set of parameters was then used to estimate the probability of false-negative disease as a function of the number of LNs examined. The probability of a false-negative LN dissection was estimated at 53%, 33%, 24%, 18%, 14%, 11%, and less than 10% for one, two, three, four, five, six, and greater than six LNs examined, respectively (Fig 2).
Fig 2.
Probability of a false-negative lymphadenectomy as a function of number of lymph nodes examined in a patient with truly lymph node–positive disease.
Step 2: Estimate True-Positive LN Prevalence
True prevalence of node-positive disease in these patients was then estimated for the overall population of 78,724 patients. The observed (reported) and derived true (reported plus estimated false negative) prevalence rates were then estimated for the population overall and by pathologic T stage (Table 2). The observed percentage of patients with positive LNs varied by T1b-4 stage (36.9% to 78.6%) and was substantially higher after accounting for false negatives (47.1% to 98.4%). Because the risk of estimated true LN-positive disease was so high in patients with pathologic T4 disease, these patients were removed from subsequently presented estimates of occult nodal disease.
Table 2.
Estimated True Prevalence of Lymph Node–Positive Disease by Pathologic T Stage
| Prevalence | All Patients (%) | Pathologic T Stage (%) | |||
|---|---|---|---|---|---|
| T1b | T2 | T3 | T4 | ||
| Observed prevalence | 49.1 | 36.8 | 45.5 | 64.7 | 78.6 |
| Corrected (true) prevalence | 62.1 | 47.1 | 59.3 | 78.8 | 98.4 |
Step 3: Risk of Occult Nodal Disease
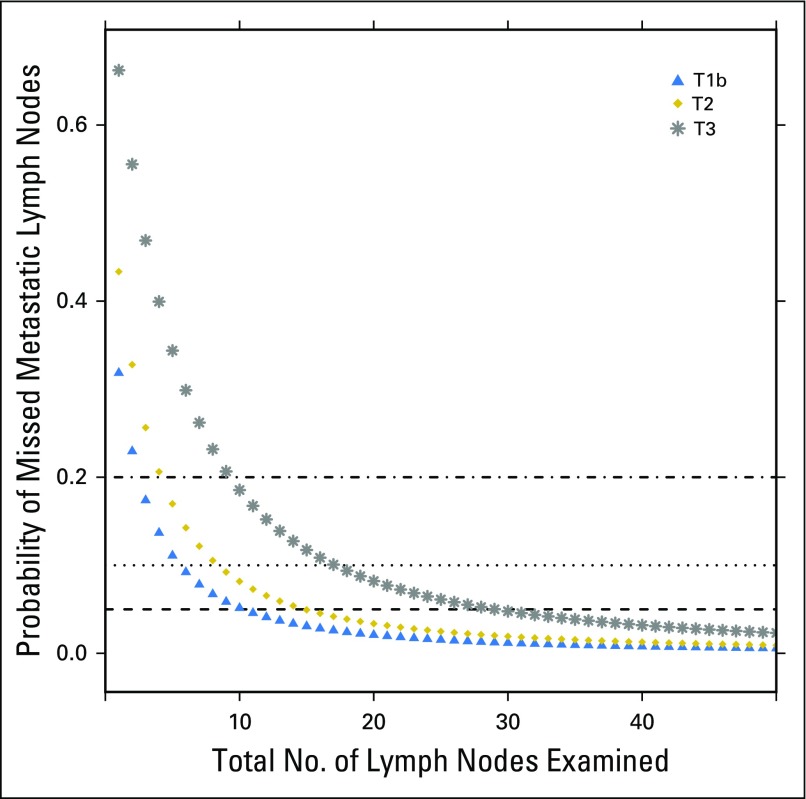
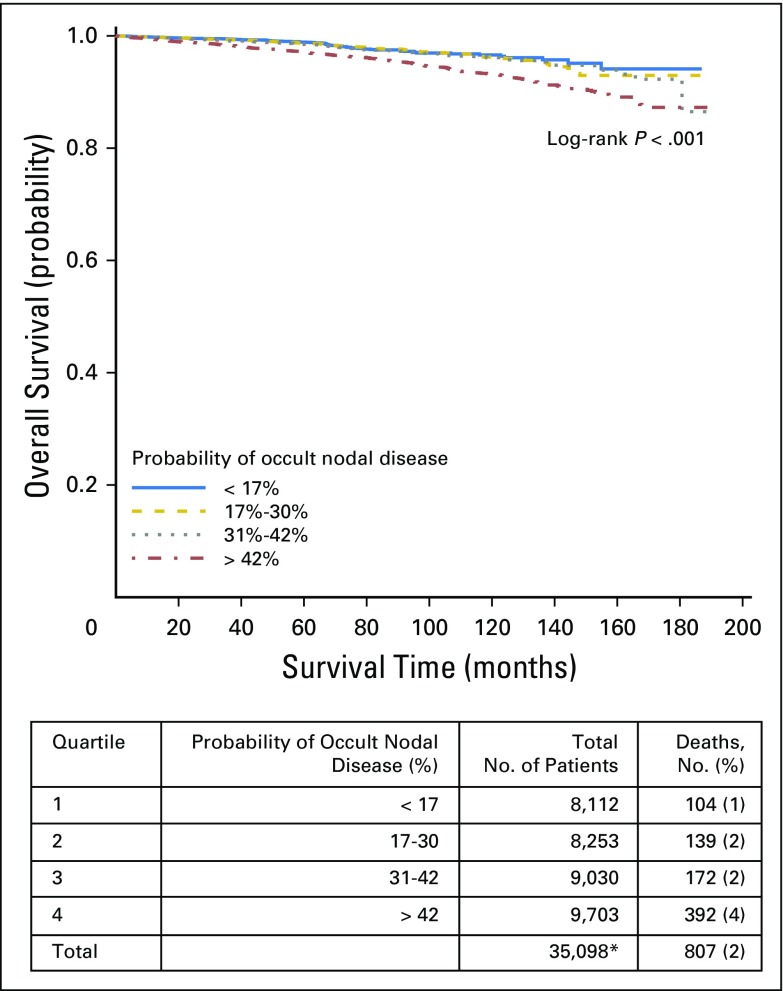
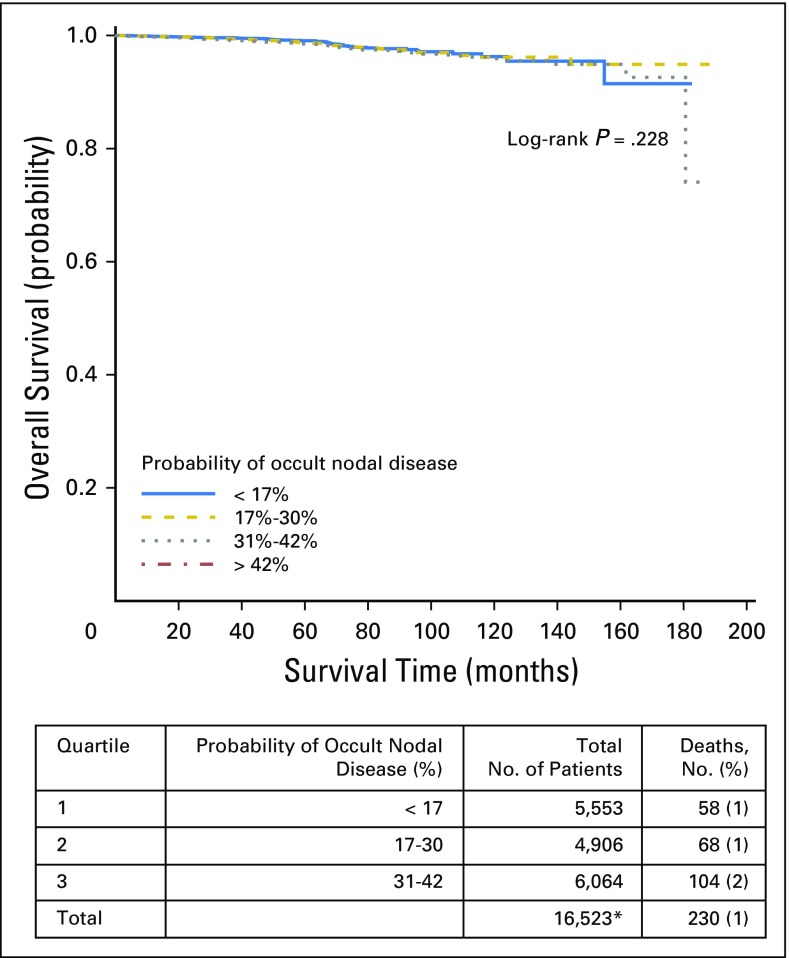
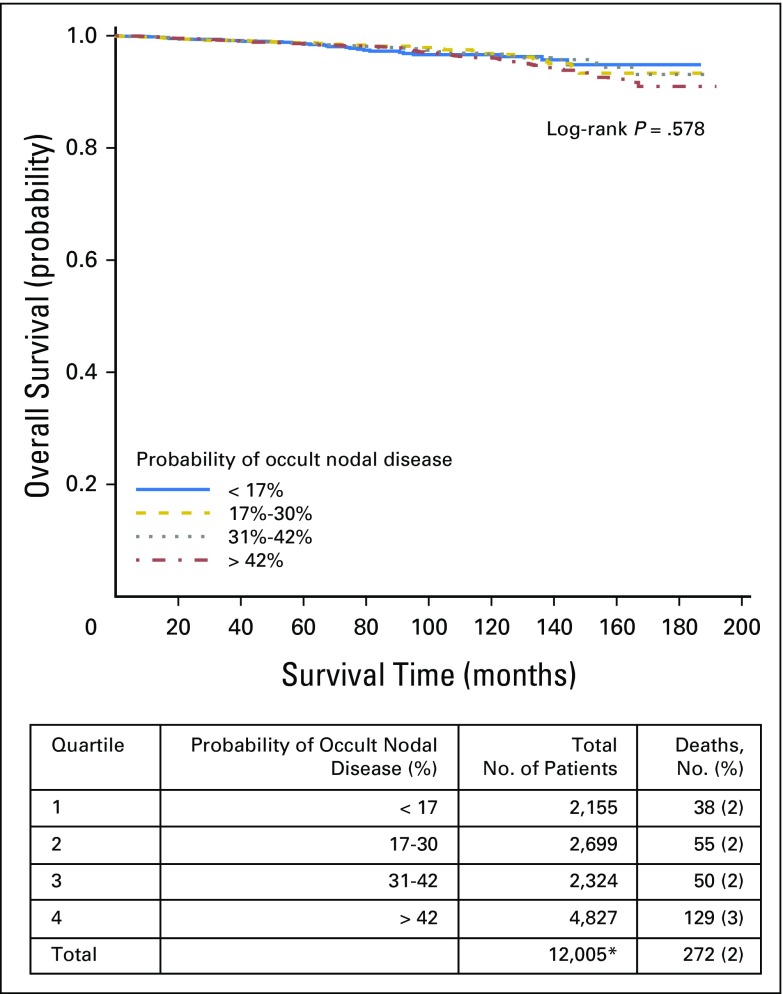
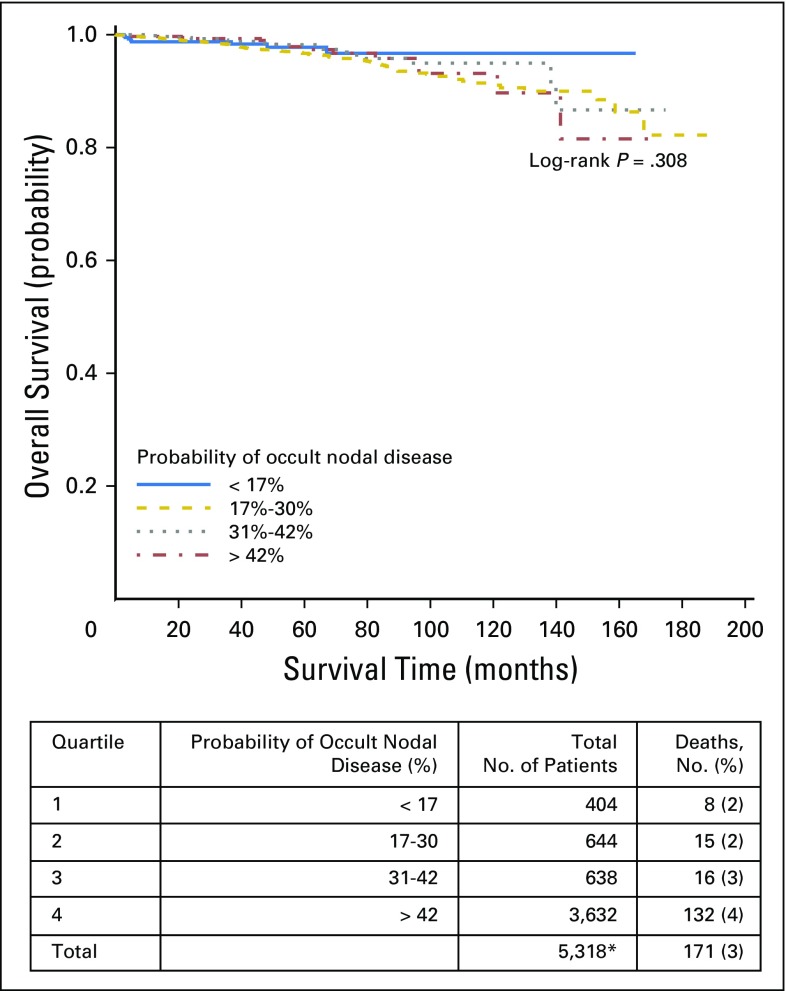
Finally, we combined our corrected estimates of the true LN-positive prevalence with the likelihood of missing a positive LN to estimate the risk of residual occult nodal disease after surgical resection. To rule out occult nodal disease with 90% confidence, a total of six, nine, and 18 nodes would need to be examined for patients with pT1b, pT2, and pT3 disease, respectively (Fig 3). Quartile of occult nodal disease risk was significantly associated with OS (log-rank P < .001). Patients with the highest quartile probability of occult nodal disease experienced an inferior 10-year OS of 93%, compared with an OS of 96% to 97% in the remaining quartiles. There was no significant difference in OS between quartiles of occult nodal disease within tumor stage. However, for both T2 and T3 tumors, patients with the lowest risk of occult nodal disease had numerically higher OS (Appendix Figs A1, A2, A3, and A4, online only).
Fig 3.
Probability of occult nodal disease on the basis of American Joint Committee on Cancer tumor stage.
Sensitivity Analyses Limited to Patients With Central Neck LNs
For patients with only pN1a nodes resected from the central compartment, our refit parameters were α = 1.59 (95% CI, 1.53 to 1.65) and β = 1.68 (95% CI, 1.62 to 1.74). Further limiting to patients to those with clinically node-negative presentation (cN0) yielded α = 1.74 (95% CI, 1.63 to 1.86) and β = 2.05 (95% CI, 1.92 to 2.20). Compared with the main analysis, both sensitivity analyses estimated that fewer LNs would need to be removed to achieve 90% confidence of having no occult residual disease as a function of T stage, with three, four, and eight LNs needed for removal for T1b, T2, and T3 stages, respectively.
DISCUSSION
To our knowledge, our study provides the first empirically based estimates of the risk of occult nodal disease in patients with papillary thyroid cancer as a function of primary tumor features and number of LNs examined in patients who have undergone surgery for localized disease ≥ 1 cm in size. Our estimates suggest that six, nine, and 18 LNs would need to be removed to ensure a reasonably adequate LN evaluation across all clinical scenarios for patients with T1b, T2, and T3 disease, respectively. As few as three, four, and eight LNs may need to be removed in patients with T1b, T2, and T3 disease, respectively, undergoing true PCLND. These estimates are meant to provide an objective determination of adequacy of the LN yield for patients with papillary thyroid cancer and to help standardize the interpretation of PCLND dissection. This may guide subsequent management in patients for whom substantial variation in clinical practice currently exists. By providing patients with additional objective risk information, patient preference may be more readily incorporated into treatment decisions.
We conservatively estimate an ability to predict the absence of any occult residual cervical nodal metastases with 90% certainty after the prophylactic surgical removal and examination of six, nine, and 18 negative nodes among patients with T1b, T2, and T3 tumors, respectively. In addition, understanding how many LNs would need to be examined is particularly important for surgeons who may be performing PCLND for low- and intermediate-risk tumors. Our analysis is not meant to advocate for strict PCLND per se for all low- and intermediate-risk tumors, nor do we advocate for extensive central neck dissections for all patients; instead, we argue against the berry-picking approach, offering physicians and patients a guide of adequacy of LN yield, if such lymphadenectomy is deemed to be necessary or indicated.
The goal of personalized medicine has been one of the major pushes in cancer care18 and has been used to provide risk-adapted treatment strategies in many areas,19,20 but it has been slow to inform the management of thyroid cancer.21,22 Our aim was to provide surgeons, pathologists, endocrinologists, and patients an objective estimate and intuitive sense of the risk of harboring occult nodal disease. These results may help inform the management of low-, intermediate-, and high-risk tumors by providing guidance for surgeons in the extent of lymphadenectomy and for pathologists in the need for careful specimen evaluation to optimize LN yield. The findings of this study are less pertinent for patients with extremely low-risk tumors, such as intrathyroidal, solitary papillary thyroid cancers with no evidence of clinical LN involvement on preoperative evaluation, because these patients are known to have excellent outcomes regardless of the extent of surgical management and RAI administration.5,14
Nearly half of patients with differentiated thyroid cancer have clinically occult LNs at diagnosis.23 Preoperative ultrasound is currently used in the majority of patients.24,25 Other imaging modalities such as magnetic resonance imaging, positron emission tomography,26 and computed tomography25 have been shown to have low sensitivity (approximately 50% to 62% or less) for detecting metastatic LNs. In the absence of accurate nonsurgical staging and objective data, there is a general clinical intuition that patients with limited LN dissections may be at higher risk for harboring occult disease.27 This concern leads some surgeons to perform a PCLND at the time of initial thyroidectomy in the absence of any clinical or radiologic evidence of nodal disease. This approach is most often used in patients in whom high risk of nodal disease or recurrence is suspected prior to surgery (ie, more advanced tumors) or in whom adjuvant RAI may not be readily available (ie, Japan). There is currently no recommended number of LNs that need to be evaluated to guide the certainty that a patient is in fact LN negative. There also is a paucity of data quantifying the risk of occult nodal disease that is needed to guide the intensity of surveillance and use of adjuvant therapy in these patients. For patients who have only had limited dissections but who have higher risk features on final pathology, physicians are left with challenging decisions regarding intensity of adjuvant care and surveillance.
Our study approach has several key distinctions. First, all equations and computational models were independently derived by two of the authors and varied slightly from the equations described in the past.15 We hope to have provided a transparent methodology that can be readily translated to other clinical scenarios in oncology where objective risk estimates are required to aid in postoperative oncologic management. Although further validation studies may be warranted, randomized trials in the adjuvant management of clinically node-negative, differentiated thyroid cancer are unlikely.
The current study has several limitations. First, the study was retrospective, and therefore, unobserved associations between key variables and other potential confounders intrinsic to retrospective studies could not be appreciated. Although prospective validation is ideal, in papillary thyroid cancer, the long natural history of the disease has made this difficult, if not impossible. In fact, few randomized trials of adjuvant management strategies have been reported, and the ability to conduct a randomized trial to address this question is not thought to be feasible.28 Second, we make several necessary assumptions in our model. We have taken care to explicitly state any assumptions made that would need to hold in order for the provided estimates to be accurate. It should be noted that the cohort used in the initial modeling was derived from patients who had at least two LNs examined. As a result, we believe this cohort, if anything, may slightly overestimate the risk of residual nodal disease. To address this point, we conducted sensitivity analyses limited to patients with central neck disease only (pathologic N1a) with and without clinically node-negative disease (cN0). Both of these analyses yielded similar α and β parameter fits as the main analysis, but suggested that patients with clinically node-negative (cN0) presentation undergoing PCLND would require fewer LNs removed to assure adequate evaluation. It should be noted that such analyses are limited to the extent that the recorded clinical nodal status within the National Cancer Database is accurate. Our work may be particularly helpful in providing clinicians the objective reassurance needed to avoid unnecessary adjuvant RAI in patients with limited PCLND and associated risks. Third, the surgical intent of patients in our study is unknown. Although our study does not directly inform the current debate surrounding PCLND in these patients,29 it does provide a more objective means with which to interpret the value of a negative dissection. Fourth, to achieve adequate power, a long study period was necessary; although it is possible that some clinical or pathologic changes occurred over 14 years, these are unlikely to be significant and are impossible to account at a patient level. In fact, it has been shown that for medullary thyroid cancer, no changes in diagnosis, treatment, or outcomes were seen in more than three decades in the United States.30 The practice of PCLND was called to attention with the 2009 American Thyroid Association guidelines,31 which were interpreted as supporting its use, even though there was a scarcity of evidence in the literature. LN berry picking and prophylactic lateral neck dissection have never been advocated; thus, we do not believe that these would affect our findings. Finally, we did not have genomic or other conventionally personalized information on patients that might impact their competing risks of recurrence versus non–cancer-related mortality.
Our study provides the first empirically based estimates of occult nodal disease in patients with papillary thyroid cancer as a function of primary tumor features and number of LNs examined. These objective risk estimates could be used to guide the determination of adequacy of the LN yield, particularly for patients with low-, intermediate-, and high-risk tumors, as well as the need for adjuvant RAI and surveillance in patients for whom treatment is not currently standardized and for whom substantial nonclinical (eg, geographic, practice level) variation exists. We hope that use of our model will allow clinicians to better determine the risk of occult disease, the benefit of LN evaluation, and the adequacy of the LN yield to reduce the rates of overtreatment for patients with low-risk tumors and undertreatment of those with higher risk tumors. In the end, this information may be used to counsel patients regarding their individual disease risk and allow them to participate more actively in decisions surrounding their management.
Appendix
Supplemental Methods
The goal of this study was to provide an objective assessment of the degree of certainty with which a surgeon could exclude the risk of occult nodal disease after neck dissection in patients with papillary thyroid cancer. To do this, we quantified the negative predictive value (NPV) of a pathologically node-negative lymph node (LN) evaluation after definitive surgery. We use the following notation and equations:
Where TN indicates true-negative lymph node evaluation, FN indicates false-negative lymph node evaluation (pathologically negative but occult disease present), and TP indicates true positive (observed LN positive).
Modeling algorithm.
Step 1 is to estimate the probability of having a false-negative neck evaluation (ie, the probability of having no positive LNs observed in a patient with truly LN-positive disease), and then step 2 is to estimate the number of false-negative patients in our sample using equation 1. These data were (step 3) used to calculate the corrected prevalence of LN disease in our sample and then (step 4) used to calculate the NPV as a function of the number of LNs examined and the primary tumor stage. We assumed that there were no false-positive patients (ie, all pathologically LN-positive patients were considered to be truly LN positive). All stage information used was pathologic stage after definitive initial surgery. Our method, although independently rederived with subsequent adjustments, is based on previous work initially described in colon cancer.15
Step 1: Estimate Probability of a (False) Negative LN Evaluation in a Patient Who Has One or More Positive LNs as a Function of the Number of LNs Examined [P (FNm)]
We estimated the probability of a false-negative LN evaluation as the probability of removing only negative LNs from a patient in whom one or more positive LNs were present. We use m to indicate the number of LNs examined.
To do this, we first modeled the percentage of LNs that were observed to be pathologically positive within the cohort of all patients with two or more LNs removed (n = 33,826 of 38,653 patients with LN-positive disease). This was completed using a β-binomial distribution to fit a flexible probability distribution of the percentage of LNs that were positive among patients with any known LN-positive disease. A maximum likelihood approach using the R package VGAM (R Foundation for Statistical Computing, Vienna, Austria) was used to estimate a distribution with shape parameters of α = 1.540 (95% CI, 1.507 to 1.574) and β = 1.708 (95% CI, 1.670 to 1.745). It should be noted that a single set of α and β parameters (reported earlier) were fit across all patients with known LN-positive disease per the original Gönen et al15 approach.
We then calculated P(FNm) at each possible value of number of nodes examined:
Step 2: Estimate Number of Patients Who Were False LN-Negative
To calculate the NPV, we next needed to estimate the number of false LN-negative patients within our cohort of all patients with one or more LNs removed (N = 78,724). We computed the number of false-negative patients at each value of m (#FNm) as follows:
Where m indicates number of nodes examined, P(FNm) indicates probability of missing nodal disease when m nodes are examined, and #TPm indicates number of patients identified as node-positive when m nodes were examined.
Step 3: Calculate Apparent and Corrected Prevalence of Patients With True LN-Positive Disease
Next, we calculated the apparent (observed) prevalence of LN disease and then estimated the true prevalence of LN disease within the overall study population by accounting for the number of false negatives derived earlier.
Apparent prevalence was calculated as:
Corrected prevalence was calculated, by summing over all m, as:
Step 4: Determine Probability of Occult LN Disease
Last, we calculated the NPV of an LN evaluation at each value of m (NPVm) as:
Fig A1.
Overall survival by quartiles of probability of occult nodal disease in all tumor stages. (*) Includes node-negative patients with available survival data only.
Fig A2.
Overall survival by quartiles of probability of occult nodal disease in patients with stage T1 disease. (*) Includes node-negative patients with available survival data only.
Fig A3.
Overall survival by quartiles of probability of occult nodal disease in patients with stage T2 disease. (*) Includes node-negative patients with available survival data only.
Fig A4.
Overall survival by quartiles of probability of occult nodal disease in patients with stage T3 disease. (*) Includes node-negative patients with available survival data only.
Footnotes
Supported by departmental funds and by P30 Cancer Center Support Grant No. P30-CA014236 (S.T. and T.H.).
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Timothy J. Robinson, Samantha Thomas
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
How Many Lymph Nodes Are Enough? Assessing the Adequacy of Lymph Node Yield for Papillary Thyroid Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Timothy J. Robinson
No relationship to disclose
Samantha Thomas
No relationship to disclose
Michaela A. Dinan
Consulting or Advisory Role: Salix, Sandoz
Research Funding: Genzyme, AstraZeneca
Sanziana Roman
No relationship to disclose
Julie Ann Sosa
Consulting or Advisory Role: Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry, which is supported by NovoNordisk, GlaxoSmithKline, AstraZeneca, and Eli Lilly through UBC.
Terry Hyslop
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca
Patents, Royalties, Other Intellectual Property: Targeted Diagnostics and Therapeutics
REFERENCES
- 1.Cancer Genome Atlas Network : Comprehensive molecular characterization of human colon and rectal cancer Nature 487:330–337,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alizadeh AA Eisen MB Davis RE, etal: Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling Nature 403:503–511,2000 [DOI] [PubMed] [Google Scholar]
- 3.Adam MA Pura J Goffredo P, etal: Impact of extent of surgery on survival for papillary thyroid cancer patients younger than 45 years J Clin Endocrinol Metab 100:115–121,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beadle GW, Tatum EL: Genetic control of biochemical reactions in neurospora Proc Natl Acad Sci U S A 27:499–506,1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdueva D Wing MR Schaub B, etal: Experimental comparison and evaluation of the Affymetrix exon and U133Plus2 GeneChip arrays PLoS One 2:e913,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goffredo P Thomas SM Dinan MA, etal: Patterns of use and cost for inappropriate radioactive iodine treatment for thyroid cancer in the United States: Use and misuse JAMA Intern Med 175:638–640,2015 [DOI] [PubMed] [Google Scholar]
- 7.Basu A Raghunath M Bishayee S, etal: Inhibition of tyrosine kinase activity of the epidermal growth factor (EGF) receptor by a truncated receptor form that binds to EGF: Role for interreceptor interaction in kinase regulation Mol Cell Biol 9:671–677,1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho SY Lee TH Ku YH, etal: Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia Surgery 157:111–118,2015 [DOI] [PubMed] [Google Scholar]
- 9.Laird AM Gauger PG Miller BS, etal: Evaluation of postoperative radioactive iodine scans in patients who underwent prophylactic central lymph node dissection World J Surg 36:1268–1273,2012 [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1002/bjs.10036. Nixon IJ, Wang LY, Ganly I, et al: Outcomes for patients with papillary thyroid cancer who do not undergo prophylactic central neck dissection. Br J Surg 103:218-225, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang TS Cheung K Farrokhyar F, etal: A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer Ann Surg Oncol 20:3477–3483,2013 [DOI] [PubMed] [Google Scholar]
- 12.Adamson ED, Wiley LM: The EGFR gene family in embryonic cell activities Curr Top Dev Biol 35:71–120,1997 [DOI] [PubMed] [Google Scholar]
- 13. Affymetrix: Alternative transcript analysis methods for exon arrays. Affymetrix Whitepaper 2005. http://www.affymetrix.com/sup port/technical/whitepapers.affx.
- 14.Anscher MS: Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy Oncologist 15:350–359,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gönen M, Schrag D, Weiser MR: Nodal staging score: A tool to assess adequate staging of node-negative colon cancer J Clin Oncol 27:6166–6171,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edge S, Byrd DR, Compton CC, et al (eds): American Joint Committee on Cancer Staging Manual (ed 7). New York, NY, Springer, 2011.
- 17.Bemmo A Benovoy D Kwan T, etal: Gene expression and isoform variation analysis using Affymetrix exon arrays BMC Genomics 9:529,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RS, Willard HF, Snyderman R: Personalized health planning Science 300:549,2003 [DOI] [PubMed] [Google Scholar]
- 19.Anscher MS, Vujaskovic Z: Mechanisms and potential targets for prevention and treatment of normal tissue injury after radiation therapy Semin Oncol 32:S86–S91,2005. suppl 3 [DOI] [PubMed] [Google Scholar]
- 20.Antonarakis ES Lu C Wang H, etal: AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer N Engl J Med 371:1028–1038,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball CA Brazma A Causton H, etal: Submission of microarray data to public repositories PLoS Biol 2:E317,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbee MS Ogunniyi A Horvat TZ, etal: Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology Ann Pharmacother 49:907–937,2015 [DOI] [PubMed] [Google Scholar]
- 23.Alroy I, Yarden Y: The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions FEBS Lett 410:83–86,1997 [DOI] [PubMed] [Google Scholar]
- 24.Anczuków O, Krainer AR: The spliceosome, a potential Achilles heel of MYC-driven tumors Genome Med 7:107,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger MF Levin JZ Vijayendran K, etal: Integrative analysis of the melanoma transcriptome Genome Res 20:413–427,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.André F Michiels S Dessen P, etal: Exonic expression profiling of breast cancer and benign lesions: A retrospective analysis Lancet Oncol 10:381–390,2009 [DOI] [PubMed] [Google Scholar]
- 27.Anscher MS Thrasher B Zgonjanin L, etal: Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury Int J Radiat Oncol Biol Phys 71:829–837,2008 [DOI] [PubMed] [Google Scholar]
- 28.Carling T Carty SE Ciarleglio MM, etal: American Thyroid Association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma Thyroid 22:237–244,2012 [DOI] [PubMed] [Google Scholar]
- 29. doi: 10.1089/thy.2015.0020. Haugen BRM, Alexander EK, Bible KC, et al: 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid 26:1-133, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roman S, Lin R, Sosa JA: Prognosis of medullary thyroid carcinoma: Demographic, clinical, and pathologic predictors of survival in 1252 cases Cancer 107:2134–2142,2006 [DOI] [PubMed] [Google Scholar]
- 31.Cooper DS Doherty GM Haugen BR, etal: Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer Thyroid 19:1167–1214,2009 [DOI] [PubMed] [Google Scholar]