Abstract
Purpose
There is a considerable variability in the level of molecular responses achieved with imatinib therapy in patients with chronic myeloid leukemia (CML). These differences could result from variable therapy adherence.
Methods
Eighty-seven patients with chronic-phase CML treated with imatinib 400 mg/d for a median of 59.7 months (range, 25 to 104 months) who had achieved complete cytogenetic response had adherence monitored during a 3-month period by using a microelectronic monitoring device. Adherence was correlated with levels of molecular response. Other factors that could influence outcome were also analyzed.
Results
Median adherence rate was 98% (range, 24% to 104%). Twenty-three patients (26.4%) had adherence ≤ 90%; in 12 of these patients (14%), adherence was ≤ 80%. There was a strong correlation between adherence rate (≤ 90% or > 90%) and the 6-year probability of a 3-log reduction (also known as major molecular response [MMR]) in BCR-ABL1 transcripts (28.4% v 94.5%; P < .001) and also complete molecular response (CMR; 0% v 43.8%; P = .002). Multivariate analysis identified adherence (relative risk [RR], 11.7; P = .001) and expression of the molecular human organic cation transporter-1 (RR, 1.79; P = .038) as the only independent predictors for MMR. Adherence was the only independent predictor for CMR. No molecular responses were observed when adherence was ≤ 80% (P < .001). Patients whose imatinib doses were increased had poor adherence (86.4%). In this latter population, adherence was the only independent predictor for inability to achieve an MMR (RR, 17.66; P = .006).
Conclusion
In patients with CML treated with imatinib for some years, poor adherence may be the predominant reason for inability to obtain adequate molecular responses.
INTRODUCTION
Chronic myeloid leukemia (CML) is characterized by a consistent chromosomal abnormality (the Philadelphia [Ph] chromosome), which carries a unique fusion gene, termed BCR-ABL1.1 In the absence of treatment, CML is inexorably fatal. Imatinib is an adenosine triphosphate analog that selectively inhibits the enhanced tyrosine kinase activity of the Bcr-Abl1 oncoprotein and induces durable cytogenetic responses in the majority of patients with a relatively benign adverse effect profile.1 In approximately 75% of patients, the Ph chromosome is no longer detectable after 2 years of therapy (for a status referred to as complete cytogenetic response [CCyR]).2,3 The achievement of CCyR is the major objective of therapy, because it is associated with prolonged survival.2,3 In patients who achieve CCyR, BCR-ABL1 transcript levels may be monitored to assess the quantity of residual leukemia, and results are often expressed as the log10 reduction from a standardized value for untreated patients. It is generally accepted that CCyR corresponds to an approximately 2-log reduction in transcript levels.4 By 5 years, approximately 50% of patients will have achieved a 3-log reduction in transcript levels (defined as a major molecular response [MMR])3; this confers additional clinical benefit,5 and it is also considered an important therapeutic target.6 With continued treatment, approximately 20% to 30% of patients eventually achieve a 4-log reduction; in at least 10% of patients, the transcripts will become undetectable (ie, complete molecular response [CMR]).3 In some instances, durable CMR may be the equivalent of cure, as it is possible to discontinue the imatinib in some of these patients without subsequent relapse.7 The reasons underlying the different responses in different patients are unclear, but they may be caused in part by the intrinsic heterogeneity of CML.8,9
The extent to which patients with cancer comply with prescribed oral anticancer therapy ranges between 16% and 100%, depending on the specific therapy and method used to measure adherence.10 The methods used to monitor drug adherence include self reporting, frequency of repeat prescriptions, pill counts, drug plasma levels, and various microelectronic monitoring systems (MEMS). These systems consist of an electronic device fitted in the cap of a normal-looking medication bottle that automatically records each time the bottle is opened. Although, when using MEMS, one cannot be certain that the specified daily dose is actually taken each time the patient opens the bottle, MEMS are considered the gold standard for measuring adherence.11–14 The other methods are less accurate, as people may be reluctant to admit bad behavior or may remove unused tablets before returning the bottle. As adherence declines, pill counts become even less accurate.12,13 Moreover, patients who are persistently noncompliant tend to take their medication on the day before they visit their physician, thereby giving a false impression of adherence when drug levels are measured.12–14 In two different studies performed in heterogeneous cohorts of patients with CML, adherence to imatinib was estimated at approximately 75%15 (using refilling prescriptions) and 90%16 (using pill counts). In the latter study, higher adherence rates were associated with better outcome.16
We designed a clinical study to determine whether imatinib adherence correlates with degree of molecular response in which adherence was monitored by using MEMS. We also considered clinical variables previously shown to predict response together with other factors identified more recently (ie, expression of human organic cation transporter-1 [hOCT1],17,18 polymorphisms in multidrug resistance gene-1 [MDR1, or ABCB1],19 mutations in the tyrosine kinase domain [KD] of BCR-ABL1,20 and imatinib plasma levels).21,22
METHODS
Patient Variables and Treatment
Between April 2008 and February 2009, 99 consecutive adults with BCR-ABL1- positive CML in chronic phase who had received imatinib as first therapy for some years were offered enrollment on the study at Hammersmith Hospital. Three refused participation. Of the 96 patients enrolled, two were lost to follow-up, and seven others were excluded from the study because they could not be monitored with MEMS (because four patients were using dosing boxes and because the MEMS malfunctioned for three patients). Eighty-seven patients constituted the basis of this report. The study protocol was approved by the research ethics committee, and patients gave written informed consent. Patients were eligible if imatinib was started within 6 months of diagnosis while in first chronic phase.2 Imatinib was prescribed initially as 400 mg daily to be taken in a single dose. Patients were eligible for the study if they had been treated with imatinib for 2 years or longer (median, 59.7 months; range, 25 to 104 months) and were able to tolerate at least 400 mg daily. All patients were in CCyR at the time of enrollment. Before inclusion on the study, patients had been monitored in our center, as described elsewhere.3 For patients who failed to achieve MMR but who were tolerating the 400-mg dose well (ie, grade 1 or lower toxicity), the dose had been increased to 600 mg daily between 18 and 24 months after starting imatinib. Table 1 lists the patient characteristics.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | Patients (N = 87) |
|---|---|
| Age, years | |
| At diagnosis | |
| Median | 45.4 |
| Range | 20.9-86.4 |
| At enrollment | |
| Median | 50.7 |
| Range | 25.5-89.0 |
| Sex, % | |
| Male | 56.3 |
| Weight, kg | |
| Median | 74.0 |
| Range | 40.0-119.7 |
| Sokal risk group, % | |
| Low | 37.9 |
| Intermediate | 36.8 |
| High | 25.3 |
| Hemoglobin at diagnosis, g/L | |
| Median | 116 |
| Range | 69-160 |
| Leukocyte count at diagnosis, × 109/L | |
| Median | 139.5 |
| Range | 5.1-410.9 |
| BCR-ABL1 transcript type, % | |
| e13a2 | 37.9 |
| e14a2 | 46.0 |
| e13a2 and e14a2 | 16.1 |
| BCR-ABL1/ABL1 ratio at diagnosis, % | |
| Median | 73.2 |
| Range | 10.1-334.3 |
| Tyrosine kinase domain mutations at enrollment* | 1.2 |
| Imatinib plasma level at end of trial, μg/mL | |
| On 400 mg daily | |
| Median | 0.9 |
| Range | 0.4-1.6 |
| On 600 mg daily | |
| Median | 1.3 |
| Range | 0.6-3.5 |
| MDR1 polymorphism, % | |
| C/C | 86.2 |
| T/C | 13.8 |
| hOCT1/GUSB transcript ratio at diagnosis | |
| Median | 0.16 |
| Range | 0.013-3.5 |
| Time from diagnosis to imatinib therapy, months | |
| Median | 2.2 |
| Range | 0-5.1 |
| Time from imatinib therapy to enrollment, months | |
| Median | 59.7 |
| Range | 25-104 |
| Patients with MMR | |
| % | 65.5 |
| Probability at 6 years | 69.7 |
| Time to MMR, months | |
| Median | 20.4 |
| Range | 9-63 |
| Patients with a 4-log reduction | |
| % | 42.5 |
| Probability at 6 years | 55.0 |
| Time to 4-log reduction, months | |
| Median | 33 |
| Range | 9-63 |
| Patients with CMR | |
| % | 25.3 |
| Probability at 6 years | 32.1 |
| Time to CMR, months | |
| Median | 45.6 |
| Range | 9-69 |
Abbreviations: MMR, major molecular response; CMR, complete molecular response; MDR1, multidrug resistance gene-1; hOTC1, human organic cation transporter-1; GUS, glucuronidase.
One patient had the kinase domain mutation Q252H at the beginning of the monitoring period. In a second patient, the mutation T315I was found at the end of the monitoring period. Both patients had a low adherence rate (87 and 79%, respectively).
Adherence Measures
Patients were monitored for a median of 91 days (range, 84 to 120 days) by using MEMS (Aardex, Zug, Switzerland). Patients were told that their adherence was going to be monitored by counting the number of imatinib tablets returned, but they were not told about the monitoring system in the bottle caps. Because the half-life of imatinib is long, the adherence rate for each patient was defined as the dose that was taken according to the MEMS reading expressed as a percentage of the dose prescribed during the total duration of the study.
Laboratory Assessments
BCR-ABL1 transcripts were measured in the blood at 6- to 12-week intervals from diagnosis and at the beginning and end of the monitoring period by using real-time quantitative polymerase chain reaction (Q-PCR).3,23–25 Results were expressed as log10 reductions from a standardized baseline according to the international scale.26 BCR-ABL1 KD mutation analysis was performed as described elsewhere.20
Trough imatinib plasma levels were measured as previously reported.21 Patients who took the imatinib in the evenings were instructed to take it in the mornings for the 3 days before the clinic visit. This was verified with the MEMS. The levels of hOCT1 transcripts were measured by Q-PCR in a peripheral-blood sample obtained at diagnosis. Briefly, primers and probes for quantitating hOCT1 were designed by using ABI Gene Express 1.5 software (ABI, Warrington, United Kingdom; Appendix Table A1, online only). Expression was measured in triplicate using the Taqman system on a 7,500 platform (ABI) with standard thermal cycling conditions, and β-glucuronidase (GUSB) was the endogenous control. The hOCT1 levels were expressed as the ratio of OCT1 to GUSB from the same sample. The polymorphism 1236C/T in ABCB1 was genotyped by using pyrosequencing.20,27 Oligonucleotide primers for genotyping were designed with PSQ Assay Design software (Biotage, Uppsala, Sweden; Appendix Table A1). Results were analyzed by using PyroMark Q24 software (Biotage, Uppsala, Sweden).
Statistical Methods
The probabilities of molecular response were calculated with the Kaplan-Meier method. Univariate analyses to identify prognostic factors for molecular response were carried out with the log-rank test. Variables significant at P < .20 were entered into a proportional hazards regression analysis; a forward-stepping procedure was employed to find the best model. The proportional hazards assumption was confirmed by adding a time-dependent covariate for each covariate. Tests for interactions were carried out, but none was found to have statistical significance. The relation between the different prognostic factors and the response at 18 months was explored by using a logistic regression model. Groups were compared by using Fisher's exact test for categoric data and the Mann-Whitney Test for quantitative data. P values were two sided.
RESULTS
Long-Term Adherence to Imatinib
For the 87 evaluable patients, the median adherence measured by MEMS was 97.6% (range, 22.6% to 103.8%). Tables 2 and 3 show the proportion of patients with different adherence rates. The adherence rates did not differ significantly in patients who had been taking the imatinib for different lengths of time (always beyond the second year); for example, the median adherence rate for the 11 patients monitored during the third year of therapy was 98.8%, a value similar to the 99.4% for the 12 patients monitored during the eighth year (Data Supplement, online only).
Table 2.
Six-Year Probability of MMR, 4-Log Reduction in Transcript Levels, and CMR and Degree of Adherence
| Adherence Rate (%) | No. of Patients | Six-Year Probability of Response |
|||||
|---|---|---|---|---|---|---|---|
| MMR |
4-Log Reduction |
CMR |
|||||
| % | P | % | P | % | P | ||
| ≥100 | 36 | 91.1 | .01 | 79.9 | .02 | 46.7 | .02 |
| ≤ 99 | 51 | 58.6 | 38.6 | 22.7 | |||
| > 95 | 57 | 94.5 | < .001 | 77.2 | < .001 | 45.2 | .002 |
| ≤ 95 | 30 | 29.3 | 15.0 | 8.2 | |||
| > 90 | 64 | 93.7 | < .001 | 76.0 | < .001 | 43.8 | .002 |
| ≤ 90 | 23 | 13.9 | 4.3 | 0 | |||
| > 85 | 69 | 85.8 | < .001 | 69.2 | .001 | 40.8 | .007 |
| ≤ 85 | 18 | 11.8 | 5.6 | 0 | |||
| > 80 | 75 | 81.2 | .001 | 63.8 | .005 | 37.1 | .04 |
| ≤ 80 | 12 | 0 | 0 | 0 | |||
NOTE. The median adherence rates for patients with a rate of ≤ 99%, ≤ 95%, ≤ 90%, ≤ 85%, and ≤ 80% were 93.5%, 81.7%, 76.0%, 73.9%, and 63.1%, respectively.
Abbreviations: MMR, major molecular response; CMR, complete molecular response.
Table 3.
Relation Between the 18-Month Responses and the Degree of Adherence
| Adherence Rate (%) | No. of Patients | Patients With Responses at 18 Months |
|||||
|---|---|---|---|---|---|---|---|
| MMR |
4-Log Reduction |
||||||
| No. | % | P | No. | % | P | ||
| ≥ 100 | 36 | 20 | 55.5 | .1 | 14 | 38.8 | .4 |
| ≤ 99 | 51 | 19 | 37.2 | 15 | 29.4 | ||
| > 95 | 57 | 34 | 59.6 | .002 | 25 | 43.9 | .013 |
| ≤ 95 | 30 | 5 | 16.7 | 4 | 13.3 | ||
| > 90 | 64 | 37 | 57.8 | < .001 | 28 | 43.7 | .004 |
| ≤ 90 | 23 | 2 | 8.7 | 1 | 5.6 | ||
| > 85 | 69 | 38 | 55.1 | < .001 | 28 | 40.6 | .001 |
| ≤ 85 | 18 | 1 | 5.6 | 1 | 5.6 | ||
| > 80 | 75 | 39 | 52 | < .001 | 29 | 38.7 | .007 |
| ≤ 80 | 12 | 0 | 0 | 0 | 0 | ||
NOTE. The median adherence rates for patients with a rate of ≤ 99%, ≤ 95%, ≤ 90%, ≤ 85%, and ≤ 80% were 93.5%, 81.7%, 76.0%, 73.9%, and 63.1%, respectively.
Abbreviation: MMR, major molecular response.
Achievement of a Molecular Response Is Related to the Adherence to Imatinib Therapy
The adherence rate was strongly associated with prior achievement of MMR (RR, 1.093; P < .001), with achievement of 4-log reduction (RR, 1.104; P = .002) and with achievement of CMR (RR, 1.135; P = .012). Table 2 and Figure 1 show the 6-year probability of MMR, 4-log reduction, and CMR according to adherence rates. Similar results were found when patients taking imatinib 400 and 600 mg/d were considered separately. We also correlated adherence rates with the specific molecular responses achieved at the 18-month time point, as shown in Table 3.
Fig 1.
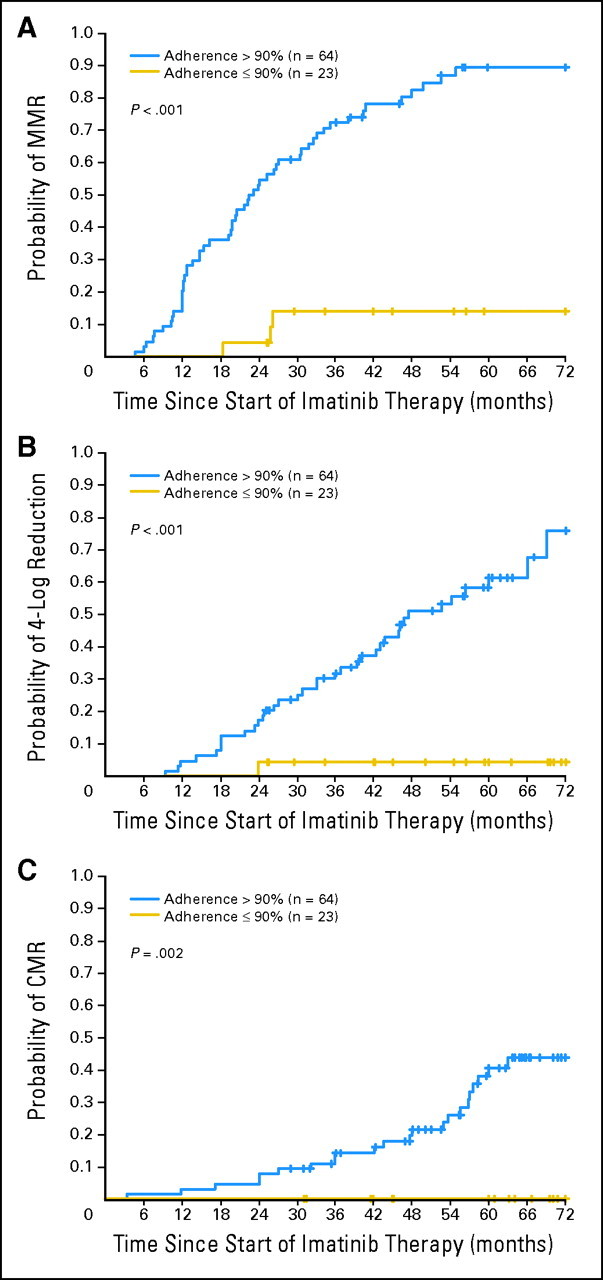
Six-year probability of major molecular response (MMR), 4-log reduction in transcript levels, and complete molecular response (CMR) in the 87 enrolled patients according to the measured adherence rate. The probability of MMR for the 23 patients with an adherence rate ≤ 90% was 13.9%, whereas the probability was 93.7% for the 64 patients with an adherence rate greater than 90% (P < .001). Similarly, the probability of a 4-log reduction was 4.3% versus 76% (P < .001), and the probability of CMR was 0% versus 43.8% (P = .002).
Adherence to Therapy Is the Critical Factor for Achieving Molecular Responses
We studied the influence on achievement of MMR, 4-log reduction, and CMR of most of the important prognostic factors recognized to date. Tables 4 and 5 show results of the univariate analysis.
Table 4.
Patient Characteristics and 6-Year Probability of MMR, 4-Log Reduction in Transcript Levels, and CMR at Diagnosis
| Variable at Diagnosis | No. of Patients | Response |
|||||
|---|---|---|---|---|---|---|---|
| MMR |
4-Log Reduction |
CMR |
|||||
| % | P | % | P | % | P | ||
| Sex | |||||||
| Male | 49 | 68.1 | .45 | 53.8 | .2 | 26.9 | .29 |
| Female | 38 | 70.3 | 59.5 | 39.1 | |||
| Age, years | |||||||
| ≤ 45 | 42 | 66.4 | .056 | 44.2 | .07 | 21.0 | .052 |
| > 45 | 45 | 80.2 | 65.8 | 46.1 | |||
| RR | 1.020 | .06 | 1.013 | .27 | 1.015 | .15 | |
| Sokal risk group | .49 | .98 | .36 | ||||
| Low | 33 | 77.8 | 67.8 | 54.1 | |||
| Intermediate | 32 | 69.4 | 47.0 | 31.1 | |||
| High | 22 | 61.3 | 47.1 | 24.8 | |||
| Hemoglobin, g/L | |||||||
| ≤ 115 | 40 | 59.2 | .036 | 39.5 | .03 | 14.7 | .011 |
| > 115 | 47 | 80.7 | 69.1 | 47.6 | |||
| RR | 1.186 | .012 | 1.323 | .01 | 1.209 | .07 | |
| Leukocytes, × 109/L | |||||||
| ≤ 140 | 44 | 78.8 | .012 | 56.7 | .022 | 35.4 | .017 |
| > 140 | 43 | 63.1 | 37.6 | 28.1 | |||
| RR | 0.996 | .008 | 0.996 | .015 | .996 | .11 | |
| BCR-ABL1 transcript type | |||||||
| e14a2 | 40 | 78.1 | .05 | 56.9 | .024 | 34.1 | .29 |
| e13a2 | 33 | 63.5 | 35.7 | 20.3 | |||
| e13a2 and e14a2 | 14 | 76.5 | 57.6 | 38.5 | |||
| BCR-ABL1/ABL1 ratio, % | |||||||
| ≤ 100 | 44 | 71.4 | .25 | 53.0 | .038 | 32.7 | .1 |
| > 100 | 43 | 52.6 | 26.6 | 8.4 | |||
| RR | .996 | .44 | .971 | .002 | .979 | .13 | |
| hOCT1 transcript level | |||||||
| ≤ 0.16 | 30 | 55.2 | < .001 | 42.0 | .01 | 16.6 | .02 |
| > 0.16 | 30 | 81.4 | 64.8 | 45.3 | |||
| RR | 2.199 | < .001 | 1.990 | .001 | 1.665 | .04 | |
| MDR1 polymorphism | |||||||
| C/C | 75 | 71.1 | .9 | 57.8 | .35 | 30.8 | .8 |
| T/C | 12 | 68.7 | 36.5 | 37.1 | |||
Abbreviations: MMR, major molecular response; CMR, complete molecular response; RR, relative risk; hOCT1, human organic cation transporter-1; MDR1, multidrug resistance gene-1.
Samples were not available in 27 patients. For this reason, we repeated the multivariate analysis excluding this variable. The adherence rate and WBC were the only independent predictors for the achievement of MMR; adherence rate was the only independent predictor for the achievement of a 4-log reduction and CMR. In three patients, the trough plasma level was not available.
Table 5.
Patient Characteristics and 6-Year Probability of MMR, 4-Log Reduction in Transcript Levels, and CMR While on Study
| On-Study Variable | No. of Patients | Response |
|||||
|---|---|---|---|---|---|---|---|
| MMR |
4-Log Reduction |
CMR |
|||||
| % | P | % | P | % | P | ||
| Age, years | |||||||
| ≤ 50 | 42 | 62.8 | .03 | 45.5 | .06 | 16.3 | .01 |
| > 50 | 45 | 82.7 | 63.3 | 49.9 | |||
| RR | 1.021 | .037 | 1.015 | .23 | 1.021 | .06 | |
| Weight, kg | |||||||
| ≤ 74 | 40 | 72.7 | .45 | 53.6 | .99 | 26.2 | .49 |
| > 74 | 47 | 68.2 | 56.4 | 43.9 | |||
| RR | .992 | .34 | 1.000 | .99 | 1.004 | .76 | |
| Imatinib plasma level, μg/mL*† | |||||||
| ≤ 1 | 43 | 60.1 | .02 | 53.0 | .07 | 23.3 | .14 |
| > 1 | 41 | 83.2 | 68.0 | 44.4 | |||
| RR | 2.11 | .01 | 2.50 | .06 | 2.25 | .09 | |
| Adherence rate, % | |||||||
| > 90 | 64 | 93.7 | < .001 | 76.0 | < .001 | 43.8 | .002 |
| ≤ 90 | 23 | 13.9 | 4.3 | 0 | |||
| RR | 1.093 | < .001 | 1.104 | .002 | 1.135 | .012 | |
NOTE. The relative risks and their P values are provided for quantitative variables.
Abbreviations: MMR, major molecular response; CMR, complete molecular response; RR, relative risk.
In three patients, the trough plasma level was not available.
When we considered only the patients receiving imatinib 400 mg/d, the RRs for MMR, 4-log reduction, and complete cytogenic response were 2.62 (P = .01), 2.83 (P = .046), and 2.38 (P = .08), respectively.
Imatinib plasma levels measured at the end of the study were significantly associated with prior MMRs (RR, 2.11; P = .01; Table 4). When we subclassified the patients according to the plasma level, as previously reported,21 we found that patients with imatinib levels less than 1 μg/mL had a lower probability of being in MMR (Table 5). Similar results were found when we considered only the patients taking imatinib 400 mg (Table 5). Imatinib plasma levels were not correlated with outcome when we considered only the patients receiving imatinib 600 mg.
Higher levels of hOCT1 transcripts at diagnosis significantly predicted for the achievement of MMR (RR, 2.199; P < .001), 4-log reduction (RR, .69; P = .001), and CMR (RR, 1.665; P = .045). The patients with an hOCT1 transcript level less than the median value (ie, 0.16) had a lower probability of 6-year MMR, of 4-log reduction, and of CMR that the patients with higher levels (Table 4).
We analyzed the relative influence of the various factors in multivariate analysis. The degree of adherence to imatinib therapy (ie, greater or less than 90%) and hOCT1 transcript level (ie, greater or less than the median) were the only two independent factors for MMR in the multivariate analysis (RRs, 11.17 [P = .001] and 1.79 [P = .038], respectively). The degree of adherence to therapy and the hOCT1 transcript level were the only independent factors for the achievement of a 4-log reduction; RRs were 19.35 (P < .001) and 1.74 (P = .048), respectively. The degree of adherence to therapy was the only independent factor for achieving CMR (RR, 19.35; P = .004). Similar results were found when the variables were considered as continuous and when analysis was limited to patients receiving imatinib 400 mg daily (data not shown).
We also considered the prognostic influence of the achievement of an early molecular response (EMR), defined as having achieved a 1-log reduction (BCR-ABL/ABL ratio ≤ 10%) by 3 months.28,29 The 32 patients with EMR had a superior probability of achieving MMR, a 4-log reduction in transcript levels, and CMR compared with the 55 patients without EMR (namely, 81.8% v 62% [P < .001]; 76.7% v 42.7% [P = .001], and 46.5% v 22.4% [P = .006]). When we included EMR in the multivariate model presented in the Methods, we found that adherence rate and EMR were the only independent factors for MMR (RR, 14.2 [P < .001] and 2.8 [P < .001], respectively) and a 4-log reduction in transcript levels (RR, 18.9 [P < .001] and 2.6 [P = .004], respectively), but the adherence rate remained the only independent factor for CMR. Patients with high hOCT1 expression had a greater probability of EMR that patients with low expression (52.1% v 17.1; P = .006).
Adherence to Imatinib Is the Most Important Factor Contributing to Molecular Responses but Is Poor After Dose Increase
The median adherence rate in the 32 patients who had their dose of imatinib increased was 86% (range, 57.3% to 103.6%), which is significantly lower that that observed in the 55 patents who remained on imatinib 400 mg (98.8%; P = .021). Higher adherence rates were associated with achievement of MMR and a 4-log reduction (Fig 2).
Fig 2.
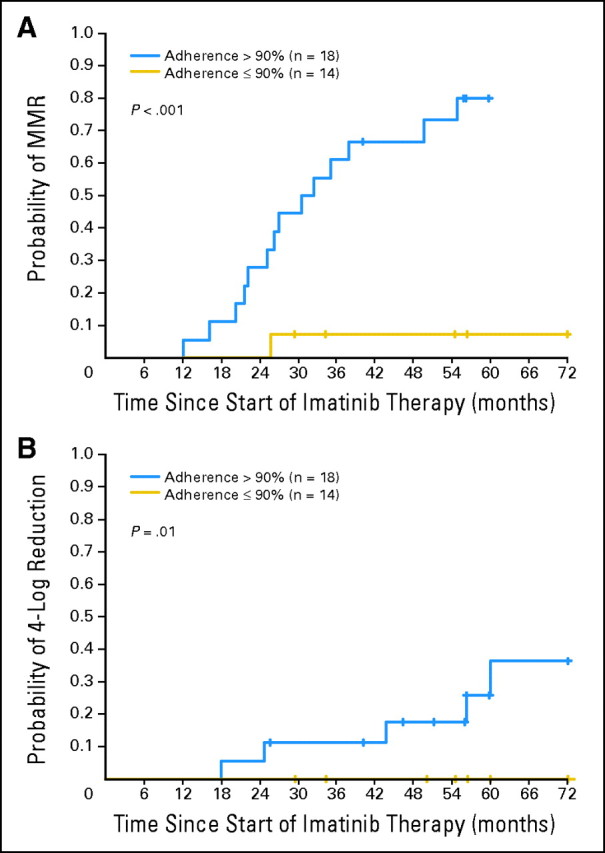
Six-year probability of major molecular response (MMR) and 4-log reduction in the transcript level in the 32 patients who had their dose of imatinib increased according to the measured adherence rate. Higher adherence rates were associated with achievement of MMR (relative risk [RR], 1.105; P = .008) and 4-log reduction (RR, 1.095; P = .026). Only three patients receiving imatinib 600 mg achieved complete molecular response (CMR), so we did not perform an analysis for this outcome. The 14 patients with an adherence rate ≤ 90% had a lower 6-year probability of MMR and 4-log reduction in transcript levels than the 18 patients with greater than 90% adherence (7.1% v 80% [P = .002] and 0% v 36.3% [P = .01]). Similar results were achieved when we considered other cutoff points for the adherence rate. The 6-year probabilities of MMR were 18.6% for the 17 patients with an adherence rate ≤ 95% versus 88.2% for the 15 patients with a rate greater than 95% (P < .001); 9.1% for the 11 patients with an adherence rate ≤ 85% versus 67.9% for the 21 patients with a rate greater than 85% (P = .006); and 0% for the seven patients with an adherence rate ≤ 80% versus 60.7% for the 25 patients with a rate greater than 80% (P = .02).
We repeated the univariate analysis limited to the patients on imatinib 600 mg and included the variables listed in Tables 4 and 5 and included EMR (data not shown). The degree of adherence to imatinib (greater or less than 90%) and hOCT1 transcript levels (greater or less than the median) were the only two factors for MMR in the univariate analysis, and RRs were 17.66 (P = .006) and 1.89 (P = .03), respectively. Adherence was the only independent predictor for MMR. Similar results were found when the variables were considered continuous (data not shown).
Low Adherence Rate is More Frequent in Young Patients, in Patients With Adverse Effects, and in Patients With Unexplained Increases in BCR-ABL1 Transcript Levels
Younger patients were more likely to have a lower adherence rate. The median age for patients with an adherence rate ≤ 90% was 43.8 years compared with 53.8 years for patients with a rate greater than 90% (P = .004). We found significantly lower adherence rates in patients with asthenia, nausea, muscle cramps, and bone or joint pains and in patients who took imatinib independently of the meals (data not shown).
Unexplained five-fold increases in BCR-ABL1 transcript levels at any time during follow-up were predictive for poor adherence. Ten (44%) of 23 patients with unexplained increases had adherence rates ≤ 90%, whereas only 10 (16%) of 64 patients with no significant change in transcript levels had an adherence rate ≤ 90% (P = .01).
DISCUSSION
Patients with CML vary greatly in their responses to therapy, as demonstrated originally by Sokal et al9 in 1984, and the same variation is seen in patients treated with imatinib in the modern era. The basis for this variation is unknown, but it has been attributed to the intrinsic biologic heterogeneity of the leukemia. Lack of adherence to oral therapy for chronic diseases is a well-recognized problem,10–12,30 and we have confirmed this, because 26.4% of patients with a potentially fatal disease fail to adhere to optimal dosing at a median of 5 years from diagnosis. Ideally, a study of the influence of adherence on prognosis would be performed in newly diagnosed patients and would require prolonged follow-up to ascertain the interactions between prognostic features, adherence, and overall outcome. To conduct such a study with the MEMS would be difficult, if not impossible. We therefore chose to conduct our study on a group of patients in stable CCyR in whom we could study drug behavior together with other known prognostic factors and could evaluate any possible effect on molecular responses. In doing so, we accepted that our study could not address the impact of adherence on early failure of imatinib, in which patients destined to experience progression early in the disease would do so irrespective of drug compliance. Conversely, it is quite possible that some of the patients who did not respond to imatinib in the first 2 years failed to respond or lost an initial response primarily because the adherence was poor; in that case, our study underestimates the overall level of adherence in a total population of patients who start treatment with imatinib. Although we accept these limitations, we have clearly shown that adherence to therapy at a median of 5 years from diagnosis is associated with the molecular response at 18 months and, indeed, is the most important factor influencing the depth of response in patients in CCyR. In practice, no CMRs were observed when adherence was ≤ 90%, and no MMRs were observed when adherence was ≤ 80%.
In this study, the only other factor influencing molecular responses was the level of expression of hOCT1 at diagnosis. The molecular transporter hOCT1 is responsible for the active intracellular intake of imatinib, and low levels have been associated with poor cytogenetic and molecular responses17,18,31,32 Our study confirms these findings. We also showed that EMR was predictive for subsequent achievement of greater degrees of molecular response28,29 and that patients with higher levels of hOCT1 were more likely to achieve EMR. We could not confirm the previously reported association between polymorphism in the MDR1 gene and achievement of MMR,19 possibly because we only studied patients with chronic phase disease in CCyR. We found KD mutations in two patients (Table 1); in both instances, the patients had low adherence rates. However, this did not allow us to establish a clear relation between the degree of adherence and the development of KD mutations. We did confirm the previously reported association between imatinib plasma levels and achievement of MMR21,22; we also found a trend toward higher probability of 4-log reduction and CMR in patients with higher imatinib plasma levels, although the predictive value of imatinib levels disappeared when adherence was taken into account.
Adherence in patients who had their imatinib dose increased was significantly lower than in the patients who remained on 400 mg/d; in many patients on the higher dose, adherence was ≤ 85%. Adherence was the only independent factor associated with molecular response after the dose of imatinib was increased. Moreover, responses to dose increases were rare when the adherence rate was ≤ 90%. It is not clear from our study whether the low adherence behavior had developed before the dose of imatinib was increased (and had, therefore, been the cause of the initial poor response) or had developed thereafter.
We found lower adherence was associated with younger age. It was also associated with adverse effects, though many patients with mild adverse effects had good adherence rates. Psychological differences between patients or differing perceptions patients have about therapy may account for a significant proportion of nonadherence. It is unclear how adherence could be improved for patients on chronic medication, but various efforts have been reported.33–35 Interestingly we found a significant association between unexplained increases in transcripts observed before enrollment and low adherence. This association is likely to be higher in practice, as some patients may have improved their adherence when they were told that their transcript levels were increasing.
In summary, a substantial proportion of patients with CML treated with imatinib for more than 2 years fail to take a drug that can unequivocally prolong their life and may, in some instances, cure their leukemia. Unfortunately, the relatively poor adherence to imatinib that we have described in this article may apply equally to patients receiving second-generation tyrosine kinase inhibitors.
Acknowledgment
We thank the patients who participated in this study; and Lorraine Armstrong and Robert P. Gale.
Appendix
Comparison of the different methods used to assess adherence.
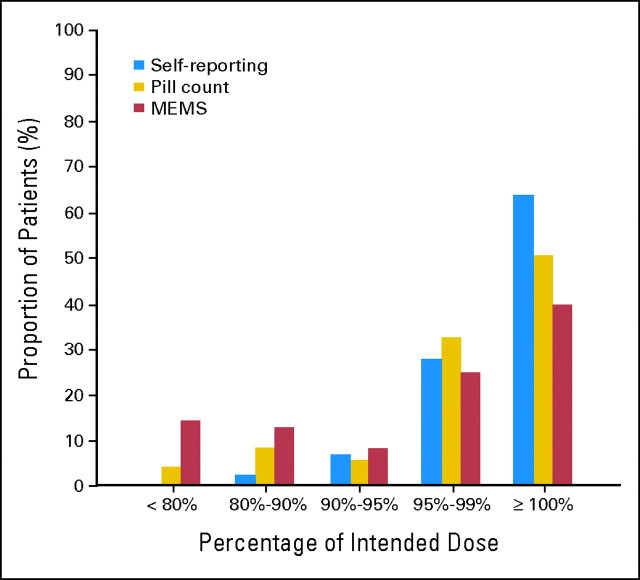
Adherence was measured using microelectronic monitoring system (MEMS; see Methods); with pill recounts; and by questions posed by the treating physician or nurse, who asked the patient to estimate the amount of drug that he or she had missed (ie, self reporting). As expected, pill counts and self reporting underestimated the number of patients with poor adherence when compared with the MEMS (Appendix Fig A1, online only). For example 26.4% of the patients had an adherence rate ≤ 90% according to the MEMS, whereas the pill recount indicated that only 11.5% of the patients had an adherence rate ≤ 90%; the proportion was even lower for self reporting (ie, 3.4%; P for the comparison between MEMS and pill count was < .001 and for the comparison between pill count and self reporting was < .0001). Self reporting and pill count overestimated the adherence rate compared with the MEMS reading even more when the threshold was changed from 90% to 85% or 80%; for example 14% had an adherence rate lower than 80% according to the MEMS reading, and only 4% had that rate according to the pill recount (P = .001). No patient admitted having taken ≤ 80% of the intended dose (Appendix Fig A1). Our study confirms the already-known fact that it is not possible to assess adherence by asking the patient, in particular if the question is posed by people involved in the day-to-day care.11–14 As reported by others,12,13 pill counts were more accurate but did not properly identified the patients with the lowest levels of adherence; for example, nine of the 18 patients with an MEMS adherence rate ≤ 85% and seven of the 12 patients with an MEMS adherence rate ≤ 80% appeared fully compliant according to the pill count.
We found major limitations in the use of imatinib plasma levels to measure adherence.12–14 For example, the median imatinib plasma level in the 18 patients with an adherence rate ≤ 85% was 1.4 μg/mL (range, 0.2 to 4.8 μg/mL). Only three of these patients had a level less than 1 μg/mL. This could be explained by MEMS record, which showed that 13 of these 18 patients had been taking more than one dose daily in the days before the clinic appointment.
We investigated whether the adherence rate when measured by self reporting or pill counts was statistically predictive of the degree of molecular response. The adherence rate when measured by self reporting did not predict for any of the molecular outcomes (data not shown). The adherence rate measured by pill count did predict for the achievement of the various molecular outcomes; the 11 patients with an adherence rate ≤ 90% had a lower 6-year probability of MMR, 4-log reduction in transcript level, and CMR than the 76 patients with an adherence rate greater than 90% (ie, 20.4% v 76.8% [P = .003], 9.1% v 62.7% [P = .03], and 0% v 37.4% [P = .04], respectively). An adherence rate ≤ 90% was an independent predictor for MMR (RR, 6.2; P = .01; RR together with OCT1, 2.1; P = .007) but not for a 4-log reduction or CMR. The adherence rate measured by pill count was not an independent predictor when adherence measured by MEMS was also taken in account.
Fig A1.
Comparison of the three methods utilized to measure adherence. MEMS, microelectronic monitoring systems.
Table A1.
Oligonucleotide Sequences for hOCT1 and MDR1 Primers
| Primer | Sequence |
|---|---|
| hOCT1 | |
| Forward | 5′-GGGCAGCCTGCTCGT-3′ |
| Reverse | 5′-CGGCCAACACACTGATTATG-3′ |
| Probe | 5′-FAM-ATGATTTTTATCTCACCTGACCTGCACTGGTTAA-TAMRA-3′ |
| GUS | |
| Forward | 5′-GAAAATATGTGGTTGGAGAGCTCATT-3′ |
| Reverse | 5′-CCGAGTGAAGATCCCCTTTTTA-3′ |
| Probe | 5′-FAM-CCAGCACTCTCGTCGGTGACTGTTCA-TAMRA-3′ |
| MDR1 | |
| Forward | 5′-TATTCGAAGAGTGGGCACAAA-3′ |
| Reverse | 5′-[Btn]-AACTTCAAGGCAATTCACAGACA-3′ |
| Sequencing | 5′-GGAATTCAGAAATGTTCACT-3′ |
Abbreviations: Btn, biotinylated; hOCT1, human organic cation transporter-1; GUS, glucuronidase; MDR1, multidrug resistance gene-1.
Footnotes
Supported in part from the NIHR Biomedical Research Centre Funding Scheme.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00632255.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: David Marin, Novartis (C), Bristol-Myers Squibb (U); Francois-Xavier Mahon, Novartis Pharma (C), Bristol-Myers Squibb (C); Jane F. Apperley, Novartis (C), Bristol-Myers Squibb (C); Mathieu Molimard, Novartis Pharma (C); John Goldman, Novartis (C), Bristol-Myers Squibb (C) Stock Ownership: None Honoraria: Francois-Xavier Mahon, Bristol-Myers Squibb, Novartis; Dragana Milojkovic, Novartis, Bristol-Myers Squibb Research Funding: David Marin, Novartis; Francois-Xavier Mahon, Novartis, Abbot Expert Testimony: None Other Remuneration: Ritti Desai, Novartis-funded attendance at European Haematology Association conference, June 2009
AUTHOR CONTRIBUTIONS
Conception and design: David Marin, Cristina Guallar
Administrative support: David Marin
Provision of study materials or patients: David Marin, Alexandra Bazeos, Francois-Xavier Mahon, Lina Eliasson, Dragana Milojkovic, Marco Bua, Jane F. Apperley, Ritti Desai, Kasia Kozlowski, Victoria Latham, Letizia Foroni, Mathieu Molimard, Alistair Reid, Katy Rezvani, Hugues de Lavallade, Jamshid S. Khorashad
Collection and assembly of data: David Marin, Lina Eliasson, Kasia Kozlowski, Christos Paliompeis
Data analysis and interpretation: David Marin, Richard Szydlo, John Goldman
Manuscript writing: David Marin, John Goldman
Final approval of manuscript: David Marin, Alexandra Bazeos, Francois-Xavier Mahon, Lina Eliasson, Dragana Milojkovic, Marco Bua, Jane F. Apperley, Richard Szydlo, Ritti Desai, Kasia Kozlowski, Christos Paliompeis, Victoria Latham, Letizia Foroni, Mathieu Molimard, Alistair Reid, Katy Rezvani, Hugues de Lavallade, Cristina Guallar, John Goldman, Jamshid S. Khorashad
REFERENCES
- 1. Goldman JM, Melo JV: Chronic myeloid leukemia: Advances in biology and new approaches to treatment N Engl J Med 349: 1451– 1464,2003. [DOI] [PubMed] [Google Scholar]
- 2. Druker B Guilhot F O'Brien S , etal : Five-year follow-up of imatinib therapy for newly diagnosed chronic myelogenous leukemia in chronic-phase shows sustained responses and high overall survival N Engl J Med 355: 2408– 2417,2006. [DOI] [PubMed] [Google Scholar]
- 3. de Lavallade H Apperley JF Khorashad JS , etal : Imatinib for newly diagnosed patients with chronic myeloid leukemia: Incidence of sustained responses in an intention-to-treat analysis J Clin Oncol 26: 3358– 3363,2008. [DOI] [PubMed] [Google Scholar]
- 4. Lin F Chase A Bungey J , etal : Correlation between the proportion of Philadelphia chromosome-positive metaphase cells and levels of BCR-ABL mRNA in chronic myeloid leukaemia Genes Chromosomes Cancer 13: 110– 114,1995. [DOI] [PubMed] [Google Scholar]
- 5. Marin D Milojkovic D Olavarria E , etal : European LeukemiaNet criteria for failure or sub-optimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor Blood 112: 4437– 4444,2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baccarani M Cortes J Pane F , etal : Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet J Clin Oncol 27: 6041– 6051,2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rousselot P Huguet F Rea D , etal : Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years Blood 109: 58– 60,2007. [DOI] [PubMed] [Google Scholar]
- 8. Gordon MY Marley SB Apperley JF , etal : Clinical heterogeneity in chronic myeloid leukaemia reflecting biological diversity in normal persons Br J Haematol 122: 424– 429,2003. [DOI] [PubMed] [Google Scholar]
- 9. Sokal JE Cox EB Baccarani M , etal : Prognostic discrimination in “good-risk” chronic granulocytic leukemia Blood 63: 789– 799,1984. [PubMed] [Google Scholar]
- 10. Ruddy K, Mayer E, Partridge A: Patient adherence and persistence with oral anticancer treatment CA Cancer J Clin 59: 56– 66,2009. [DOI] [PubMed] [Google Scholar]
- 11. Osterberg L, Blaschke T: Adherence to medication N Engl J Med 353: 487– 497,2005. [DOI] [PubMed] [Google Scholar]
- 12. Partridge AH, Avorn J, Wang PS, Winer EP: Adherence to therapy with oral antineoplastic agents J Natl Cancer Inst 94: 652– 661,2002. [DOI] [PubMed] [Google Scholar]
- 13. Waterhouse DM, Calzone KA, Mele C, Brenner DE: Adherence to oral tamoxifen: A comparison of patient self-report, pill counts, and microelectronic monitoring J Clin Oncol 11: 1189– 1197,1993. [DOI] [PubMed] [Google Scholar]
- 14. Cramer JA, Scheyer RD, Mattson RH: Compliance declines between clinic visits Arch Intern Med 150: 1509– 1510,1990. [PubMed] [Google Scholar]
- 15. Darkow T Henk HJ Thomas SK , etal : Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukaemia Pharmacoeconomics 25: 481– 496,2007. [DOI] [PubMed] [Google Scholar]
- 16. Noens L van Lierde MA De Bock R , etal : Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study Blood 113: 5401– 5411,2009. [DOI] [PubMed] [Google Scholar]
- 17. White DL Saunders VA Dang P , etal : Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: Higher doses of imatinib may overcome the negative impact of low OCT-1 activity Blood 110: 4064– 4072,2007. [DOI] [PubMed] [Google Scholar]
- 18. Wang L Giannoudis A Lane S , etal : Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia Clin Pharmacol Ther 83: 258– 264,2008. [DOI] [PubMed] [Google Scholar]
- 19. Dulucq S Bouchet S Turcq B , etal : Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia Blood 112: 2024– 2027,2008. [DOI] [PubMed] [Google Scholar]
- 20. Khorashad J de Lavallade H Apperley J , etal : The finding of kinase domain mutations in chronic phase CML patients responding to imatinib may identify those at high risk of disease progression J Clin Oncol 26: 4806– 4813,2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Picard S Titier K Etienne G , etal : Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia Blood 109: 3496– 3499,2007. [DOI] [PubMed] [Google Scholar]
- 22. Larson RA Druker BJ Guilhot F , etal : Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: A subanalysis of the IRIS study Blood 111: 4022– 4028,2008. [DOI] [PubMed] [Google Scholar]
- 23. Marin D Kaeda J Szydlo R , etal : Monitoring patients in complete cytogenetic remission after treatment of CML in chronic phase with imatinib: Patterns of residual leukaemia and prognostic factors for cytogenetic relapse Leukemia 19: 507– 512,2005. [DOI] [PubMed] [Google Scholar]
- 24. Kaeda J O'Shea D Szydlo RM , etal : Serial measurement of BCR-ABL transcripts in the peripheral blood after allogeneic stem cell transplantation for chronic myeloid leukemia: An attempt to define patients who may not require further therapy Blood 107: 4171– 4176,2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes T Deininger M Hochhaus A , etal : Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results Blood 108: 28– 37,2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cross NC Hughes TP Hochhaus A , etal : International standardisation of quantitative real-time RT-PCR for BCR-ABL Leuk Res 32: 505– 506,2008. [DOI] [PubMed] [Google Scholar]
- 27. Khorashad JS Anand M Marin D , etal : The presence of a BCR-ABL mutant allele in CML does not always explain clinical resistance to imatinib Leukemia 20: 658– 663,2006. [DOI] [PubMed] [Google Scholar]
- 28. Hughes TP Hochhaus A Branford S , etal : Reduction of BCR-ABL Transcript Levels at 6, 12, and 18 months correlates with long-term outcomes on Imatinib at 72 months: An analysis from the international randomized study of interferon versus STI571 (IRIS) in patients with chronic phase chronic myeloid leukemia 2008. ASH Annual Meeting Abstracts 112 abstr 334 [Google Scholar]
- 29. Branford S Rudzki Z Harper A , etal : Imatinib produces significantly superior molecular responses compared to interferon alfa plus cytarabine in patients with newly diagnosed chronic myeloid leukemia in chronic phase Leukemia 17: 2401– 2409,2003. [DOI] [PubMed] [Google Scholar]
- 30. Partridge AH Wang PS Winer EP , etal : Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer J Clin Oncol 21: 602– 606,2003. [DOI] [PubMed] [Google Scholar]
- 31. Thomas J Wang L Clark RE , etal : Active transport of imatinib into and out of cells: Implications for drug resistance Blood 104: 3739– 3745,2004. [DOI] [PubMed] [Google Scholar]
- 32. White DL Saunders VA Dang P , etal : OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): Reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib Blood 108: 697– 704,2006. [DOI] [PubMed] [Google Scholar]
- 33. Clifford S Barber N Elliott R , etal : Patient-centred advice is effective in improving adherence to medicines Pharm World Sci 28: 165– 170,2006. [DOI] [PubMed] [Google Scholar]
- 34. Stone VE: Strategies for optimizing adherence to highly active antiretroviral therapy: Lessons from research and clinical practice Clin Infect Dis 33: 865– 872,2001. [DOI] [PubMed] [Google Scholar]
- 35. Haynes RB Ackloo E Sahota N , etal : Interventions for enhancing medication adherence Cochrane Database Syst Rev 2008. 2: CD000011 [DOI] [PubMed] [Google Scholar]