Abstract
Purpose
To study correlations of crystalline lens anatomy and position parameters obtained using intraoperative spectral-domain (SD) optical coherence tomography (OCT) in cataract patients.
Methods
This retrospective study evaluated biometry data from 600 eyes of 399 cataract patients (mean age: 69±8.4 years) using intraoperative anterior segment SD-OCT during femtosecond laser-assisted cataract surgery. Lens anatomy and position parameters (anterior chamber depth [ACD] – center of the anterior cornea to the anterior lens capsule, lens thickness [LT] – distance between anterior and posterior lens capsules, and lens meridian position [LMP] – distance from center of the anterior cornea to intersection of the anterior and posterior lens) obtained with intraoperative SD-OCT, were correlated among themselves and with noncontact axial length (AL). Equatorial plane position (EPP) (distance between the plane of the lens equator and anterior capsule) was also studied. Pearson’s coefficients (r-values) were determined for all correlation pairs.
Results
There was a moderate correlation between AL and ACD (r=0.451; P<0.001). LMP was found to correlate strongly with ACD (r=0.77; P<0.001) but very weakly with AL (r=0.089; P=0.04). There was a moderately strong inverse correlation between LT and ACD (r=−0.586; P<0.001) but the correlation between LT and AL and LT and LMP was found to be weak (r=−0.155; P<0.001 and r=−0.121, P=0.003, respectively). Correlation of the ratio of EPP/LT and LT was weakly positive (r=0.267; P<0.001).
Conclusion
LMP correlated strongly with ACD but only minimally with AL. LT correlated fairly strongly with ACD but only minimally with LMP. This should stimulate additional research into the relationships among ocular and crystalline lens anatomy and IOL position after cataract surgery.
Keywords: SD-OCT, femtosecond laser-assisted cataract surgery, Catalys, lens meridian position, LMP, equatorial plane position
Introduction
Advancements in refractive cataract surgery in recent years have included improved preoperative biometry, introduction of femtosecond laser technology, and development of new toric and presbyopia-correcting intraocular lenses (IOLs). These technological innovations have improved refractive outcomes and allowed patients to achieve greater spectacle independence after surgery. Improved understanding of the anatomy of the anterior segment of the eye and crystalline lens is vital to improving refractive predictability of IOL implantation procedures.1,1 Optical coherence tomography (OCT) is a high-resolution advanced imaging technology, capable of providing full quantitative 3-dimensional (3-D) analysis of the anterior segment and lenticular biometry in individual eyes.1–4 Several crystalline lens geometrical parameters have been quantified previously with OCT, such as anterior chamber depth (ACD),5 curvature radius and asphericity of the anterior and posterior lens surfaces,1 lens thickness6 (LT), and lens alignment (tilt and decentration).7 Additionally, Martinez-Enriquez et al presented and validated a custom-developed quantitative OCT model for accurate estimation of the entire lens geometry, including parameters like lens volume, equatorial lens diameter, and equatorial plane position (EPP) (distance from the anterior lens apex to the lens equator).3
Spectral-domain (SD) OCT technology integrated into a femtosecond laser provides 3-D imaging of the anterior segment and helps to delineate anterior chamber structures. In addition to biometry data that can be obtained from devices used for preoperative IOL power calculations, it provides additional qualitative and quantitative lens anatomy parameters, which may have a potential role to play in improving IOL power calculations.
The purpose of this study is to determine correlations among ocular anatomy, lens position, and lens anatomy parameters in cataract patients.
Methods
This retrospective study analyzed biometry data from 600 eyes of 366 cataract patients (with a mean age of 69±8.4 years) who underwent femtosecond laser-assisted cataract surgery (FLACS) between February 2015 and May 2017. The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the Medical University of South Carolina institutional review board with a waiver of consent because the data were collected as a part of normal practice care provision.
Exclusion criteria included corneal scars or any other opacity that is a contraindication for femtosecond laser, corneal ulcer, diabetic retinopathy, macular degeneration, severe glaucoma, and other pathologies.
Study variables
Lens anatomy and position parameters obtained with the Catalys laser (Johnson & Johnson Vision, Santa Ana, CA, USA) included ACD – the center of the anterior cornea to the anterior lens capsule, LT – the distance between the anterior and posterior lens capsules, and lens meridian position (LMP) – the distance from the center of the anterior cornea to the intersection of the anterior and posterior lens (equator or meridian of the lens). The EPP, or the distance between the plane of the lens equator and anterior capsule (Figure 1), and the correlation of the ratio of EPP/LT and LT were analyzed. Another study variable was noncontact optical axial length (AL) (IOLMaster 500; Carl Zeiss Meditec, Dublin, CA, USA).
Figure 1.
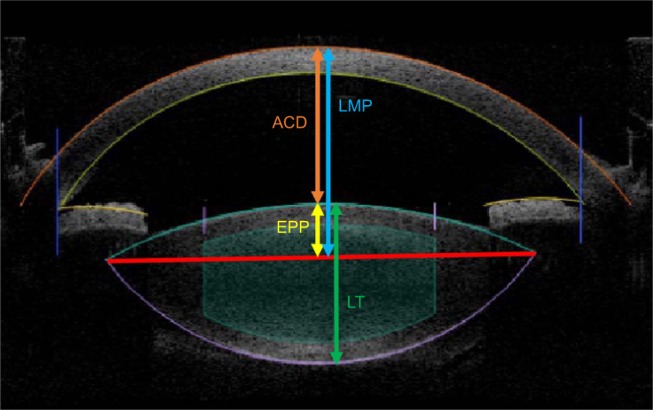
Spectral-domain anterior segment optical coherence tomography (OCT) imaging can be used to measure anterior chamber depth (ACD), equatorial plane position (EPP), lens thickness (LT), and lens meridian position (LMP).
Optical coherence tomography imaging
The Catalys femtosecond laser system uses OCT to create a 3-D model of the anterior portion of the eye to guide the laser treatment. The OCT system employs SD central wavelengths of 820–930 nm to create 3-D images of anterior ocular structures and identify the anterior and posterior surfaces of the cornea and the lens, as well as the iris border and limbus border. The reported resolution of the OCT system is 15 µm or better laterally and 30 µm or better axially.
For lens fragmentation, this software algorithm analyzes the backscattered light from the OCT system to identify the posterior lens surface and presents that information graphically for the operating physician’s consideration. Streaming X- and Y-axis cross-sectional OCT images are displayed with computer modeling of potential laser cut patterns overlaid for verification by the operating physician. If the imaging system cannot detect the posterior lens surface and the LT of the patient is not known, it can default to a conservative LT value of 2.5 mm. However, there was no such case in the current study.
Statistical analysis
Data analysis was performed using SPSS software for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). Pearson’s coefficients (r values) were determined for all correlation pairs. The strength of correlation was graded as follows: r=0: no correlation; 0<r<0.2: very weak correlation; 0.2≤r<0.4: weak correlation; 0.4≤r<0.6: moderately strong correlation; 0.6≤r<0.8: strong correlation; 0.8≤r<1: very strong correlation; r=1: perfect correlation. A P-value of <0.05 was considered statistically significant.
Results
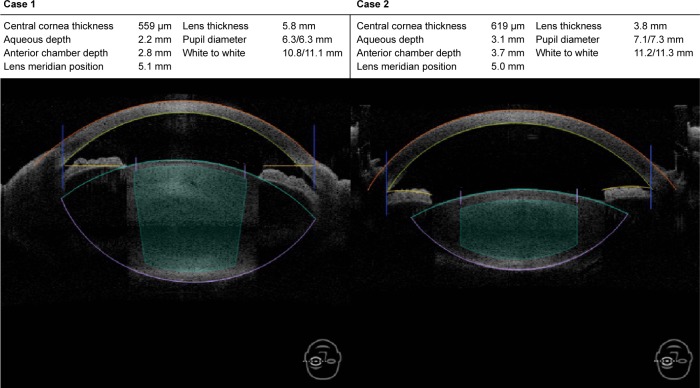
Table 1 provides mean values and ranges for the study variables. There was wide variation in LT in eyes with normal AL, with LT ranging from 3.1 mm to as much as 5.8 mm (Figure 2).
Table 1.
Patient demographics and anterior segment biometry parameters
| Parameter | Eyes (n) | Mean±SD | Range |
|---|---|---|---|
| Age (years) | 379 | 69.5±8.40 | 19–90 |
| AL (mm) | 528 | 24.13±1.49 | 20.22–31.58 |
| ACD (mm) | 600 | 3.46±0.38 | 2.40–4.60 |
| LT (mm) | 600 | 4.72±0.41 | 3.10–5.80 |
| LMP (mm) | 600 | 5.11±0.32 | 3.80–6.10 |
Abbreviations: ACD, anterior chamber depth; AL, axial length; LMP, lens meridian position; LT, lens thickness.
Figure 2.
Anterior segment optical coherence tomography (OCT) of two eyes representing wide variation in LT in cataract eyes, ranging from as high as 5.8 mm in case 1 (on the left) to 3.1 mm in case 2 (on the right). The large difference in capsular bag size between these two cases is also evident.
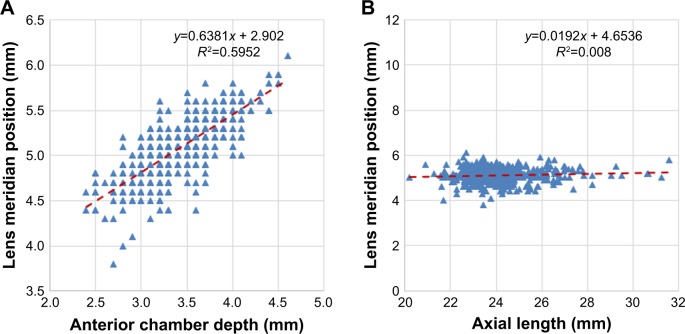
The correlation of AL with ACD was found to be moderate (r=0.451; P<0.001) (Figure 3). LMP was found to correlate strongly with ACD (r=0.771; P<0.001) (Figure 4A) but very weakly with AL (r=0.089; P=0.04) (Figure 4B). When eyes were grouped by AL, the correlation with LMP was weak in eyes with normal AL (22–25) (r=0.009; P=0.861), short AL (<22), and long AL (>25) (r=−0.113; P=0.699 and r=0.123; P=0.203, respectively) (Table 2).
Figure 3.
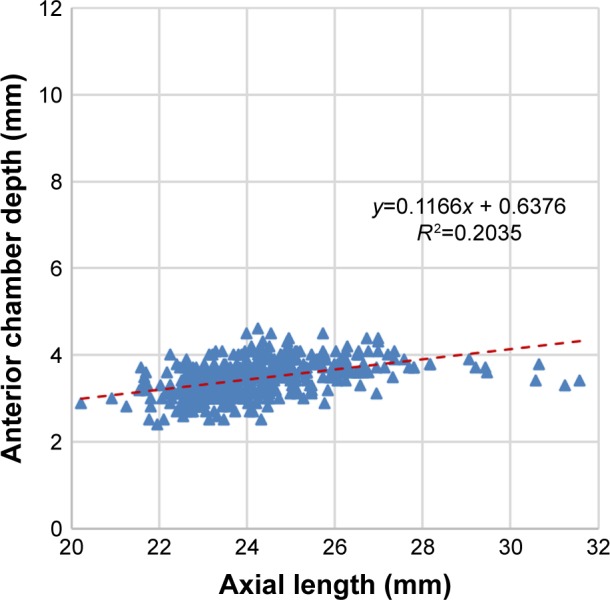
Scatter plot showing correlation between axial length (AL) and anterior chamber depth (ACD) (r=0.45).
Note: The dotted line represents the regression line.
Figure 4.
Scatter plots showing the correlation of lens meridian position (LMP) with (A) anterior chamber depth (ACD) (r=0.77) and (B) axial length (AL) (r=0.09).
Note: The dotted line represents the regression line.
Table 2.
Correlation of LMP with AL, by AL group
| Group | Eyes (n) | r | P-value | Mean±SD | Range |
|---|---|---|---|---|---|
| AL (<22) (mm) | 14 | −0.113 | 0.699 | 5.05±0.39 | 4.4–5.6 |
| AL (22–25) (mm) | 385 | 0.009 | 0.861 | 5.1±0.32 | 3.8–6.1 |
| AL (>25) (mm) | 109 | 0.123 | 0.203 | 5.16±0.29 | 4.4–5.9 |
Abbreviations: AL, axial length; LMP, lens meridian position.
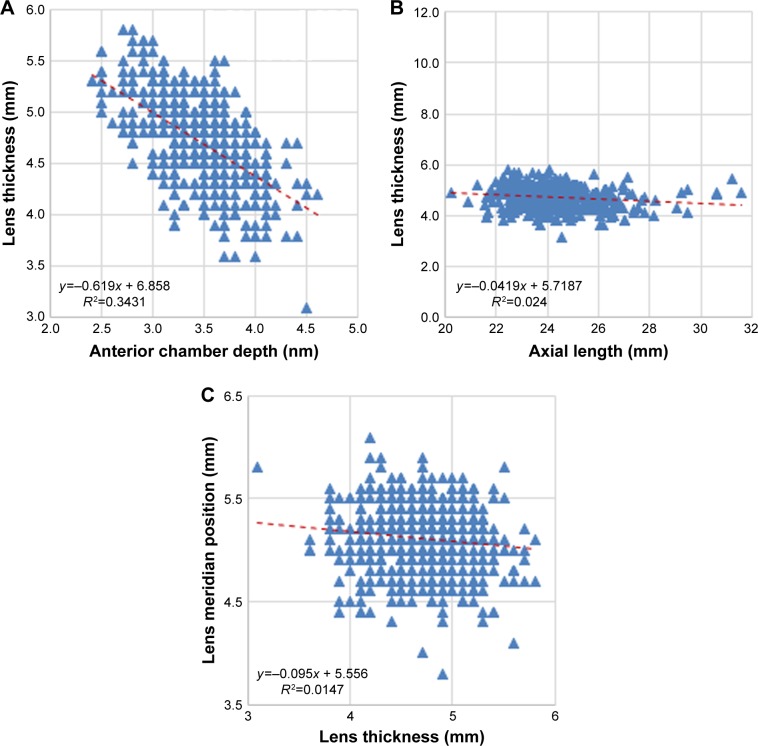
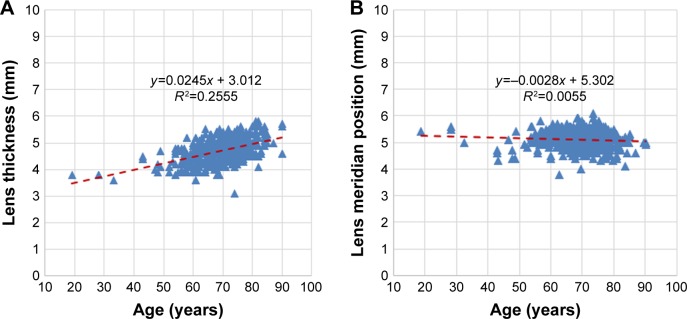
There was a moderate inverse correlation between LT and ACD (r=−0.586; P<0.001) but the correlation between LT and AL was weak (r=−0.155; P<0.001) (Figure 5A and B, respectively). When eyes were grouped according to normal, short, or long AL, the correlation between LT and AL was weak in eyes with normal AL (r=−0.166; P=0.001) and almost absent in eyes with short AL (r=−0.025; P=0.931) and long AL (r=−0.03; P=0.751) (Table 3). The correlation of LT and LMP was also very weak (r=−0.121, P=0.003) (Figure 5C). While LT was found to correlate moderately positively with age (r=0.505; P<0.001) (Figure 6A), LMP correlated very weakly with age (r=−0.075; P=0.067) (Figure 6B).
Figure 5.
Scatter plots showing the correlation of lens thickness (LT) with (A) anterior chamber depth (ACD) (r=−0.59); (B) axial length (AL) (r=−0.15); and (C) lens meridian position (LMP) (r=−0.121).
Note: The dotted line represents the regression line.
Table 3.
Correlation of LT with AL, by AL group
| Group | Eyes (n) | r | P-value | Mean±SD | Range |
|---|---|---|---|---|---|
| AL (<22) (mm) | 14 | −0.025 | 0.931 | 4.69±0.39 | 3.9–5.3 |
| AL (22–25) (mm) | 399 | −0.166 | 0.001 | 4.73±0.40 | 3.1–5.8 |
| AL (>25) (mm) | 114 | −0.030 | 0.751 | 4.62±0.39 | 3.6–5.6 |
Abbreviations: AL, axial length; LT, lens thickness.
Figure 6.
Scatter plots showing the correlation of age with (A) lens thickness (LT) (r=0.505) and (B) lens meridian position (LMP) (r=−0.075).
Note: The dotted line represents the regression line.
EPP, or the distance between the plane of the lens equator and anterior capsule, provides the relative position of the lens equator within the lens. The correlation of the ratio of EPP/LT and LT was studied and shown to be weakly positive (r=0.267; P<0.001) (Figure 7).
Figure 7.
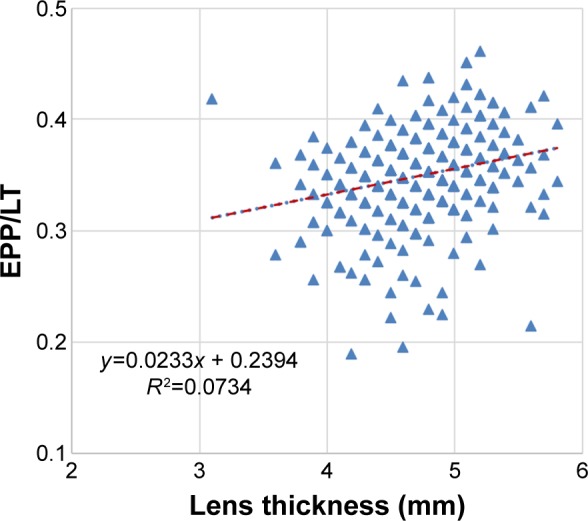
Scatter plot showing the correlation of ratio of equatorial plane position/lens thickness (EPP/LT) with LT (r=0.267).
Note: The dotted line represents the regression line.
Discussion
Third-generation IOL power formulas estimated postoperative ELP based on keratometry and AL, but this approach resulted in suboptimal refractive outcomes. Later, Olsen et al8 showed that postoperative ACD correlated significantly with preoperative lens anatomy parameters, such as ACD and LT (P<0.05). Consequently, in an attempt to improve ELP estimation and IOL power calculations, direct ACD measurements were incorporated into the IOL power calculations.9
The final position of an implanted IOL may be subject to a number of variables, including the size and centration of the capsulotomy and the size of the IOL relative to the capsular bag.10–12 Most commonly, however, we would expect the IOL to seat at or near the plane of the lens equator, where the anterior and posterior lens capsule meet and are subjected to zonular forces. The more 3-D information about the anterior segment of the eye we have, the better we can predict the postoperative ELP. A FLACS system with integrated SD-OCT enables 3-D imaging of anterior chamber ocular structures and provides a new parameter, the LMP, which closely approximates the position of the lens equator.
We found that LMP was strongly correlated with ACD (r=0.77; P<0.001) but weakly correlated with AL (r=0.09; P<0.05), indicating that LMP changes significantly relative to changes in ACD, but only minimally relative to AL. To determine if the correlation between LMP and AL is affected by the length of the eye, we studied this correlation in different AL groups; LMP was found to correlate weakly in normal eye (r=0.009; P=0.861), short eye (r=−0.113; P=0.699), and long eye (r=0.123; P=0.203). The finding that LMP changes only minimally relative to AL is surprising. The poor correlation between AL and ACD or LMP may be attributed to the fact that myopia is due to elongation of the posterior sclera during puberty, not really affecting the anterior segment (cornea and anterior sclera), which serves to anchor the zonules and thus the position of the lens. As such, this finding introduces a new dimension to our understanding of ELP and raises questions about the accuracy of factors previously thought to have affected ELP. This should stimulate additional research into the relationships among ocular and crystalline lens anatomy and IOL position after cataract surgery. The correlation of preoperative lens anatomy parameters with postoperative position of the IOL (ACD) will be evaluated in our subsequent publications.
The distance from the plane of the lens equator to the anterior capsule or to the anterior/posterior surfaces of the cornea has been studied by several investigators who suggest that lens equator or meridian position may have a role to play in improving IOL power calculations – although little of this has yet appeared in the peer-reviewed literature.3,29–32 Joo et al30 presented evidence at the 2016 ESCRS annual meeting supporting the role of LMP in improving ELP prediction. In that study, LMP from the Catalys OCT was found to fairly strongly correlate with postoperative ACD (r=0.67; P<0.01). Pyfer et al31 reported that LMP measured by Catalys closely approximated the ELP calculated from the Haigis and Sanders, Retzlaff and Kraff (theoretical) formulas. These findings indicate that LMP may be an effective parameter to predict ELP.
Olsen et al13 proposed the concept of a C constant (referring to the fraction of LT by which the center of the IOL will locate itself after surgery) to predict the postoperative position of an IOL from the preoperative thickness and position of the crystalline lens. Hwang et al29 reported that pre-LED (lens equator depth), a parameter that seems to be closely related to LMP, was positively correlated to postoperative ACD (r2=0.47; P<0.001). LED was defined as the depth from the back surface of the central cornea to the line connecting the intersection points between the anterior and posterior lens. Similarly, Martinez et al found that utilizing the EPP (or distance from the anterior lens apex to the lens equator) in IOL power calculations may lower ELP prediction error, potentially leading to improved IOL power calculations.3
In contrast, Tucker et al32 did not find that using LMP improved ELP estimation more than using ACD. As such, future studies are warranted to gain a better understanding of the potential use of LMP in improving the accuracy of ELP estimation.
We studied the correlation between LT and LMP and found a weak negative correlation (r=−0.121; P=0.003). In contrast, the correlation between LT and ACD was fairly strong (r=−0.59; P<0.001), and this corroborates the prior literature in eyes with cataract (r=0.57).14 To better understand this, we calculated mathematically the distance from the plane of the lens equator to the anterior capsule as LMP-ACD. We then determined the correlation between ratio of EPP/LT with LT and found that with increasing LT, the ratio of EPP/LT increases (r=0.267), suggesting that as LT increases, the majority of the growth occurs anterior to LMP rather than symmetrically (anterior/posteriorly) in relation to LMP (Figure 7). This seems to explain the lower r value of LT vs LMP compared with LT vs ACD. It is important to note that LMP and EPP are based on the extrapolation of OCT image of the lens (visible through the dilated pupil) to the rest of the lens behind the iris. Hence, LMP and EPP may not perfectly determine the true lens equator position because these are based on presumed and computer-generated position of equator, and not actual localization of the equator of the lens.
The moderately positive correlation we found between ACD and AL (r=0.451; P<0.001) seems to corroborate prior studies.15,16 Of note, a separate analysis from our unpublished data had previously revealed a high degree of correlation between ACD measurements obtained with the IOLMaster and those obtained with the Catalys (r=0.79; P<0.001), indicating good validity and reproducibility of the intraoperative SD-OCT measurements. This also suggests that the suction induced by the docking procedure does not affect the ACD measurements. Also, current study measurements were obtained as a part of routine FLACS procedure; therefore, all such measurements were obtained after pupil mydriasis. Since pupil mydriasis has been associated with increase in ACD17–20 and decrease in LT,17,20 due care should be taken for making such comparisons.
We found a weak negative correlation between LT and AL in the current study (r=−0.15; P<0.001). Subgroup analyses were done to understand if this correlation worked differently in short, normal, and long eyes; LT was found to correlate weakly in normal eyes (r=−0.16; P=0.001) and essentially showed no correlation in short (r=−0.02; P=0.931) and long eyes (r=−0.03; P=0.751). Although Mashige et al21 reported a stronger negative correlation in normal eyes (r=−0.52) of black South Africans, the correlation has been shown to be weak (r=0.06–0.18) in study populations more similar to ours, ie, eyes with cataract.14,15,22
While the variability in LT in noncataractous eyes (3.69–4.87 mm) was well known,23 an even wider variation in cataractous eyes with normal AL has been observed in the current study (3.1–5.8 mm). Given that IOLs are essentially one-size-fits-all, the variability in LT could be a potential factor in cases of toric IOL rotation or refractive surprise. The size of the IOL relative to the capsular bag is an important characteristic affecting postoperative alignment and rotational stability of toric IOLs.24 Capsular bag diameter (CBD) has been found to correlate positively with AL,25 suggesting a greater chance of toric IOL rotation in eyes with larger capsular bags and longer AL.24 For this reason, capsular tension ring (CTR) use is often guided by AL and is recommended in patients with high-axial myopia.26,27 While this has been helpful, direct imaging of the size of the capsular bag by SD-OCT could potentially be used to more accurately determine the need for a CTR to prevent rotation. Visual comparison of the OCT images in Figure 2 suggests that eyes with greater LT may have a correspondingly larger capsular bag size. Previous literature also documents an increase in lens equatorial diameter with increase in LT.28 However, the correlation between LT and CBD is not fully understood and requires further study.
Catalys OCT imaging and the associated parameters of LMP and LT provide the surgeon with valuable information about lens position and anatomy that may already have clinical implications for surgical planning and IOL power selection. Further analysis of these OCT imaging parameters and their association with ELP may lead to the development of custom-sized IOLs for patients with larger capsular bags, improved protocols for ensuring the stability of toric IOLs, and more robust IOL power calculations that incorporate accurate estimates of ELP and improve refractive outcomes after cataract surgery.
Conclusion
Our study found that LMP correlates strongly with ACD but only minimally with AL and the trend was similar for short, normal, or long eyes. LT changes fairly strongly with ACD but only minimally with LMP. This should stimulate additional research into the relationships among ocular and crystalline lens anatomy and IOL position after cataract surgery. Of note, the findings of the current study are specific to cataractous eyes and may not be applicable to eyes undergoing clear lens exchange. Future research is warranted to evaluate if the correlation of LMP with preoperative parameters holds true for presbyopic but clear lenses.
Acknowledgments
The authors thank Raman Bedi, MD (IrisARC, Analytics, Research & Consulting, Chandigarh, India), and Jan Beiting (Wordsmith Consulting, Cary, NC, USA) for statistical analysis and editorial assistance.
Footnotes
Disclosure
Dr Haddad and Dr Yeh have no financial or proprietary interest in any product mentioned herein. Dr Waring and Dr Rocha are consultants for Johnson & Johnson Vision. The authors report no other conflicts of interest in this work.
References
- 1.Ortiz S, Pérez-Merino P, Gambra E, de Castro A, Marcos S. In vivo human crystalline lens topography. Biomed Opt Express. 2012;3(10):2471–2488. doi: 10.1364/BOE.3.002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Enriquez E, Pérez-Merino P, Velasco-Ocana M, Marcos S. OCT-based full crystalline lens shape change during accommodation in vivo. Biomed Optics Express. 2017;8(2):918–933. doi: 10.1364/BOE.8.000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Enriquez E, Sun M, Velasco-Ocana M, Birkenfeld J, Pérez-Merino P, Marcos S. Optical coherence tomography based estimates of crystalline lens volume, equatorial diameter, and plane position. Invest Opthalmol Vis Sci. 2016;57(9):OCT600–OCT610. doi: 10.1167/iovs.15-18933. [DOI] [PubMed] [Google Scholar]
- 4.Sun M, Pérez-Merino P, Martinez-Enriquez E, Velasco-Ocana M, Marcos S. Full 3-D OCT-based pseudophakic custom computer eye model. Biomed Opt Express. 2016;7(3):1074–1088. doi: 10.1364/BOE.7.001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth G, Vajas A, Tsorbatzoglou A, Kolozsvari B, Modis L, Berta A. Assessment and reproducibility of anterior chamber depth measurement with anterior segment optical coherence tomography compared with immersion ultrasonography. J Cataract Refract Surg. 2007;33(3):443–447. doi: 10.1016/j.jcrs.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Hamzeh N, Moghimi S, Latifi G, Mohammadi M, Khatibi N, Lin SC. Lens thickness assessment: anterior segment optical coherence tomography versus A-scan ultrasonography. Int J Ophthalmol. 2015;8(6):1151–1155. doi: 10.3980/j.issn.2222-3959.2015.06.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar DA, Agarwal A, Prakash G, Jacob S, Saravanan Y, Agarwal A. Evaluation of intraocular lens tilt with anterior segment optical coherence tomography. Am J Ophthalmol. 2011;151(3):e402:406–412. doi: 10.1016/j.ajo.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Olsen T. Prediction of intraocular lens position after cataract extraction. J Cataract Refract Surg. 1986;12(4):376–379. doi: 10.1016/s0886-3350(86)80099-2. [DOI] [PubMed] [Google Scholar]
- 9.Olsen T. Prediction of the effective postoperative (intraocular lens) anterior chamber depth. J Cataract Refract Surg. 2006;32(3):419–424. doi: 10.1016/j.jcrs.2005.12.139. [DOI] [PubMed] [Google Scholar]
- 10.Filkorn T, Kovács I, Takács A, Horváth E, Knorz MC, Nagy ZZ. Comparison of IOL power calculation and refractive outcome after laser refractive cataract surgery with a femtosecond laser versus conventional phacoemulsification. J Refract Surg. 2012;28(8):540–544. doi: 10.3928/1081597X-20120703-04. [DOI] [PubMed] [Google Scholar]
- 11.Cekiç O, Batman C. The relationship between capsulorhexis size and anterior chamber depth relation. Ophthalmic Surg Lasers. 1999;30(3):185–190. [PubMed] [Google Scholar]
- 12.Kránitz K, Takacs A, Miháltz K, Kovács I, Knorz MC, Nagy ZZ. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011;27(8):558–563. doi: 10.3928/1081597X-20110623-03. [DOI] [PubMed] [Google Scholar]
- 13.Olsen T, Hoffmann P. C constant: new concept for ray tracing-assisted intraocular lens power calculation. J Cataract Refract Surg. 2014;40(5):764–773. doi: 10.1016/j.jcrs.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Shammas HJ, Shammas MC. Measuring the cataractous lens. J Cataract Refract Surg. 2015;41(9):1875–1879. doi: 10.1016/j.jcrs.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Jivrajka R, Shammas MC, Boenzi T, Swearingen M, Shammas HJ. Variability of axial length, anterior chamber depth, and lens thickness in the cataractous eye. J Cataract Refract Surg. 2008;34(2):289–294. doi: 10.1016/j.jcrs.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Sedaghat MR, Azimi A, Arasteh P, Tehranian N, Bamdad S. The relationship between anterior chamber depth, axial length and intraocular lens power among candidates for cataract surgery. Electr Phys. 2016;8(10):3127–3131. doi: 10.19082/3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arriola-Villalobos P, Almendral-Gómez J, Garzón N, et al. Effect of pharmacological pupil dilation on measurements and iol power calculation made using the new swept-source optical coherence tomography-based optical biometer. J Fr Ophtalmol. 2016;39(10):859–865. doi: 10.1016/j.jfo.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Khambhiphant B, Sasiwilasagorn S, Chatbunchachai N, Pongpirul K. Effect of pupillary dilation on Haigis formula-calculated intraocular lens power measurement by using optical biometry. Clin Ophthalmol. 2016;10:1405–1410. doi: 10.2147/OPTH.S109797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Raton A, Jimenez-Alvarez M, Arteche-Limousin L, Mediavilla-Peña E, Larrucea-Martinez I. Effect of pupil dilation on biometry measurements with partial coherence interferometry and its effect on IOL power formula calculation. Eur J Ophthalmol. 2015;25(4):309–314. doi: 10.5301/ejo.5000568. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Dong J, Tang M, Wang X, Wang H, Zhang S. Effect of pupil dilation on biometric measurements and intraocular lens power calculations in schoolchildren. PLoS One. 2018;13(9):e0203677. doi: 10.1371/journal.pone.0203677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashige KP, Oduntan OA. Axial length, anterior chamber depth and lens thickness: Their intercorrelations in black South Africans. Afr Vis Eye Health. 2017;76(1):1–7. [Google Scholar]
- 22.Hoffer KJ. Axial dimension of the human cataractous lens. Arch Ophthalmol. 1993;111(7):914–918. doi: 10.1001/archopht.1993.01090070032014. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi H, Khabazkhoob M, Miraftab M, et al. The distribution of axial length, anterior chamber depth, lens thickness, and vitreous chamber depth in an adult population of Shahroud, Iran. BMC Ophthalmol. 2012;12(1):50. doi: 10.1186/1471-2415-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dardzhikova A, Shah CR, Gimbel HV. Early experience with the AcrySof toric IOL for the correction of astigmatism in cataract surgery. Can J Ophthalmol. 2009;44(3):269–273. doi: 10.3129/i09-048. [DOI] [PubMed] [Google Scholar]
- 25.Vass C, Menapace R, Schmetterer K, Findl O, Rainer G, Steineck I. Prediction of pseudophakic capsular bag diameter based on biometric variables. J Cataract Refract Surg. 1999;25(10):1376–1381. doi: 10.1016/s0886-3350(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 26.Safran SG. Use of a capsular tension ring to prevent early postoperative rotation of a toric intraocular lens in high axial myopia. JCRS Online Case Reports. 2015;3(2):41–43. [Google Scholar]
- 27.Sagiv O, Sachs D. Rotation stability of a toric intraocular lens with a second capsular tension ring. J Cataract Refract Surg. 2015;41(5):1098–1099. doi: 10.1016/j.jcrs.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis. 2011;11(3):19. doi: 10.1167/11.3.19. [DOI] [PubMed] [Google Scholar]
- 29.Hwang K, Yoo Y, Joo C, Yoon G. New parameter for predicting the postoperative IOL position: preoperative lens equator depth measured by three-dimensional anterior segment optical coherence tomography. Abstract presented at ARVO Annual Meeting; Denver. 2015; [Accessed January 8, 2019]. Available from: https://iovs.arvojournals.org/article.aspx?articleid=2331049&resultClick=1. [Google Scholar]
- 30.Joo C, Kang M, Whang W, Chung S. Predicting effective lens position with biometric measurements using 3D-OC. Abstract presented at XXXIV CONGRESS of the ESCRS; Copenhagen. 2016; [Accessed January 8, 2019]. Available from: http://www.escrs.org/Copenhagen2016/programme/free-papers-details.asp?id=27085&day=0. [Google Scholar]
- 31.Pyfer M, Arlow T, Hufnagel E. Improving intraocular lens power calculation with femtosecond laser biometry data. Abstract presented at XXXV CONGRESS of the ESCRS; Lisbon. 2017; [Accessed January 8, 2019]. Available from: http://www.escrs.org/Lisbon2017/programme/free-papers-details.asp?id=27835&day=0. [Google Scholar]
- 32.Tucker J, Castro HM, Garg S, Farid M, Steinert R, Wade M. Biometric Analysis of the Lens Meridian Position; Abstract presented at ASCRS ASOA Symposium and Congress; Los Angeles. 2017; [Accessed January 8, 2019]. Available from: http://ascrs.org/node/28793. [Google Scholar]