Abstract
Purpose
The genetic basis of myelodysplastic syndromes (MDS) is heterogeneous, and various combinations of somatic mutations are associated with different clinical phenotypes and outcomes. Whether the genetic basis of MDS influences the outcome of allogeneic hematopoietic stem-cell transplantation (HSCT) is unclear.
Patients and Methods
We studied 401 patients with MDS or acute myeloid leukemia (AML) evolving from MDS (MDS/AML). We used massively parallel sequencing to examine tumor samples collected before HSCT for somatic mutations in 34 recurrently mutated genes in myeloid neoplasms. We then analyzed the impact of mutations on the outcome of HSCT.
Results
Overall, 87% of patients carried one or more oncogenic mutations. Somatic mutations of ASXL1, RUNX1, and TP53 were independent predictors of relapse and overall survival after HSCT in both patients with MDS and patients with MDS/AML (P values ranging from .003 to .035). In patients with MDS/AML, gene ontology (ie, secondary-type AML carrying mutations in genes of RNA splicing machinery, TP53-mutated AML, or de novo AML) was an independent predictor of posttransplantation outcome (P = .013). The impact of ASXL1, RUNX1, and TP53 mutations on posttransplantation survival was independent of the revised International Prognostic Scoring System (IPSS-R). Combining somatic mutations and IPSS-R risk improved the ability to stratify patients by capturing more prognostic information at an individual level. Accounting for various combinations of IPSS-R risk and somatic mutations, the 5-year probability of survival after HSCT ranged from 0% to 73%.
Conclusion
Somatic mutation in ASXL1, RUNX1, or TP53 is independently associated with unfavorable outcomes and shorter survival after allogeneic HSCT for patients with MDS and MDS/AML. Accounting for these genetic lesions may improve the prognostication precision in clinical practice and in designing clinical trials.
INTRODUCTION
Myelodysplastic syndromes (MDS) are myeloid neoplasms that range from conditions with a near-normal life expectancy to forms that are close to acute myeloid leukemia (AML).1 Their clinical heterogeneity reflects different somatic mutations that cause clonal proliferation and evolution of myelodysplastic cells.2-4
The fact that MDS have highly variable clinical courses makes risk stratification of crucial importance in clinical decision making.1,5 Several prognostic scoring systems based on clinical/hematologic parameters have been developed.6 In 2012, an international collaborative group created the revised International Prognostic Scoring System (IPSS-R), which defines five risk groups with different probabilities of survival and leukemic evolution.7
The only curative treatment for patients with MDS is allogeneic hematopoietic stem-cell transplantation (HSCT), which is considered as a therapeutic option until the age of 65 to 70 years in eligible patients.5 The long-term survival rate is currently approximately 30%.8-12 Reasons for transplantation failure include toxicity of the procedure and disease relapse.
Prognostic scoring systems are currently used to predict the outcome after HSCT.5,12 In particular, disease burden and cytogenetic abnormalities provide information on the risk of disease relapse after the procedure.8-12 Major limitations of the use of these scores include the reliability of some variables based on morphologic evaluation and the fact that cytogenetics is not informative in a large proportion of patients and includes secondary, late genomic events, deriving from the genome instability caused by the founding genetic mutation.4,13
Mutations in several genes have been reported to influence the risk of disease progression and to affect clinical decision making in MDS.14-17 Preliminary data suggest that mutations in TP53, TET2, and DNMT3A genes are associated with a high probability of relapse after HSCT.18
Comprehensive analyses in large patient populations are warranted to correctly estimate the independent effect of each mutation on posttransplantation outcome. To address this question, we used massively parallel sequencing to examine tumor samples for coding mutations in 34 recurrently mutated genes in myeloid neoplasms. Samples were collected from 401 patients with MDS or MDS/AML before HSCT.
PATIENTS AND METHODS
Patients and Study Design
These investigations were approved by the ethics committee of the Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. We studied 401 patients who underwent allogeneic HSCT for primary MDS or AML evolving from MDS (MDS/AML) between 1997 and 2013 and were reported to the Gruppo Italiano Trapianto di Midollo Osseo registry. Diagnosis of MDS was made or revised according to the 2008 WHO criteria.19 Clinical characteristics of patients and transplant procedures are reported in Table 1.20
Table 1.
Demographic Data, Clinical Characteristics, and Transplant-Related Features of Patients With MDS or Acute Myeloid Leukemia Evolving From MDS Who Underwent Allogeneic Hematopoietic Stem-Cell Transplantation (N = 401)
| Variable* | MDS | MDS/AML† | Comparison Between MDS and MDS/AML P |
|---|---|---|---|
| Demographic data and clinical characteristics | |||
| No. of patients (%) | 274 (68) | 127 (32) | |
| Age, years, median (range) | 54 (18-72) | 52 (19-69) | NS |
| Sex, male/female | 156 (57)/118 (43) | 75 (59)/52 (41) | NS |
| WHO classification | |||
| RCUD/RARS/MDS del(5q) | 27 (10) | — | |
| RCMD | 63 (23) | — | |
| RAEB-1 | 69 (25) | — | |
| RAEB-2 | 115 (42) | — | |
| Hemoglobin, g/dL, median (range) | 9.1 (5.8-11.3) | 8.9 (6.2-11.9) | NS |
| Leukocyte count, × 109/L, median (range) | 3.17 (0.2-42.8) | 2.71 (0.12-38.9) | NS |
| Neutrophil count, × 109/L, median (range) | 1.9 (0.09-32.3) | 1.69 (0.1-9.1) | NS |
| Platelet count, × 109/L, median (range) | 87 (2-862) | 71 (3-433) | NS |
| Transfusion dependency‡ | 159 (58) | 85 (67) | NS |
| IPSS-R risk | 260 (95) | 119 (94) | |
| Low | 47 (18) | — | < .001 |
| Intermediate | 75 (29) | 7 (6) | |
| High | 96 (37) | 64 (54) | |
| Very high | 42 (16) | 48 (40) | |
| Transplant-related features | |||
| Time from diagnosis to HSCT, months (range) | 10.1 (2-189.3) | 8.9 (2-20.5) | NS |
| Type of donor | |||
| Sibling | 151 (55) | 77 (61) | NS |
| Unrelated donor (MUD)§ | 123 (45) | 50 (39) | |
| Source of hematopoietic stem cells | |||
| Peripheral blood/cord blood | 181 (66) | 75 (61) | NS |
| Bone marrow | 93 (34) | 52 (41) | |
| Remission-induction chemotherapy | 85 (31) | 121 (95) | < .001 |
| Complete remission | 42 (49) | 69 (57) | NS |
| Conditioning regimen‖ | |||
| Standard conditioning regimen | 175 (64) | 77 (61) | NS |
| Reduced-intensity conditioning | 99 (36) | 50 (39) |
NOTE. All values are expressed as the number of patients (%), unless otherwise specified.
Abbreviations: HSCT, hematopoietic stem-cell transplantation; IPSS-R, revised International Prognostic Scoring System; MDS, myelodysplastic syndromes; MDS/AML, acute myeloid leukemia evolving from MDS; MDS (del)5q, MDS associated with isolated del(5q); MUD, matched unrelated donor; NS, not significant; RAEB-1, refractory anemia with excess blasts-1; RAEB-2, refractory anemia with excess blasts-2; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCUD, refractory cytopenia with unilineage dysplasia
All variables were analyzed at the time of transplantation in patients undergoing up-front allogeneic HSCT, and at the time of remission-induction chemotherapy in those receiving treatment before transplantation.
MDS/AML included patients with AML evolving from MDS, and those affected with RAEB-t (refractory anemia with excess blasts in transformation) according to the French-American-British classification.
Transfusion dependency was defined as having at least one red blood cell transfusion every 8 weeks over a period of 4 months.20
Criteria for selection of human leukocyte antigen (HLA)–matched unrelated donors before 2002 included low-resolution typing for HLA class I (A, B) and high-resolution typing for HLA-DRB1. Since 2002, criteria have included high-resolution typing for both HLA class I (A, B, C) and class II alleles (DRB1/3/4/5, DQA1, DPB1).
The most frequent conditioning regimens included the following: total body irradiation (TBI) and cyclophosphamide, TBI and fludarabine, busulfan and cyclophosphamide, thiotepa and cyclophosphamide, and thiotepa and fludarabine. In most patients, graft-versus-host disease prophylaxis was combined cyclosporine and methotrexate.
Sample Processing, DNA Sequencing, and Mutation Analysis
We analyzed bone marrow mononuclear cells collected at the time of transplant in patients receiving allogeneic HSCT upfront, and at the time of remission-induction therapy in those receiving treatment before transplantation. In nine patients, samples at the time of disease relapse after HSCT were also studied. DNA was isolated from tumor cells using the Gentra Puregene Blood Kit (Qiagen, Germantown, MD).
A TruSeq Custom Amplicon panel (TSCA; Illumina, San Diego, CA) targeting complete coding exons and their adjacent splice junctions from 34 genes was designed using Illumina Design Studio software. Genes were selected based on the available evidence in myeloid neoplasms (Appendix Table A1, online only). The TSCA panel consisted of 886 amplicons, 425 bp in length, for a total of 205 kb targeted DNA. Dual-barcoded TSCA libraries were created from 250 ng of high-quality DNA according to the manufacturer’s protocol. Libraries were multiplexed and underwent 2 × 250–bp paired-end sequencing on a MiSeq sequencing system using MiSeq Reagent Kit v3 (Illumina).
Mutational analysis of low-performer regions (ie, regions with inadequate coverage [< 100×]) was carried out using Nextera XT sample preparation kit (Illumina), and sequencing reactions were performed using the MiSeq v2 (2 × 150 bp) chemistry. The resulting average depth of coverage for the 886 amplicons was 980×. Sequence reads were initially aligned to the human genome (GRCh37/hg19) using the Burrows-Wheeler aligner. The Genome Analysis Toolkit (www.broadinstitute.org/gatk/) was later used to clean up reads and to make alignment data more reliable for the variant calling (Genome Analysis Toolkit data clean up best practice). Single nucleotide variants and small insertions and deletions were identified by MuTect and UnifiedGenotyper, respectively.
Functionally annotated variants were filtered accordingly to the following criteria. Synonymous variants and variants located outside protein-coding regions were filtered. Polymorphisms described in dbSNP (version 138) and in 1000 Genomes Project with a population frequency > 1% and 0.14%, respectively, were removed. Finally, variants with coverage < 30× and < 10 supporting reads, and variants with an allelic fraction (VAF) lower than 1%, were filtered. The remaining variants, evaluated as candidate somatic mutations, were finally tagged as oncogenic using different criteria based on information retrieved from literature, sequence conservation, and in silico prediction effect.21-23
Statistical Analysis
Comparison of numerical variables between groups was carried out using a nonparametric approach (Mann-Whitney test or Kruskal-Wallis analysis of variance). Comparison of the distribution of categorical variables in different groups was performed with either Fisher’s exact test (2 × 2 tables) or the χ2 test (larger tables). Overall survival (OS) was defined as the time between transplantation and death (from any cause) or last follow-up (for censored observations). When estimating nonrelapse mortality, any death in the absence of disease relapse was considered an event. The probability of relapse was estimated considering transplant as a failure at the time of hematologic relapse (evaluated according to standardized criteria).24 The cumulative probability of surviving was estimated using the Kaplan-Meier product limit method. The cumulative incidence of relapse and nonrelapse mortality were estimated with a competing-risks approach.25 Univariable and multivariable survival analyses were performed using Cox proportional hazards regression. To compare different statistical models, we used the likelihood ratio test. Analyses were performed using STATA 11.2 SE (STATA, College Station, TX) and Statistica 7.0 (StatSoft Inc., Tulsa, OK) software.
RESULTS
Gene Mutations in Patients With MDS and MDS/AML Receiving HSCT
Oncogenic mutations were identified in 34 genes in the whole study population. RUNX1 was the most frequently mutated gene (23%), followed by SRSF2 (17%), ASXL1 (17%), SF3B1 (16%), KRAS/NRAS (16%), DNMT3A (15%), TP53 (13%), and TET2 (10%) (Fig 1A).
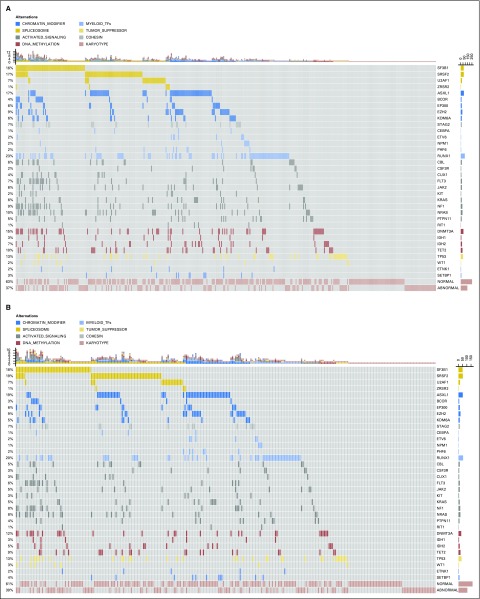
Fig 1.
Mutation patterns observed in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia evolving from MDS, who were treated with allogeneic hematopoietic stem-cell transplantation. (A) Whole patient population (N = 401); (B) patients with MDS diagnosed according to WHO criteria (n = 274); and (C) patients with acute myeloid leukemia evolving from MDS (n = 127). The plot represents a graphical summary of the distribution of somatic lesions in sequenced genes across the set of patients, grouped in categories as labeled in the legend. Columns represent samples and rows represent genes. Mutations are depicted by colored glyphs whose colors are used to distinguish different pathways, and their number per sample and per gene is summarized on the top and on the right side of the plot, respectively. In panel C, colors reflect ontogeny specificity of mutated genes, and genetic ontogeny groups are labeled on the top.
In total, 318 of 401 patients (79%) had at least one oncogenic point mutation, whereas cytogenetic studies identified abnormalities in 149 patients (37%). When sequencing and cytogenetics were combined, the number of patients with MDS-related oncogenic lesions increased to 87%. Indeed, 97 patients had one oncogenic point mutation (24%), 123 had two or three mutations (31%), and 98 had greater than four mutations (24%). No significant relationship was observed between WHO category (reflecting disease burden) and the prevalence of patients carrying driver mutation(s). Moreover, the sample quality and the average depth read in wild-type patients were comparable to those of mutated cases.
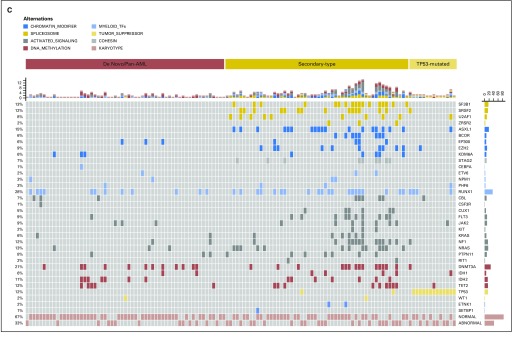
A significantly higher prevalence of mutations in splicing factors were observed in MDS compared with MDS/AML (P = .021), whereas mutations in DNA methylators were more frequent in MDS/AML than in MDS (P = .001; Fig 1B and C). We then focused on MDS/AML and stratified these 127 patients according to three distinct genetic subtypes, as previously defined25: secondary-type AML (including patients carrying mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 genes), TP53-mutated AML, and de novo AML (mainly including patients with NPM1 mutations, MLL/11q23 and CBF rearrangements). Accordingly, 55 subjects were classified as secondary-type AML (43%), 13 as TP53-mutated AML (10%), and 59 as de novo AML (47%; Fig 1C).
Genetic Predictive Features for the Outcomes of Allogeneic HSCT in Patients With MDS and MDS/AML
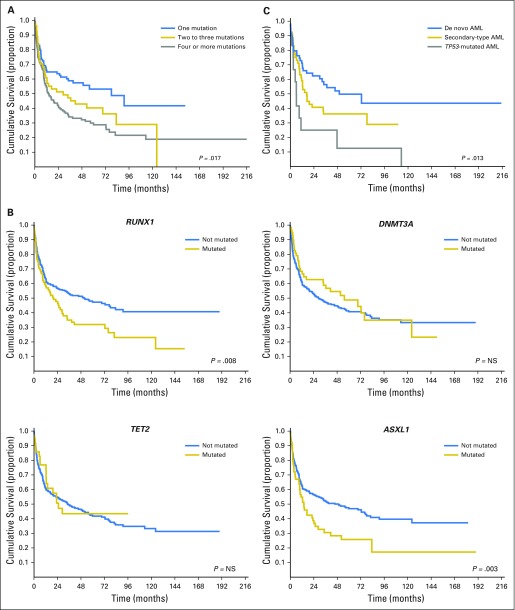
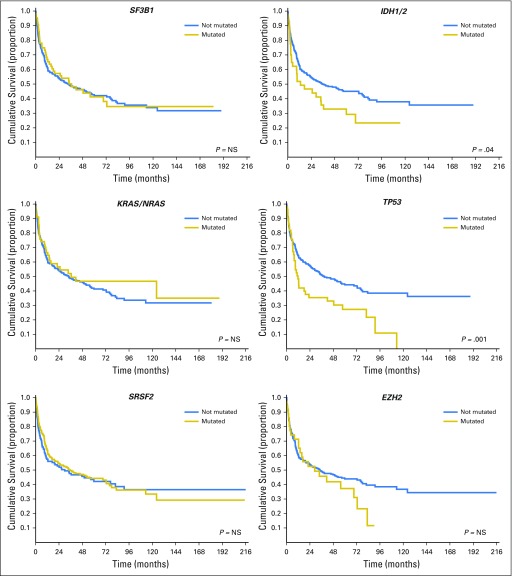
In our study, the number of somatic mutations was found to have a significant effect on probability of relapse and OS after HSCT (P < .001 and P = .017, respectively; Fig 2A). We then examined the hazard ratio (HR) of death associated with mutations in the genes mutated in ≥ 5% of patients in this cohort. In univariable analysis, mutations in RUNX1, ASXL1, IDH1/2, and TP53 were associated with increased probability of relapse (HR, 1.78 [95% CI, 1.26 to 2.27], P = .001; HR, 1.89 [95% CI, 1.34 to 2.56], P < .001; HR, 1.74 [95% CI, 1.17 to 2.38], P = .002; and HR, 1.95 [95% CI, 1.54 to 2.57], P < .001, respectively) and shorter OS (HR, 1.69 [95% CI, 1.1 to 2.23], P = .008; HR, 1.73 [95% CI, 1.23 to 2.18], P = .003; HR, 1.42 [95% CI, 0.95 to 2.16], P = .04; and HR, 1.92 [95% CI, 1.48 to 2.37], P = .001, respectively; Fig 2B and Table 2).
Fig 2.
Relationship between (A) number of mutations and (B) type of oncogenic mutations and overall survival of patients with myelodysplastic syndromes (MDS) receiving allogeneic hematopoietic stem-cell transplantation. (C) Posttransplantation overall survival among patients with acute myeloid leukemia (AML) evolving from MDS according to genetic ontogeny group. NS = not significant.
Table 2.
Prognostic Value of Gene Mutations for Posttransplantation Outcomes in Univariable and Multivariable Analyses
| Analysis Type | Probability of Relapse | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariable analysis | ||||||
| Whole study population | ||||||
| Variable | ||||||
| ASXL1 | 1.89 | 1.34 to 2.56 | < .001 | 1.73 | 1.23 to 2.18 | .003 |
| RUNX1 | 1.78 | 1.26 to 2.27 | .001 | 1.69 | 1.1 to 2.23 | .008 |
| IDH1/2 | 1.74 | 1.17 to 2.38 | .002 | 1.42 | 0.95 to 2.16 | .04 |
| TP53 | 1.95 | 1.54 to 2.57 | < .001 | 1.92 | 1.48 to 2.37 | .001 |
| Multivariable analysis | ||||||
| Patients with MDS | ||||||
| Variable | ||||||
| ASXL1 | 1.89 | 1.41 to 2.46 | .003 | 1.72 | 1.39 to 2.23 | .008 |
| RUNX1 | 1.67 | 1.31 to 2.37 | .02 | 1.59 | 1.29 to 2.18 | .035 |
| TP53 | 1.90 | 1.52 to 2.39 | .019 | 1.82 | 1.48 to 2.47 | .022 |
| Patients with MDS/AML | ||||||
| Variable | ||||||
| ASXL1 | 2.41 | 1.59 to 4.41 | .029 | 2.09 | 1.64 to 3.89 | .021 |
| RUNX1 | 2.46 | 1.69 to 4.52 | .038 | 1.96 | 1.47 to 4.08 | .031 |
| TP53 | 3.12 | 1.77 to 5.11 | .003 | 2.54 | 1.61 to 4.09 | .004 |
Abbreviations: HR, hazard ratio; MDS, myelodysplastic syndromes; MDS/AML, acute myeloid leukemia evolving from MDS.
The prognostic effect of gene mutations was maintained when considering patients with MDS and patients with MDS/AML separately (P values ranging from .039 to < .001). RUNX1 mutations were significantly associated with multilineage dysplasia (P = .012), excess blasts (P = .024), and decreased level of platelets (P = .031). A borderline association was found between ASXL1 mutations and poor/very poor cytogenetic risk according to IPSS-R criteria (P = .052). IDH1/2 mutations were associated with excess blasts (P = .018) and multilineage dysplasia (P = .009). TP53 mutations were associated with poor/very poor cytogenetic risk (P < .001), transfusion dependency (P = .042), and decreased level of neutrophils (P = .033).
As a next step, we fitted a Cox multivariate model to evaluate the prognostic effect of somatic mutations on posttransplantation outcome, considering as covariates the factors age and sex of recipient; hemoglobin, neutrophil, and platelet levels; percentage of marrow blasts; cytogenetics (according to IPSS-R criteria), disease stage at transplantation (complete remission v active/progressive disease), source of hematopoietic stem cells (peripheral blood v bone marrow), type of donor (human leukocyte antigen–identical sibling v matched unrelated donor); and type of conditioning (reduced-intensity v standard conditioning).
In the analysis performed on patients with MDS, mutations in ASXL1, RUNX1, and TP53 genes showed an independent effect on probability of relapse and OS after transplantation (ASXL1: HR, 1.89 [95% CI, 1.41 to 2.46], P = .003 and HR, 1.72 [95% CI, 1.39 to 2.23], P = .008; RUNX1: HR, 1.67 [95% CI, 1.31 to 2.37], P = .020 and HR, 1.59 [95% CI, 1.29 to 2.18], P = .035; TP53: HR, 1.90 [95% CI, 1.52 to 2.39], P = .019 and HR, 1.82 [95% CI, 1.48 to 2.47], P = .022, respectively; Table 2).
To account for the long period of recruitment, we analyzed the effect of year of transplantation on clinical outcome. Year of transplantation showed a significant effect on transplant-related mortality (P = .011) and a borderline effect on OS (P = .062), whereas probability of relapse was not significantly affected.
We then stratified mutations according to VAF. Patients with mutated ASXL1, RUNX1, and TP53 genes with VAF ≤ 10% versus > 10% were 14% versus 86%, 55% versus 45%, and 45% versus 55%, respectively. The negative effect of gene mutations on posttransplantation outcome was maintained when performing separate analyses on patients with VAF ≤ 10% versus > 10% (data not shown).
To verify whether somatic mutations could improve the prognostic stratification of patients with MDS who underwent HSCT, we fitted two separate multivariable analyses, including and not including ASXL1, RUNX1, and TP53 mutations, respectively, and compared them using the likelihood ratios test. The model comparison resulted in a significant P value (P < .001), thus confirming the importance of accounting for gene mutations in the prognostic model.
We then focused on patients with MDS/AML. Mutations in ASXL1, RUNX1, and TP53 genes confirmed an independent effect on probability of relapse and OS after transplantation (ASXL1: HR, 2.41 [95% CI, 1.59 to 4.41], P = .029 and HR, 2.09 [95% CI, 1.64 to 3.89], P = .021; RUNX1: HR, 2.46 [95% CI, 1.69 to 4.52], P = .038 and HR, 1.96 [95% CI, 1.47 to 4.08], P = .031; and TP53: HR, 3.12 [95% CI, 1.77 to 5.11], P = .003 and HR, 2.54 [95% CI, 1.61 to 4.09], P = .004, respectively; Table 2).
We stratified patients with MDS/AML according to three distinct genetic subtypes (ie, de novo AML [reference group], secondary-type AML, and TP53-mutated AML).26 Genetic AML subgroups were significantly associated with a different probability of relapse and survival after transplantation (P = .003 and P = .013, respectively; Fig 2C). In multivariable analysis, AML ontogeny maintained an independent effect on probability of relapse and survival after transplantation (HR, 1.78 [95% CI, 1.36 to 3.63], P = .028 and HR, 1.74 [95% CI, 1.25 to 3.87], P = .042, respectively).
Clinical Impact of Somatic Mutations in Patients With MDS Receiving HSCT, Stratified According to IPSS-R
First, we evaluated the prognostic effect of the IPSS-R score using a multivariate regression model. In this analysis, focused on patients with MDS, IPSS-R significantly affected probability of relapse (HR, 1.53 [95% CI, 1.18 to 2.16], P < .001) and OS (HR, 1.41 [95% CI, 1.11 to 2.05], P = .001). We then introduced somatic mutations in ASXL1, RUNX1, and TP53 genes as covariables in the model. Both IPSS-R and gene mutations maintained an independent effect on posttransplantation outcome (IPSS-R: probability of relapse HR, 1.37 [95% CI, 1.02 to 1.99], P < .001 and OS HR, 1.29 [95% CI, 1.04 to 2.21], P = .001; ASXL1: probability of relapse HR, 1.95 [95% CI, 1.16 to 3.14], P = .015 and OS HR, 1.69 [95% CI, 1.26 to 2.35], P = .007; RUNX1: probability of relapse HR, 1.72 [95% CI, 0.98 to 2.77 ], P = .041 and OS HR, 1.69 [95% CI, 1.06 to 1.97], P = .017; and TP53: probability of relapse HR, 1.79 [95% CI, 1.25 to 2.59], P = .030 and OS HR, 1.48 [95% CI, 1.08 to 2.37], P = .036).
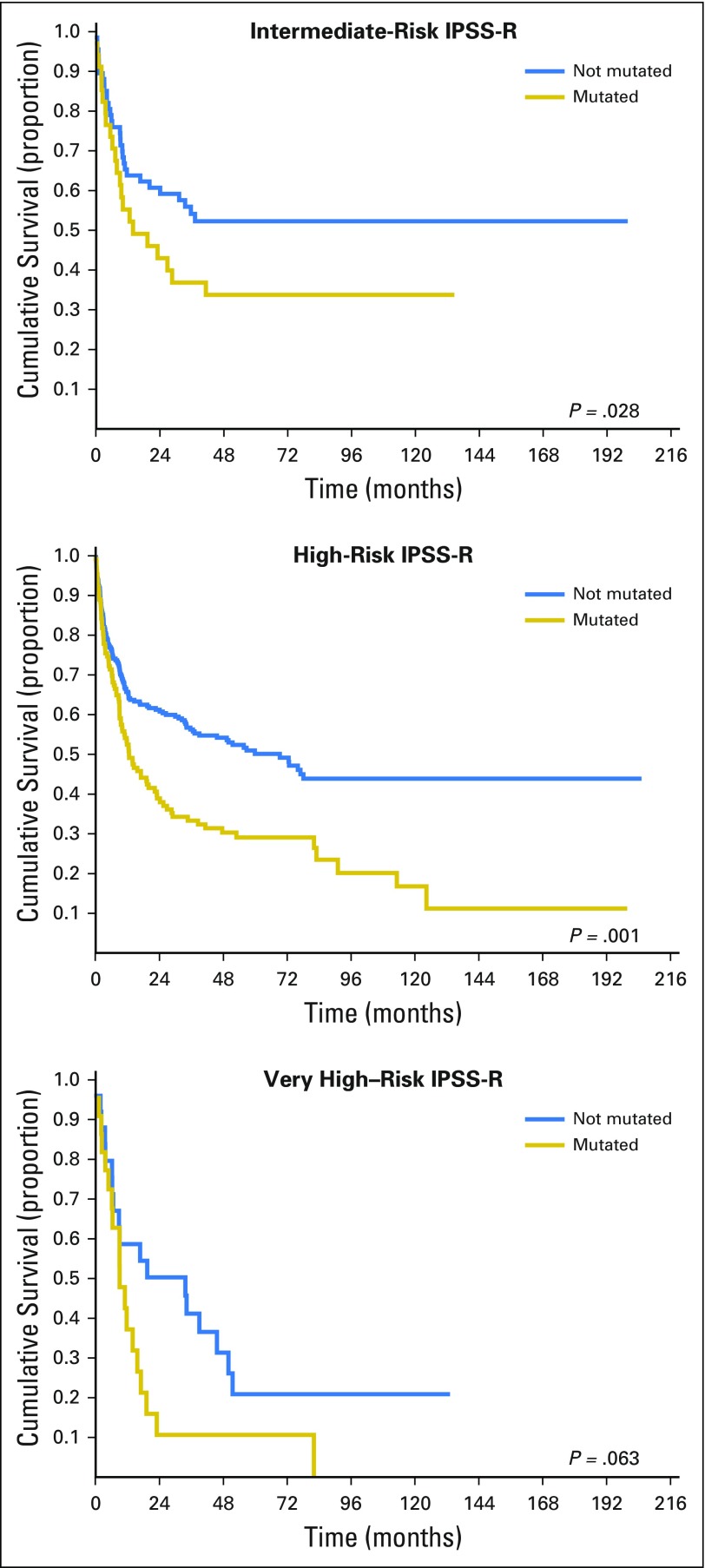
In prognostic terms, because the HRs of IPSS-R score and of ASXL1, RUNX1, and TP53 mutations are comparable in size, the increase in risk resulting from the presence of mutated genes is equivalent to the increase resulting from a one-step shift into a more advanced IPSS-R risk group. Posttransplantation outcomes in patients with MDS classified by IPSS-R and stratified according to the presence of mutations in ASXL1, RUNX1, and TP53 genes are reported in Fig 3. Accounting for various combinations of a patient’s IPSS-R category and mutational status, 5-year probability of survival and cumulative incidence of relapse after allogeneic HSCT ranged from 0% to 73% and from 4% to 77%, respectively. Compared with the IPSS-R–based stratification, when introducing gene mutations, the prediction of posttransplantation outcome would significantly change for 34% of patients.
Fig 3.
Posttransplantation overall survival of patients with myelodysplastic syndromes classified by the revised International Prognostic Scoring System (IPSS-R) and stratified according to the presence of mutations in the ASXL1, RUNX1, and TP53 genes.
Finally, to verify whether gene mutation could improve the IPSS-R prognostic stratification of patients with MDS who underwent allogeneic HSCT, we fitted two separate multivariable analyses including and not including gene mutations as covariables, respectively, and compared them using the likelihood ratios test. The model comparison resulted in a significant P value (P < .001), thus confirming the importance of accounting for gene mutations in the prognostic model.
Mutation Pattern at Disease Relapse After HSCT in Patients With MDS and MDS/AML
We used massively parallel sequencing to examine paired tumor samples collected from nine patients before HSCT and at the time of disease relapse after the procedure. Different types of clonal evolution occurred at relapse. In seven patients, the founder clone recurred, whereas in two patients a subclone of the founder clone escaped and expanded at relapse. In all patients, additional mutations not detected at diagnosis were observed at the time of relapse (Table 3). Focusing on the three genes associated with negative posttransplantation outcomes in our study, in patient 3, the founder clone carrying RUNX1 mutation recurred at the time of relapse, whereas in patients 7 and 9, mutations of RUNX1 and ASXL1 (both with low VAF before transplant) expanded at the time of disease recurrence, respectively.
Table 3.
Mutation Pattern at Disease Relapse After Transplantation in Patients With Myelodysplastic Syndromes and Acute Myeloid Leukemia Evolving From Myelodysplastic Syndromes
| Patient | WHO Category (before HSCT) | Founding Clone (before HSCT) | Clonal Evolution (disease relapse) | Comparison of New Mutations Not Detected Before HSCT |
|---|---|---|---|---|
| GITMO 1 | RAEB-2 | PTPN11 | Founder clone recurs | Yes |
| GITMO 2 | MDS/AML | NPM1 | Founder clone recurs | Yes |
| GITMO 3 | RAEB-1 | RUNX1 | Founder clone recurs | Yes |
| GITMO 4 | RAEB-2 | DNMT3A | A subclone expands (IDH1) | Yes |
| GITMO 5 | RAEB-1 | STAG2 | Founder clone recurs | Yes |
| GITMO 6 | MDS/AML | SRSF2 | Founder clone recurs | Yes |
| GITMO 7 | RAEB-2 | EZH2 | A subclone expands (RUNX1) | Yes |
| GITMO 8 | RCMD | SRSF2 | Founder clone recurs | Yes |
| GITMO 9 | RAEB-2 | SRSF2 | Founder clone recurs | Yes |
Abbreviations: GITMO, Gruppo Italiano Trapianto di Midollo Osseo; HSCT, hematopoietic stem-cell transplantation; MDS, myelodysplastic syndromes; MDS/AML, acute myeloid leukemia evolving from MDS; RAEB-1, refractory anemia with excess blasts-1; RAEB-2, refractory anemia with excess blasts-2; RCMD, refractory cytopenia with multilineage dysplasia.
DISCUSSION
Disease relapse is a common cause of HSCT failure in patients with MDS or MDS/AML.8-11 We tested the hypothesis that driver mutations may have an effect in predicting posttransplantation outcomes in these patients.18 Mutations in ASXL1, RUNX1, and TP53 genes were found to be independent predictors of relapse and OS after HSCT. The integration of mutations into currently available predictive models was found to increase the ability to capture prognostic information at the individual patient level.27
In this study, gene sequencing significantly increased the proportion of patients with information on disease biology with respect to conventional cytogenetics.14 The genotype of MDS treated with HSCT was consistent with a patient subgroup at high risk of clonal evolution, as indicated by a decreased frequency of SF3B1 mutations and increased frequency of mutations in transcription factors (RUNX1) and TP53 with respect to the whole MDS population.14 We observed in addition that at least three distinct genetic subtypes may account for unique MDS/AML clinical phenotypes: secondary-type AML (including patients carrying mutations in MDS-related genes), TP53-mutated AML, and de novo AML,26 thus suggesting that gene ontology may provide more objective diagnostic criteria with respect to clinical classification in these patients. Finally, massively parallel sequencing provided information on clonal evolution occurring at relapse after HSCT. In some patients, the founder clone recurred, whereas in other patients a subclone of the founder clone escaped and expanded at relapse.28 In all patients, additional mutations not detected at the time of diagnosis were observed at the time of relapse.
We provided evidence of clinical utility in considering mutation screening to predict survival after transplantation in patients with MDS and MDS/AML. In clinical practice, IPSS-R score identified four groups of patients with different probabilities of survival and disease relapse after HSCT.12 A major contribution to the improvement of posttransplantation outcome prediction by IPSS-R was provided by the refinement of the prognostic role of chromosomal abnormalities.7 Nevertheless, cytogenetics is not informative in a large proportion of patients and reveals secondary genetic events.4 Accounting for various combinations of a patient’s IPSS-R category and mutational status of ASXL1, RUNX1, and TP53 genes, 5-year probability of survival and relapse after allogeneic HSCT ranged from 0% to 73% and from 4% to 77%, respectively. In direct comparison, a predictive model accounting for gene mutations was found to be more likely to capture prognostic information with respect to IPSS-R alone.
In patients with MDS/AML, we observed that gene ontology predicts survival after transplantation. Secondary-type AML was associated with a lower probability of survival after transplant compared with patients with de novo AML. Moreover, TP53 mutations identified a group of patients with dismal outcomes after transplantation.
Overall, these results serve as a proof of concept that the integration of somatic mutations significantly increase the ability to capture prognostic information in patients with MDS and MDS/AML who are receiving allogeneic HSCT, and may provide a basis for improving clinical decision making.4,27 Possible interventions in patients with high risk of disease relapse after HSCT according to genotype may include the anticipation of the transplant procedure in early disease phase, the use of innovative conditioning regimens to increase the probability of eradicating the MDS clone, and prophylaxis of disease recurrence after transplantation by donor leukocyte infusions and targeted/novel therapies.29,30
There are potential weaknesses in our work, mainly related to the retrospective nature of this registry-based study. These include patient selection, missing data in a proportion of patients, a long period of recruitment, and different types of transplantation and of pretransplantation treatment. Moreover, in the absence of a matched control sample, it is challenging to distinguish with perfect accuracy between somatic and germline variants. Despite these limitations, clinical and hematologic data were available in the great majority of the original patient population, and analyses were adjusted for all known potential confounding factors. Furthermore, samples for mutation screening were homogeneously collected from bone marrow before treatment, and the landscape of truly somatic mutations in tested genes has been well established from large-scale genomics studies,2,3,14-16,18 allowing confident predictions to be made. Although we are aware that a prospective validation of our observations is needed, we believe that the findings of this study may contribute to improving prognostic counseling of patients with MDS and the design of clinical trials.
Appendix
Table A1.
Target Gene List
| Gene | Pathway | NCBI ID | Position |
|---|---|---|---|
| SF3B1 | RNA splicing | 23451 | 2q33.1 |
| SRSF2 | RNA splicing | 6427 | 17q25.1 |
| U2AF1 | RNA splicing | 7307 | 21q22.3 |
| ZRSR2 | RNA splicing | 8233 | Xp22.1 |
| DNMT3A | DNA methylation | 1788 | 2p23 |
| IDH1 | DNA methylation | 3417 | 2q33.3 |
| IDH2 | DNA methylation | 3418 | 15q26.1 |
| TET2 | DNA methylation | 54790 | 4q24 |
| ASXL1 | Chromatin and histones | 171023 | 20q11.1 |
| BCOR | Chromatin and histones | 54880 | Xp11.14 |
| EP300 | Chromatin and histones | 2033 | 22q13 |
| EZH2 | Chromatin and histones | 2146 | 7q35-36 |
| KDM6A | Chromatin and histones | 7403 | Xp11.2 |
| CBL | Signaling | 867 | 11q23.3 |
| CSF3R | Signaling | 1441 | 1p35-p34.3 |
| FLT3 | Signaling | 2322 | 13q12 |
| JAK2 | Signaling | 3717 | 9p24 |
| KIT | Signaling | 3815 | 4q12 |
| KRAS | Signaling | 3845 | 12p12.1 |
| NF1 | Signaling | 4763 | 17q11.2 |
| NRAS | Signaling | 4893 | 1p13.2 |
| PTPN11 | Signaling | 5781 | 12q24.1 |
| RIT1 | Signaling | 6016 | 1q22 |
| CEBPA | Transcriptional regulation | 1050 | 19q13.1 |
| CUX1 | Transcriptional regulation | 1523 | 7q22.1 |
| ETV6 | Transcriptional regulation | 2120 | 12p13.2 |
| NPM1 | Transcriptional regulation | 4869 | 5q35 |
| PHF6 | Transcriptional regulation | 84295 | Xq26.2 |
| RUNX1 | Transcriptional regulation | 861 | 21q22.3 |
| STAG2 | Cohesin complex | 10735 | Xq25 |
| TP53 | Tumor suppressor gene | 7157 | 17p13.1 |
| WT1 | Tumor suppressor gene | 7490 | 1p13 |
| SETBP1 | Genetic cancer susceptibility | 26040 | 18q21.1 |
| ETNK1 | Metabolic process | 55500 | 12p12.1 |
Abbreviations: ID, identification; NCBI, National Center for Biotechnology Information.
Footnotes
Written on behalf of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO; www.gitmo.it).
Supported by AIRC (Associazione Italiana Per la Ricerca sul Cancro, IG_17554), Milan; Fondazione Veronesi, Milan; Fondazione Cariplo & Regione Lombardia, Milan (Grant No. 42916996); Beat Leukemia Foundation, Milan; Fondazione Costa, Ivrea, Italy; Worldwide Cancer Research (Grant No. 15-1226), St Andrews, Scotland to Matteo G. Della Porta; and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy to Matteo G. Della Porta and Emilio P. Alessandrino.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Matteo G. Della Porta, Emilio P. Alessandrino, Alessandro Rambaldi, Mario Cazzola
Provision of study materials or patients: Anna Gallì, Chiara Milanesi
Collection and assembly of data: Matteo G. Della Porta, Anna Gallì, Andrea Bacigalupo, Silvia Zibellini, Massimo Bernardi, Bernardino Allione, Maria Teresa van Lint, Pietro Pioltelli, Paola Marenco, Alberto Bosi, Maria Teresa Voso, Simona Sica, Mariella Cuzzola, Emanuele Angelucci, Marianna Rossi, Marta Ubezio, Orietta Spinelli, Cristina Tresoldi, Sarah Pozzi, Silvia Luchetti, Laura Pezzetti, Silvia Catricalà, Chiara Milanesi, Benedetto Bruno, Fabio Ciceri, Francesca Bonifazi, Elli Papaemmanuil, Armando Santoro, Emilio P. Alessandrino, Alessandro Rambaldi, Mario Cazzola
Data analysis and interpretation: Matteo G. Della Porta, Ettore Rizzo, Alberto Malovini, Ivan Limongelli, Virginia V. Ferretti, Alberto Riva, Riccardo Bellazzi, Emilio P. Alessandrino, Alessandro Rambaldi, Mario Cazzola
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients With Myelodysplastic Syndromes Treated With Allogeneic Hematopoietic Stem-Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Matteo G. Della Porta
No relationship to disclose
Anna Gallì
No relationship to disclose
Andrea Bacigalupo
Speakers’ Bureau: Therakos, Pfizer, Merck Sharp & Dohme, Sanofi, Pierre Fabre, Miltenyi Biotec, Amgen
Silvia Zibellini
No relationship to disclose
Massimo Bernardi
No relationship to disclose
Ettore Rizzo
No relationship to disclose
Bernardino Allione
No relationship to disclose
Maria Teresa van Lint
No relationship to disclose
Pietro Pioltelli
No relationship to disclose
Paola Marenco
No relationship to disclose
Alberto Bosi
No relationship to disclose
Maria Teresa Voso
Speakers’ Bureau: Celgene
Research Funding: Celgene (Inst)
Simona Sica
No relationship to disclose
Mariella Cuzzola
No relationship to disclose
Emanuele Angelucci
Consulting or Advisory Role: Novartis Oncology
Travel, Accommodations, Expenses: Binding Site, Novartis
Marianna Rossi
No relationship to disclose
Marta Ubezio
No relationship to disclose
Alberto Malovini
No relationship to disclose
Ivan Limongelli
No relationship to disclose
Virginia V. Ferretti
No relationship to disclose
Orietta Spinelli
No relationship to disclose
Cristina Tresoldi
No relationship to disclose
Sarah Pozzi
No relationship to disclose
Silvia Luchetti
No relationship to disclose
Laura Pezzetti
No relationship to disclose
Silvia Catricalà
No relationship to disclose
Chiara Milanesi
No relationship to disclose
Alberto Riva
No relationship to disclose
Benedetto Bruno
No relationship to disclose
Fabio Ciceri
No relationship to disclose
Francesca Bonifazi
No relationship to disclose
Riccardo Bellazzi
Stock or Other Ownership: Biomeris S.r.l., Engenome S.r.l.
Elli Papaemmanuil
No relationship to disclose
Armando Santoro
Consulting or Advisory Role: Roche, Celgene, Takeda, Bristol-Myers Squibb, ArQule
Emilio P. Alessandrino
No relationship to disclose
Alessandro Rambaldi
No relationship to disclose
Mario Cazzola
No relationship to disclose
REFERENCES
- 1.Adès L, Itzykson R, Fenaux P: Myelodysplastic syndromes Lancet 383:2239–2252,2014 [DOI] [PubMed] [Google Scholar]
- 2.Papaemmanuil E Cazzola M Boultwood J, etal: Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts N Engl J Med 365:1384–1395,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida K Sanada M Shiraishi Y, etal: Frequent pathway mutations of splicing machinery in myelodysplasia Nature 478:64–69,2011 [DOI] [PubMed] [Google Scholar]
- 4.Cazzola M, Della Porta MG, Malcovati L: The genetic basis of myelodysplasia and its clinical relevance Blood 122:4021–4034,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malcovati L Hellström-Lindberg E Bowen D, etal: Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet Blood 122:2943–2964,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Porta MG Tuechler H Malcovati L, etal: Validation of WHO classification-based Prognostic Scoring System (WPSS) for myelodysplastic syndromes and comparison with the revised International Prognostic Scoring System (IPSS-R). A study of the International Working Group for Prognosis in Myelodysplasia (IWG-PM) Leukemia 29:1502–1513,2015 [DOI] [PubMed] [Google Scholar]
- 7.Greenberg PL Tuechler H Schanz J, etal: Revised International Prognostic Scoring System for myelodysplastic syndromes Blood 120:2454–2465,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deeg HJ Shulman HM Anderson JE, etal: Allogeneic and syngeneic marrow transplantation for myelodysplastic syndrome in patients 55 to 66 years of age Blood 95:1188–1194,2000 [PubMed] [Google Scholar]
- 9.Sierra J Pérez WS Rozman C, etal: Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia Blood 100:1997–2004,2002 [PubMed] [Google Scholar]
- 10.Alessandrino EP Della Porta MG Bacigalupo A, etal: WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: A study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood 112:895–902,2008 [DOI] [PubMed] [Google Scholar]
- 11.Lim Z Brand R Martino R, etal: Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia J Clin Oncol 28:405–411,2010 [DOI] [PubMed] [Google Scholar]
- 12.Della Porta MG Alessandrino EP Bacigalupo A, etal: Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R Blood 123:2333–2342,2014 [DOI] [PubMed] [Google Scholar]
- 13.Della Porta MG Travaglino E Boveri E, etal: Minimal morphological criteria for defining bone marrow dysplasia: A basis for clinical implementation of WHO classification of myelodysplastic syndromes Leukemia 29:66–75,2015 [DOI] [PubMed] [Google Scholar]
- 14.Papaemmanuil E Gerstung M Malcovati L, etal: Clinical and biological implications of driver mutations in myelodysplastic syndromes Blood 122:3616–3627,2013; quiz 3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejar R Stevenson K Abdel-Wahab O, etal: Clinical effect of point mutations in myelodysplastic syndromes N Engl J Med 364:2496–2506,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerstung M Pellagatti A Malcovati L, etal: Combining gene mutation with gene expression data improves outcome prediction in myelodysplastic syndromes Nat Commun 6:5901,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jädersten M Saft L Smith A, etal: TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression J Clin Oncol 29:1971–1979,2011 [DOI] [PubMed] [Google Scholar]
- 18.Bejar R Stevenson KE Caughey B, etal: Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation J Clin Oncol 32:2691–2698,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vardiman JW Thiele J Arber DA, etal: The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes Blood 114:937–951,2009 [DOI] [PubMed] [Google Scholar]
- 20. doi: 10.1200/JCO.2006.08.5696. Malcovati L, Germing U, Kuendgen A, et al: Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 25:3503-3510, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Durbin R: Fast and accurate long-read alignment with Burrows-Wheeler transform Bioinformatics 26:589–595,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DePristo MA Banks E Poplin R, etal: A framework for variation discovery and genotyping using next-generation DNA sequencing data Nat Genet 43:491–498,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cibulskis K Lawrence MS Carter SL, etal: Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples Nat Biotechnol 31:213–219,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD Bennett JM Kantarjian H, etal: Report of an international working group to standardize response criteria for myelodysplastic syndromes Blood 96:3671–3674,2000 [PubMed] [Google Scholar]
- 25.Logan BR, Zhang MJ, Klein JP: Regression models for hazard rates versus cumulative incidence probabilities in hematopoietic cell transplantation data Biol Blood Marrow Transplant 12:107–112,2006. 1 Suppl 1 [DOI] [PubMed] [Google Scholar]
- 26.Lindsley RC Mar BG Mazzola E, etal: Acute myeloid leukemia ontogeny is defined by distinct somatic mutations Blood 125:1367–1376,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballman KV: Biomarker: Predictive or prognostic? J Clin Oncol 33:3968–3971,2015 [DOI] [PubMed] [Google Scholar]
- 28.Walter MJ Shen D Ding L, etal: Clonal architecture of secondary acute myeloid leukemia N Engl J Med 366:1090–1098,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kröger N: Allogeneic stem cell transplantation for elderly patients with myelodysplastic syndrome Blood 119:5632–5639,2012 [DOI] [PubMed] [Google Scholar]
- 30.Stone RM: How I treat patients with myelodysplastic syndromes Blood 113:6296–6303,2009 [DOI] [PubMed] [Google Scholar]