Abstract
Context: Epilepsy is a common life-threatening neurological disorder that is often drug-resistant and associated with cognitive impairment. The traditional Chinese patent medicine Songling Xuemaikang capsules (SXC) is clinically used for epilepsy therapy and alleviation of cognitive impairment.
Objective: This study investigates the neuronal protective effect of SXC combined with carbamazepine (CBZ) on epilepsy and cognitive impairment in kainic acid-kindled SD rats.
Materials and methods: Kainic acid-kindled rats were established by injection of 0.45 μg kainic acid and randomly divided into 5 groups (n = 14): saline (sham-operated), control, CBZ, SXC and CBZ + SXC combined group. Rats in the treatment groups received CBZ (50 mg/kg/d), SXC (600 mg/kg/d) or combined CBZ (50 mg/kg/d) + SXC (600 mg/kg/d) via intragastric injection for 60 days. Epileptic behaviours, cognitive impairment, neuronal apoptosis and expression of p-Akt, Akt and caspase-9 were measured, and the alleviation of cognitive damage and neuronal apoptosis was analyzed.
Results: The combined administration of SXC and CBZ significantly decreased the frequency of seizures (1.2 ± 0.3) and the number of episodes (1.3 ± 0.5) above stage III (p < 0.05). Neuronal apoptosis was improved (p < 0.01), and cognitive damage was ameliorated (p < 0.05).The level of p-Akt was enhanced (p < 0.01) whereas the expression of caspase-9 was evidently inhibited (p < 0.01) in the combined group.
Conclusions: The present findings confirm that the combined use of SXC with CBZ can effectively control epileptic seizures, alleviate damage to hippocampal neurons and protect against cognitive impairment. The mechanism of action might be related to the upregulation of p-Akt and inhibition of caspase-9 expression.
Keywords: Cognitive impairment, neuron apoptosis, p-Akt, caspase-9
Introduction
Epilepsy is one of the most chronic neurological disorders affecting millions of people worldwide (Caraballo and Fejerman 2015). Neuron damage, degeneration and apoptosis are the major consequences of status epilepticus (Hoppe et al. 2013), which can lead to cognitive impairment in the human brain (Laxer et al. 2014). Approximately one-third of patients suffer from cognitive deficits and present with serious neuropsychological problems (Bell et al. 2011), such as slow response and poor concentration and memory (Maschio et al. 2015).
To date, first-line antiepileptic drugs (AEDs) have been given priority in the clinical treatment of epileptic seizures (Stafstrom and Carmant 2015). Among these AEDs, carbamazepine (CBZ) is commonly used and shows prominent efficacy in decreasing epileptogenesis (Waisburg and Alvarez 1998). Long-term use of CBZ, however, can bring drug resistance, reduce its clinical curative effect and induce cognitive dysfunction (Gierbolini et al. 2016). As studies have shown, it is feasible to use traditional Chinese medicine in epileptic patients (Wu et al. 2012), for its better acceptability in the human body and fewer side effects compared with chemical drugs, thus providing a new method of epileptic therapy (Hijikata et al. 2006). Several traditional Chinese medicines have been used in combination with CBZ in clinical practice, such as Compound Danshen Dripping Pills (Jia et al. 2018), Gastrodia capsules and Songling Xuemaikang capsules (SXC), which show distinctive superiority in antiepileptic treatment (Hu et al. 2004).
SXC is a traditional Chinese patent medicine mainly containing the commonly used herbal medicines Radix Puerariae lobatae Ohwi (Leguminosae.), Pinus densiflora Sieb. et Zucc. (Pinaceae) and has been authorized by the Chinese Pharmacopoeia (Yang et al. 2015). Puerarin, which is the active pharmaceutical ingredient in Radix Puerariae lobatae, can protect the vascular endothelium and alleviate ischaemic brain injury (Wu et al. 2007). Its mechanism may be related to downregulation of neuronal apoptosis from activation of the phosphatidyl-inositol 3 kinase/Akt (PI3K/Akt) signal pathway (Xie et al. 2014). PI3K is an intracellular phosphatidyl-inositol kinase whose products activate the expression of Akt (Poornima et al. 2013). Increased expression of phosphorylated Akt protein has been observed to be beneficial in reducing the apoptosis of hippocampal neurons (Lin et al. 2015). Caspase-9 is a start-up enzyme that is considered to be an initiator and central part of the cell apoptosis pathway (Würstle et al. 2012). The activated kinase Akt can phosphorylate caspase-9 (Zhang et al. 2013), thus blocking the cell apoptosis process and protecting the hippocampus from neuronal damage (Kawamoto et al. 2016). Studies (Wei et al. 2014) have found that the administration of SXC together with CBZ can increase the concentration of CBZ in the brain, suggesting that it is preferable to treat epileptic seizures by the combined administration of SXC and CBZ.
To verify the antiepileptic effects of SXC and CBZ, kainic acid-kindled rats were established and treated with SXC, CBZ and their combination, after which the behaviour of epileptic seizures and cognitive changes were carefully observed. In this study, we also investigated neuronal protection and the role the administration of SXC and CBZ could play in regulating p-Akt, Akt and caspase-9, thereby clinically providing an experimental basis for application of the medication.
Materials and methods
Animals and treatment
Seventy adult male SD rats (220 ± 20 g) were obtained from the GLP experimental centre of Lanzhou University. The animals were raised in a temperature-controlled room (19 ± 1 °C) with a 12 h light/dark cycle and were allowed free access to food and water. All animal experimental procedures were performed in accordance with the animal care guidelines of the Institutional Animal Care and Use Committee and approved by the Animal Ethics Committee of Lanzhou University Second Hospital.
All of the rats were anaesthetized with 10% chloral hydrate intraperitoneally (0.35 g/kg body weight) and fixed into a stereotaxic apparatus (Brain Stereotaxic Instrument, RWD Life Science Co., Ltd., China). The dorsal surface of the skull was exposed with a midline incision. Based on the stereotaxic atlas, a burr hole was drilled at the following location (coordinates from the bregma: anterior-posterior = −0.85 mm, lateral = −1.90 mm, ventral = −5.5 mm). The SD rats were randomly divided into five groups (n = 14): a control group, a CBZ group, a SXC group, a combination group of SXC + CBZ and a saline sham-operated group. The first four groups were injected with 0.45 μL kainic acid slowly over 10 min (1.0 μg/μL, #K0250; Sigma-Aldrich, St. Louis, MO), whereas the saline group was injected with normal saline. All surgeries were performed under anaesthesia with chloral hydrate and efforts were made to minimize animal suffering. Additional care was taken the days following an operation by providing the animals with comfortable space and enriched soft food.
The epileptic rats were continuously treated with their respective substances for 60 days, the CBZ group with CBZ (50 mg/kg/d), the SXC group with SXC (600 mg/kg/d) and the combined group with SXC (600 mg/kg/d)+CBZ (50 mg/kg/d), whereas the rats in the saline and control groups were treated with saline solution (2.5 mL/kg/d). The doses of drugs administered to animals were calculated accordingly to the body surface area by Meeh-Rubner Equation (Wu et al. 2015). The CBZ (0.1 g/tablet, #4080232LA; Shanghai Zhongxi Pharmaceutical Co., Ltd., China) and SXC (0.5 g/capsule, #20150125; Kanghong Pharmaceutical Co., Ltd., China) were ground and dissolved in normal saline solution before use.
Behavioural observation
Rat seizure behaviour was determined according to Racine’s scale (Phelan et al. 2015): stage 0, no response or seizure behaviour; stage I, facial twitches such as chewing and winking; stage II, chewing and head nodding; stage III, unilateral forelimb clonus convulsion; stage IV, bilateral forelimb convulsion and rearing; and stage V, bilateral forelimb clonus, rearing and rolling. All of the behavioural changes were carefully observed by the same observer, and the number of behaviours above stage III was recorded.
Morris water maze test
The Morris water maze consisted of a round pool 100 cm in diameter and 50 cm in depth (WMT-100; Taimeng Technology Co., Ltd., China). The pool was filled with water at 21 ± 1 °C and divided into four quadrants: northeast (NE), southeast (SE), southwest (SW) and northwest (NW). A hidden escape platform of 10 cm in diameter was submerged with 1 cm beneath the liquid surface in the centre of the NE quadrant. The water was opacified by the addition of powdered high-dispersion titanium dioxide. The rats were placed in the water facing the pool wall at one of four randomly selected starting locations (Vorhees and Williams 2006).
The test included two parts: the place navigation test and a spatial probe test. The place navigation test was performed with four consecutive days of training four times per day. The rat was placed in the water facing the wall of the pool at one of three uniformly spaced starting points other than on the platform in the NE quadrant. The trial began when the rat was manually placed in the water facing the wall and terminated when the rat reached the platform. The rats were given 120 s to locate the hidden platform, and any rat that did not find the platform within 120 s was guided to the platform with its escape latency recorded as 120 s. All of the rats were left on the platform for 15 s before the next trial was initiated. On the last day of the test, probe trials without the platform were assessed with a specific starting point (SW quadrant), and the platform-crossing frequency and time spent in the target quadrant were recorded.
Histological staining
After the 60-day treatment, the rats were anaesthetized, placed in the supine position and perfused through the heart with saline followed by 4% formaldehyde. The brain was then removed through the cranial cavity and fixed in 4% formaldehyde. Brain sections were transferred to ethanol of different concentrations and then to dimethylbenzene. The sections were embedded with paraffin and stained with Nissl stain and HE stain. Two slide preparations were made. (A) The slides were immersed in 1% toluidine blue solution (#89640; Sigma-Aldrich) for 6 min, then washed in distilled water, and 70% and 95% ethanol, and finally treated with 100% ethanol, three times for 1 min each and dimethylbenzene twice for 5 min each. Neuronal loss in the CA3 area of the hippocampus was observed under a light microscope (BX-50; Olympus, Japan), and the number of neurons was recorded. (B) The slides were treated with 1% toluidine blue solution for 5 min and then washed in a series of ethanol washes along with a concentration gradient and distilled water. Haematein solution was added, and the slides were maintained for 5 min following awash with water and hydrochloric acid alcohol. Eosin solution was then added and the slides were immersed for another 5 min at 37 °C. Dimethylbenzene was added after three washes with distilled water and the slides were incubated at room temperature for 10 min. Finally, the slides were placed under glass coverslips and analyzed under the light microscope.
Western blotting
The hippocampus was carefully removed before extracting the total protein on ice. After adding 360 μL RIPA and 3.6 μL PMSP solution (both from Biyuntian Technologic Inc., China), the homogenate was centrifuged at 12,000 rpm for 10 min. The supernatant was collected and the concentration of protein was determined using a BCA protein assay kit (Biyuntian Technologic Inc.). Equal amounts of protein were separated by SDS-polyacrylamide gels and then transferred onto PVDF membranes (Solarbio Science & Technology Co., Ltd., China). The membranes containing P-Akt were blocked in 5% bovine serum albumin (BSA), whereas the others were blocked with 5% non-fat milk for 2 h at room temperature and then incubated overnight at 4 °C with the following primary antibodies: anti-p-Akt (1:1000, #AF0016; Affinity Bioscience, China), anti-Akt (1:1000, #AF6261; Affinity Bioscience), anti-caspase-9 (1:1000, #AF6348; Affinity Bioscience) and anti-β-actin (1:1000, #TA-09;Zhongshan Biotech, China). The membranes were washed with TBST (4 × 8 min) and incubated for 2 h with HRP-conjugated secondary antibodies (#ZB2301; Zhongshan Biotech): goat anti-mouse IgG (1:5000) and goat anti-rabbit IgG (1:8000). The blots were developed using an enhanced chemiluminescence kit and exposed to X-ray film. The protein expression levels were analyzed using Image J software (NIH, Bethesda, MD, USA).
Data analysis
Results are expressed as means ± SD and quantitative variables were compared by ANOVA. A p value less than 0.05 or 0.01was regarded as a significant difference. The data analysis was performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Establishment and degree of seizures by administration of kainic acid
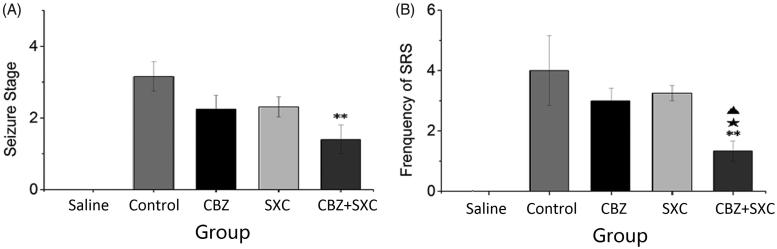
Within 0.5 h after kainic acid injection, the rats began to experience epileptic seizures, such as chewing, continuous nodding, forelimb clonus, rolling and rearing, and then gradually stopped the seizures and regained their autonomic activities. The animals showed chronic epilepsy of grades I–V after the latency period. After 60 days of administration, kainic acid-kindled rats in the control group exhibited a gradual increase in seizure intensity from grades I to V, whereas seizure episodes in the groups receiving AEDs varied from grades I to IV (Figure 1(A)). The combined group had significantly lower scores for grades of seizure episode (1.3 ± 0.5) and frequency (1.2 ± 0.3) than the control group (3.2 ± 0.4 in grade, p < 0.01; 4.0 ± 1.1 in frequency, p < 0.01). Although the frequency and extent of epileptic seizures in the SXC and CBZ groups were lower than those of the control group, the differences were not significant. Therefore, the seizures could be effectively controlled with the combined administration of SXC and CBZ.
Figure 1.
Effects of SXC and its combined administration with CBZ on epileptic seizures. (A) Stages of epileptic seizures. (B) Frequency of spontaneous recurrent seizures in each group. Observations were made three times/day for one week, and only seizures of stage III or higher were recorded. Results are presented as means ± SEM. **p < 0.01 vs. control group; ★p < 0.05 vs. CBZ group; ▲p < 0.05 vs. SXC group (n = 14 per group).
Effect of SXC and its combined administration with CBZ on cognitive impairment
The Morris water maze was used to assess spatial learning and memory abilities in the animals with chronic epilepsy. As shown in Table 1, the escape latencies in the groups receiving AEDs were decreased, and there were significant differences compared with the saline group (p < 0.05 and p < 0.01). Although latency in the CBZ group decreased, it was not significantly different from that in the control group. However, latency in the SXC group (p < 0.05) and the combined group (p < 0.01) was significantly lower than that in the control group.
Table 1.
Average escape latencies for finding the hidden platform on each trial day.
| Group | Dose | Latency(s) |
Frequency across the platform | |||
|---|---|---|---|---|---|---|
| (mg/kg) | Day 1 | Day 2 | Day 3 | Day 4 | ||
| Saline | – | 31.3 ± 6.5 | 20.8 ± 6.4 | 16.7 ± 6.1 | 10.2 ± 1.9 | 6.3 ± 1.0 |
| Control | – | 81.4 ± 7.1‡ | 64.1 ± 7.8‡ | 57.5 ± 7.8† | 56.1 ± 7.5† | 1.2 ± 0.6‡ |
| CBZ | 50 | 80.2 ± 6.4‡ | 41.6 ± 4.4 | 37.4 ± 6.0 | 27.7 ± 6.3 | 3.3 ± 0.5 |
| SXC | 600 | 69.1 ± 5.3† | 30.2 ± 4.3* | 17.9 ± 5.7** | 18.1 ± 5.1** | 5.8 ± 0.8** |
| CBZ + SXC | 50 + 600 | 71.7 ± 7.5† | 34.1 ± 3.9 | 22.5 ± 2.3* | 19.4 ± 4.0* | 4.5 ± 0.9* |
Results are presented as means ± SEM. †p < 0.05, ‡p < 0.01 vs. saline group; *p < 0.05, **p < 0.01 vs. control group (n = 6 per group).
The spatial probe test indicated that compared with that of the saline group, the frequency of swimming across the platform in the other four groups was decreased, whereas it was significantly different in the control group (p < 0.01). Rats in the SXC group (p < 0.01) and combined group (p < 0.05) significantly improved their performances in crossing the target quadrant compared with those in the control group. Compared with rats in the CBZ group, those in the SXC group spent significantly more time in the target quadrant (p < 0.05). These results indicated that along with the extension of epileptic seizures, there was a declining trend in the cognitive competence of the rats. The results after treatment with the AEDs showed that CBZ alone could not significantly improve the learning and memory ability of the rats, whereas the administration of SXC and the combined administration of SXC + CBZ better ameliorated the changes in cognitive competence of the rats.
Effect of the combined administration of SXC + CBZ on histopathological damage
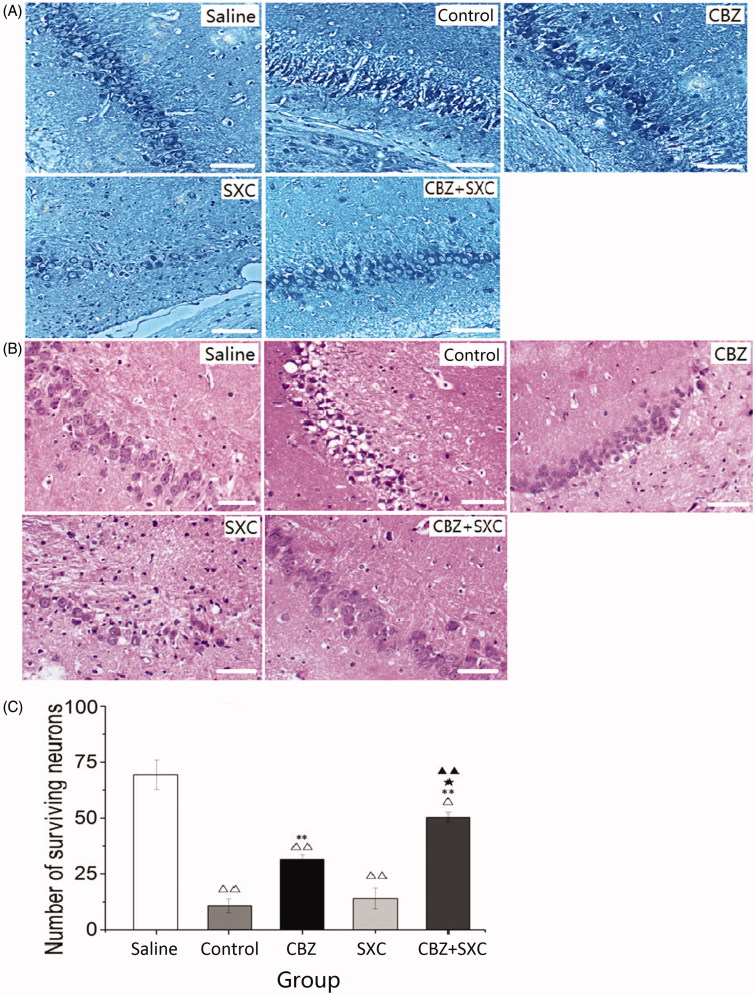
Observation of the Nissl- and HE-stained sections distinctly revealed that the neurons in the CA3 region of the hippocampus in the saline group were finely clustered together, with their contour clear and cytoplasm transparent (Figure 2(A)). In the control group, however, the nucleoli were pyknotic, and missing and disordered arrangement of neurons was evident (Figure 2(B)). Such damage was much less severe in the AED-treated rats. Alterations of the structure in the hippocampus were significantly reduced in the CBZ and combined groups. There was also a significant difference in the amounts of neurons in the CBZ group (p < 0.01) and combined group (p < 0.01) compared with the control group (Figure 2(C)).The combined administration group (p < 0.05) showed evident superiority over the CBZ group. However, the SXC treatment group per se did not show any overt improvement in the neurons and hippocampus. These figures indicated that the combined administration of SXC and CBZ provided superior neuronal protection in epilepsy.
Figure 2.
Effects of SXC and its combined administration with CBZ on kainic acid-induced death. (A) Nissl staining of the CA3 region in the hippocampus of each group (magnification × 200). (B) HE staining of the CA3 region in the hippocampus of each group (magnification × 200). (C) The number of surviving neurons in each group. Results are presented as means ± SEM. △p < 0.05, △△p < 0.01 vs. saline group; **p < 0.01 vs. control group; ★p < 0.05 vs. CBZ group; ▲▲p < 0.01 vs. SXC group (n = 6 per group, scale bars: 100 μm).
Effect of SXC and its combined administration with CBZ on the expression of p-Akt, Akt and caspase-9 in the hippocampal CA3 region
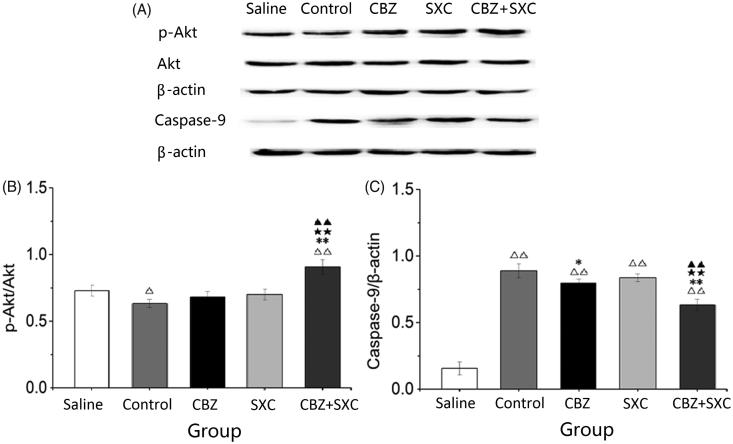
The expression of p-Akt in the groups receiving AEDs was increased, and it was significantly upregulated in the SXC group compared with the saline group (Figure 3). Simultaneously, p-Akt expression was obviously enhanced in the combined group compared with the CBZ group (p < 0.01). Based on statistical analysis, although the expression of caspase-9 decreased in the CBZ group, it decreased significantly in the SXC and combined groups compared with the control group (p < 0.01). The expression of caspase-9 in the combined administration group (p < 0.01) also significantly decreased compared with that in the CBZ group. These results indicated that the expression of p-Akt was obviously enhanced and that of caspase-9 significantly decreased in the group treated with CBZ and SXC together, which might have inhibited the apoptosis of neuronal cells.
Figure 3.
Effects of SXC and its combined administration with CBZ on the expression of p-Akt, Akt and caspase-9 in the hippocampus of each group. (A) Western blotting was performed to evaluate the protein expression of p-Akt, Akt and caspase-9. β-actin was used as an internal control. (B and C) Densitometry analysis was performed with Image J software. Results are presented as means ± SEM. △p < 0.05, △△p < 0.01 vs. saline group; *p < 0.05, **p < 0.01 vs. control group; ★★p < 0.01 vs. CBZ group; ▲▲p < 0.01 vs. SXC group (n = 6 per group).
Discussion
Epilepsy is a common chronic relapsing disease of the nervous system (Huberfeld et al. 2015), for which combined treatment with SXC and CBZ is used clinically to treat epileptic seizures. Studies have found that SXC can prominently reduce cell apoptosis and improve the ultrastructure of the rat hippocampus (Zheng and Liang 2010). The present study showed that after 60 days of treatment, the administration of SXC or CBZ alone could not effectively control epileptic seizures, yet combination therapy with SXC and CBZ showed significant curative effects against epilepsy. The results indicated that the combined use of SXC and CBZ could improve the antiepileptic effects in kainic acid-kindled rats.
Chronic epilepsy greatly influences brain function. Status epilepticus can cause abnormal brain structures, especially in the CA1 and CA3 areas of the hippocampus that can lead to the loss or even necrosis of neurons (Allen et al. 2017). Cognitive impairment has also been reported as a main neurobehavioural comorbidity of chronic epilepsy (Zhao et al. 2014), and its damage to learning and memory functions has become a non-ignorable part of epilepsy treatment that significantly affects the outcome and living quality of epileptic patients. Studies have confirmed that the structure of the hippocampus is closely related to cognitive function (Gilbert et al. 2000), animals whose hippocampal formation has been destroyed show a decline in learning and memory ability, and patients with the hippocampus surgically resected suffer from cognitive dysfunction (Titiz et al. 2014). Rat models of status epilepticus have proved that damage to the cyclic adenosine monophosphate/protein kinase A signal transduction pathway can also lead to cognitive dysfunction in pubescent rats (Vã z-Lp et al. 2005). Results of the Morris water maze experiment in the present study illustrated that with the extension of epileptic seizures, the learning and memory ability of rats decreased and administration of CBZ alone for 60 days could not effectively improve the cognitive ability of the rats. For the groups receiving SXC, however, the number of rats traversing the original platform position increased, and the escape latency shortened, indicating that the learning and memory abilities of the epileptic rats significantly improved. It can be inferred that the administration of SXC and CBZ alleviates the neuronal damage occurring in the epileptic hippocampus, inhibit neuronal apoptosis and improve cognitive function in the brain.
Neuronal damage and loss in the hippocampus are the main consequences of long-term epileptic seizures that lead to hippocampal sclerosis and cerebral injury (Cendes et al. 2014). Cognitive deficit is also related to neuronal cell loss (Fuentes and Smith 2015). Therefore, the protection of hippocampal neurons has become a crucial target for improving the pathological process of epilepsy and the prognosis of affected patients. In the present study, although the CA3 region of the hippocampus showed no obvious neuronal loss in the SXC group, even fewer neurons were lost in the CBZ and combined groups. The combined administration group showed the best neuronal protective effect of all of the groups. These results indicated that the combined administration of SXC and CBZ could reverse neuronal damage occurring in the CA3 region of the hippocampus by protecting neuronal cells from apoptosis.
Studies in recent years have found that the PI3K/Akt signal pathway has a certain relationship with hippocampal hypoxic-ischaemic brain injuries caused by chronic epilepsy (Kitagawa et al. 1999). The activation of the PI3K/Akt signal transduction pathways in cells downstream of its signal cascade reaction may be the key to resisting apoptosis (Zheng et al. 2013). Akt could be activated via phosphorylation at site 308 of threonine and site 473 of serine and then exert its inhibitory or activation effects on downstream proteins NF-κB, Bad and caspase, thus promoting cell survival (Shultz et al. 2010). Caspase-9 is the initiator and central part of the cell apoptosis pathway. Akt can phosphorylate and deactivate caspase-9, ultimately blocking cell apoptosis. Consistently, our study confirmed that compared with treatment with CBZ or SXC alone, the administration of CBZ together with SXC increased the expression of p-Akt and inhibited the expression of caspase-9 to significantly control the process of apoptosis and protect neuronal cells. The results indicated that the mechanism of action may be related to activation of the PI3K/Akt signal pathway, phosphorylation of Akt proteins and inhibition of caspase-9 mitochondrial apoptosis, thus ultimately resisting cell apoptosis and exerting neuronal protective effect.
In conclusion, this study on the use of SXC together with CBZ provided a theoretical basis for epileptic therapy. The combined administration of SXC and CBZ was more effective in the control of epileptogenesis, alleviated damage to hippocampal neurons and protected against cognitive impairment in kainic acid-kindled rats. Its mechanism of action may be related to the upregulation of p-Akt and the inhibition of caspase-9 expression. The combined administration of SXC with CBZ may provide a prospective therapeutic option to improve the treatment of epileptic patients.
Funding Statement
This study was supported by the Natural Science Foundation of Gansu Province [Grant no. 18JR3RA307] and National Natural Science Foundation of China [Grant no. 81550048].
Disclosure statement
No potential conflict of interest was reported by the authors..
References
- Allen LA, Harper RM, Kumar R, Guye M, Ogren JA, Lhatoo SD, Lemieux L, Scott CA, Vos SB, Rani S, et al. . 2017. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front Neurol. 8:544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell B, Lin JJ, Seidenberg M, Hermann B. 2011. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 7:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo R, Fejerman N. 2015. Management of epilepsy in resource-limited settings. Epileptic Disord. 17:13–18. [DOI] [PubMed] [Google Scholar]
- Cendes F, Sakamoto AC, Spreafico R, Bingaman W, Becker AJ. 2014. Epilepsies associated with hippocampal sclerosis. Acta Neuropathologica. 128:21–37. [DOI] [PubMed] [Google Scholar]
- Fuentes A, Smith ML. 2015. Patterns of verbal learning and memory in children with intractable temporal lobe or frontal lobe epilepsy. Epilepsy Behav. 53:58–65. [DOI] [PubMed] [Google Scholar]
- Gierbolini JR, Giarratano M, Benbadis SR. 2016. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy - a comparative review. Expert Opin Pharmacother. 17:885–888. [DOI] [PubMed] [Google Scholar]
- Gilbert TH, Hannesson DK, Corcoran ME. 2000. Hippocampal kindled seizures impair spatial cognition in the Morris water maze. Epilepsy Res. 38:115–125. [DOI] [PubMed] [Google Scholar]
- Hijikata Y, Yasuhara A, Yoshida Y, Sento S. 2006. Traditional Chinese medicine treatment of epilepsy. J Altern Complement Med. 12:673–677. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Wagner L, Hoffmann JM, Von LM, Elger CE. 2013. Comprehensive long-term outcome of best drug treatment with or without add-on vagus nerve stimulation for epilepsy: a retrospective matched pairs case-control study. Seizure. 22:109–115. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Blauwblomme T, Miles R. 2015. Hippocampus and epilepsy: findings from human tissues. Rev Neurol. 171:236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JY, Wang GQ, Cheng N, Wang X, Hong M, Han Y, Yang R. 2004. Clinical study on manifestation of hepatolenticular degeneration complicated with epilepsy and therapeutic effect of integrative Chinese and Western medicine treatment. Chin J Integr Med. 24:793–797. [PubMed] [Google Scholar]
- Jia C, Han S, Wei L, Dang X, Niu Q, Chen M, Cao B, Liu Y, Jiao H. 2018. Protective effect of compound Danshen (Salvia miltiorrhiza) dripping pills alone and in combination with carbamazepine on kainic acid-induced temporal lobe epilepsy and cognitive impairment in rats. Pharm Biol. 56:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto Y, Ayaki T, Urushitani M, Ito H, Takahashi R. 2016. Activated caspase-9 immunoreactivity in glial and neuronal cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 628:207–212. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Warita H, Sasaki C, Zhang WR, Sakai K, Shiro Y, Mitsumoto Y, Mori T, Abe K. 1999. Immunoreactive Akt, PI3-K and ERK protein kinase expression in ischemic rat brain. Neurosci Lett. 274:45–48. [DOI] [PubMed] [Google Scholar]
- Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, Resnick T, Benbadis SR. 2014. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 37:59–70. [DOI] [PubMed] [Google Scholar]
- Lin YX, Lin K, Liu XX, Kang DZ, Ye ZX, Wang XF, Zheng SF, Yu LH, Lin ZY. 2015. PI3K-AKT pathway polymerase chain reaction (PCR) array analysis of epilepsy induced by type II focal cortical dysplasia. Genet Mol Res. 14:9994–10000. [DOI] [PubMed] [Google Scholar]
- Maschio M, Dinapoli L, Fabi A, Giannarelli D, Cantelmi T. 2015. Cognitive rehabilitation training in patients with brain tumor-related epilepsy and cognitive deficits: a pilot study. J Neurooncol. 125:419–426. [DOI] [PubMed] [Google Scholar]
- Poornima P, Weng CF, Padma VV. 2013. Neferine from Nelumbo nucifera induces autophagy through the inhibition of PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food Chem. 141:3598–3605. [DOI] [PubMed] [Google Scholar]
- Phelan KD, Shwe UT, Williams DK, Greenfield LJ, Zheng F. 2015. Pilocarpine-induced status epilepticus in mice: a comparison of spectral analysis of electroencephalogram and behavioral grading using the Racine scale. Epilepsy Res. 117:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz JC, Goehe RW, Wijesinghe DS, Murudkar C, Hawkins AJ, Shay JW, Minna JD, Chalfant CE. 2010. Alternative splicing of caspase 9 is modulated by the phosphoinositide 3-kinase/Akt pathway via phosphorylation of SRp30a. Cancer Res. 70:9185–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Carmant L. 2015. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 5:a022426–a022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titiz AS, Mahoney JM, Testorf ME, Holmes GL, Scott RC. 2014. Cognitive impairment in temporal lobe epilepsy: role of online and offline processing of single cell information. Hippocampus. 24:1129–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vã z-Lp A, Sierra-Paredes G, Sierra-Marcuã OG. 2005. Role of cAMP-dependent protein kinase on acute picrotoxin-induced seizures. Neurochem Res. 30:613–618. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 1:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisburg H, Alvarez N. 1998. Carbamazepine in the treatment of epilepsy in people with intellectual disability. J Intellect Disabil Res. 42: 36–40. [PubMed] [Google Scholar]
- Wei L, Jia C, Dang X, Jiao H. 2014. Effects of Songlingxuemaikang capsules to the pharmacokinetics of carbamazepine in pentylenetetrazol (PTZ) - kinding seizures mice. Pharmacol Clin Chin Materia Med. 30:136–138. [Google Scholar]
- Wu L, Qiao H, Li Y, Li L. 2007. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomed. 14:652–658. [DOI] [PubMed] [Google Scholar]
- Wu M, Fang M, Hu Y, Wang X. 2012. Four types of traditional Chinese medicine inducing epileptic seizures. Seizure. 21:311–315. [DOI] [PubMed] [Google Scholar]
- Wu TT, Qu HH, Hu LN, Sun Y, Dai J, Sun H, Li YF, Zhao Y, Wang QG. 2015. Calculation of the conversion coefficients of equivalent dose among tree shrews, human and other species of experimental animals based on the body surface areas of tree shrews. Chin J of Trad Chin Med Phar. 30:203–205. [Google Scholar]
- Würstle ML, Laussmann MA, Rehm M. 2012. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res. 318:1213–1220. [DOI] [PubMed] [Google Scholar]
- Xie N, Wang C, Lian Y, Wu C, Zhang H, Zhang Q. 2014. Puerarin protects hippocampal neurons against cell death in pilocarpine-induced seizures through antioxidant and anti-apoptotic mechanisms. Cell Mol Neurobiol. 34:1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XC, Xiong XJ, Yang GY, Wang HR, Wang J. 2015. Songling Xuemaikang capsule for primary hypertension: a systematic review of randomized controlled trials. Chin J Integr Med. 21:312–320. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li H, Liu X, Bi J. 2013. Effect of caspase-9 inhibition on endoplasmic reticulum stress induced cortical neuronal injury in rats. Int J Clin Exp Med. 6:546–551. [PMC free article] [PubMed] [Google Scholar]
- Zhao RR, Xu XC, Xu F, Zhang WL, Zhang WL, Liu LM, Wang WP. 2014. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem Biophys Res Commun. 448:414–417. [DOI] [PubMed] [Google Scholar]
- Zheng GZ, Liang RQ. 2010. Ultrastructural effect of Songling Xuemaikang on acute cerebral ischemia-reperfusion injury in rat model. Chinese Arch Trad Chinese Med. 9:1988–1990. [Google Scholar]
- Zheng H, Wang X, Tang Z, Zheng W, Li Z. 2013. The PI3K/Akt and ERK1/2 signaling pathways mediate the erythropoietin-modulated calcium influx in kainic acid-induced epilepsy. Neuroreport. 24:335–441. [DOI] [PubMed] [Google Scholar]