Abstract
Background and Purpose:
Recent studies using cultured cells and rodent intracerebral hemorrhage (ICH) models have implicated receptor interacting protein kinase-1 (RIPK1) as a driver of programmed necrosis and secondary injury based on use of chemical inhibitors. However, these inhibitors have off target effects and cannot be used alone to prove a role for RIPK1. The aim of the current study was to examine the effect of genetic inhibition of the kinase domain of RIPK1 in a mouse ICH model.
Methods:
We subjected two lines of mice with RIPK1 point mutations of the kinase domain (K45A and D138N), rendering them kinase inactive, to autologous blood ICH and measured acute cell death and functional outcome.
Results:
Compared to wild-type controls, RIPK1K45A/K45A and RIPK1D138N/D138N had significantly less cells with plasmalemma permeability, less acute neuronal cell death, less weight loss and more rapid weight gain to baseline, and improved performance in a Morris water maze paradigm after autologous blood ICH. In addition, mice systemically administered GSK’963, a potent, specific, brain penetrant small molecule RIPK1 inhibitor, had reduced acute neuronal death at 24h after ICH.
Conclusions:
The data show that the kinase domain of RIPK1 is a disease driver of ICH, mediating both acute cell death and functional outcome, and support development of RIPK1 inhibitors as therapeutic agents for human ICH.
Keywords: Intracerebral hemorrhage, mice, necrosis, functional outcome, receptor interacting protein kinase-1, cell death
Subject Terms: Genetically Altered and Transgenic Models, Intracranial Hemorrhage, Neuroprotectants, Animal models of human disease, inflammation
Introduction
Intracerebral hemorrhage (ICH) accounts for 15% of strokes affecting 2 million people per year worldwide, with up to 70% mortality and less than 40% of survivors functioning independently at 3 months1. Clinical trials evaluating aggressive blood pressure management (INTERACT2), decompressive craniectomy, stereotactic hematoma evacuation (MISTIE II), and intraventricular thrombolysis (CLEAR III) failed to improve the neurological sequelae in ICH survivors2–5. Thus, specific treatment to improve outcome after ICH remains elusive, in part because of incomplete understanding of secondary injury mechanisms6–8.
The molecular biology of ICH-induced cell death has been a major focus of research efforts, with early studies emphasizing a role for caspase-mediated apoptosis9, 10. However, brain specimens from humans with ICH also exhibit necrosis, suggesting additional modes of neuronal death11, 12. Necroptosis is a form of necrosis mediated by the kinase domains of serine/threonine receptor interacting protein kinases (RIPK)-1 and −3, which phosphorylate and activate mixed lineage kinase domain-like (MLKL) protein in a RIPK1-RIPK3-MLKL necrosome complex13, 14. RIPK1 kinase activity can also induce apoptosis via assembly of a RIPK1-FADD-RIPK3-Caspase-8 ripoptosome15, 16 and inflammation by RIPK3-ERK-NFkB/cFos activation17 or by activation of inflammasomes18. Thus, RIPK1 is a master regulator of necrosis, apoptosis, and inflammation.
Studies using cultured neurons19, 20 and astrocytes21 show that blood derivatives can induce RIPK1 activation, necrosome assembly, and necrosis that is inhibitable by necrostatin-1, a pharmacological RIPK1 inhibitor- suggesting that brain cells may undergo necroptosis in response to ICH. Moreover, necrotic phenotypes occur in rodent collagenase and autologous blood ICH models22–26, and genetic deletion of RIPK3 inhibits plasmalemma permeability after collagenase ICH26. A growing number of studies have implicated RIPK1 and necroptosis in acute traumatic and ischemic brain injury13, 27, 28, as well as ICH19, 22–26, 29, based on protection with necrostatin-1. However, necrostatin-1 has off-target effects14 including inhibition of indoleamine-2,3-dioxygenase (IDO), and cannot be used alone to prove RIPK1 involvement in vivo30. This roadblock to understanding the role of RIPK1 in acute brain injury has persisted, until recently, in part because of lack of genetic tools to inhibit RIPK1.
Here, we used RIPK1 mutant mice with point mutations that render their kinase domain inactive (“kinase-dead”), to interrogate RIPK1 kinase function in a mouse autologous blood ICH model. We also tested systemic administration of GSK’963, a potent and selective brain penetrant next-generation RIPK1 inhibitor. The results confirm a predominant role for RIPK1 as disease driver of ICH, and validate future studies aimed at elucidating RIPK1 mechanisms in ICH, a devastating condition for which specific therapy is lacking.
Materials and Methods
All experimental protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee and conducted according to the National Institutes of Health guide for the care and use of laboratory animals and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. Studies were performed using adult (8–12 weeks, 25–30 g) male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA), RIPK1K45A/K45A (RIP1 Kinase-dead knock-in; a gift from GlaxoSmithKlein, PA)31 and RIPK1D138N/D138N (RIP1 Kinase-dead knock-in; a gift from Genentech, San Francisco, CA)32 mouse lines derived solely from C57Bl/6. Mice were housed in a pathogen-free environment with 12-hour day night cycles. Food and water were provided ad libitum. A descriptive table of experimental groups, the timeline of experimentation and total number of mice is provided in Supplemental Table I. All experiments were performed in a fully randomized and blinded design.
Autologous Blood Injection Intracerebral Hemorrhage (ICH-ABI) Model
Mice were anesthetized with isoflurane (2%) in 70% O2/30% N2O and core temperature was maintained at 37oC ± 1oC with a heating pad. A midline scalp incision was made, and a 1mm burr hole was drilled on the right side of the skull (from bregma: 0.1mm posterior, 2.5mm lateral). Non-heparinized autologous blood (30ul) collected from the facial vein, was pumped into the right striatum (3.5mm deep) using a 26-gauge Hamilton syringe and syringe pump (Stoelting, Wood Dale, IL) over one minute. The syringe was left in place for 10min then withdrawn. The burr hole was sealed with bone wax, and the scalp sutured closed. Mice were recovered at 37oC for 1h. In the sham ICH group, mice underwent the same surgical procedures but with 30μl sterile saline infusion.
Preparation of Brain Tissue and Quantitation of Propidium Iodide (PI)- and Fluoro JadeB (FJB)-Positive Cells
Propidium iodide (PI, 100μg in 400μl sterile PBS; Sigma, St. Louis, USA) was administered to mice and PI+ cells were quantitated in hemorrhagic brain as described previously26. For FJB staining, brain sections were post fixed in 4% paraformaldehyde and stained according to the manufacturer’s instructions. FJB+ cell counts were performed as described previously26.
Immunohistochemistry
High mobility group box 1 (HMGB1) and NeuN immunohistochemistry was performed as previously described26.
Western Blotting
Mice were transcardially perfused with heparin containing saline and brains were frozen on dry ice and kept at −80°C until use. 200μm coronal sections were cut on a cryostat and striatal hemorrhagic lesions were dissected along with similar amounts of contralateral striatum, and brain tissue was subjected to Western blot as previously described33 using rabbit polyclonal HMGB1 antibody (1:2000, Abcam) and mouse monoclonal NeuN antibody (1:1000, Millipore) overnight at 4°C and horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG secondary antibodies (1:3000, Cell Signaling, Cambridge, MA) for 1h at room temperature. Protein bands were normalized to beta actin (1:3000, Cell Signaling) and densitometry was performed using NIH Image J software. Cerebrospinal fluid (CSF) samples were taken from the cisterna magna and blotted as previously described34.
Grid walk test
The grid walk test was performed as previously described35.
Morris water maze (MWM)
The MWM was performed as previously described33 with a total of 7 hidden platform trials and 90s maximum latency to the platform.
Statistical Analyses
Data are mean ± standard error of the mean. PI+, FJB+, NeuN+ and Hoechst+ cell counts, densitometry, and MWM probe trial data were analyzed by t-Test. MWM hidden and visible platform trials, and weight loss data, were analyzed by repeated measures ANOVA (group × time) using GraphPad Prism 7. Behavioral tests were powered based on our previous publications. All other tests were powered assuming 50% difference between group means and estimated standard deviation 30% and power 0.8.
Results
Autologous blood ICH induces plasmalemma damage and fatal cellular injury.
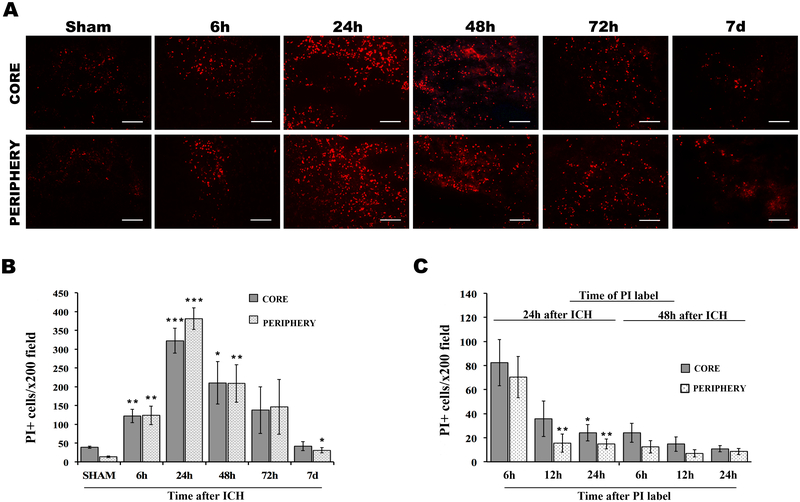
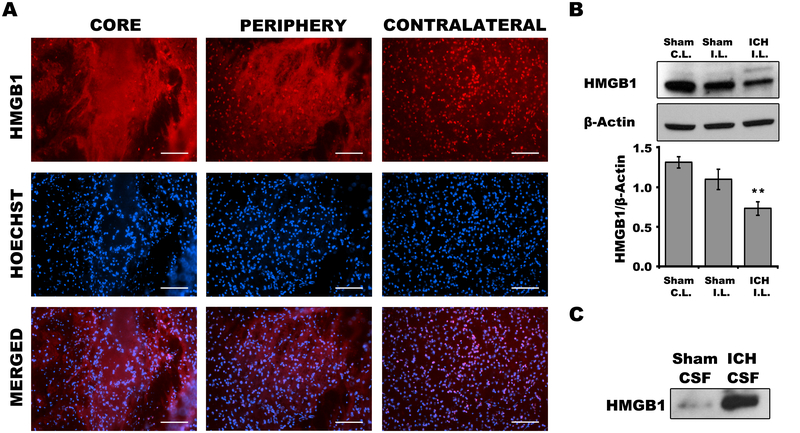
Propidium iodide labeling showed an increase in cells with plasmalemma damage beginning at 6 and peaking at 24h, with return to sham injury levels by 7d in core and peripheral hemorrhagic regions (Figure1A, B). PI+ cells represented 74.6% and 66.1% of total cells in core and peripheral hemorrhagic regions at 24h, and 56.5% and 45.7% of total cells in core and periphery at 48h after ICH; by 7d PI+ cells repented less than 5% of total cells in each hemorrhagic region (compare Figure 1B to Supplemental Figure IA). PI+ cells labeled at either 24 or 48h rapidly disappeared from injured brain (compare Figure 1C to 1B), suggesting fatal injury. Compared to contralateral hemispheres, robust nuclear to cytosol translocation and release of HMGB1 was observed in hemorrhagic brain regions at 24h, and Western blot analyses confirmed loss of brain HMGB1 and its appearance in CSF (Figure 2) consistent with necrosis and/or release from inflammatory cells. Taken together, the data suggest that necrosis occurs in our ICH model and that PI is a useful marker of cell death to interrogate RIPK1 function.
Figure 1:
Plasmalemma permeability detected by in vivo propidium iodide (PI) in core and peripheral hemorrhage regions after intracerebral hemorrhage (ICH). A, Representative photomicrographs of PI+ cells at indicated times after ICH. B, Quantitation of PI+ cells in x200 fields at 6h–7d after ICH. *p<0.05 vs. sham (24h); **p<0.005 vs. sham; ***p<0.005 vs. sham. C, Time course of disappearance of PI+ cells from hemorrhagic brain regions after pulse labeling at 24 or 48h. *p<0.05 vs. 6h; **p<0.005 vs. 6h. Scale bars: 100 um.
Figure 2:
High mobility group box 1 (HMGB1) release from brain after intracerebral hemorrhage (ICH). A, Immunohistochemical detection of HMGB1 at 24h in WT mice. B, Western blot detection of HMGB1 and densitometry results at 24h in WT mice. *p<0.05 vs. sham ICH. (C.L., contralateral, I.P., Ipsilateral) C. Representative Western blot showing release of HMGB1 into cerebrospinal fluid (CSF) of WT mice at 24h.
RIPK1 kinase-dead mutations protect against acute cell death after autologous blood ICH.
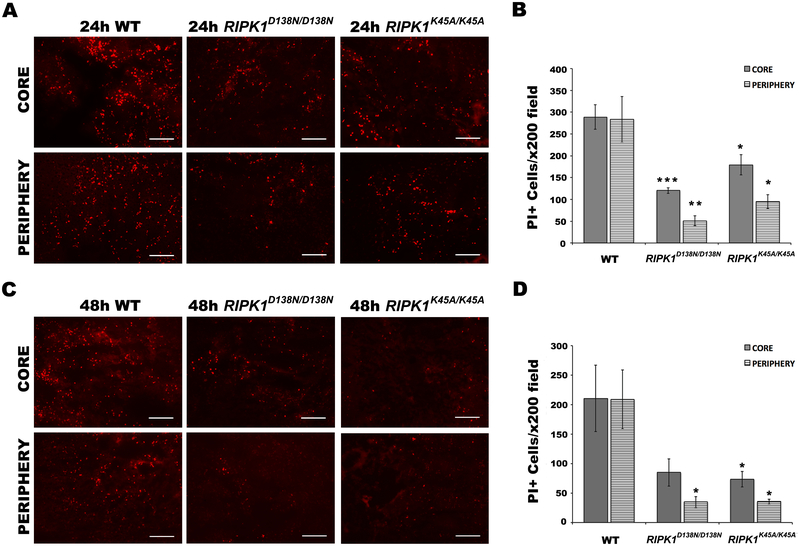
We next sought a functional role for RIPK1 by subjecting two lines of RIPK1 kinase-dead point mutant mice (RIPK1K45A/K45A, RIPK1D138N/D138N) to autologous blood ICH. Compared to wild-type, RIPK1K45A/K45A and RIPK1D138N/D138N groups had significantly reduced PI+ cells in core and peripheral hemorrhagic regions at 24 and 48h (Figure 3). In addition, RIPK1K45A/K45A and RIPK1D138N/D138N mutant mice had significantly less FJB+ neurons than wild-type in both hemorrhagic regions at both time points (Figure 4). Notably, RIPK1K45A/K45A mice had 16% less NeuN+ cells compare to wild-type at baseline, suggesting a role for this mutation in brain development (Supplemental Figure IIA). Moreover, in an analysis of cell loss after ICH, RIPK1D138N/D138N mice had significantly increased Hoechst+ cells at 24 and 48h after ICH compare to wild-type, consistent with a protective effect on cell viability whereas RIPK1K45A/K45A mice had no difference vs. WT (Supplemental Figure IB) but had 20% less Hoechst+ cells in striatum at baseline (Supplemental Figure IIB).
Figure 3.
RIPK1 kinase-dead mutations reduce PI+ cells in core and peripheral hemorrhage regions after intracerebral hemorrhage (ICH). A, C, representative photomicrographs of PI+ cells at 24h (A) and 48h (C) after ICH in wild-type (WT) and mutant mice. B, D, Quantitation of PI+ cells in x200 fields at 24h (B) and 48h (D) after ICH. *p<0.05 vs. WT; **p<0.005 vs. WT; ***p<0.0005 vs. WT. Scale bars: 100 um.
Figure 4.
RIPK1 kinase-dead mutations reduce Fluoro JadeB (FJB)+ cells in core and peripheral hemorrhage regions after intracerebral hemorrhage (ICH). A, C, representative photomicrographs of FJB+ cells at 24h (A) and 48h (C) after ICH in wild-type (WT) and mutant mice. B, D, Quantitation of FJB+ cells in x200 fields at 24h (B) and 48h (D) after ICH. *p<0.05 vs. WT; **p<0.005 vs. WT; ***p<0.0005 vs. WT. Scale bars: 100 um.
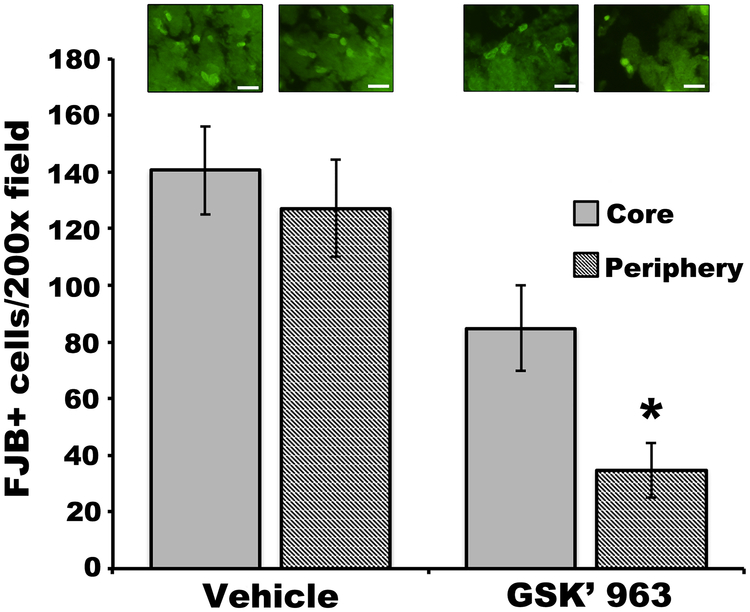
We next sought to confirm these findings with GSK’963, a pharmacological RIPK1 inhibitor36, administered intraperitoneally (25mg/kg) every three hours beginning at the time of ICH. Compared to vehicle, GSK’963 significantly reduced the numbers of FJB+ cells in peripheral hemorrhage regions, whereas the reduction in the core failed to reach significance (Figure 5). Of note, NeuN histochemistry did not prove feasible to assess neuronal cell numbers in the hemorrhagic lesion (Supplemental Figure III).
Figure 5:
GSK’963 reduces Fluoro JadeB (FJB)+ cells at 24h after ICH. *p<0.05 vs. vehicle. Scale bars: 20 um.
RIPK1 kinase-dead mutations protect against weight loss and functional deficits after ICH.
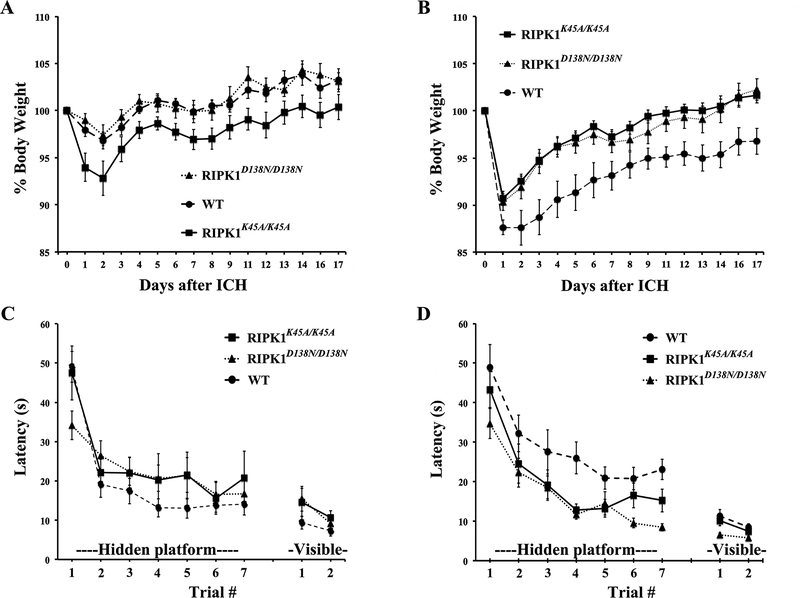
We next sought to determine whether RIPK1 kinase inhibition might protect against physiological and functional consequences of ICH. Weight loss and recovery was similar between sham injured WT and RIPK1D138N/D138N groups, but RIPK1K45A/K45A sham injured mice had increased weight loss and delayed weight recovery vs. sham injured WT (Figure 6A) suggesting that RIPK1K45A/K45A mice are vulnerable to sham injury. Following ICH, RIPK1D138N/D138N and RIPK1K45A/K45A mutants had improved weight loss and recovery vs. WT (Figure 6B). Sham injured RIPK1D138N/D138N had improved recovery of weight loss vs. injured RIPK1D138N/D138N, whereas RIPK1K45A/K45A sham and injured mice had similar weight loss and recovery, suggesting no effect of ICH in the RIPK1K45A/K45A mutants beyond the effect of sham injury.
Figure 6.
RIPK1 kinase-dead mutations improve recovery after intracerebral hemorrhage (ICH) in mice. A, weight loss and recovery of weight gain after sham ICH was not different between wild type (WT) and RIPK1K45A/K45A or WT and RIPK1D138N/D138N mice. B, Decreased weight loss and faster weight gain was observed in RIPK1K45A/K45A (p<0.001 for group) and RIPK1D138N/D138N (p=0.001) groups vs. WT after ICH. C, No difference among sham injured groups in Morris water maze performance. D, Improved hidden platform performance after ICH in RIPK1K45A/K45A and RIPK1D138N/D138N groups vs. WT (p<0.05 for group for each comparison vs. WT).
In the MWM, sham injured RIPK1K45A/K45A and RIPK1D138N/D138N groups performed similarly to WT in hidden and visible platform trials (Figure 6C) and in probe trials (Supplemental Figure IV). Following ICH, compared to WT, RIPK1K45A/K45A and RIPK1D138N/D138N performed significantly better on hidden platform trials with no differences in visible platform (Figure 6D) or probe trials (Supplemental Figure IV). No differences in swim speeds were noted among sham (Supplemental Figure VA) or injured (Supplemental Figure VB) groups, suggesting that differences in hidden platform performance were not attributable to locomotor activity. No differences were observed between RIPK1K45A/K45A and RIPK1D138N/D138N groups compare to wild-type in pre- or post-injury foot faults on a grid walk test (Supplemental Figure VI).
Discussion
Recent studies using in vitro and in vivo ICH models have suggested a role for RIPK1 as a disease driver based on studies with necrostatin-1 or siRNA mediated RIPK1 knockdown20,23–27,30. To our knowledge, this is the first study to examine RIPK1 in any acute brain injury model using genetic tools. Mice with either of two distinct kinase-inactivating RIPK1 point mutations had significantly reduced cells with plasmalemma damage, reduced acute neuronal cell death, and improved physiological (weight loss/gain after injury) and functional (Morris water maze) outcomes. In addition, systemic administration of the RIPK1 inhibitor GSK’963 also reduced neuronal death at 24h after ICH, similar to results in RIPK1 mutant mice. The data validate an important role for RIPK1 in the pathogenesis of cell death and functional outcome after ICH, and support the idea that RIPK1 is a therapeutic target for patients with ICH.
In vivo PI labeling detected cells with plasmalemma damage and presumed necrosis, or late apoptosis, as previously reported in a collagenase ICH model26. In that study, PI+ cells peaked at 48h, whereas peak numbers appeared by 24h in our autologous blood ICH model. To confirm that PI labeling signals fatal cellular injury after ICH, we performed pulse labeling experiments showing rapid disappearance of PI+ cells labeled at 24 or 48h after injury. The kinetics of PI+ cell loss obtained herein are in striking contrast to the much slower disappearance of PI+ cells from injured brain after controlled cortical impact37. The robust findings of nuclear translocation of HMGB1 is consistent with passive release from injured/necrotic cells, but is not specific for necrosis as HMGB1 may be actively released from stimulated inflammatory cells38. The findings that genetic or pharmacological RIPK1 inhibition reduced numbers of PI+ and FJB+ cells constitutes strong biochemical evidence of necroptosis, although RIPK1 can also promote apoptosis39 which can present with plasmalemma damage at later stages. Taken together, the cellular and biochemical data do not discount the possibility of apoptotic death programs in PI+ cells, but are consistent with the idea that necroptosis contributes to cell death after ICH, and that RIPK1 is an important driver of programmed neuronal death in this model. Further studies are needed to show inhibition of mechanisms specific for necroptosis in RIPK1 mutant mice such as MLKL and RIPK3 phosphorylation events17, 32.
Moreover, Hoechst+ cell count data also support a protective role for RIPK1 inhibition as suggested by the increased numbers of Hoechst+ cells remaining in the hemorrhagic lesion of RIPK1D138N/D138N mice at 24 and 48h (Supplemental Figure IB), a finding that is not completely accounted for by the increased numbers of Hoechst+ cells at baseline in these mice (Supplemental Figure IIB). Of note, RIPK1K45A/K45A mice did not show greater numbers of Hoechst+ cells at 24 or 48h after ICH vs. WT (Supplemental Figure IB) but RIPK1K45A/K45A mice had 20% less Hoechst+ cells at baseline vs. WT (Supplemental Figure IIB) which could account for their seeming lack of protection in this assay.
Genetic inhibition of RIPK1 kinase activity also improved functional recovery and physiology after ICH, as evidenced by reduced overall weight loss in both RIPK1 mutants on the first post-injury day, and faster recovery to baseline weight vs. WT. Interestingly, RIPK1K45A/K45A mutants had an exaggerated response to sham ICH that resulted in a more pronounced weight loss and prolonged weight recovery vs. WT. Despite these differences, injured RIPK1K45A/K45A mice had improved weight gain and performance in the water maze vs. injured WT, suggesting a robust degree of protection by the RIPK1K45A/K45A mutation in the context of ICH. Hidden platform performance in the Morris water maze was also improved in injured RIPK1D138N/D138N mutants vs. WT. Based on prior reports of marked phenotypic differences in various point mutant RIPK3 kinase-dead mice16, it is not surprising that RIPK1K45A/K45A and RIPK1D138N/D138N lines showed different phenotypes with sham injury in some of the outcome measures in the current study. These results may be due to slight genetic differences in background strains, but RIPK1D138N/D138N and RIPK1K45A/K45A are made in C57Bl/6 mice without DNA from other strains31, 40. More likely, the observed differences are due to differential effects of specific kinase domain mutations on RIPK1 function overall. The finding that hidden platform performance was not different between sham injured and injured RIPK1K45A/K45A and RIPK1D138N/D138N mice, whereas WT ICH mice performed significantly worse than WT shams, suggests that RIPK1 may be a therapeutic target to limit at least some functional deficits induced by ICH. Others have reported improved functional outcomes with intracerebroventricular administration of necrostatin-1, or siRNA against RIPK1, in rodent ICH models23–27,30. Our data confirm and support the involvement of RIPK1 suggested by those prior studies, but not in all functional tests as no differences among groups were observed in the grid walk test.
RIPK1 could mediate cell death and functional outcome through several distinct mechanisms. The kinase activity of RIPK1 is required for necrosome assembly via phosphorylation and activation of RIPK3, and association with MLKL. Phosphorylation of MLKL in the necrosome induces its activation and translocation to the cell membrane, where it induces necroptosis in part by permeabilizing the plasmalemma via unknown mechanisms14. In addition to necrosis, RIPK1 can assemble a RIPK1-RIPK3-FADD-caspase-8 ripoptosome complex, which can induce apoptosis via activation of caspase 8, or inflammation via inflammasome activation14, 15, 18. Our group recently reported an additional pathway by which RIPK1 mediates inflammation, by activating a RIPK3-ERKI/II-cFos/NFkB pathway distinct from necroptosis17. The NFkB domain of RIPK1 is not inhibited by kinase domain mutations, so RIPK1 mutants used herein might still be expected to exert inflammatory activity after ICH. Future studies in our laboratory are aimed at elucidating specific mechanisms of RIPK1 in the pathogenesis of cellular injury and death (e.g., apoptosis and necrosis) and inflammation after ICH.
Prior studies in mouse collagenase ICH models demonstrated that intracerebroventricular injection of necrostatin-1 prior to development of hematoma improved several clinically relevant outcomes after ICH, including PI+ cells, edema, blood-brain barrier disruption, inflammation, and neurological scores23–27,30. These studies suggested a role for RIPK1, but the high local concentrations of necrostatin-1 infused in brain, and its known off-target effects, precluded these studies from proving a role for RIPK1 in vivo. We used systemic administration of GSK’963, a next generation potent, selective small molecule RIPK1 inhibitor36, to complement the use of RIPK1 mutant mice. Systemic administration of GSK’963 reduced acute neuronal death at 24h, albeit not as robustly as seen in mutant mice. This result is expected because drug penetration to core hemorrhage regions may be inconsistent due to relative lack of blood flow, edema, or other reasons. GSK’963 inhibits RIPK1 in vitro 200-fold more potently than necrostatin-1, and inhibits endotoxin-induced hypothermia in mice to a greater extent also36. Human RIPK1 inhibitors are currently in clinical trials of inflammatory diseases of the skin, colon, and joints. Data from the current study suggest the development of RIPK1 inhibitors for humans with hemorrhagic stroke. The current study builds upon recent findings that necroptosis signaling intermediates are observed in brain after autologous blood ICH in rodents. Zille et al. (2017) recently showed that ERK phosphorylation, a key step in RIPK1 inflammatory signaling17, occurs after ICH in mice19. Shen et al. (2017) showed that following autologous blood ICH in rats, the activated form of RIPK1 (phospho-serine RIPK1) was increased in hemorrhagic brain, as well as RIPK1-RIPK3-MLKL-caspase 8 interaction20. Downregulation of RIPK1 by siRNA reduced necrosome assembly and PI+ cells, blood-brain barrier permeability to Evans blue, and brain edema. Whether or not these findings are attributable to inhibition of the kinase domain of RIPK1, other RIPK1 molecular domains, or another molecule(s) remain to be determined because siRNA reduces total levels of RIPK1 protein, and may have off target effects on other RNA species. Nonetheless, the study by Shen et al. (2017), along with our prior report showing reduced PI+ cells after ICH in RIPK3 knockout mice, complement data from the current study that strongly argue for a role for RIPK1 and RIPK3 in the pathogenesis of ICH.
Summary/Conclusions:
Genetic evidence presented herein confirms an important role for RIPK1 kinase activity as a disease driver in ICH. Future studies are needed to determine the exact mechanisms (necrosis, apoptosis, inflammation) responsible for improved outcomes with RIPK1 inhibition, and to identify additional RIPK1 signaling pathways that may serve as therapeutic targets for patients with ICH, for which no specific therapy is currently available to reduce neuronal cell death and improve outcome.
Supplementary Material
Acknowledgments:
None
Sources of Funding: This work was supported by NINDS RO1NS071072 (MJW) and 5PO1NS055104–08 (EHL).
Footnotes
Disclosures:
P.J.G. is an employee and stockholder of GlaxoSmithKline who are developing RIP1 kinase inhibitors.
A.D. is a consultant for Denali Therapeutics.
References
- 1.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365 [DOI] [PubMed] [Google Scholar]
- 3.Hanley DF, Lane K, McBee N, Ziai W, Tuhrim S, Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: Results of the randomised, multicentre, multiregion, placebo-controlled clear iii trial. Lancet. 2017;389:603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (mistie): A randomised, controlled, open-label, phase 2 trial. The Lancet. Neurology 2016;15:1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendelow AD. Surgical craniotomy for intracerebral haemorrhage. Frontiers of neurology and neuroscience. 2015;37:148–154 [DOI] [PubMed] [Google Scholar]
- 6.Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids and barriers of the CNS. 2014;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xi G, Strahle J, Hua Y, Keep RF. Progress in translational research on intracerebral hemorrhage: Is there an end in sight? Progress in neurobiology. 2014;115:45–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke. 2011;42:1781–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–882; discussion 882–873 [DOI] [PubMed] [Google Scholar]
- 10.Zhang XQ, Zhang ZM, Yin XL, Zhang K, Cai H, Ling F. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: Tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chin Med J (Engl). 2010;123:1246–1250 [PubMed] [Google Scholar]
- 11.Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041–1047; discussion 1047–1048 [PubMed] [Google Scholar]
- 12.Wang KY, Wu CH, Zhou LY, Yan XH, Yang RL, Liao LM, et al. Ultrastructural changes of brain tissues surrounding hematomas after intracerebral hemorrhage. European neurology. 2015;74:28–35 [DOI] [PubMed] [Google Scholar]
- 13.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119 [DOI] [PubMed] [Google Scholar]
- 14.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2016;18:127–136 [DOI] [PubMed] [Google Scholar]
- 15.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. Ciaps block ripoptosome formation, a rip1/caspase-8 containing intracellular cell death complex differentially regulated by cflip isoforms. Mol Cell. 2011;43:449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, et al. Rip3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najjar M, Saleh D, Zelic M, Nogusa S, Shah S, Tai A, et al. Ripk1 and ripk3 kinases promote cell-death-independent inflammation by toll-like receptor 4. Immunity. 2016;45:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, et al. Ripk3 promotes cell death and nlrp3 inflammasome activation in the absence of mlkl. Nat Commun. 2015;6:6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48:1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen HLC, Zhang D, Yao X, Zhang K, Li H, Chen, G. Role for rip1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro. Cell Death and Disease 2017;8, e2641; doi: 10.1038/cddis.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laird MD, Wakade C, Alleyne CH, Jr., Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med. 2008;45:1103–1114 [DOI] [PubMed] [Google Scholar]
- 22.Chang P, Dong W, Zhang M, Wang Z, Wang Y, Wang T, et al. Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J Mol Neurosci. 2014;52:242–249 [DOI] [PubMed] [Google Scholar]
- 23.King MD, Whitaker-Lea WA, Campbell JM, Alleyne CH, Jr., Dhandapani KM. Necrostatin-1 reduces neurovascular injury after intracerebral hemorrhage. International journal of cell biology. 2014;2014:495817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majmundar N, Kim B, Prestigiacomo CJ. Necroptosis pathway in treatment of intracerebral hemorrhage: Novel therapeutic target. World neurosurgery. 2016;89:716–717 [DOI] [PubMed] [Google Scholar]
- 25.Su X, Wang H, Kang D, Zhu J, Sun Q, Li T, et al. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting rip1/rip3 pathway. Neurochem Res. 2015;40:643–650 [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, et al. Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke. 2012;43:524–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88:1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:1564–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.S H, L C, Z D, Y X, Z K, L H, et al. Role for rip1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro Cell Death and Disease 2017;8, e2641; doi: 10.1038/cddis.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both ripk1 and ido: Consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20:185–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger SB, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, et al. Cutting edge: Rip1 kinase activity is dispensable for normal development but is a key regulator of inflammation in sharpin-deficient mice. J Immunol. 2014;192:5476–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, et al. Activity of protein kinase ripk3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360 [DOI] [PubMed] [Google Scholar]
- 33.Khuman J, Meehan WP 3rd, Zhu X, Qiu J, Hoffmann U, Zhang J, et al. Tumor necrosis factor alpha and fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J Cereb Blood Flow Metab. 2011;31:778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, et al. Spreading depression triggers headache by activating neuronal panx1 channels. Science. 2013;339:1092–1095 [DOI] [PubMed] [Google Scholar]
- 35.Gibson CL, Bath PM, Murphy SP. G-csf reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439 [DOI] [PubMed] [Google Scholar]
- 36.Berger SB, Harris P, Nagilla R, Kasparcova V, Hoffman S, Swift B, et al. Characterization of gsk’963: A structurally distinct, potent and selective inhibitor of rip1 kinase. Cell death discovery. 2015;1:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whalen MJ, Dalkara T, You Z, Qiu J, Bermpohl D, Mehta N, et al. Acute plasmalemma permeability and protracted clearance of injured cells after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28:490–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersson U, Tracey KJ. Hmgb1 is a therapeutic target for sterile inflammation and infection. Annual review of immunology. 2011;29:139–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofengeim D, Yuan J. Regulation of rip1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736 [DOI] [PubMed] [Google Scholar]
- 40.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, et al. Cutting edge: Ripk1 kinase inactive mice are viable and protected from tnf-induced necroptosis in vivo. J Immunol. 2014;193:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.