Abstract
This study examined the effect of the presence of single or multiple embryos on the transcriptome of the bovine oviduct. In experiment 1, cyclic (nonbred, n = 6) and pregnant (artificially inseminated, n = 11) heifers were slaughtered on Day 3 after estrus, and the ampulla and isthmic regions of the oviduct ipsilateral to the corpus luteum were separately flushed. Oviductal epithelial cells from the isthmus region, in which all oocytes/embryos were located, were snap-frozen for microarray analysis. In experiment 2, heifers were divided into cyclic (nonbred, n = 6) or pregnant (multiple embryo transfer, n = 10) groups. In vitro-produced presumptive zygotes were transferred endoscopically to the ipsilateral oviduct on Day 1.5 postestrus (n = 50 zygotes/heifer). Heifers were slaughtered on Day 3, and oviductal isthmus epithelial cells were recovered for RNA sequencing. Microarray analysis in experiment 1 failed to detect any difference in the transcriptome of the oviductal isthmus induced by the presence of a single embryo. In experiment 2, following multiple embryo transfer, RNA sequencing revealed 278 differentially expressed genes, of which 123 were up-regulated and 155 were down-regulated in pregnant heifers. Most of the down-regulated genes were related to immune function. In conclusion, the presence of multiple embryos in the oviduct resulted in the detection of differentially expressed genes in the oviductal isthmus; failure to detect changes in the oviduct transcriptome in the presence of a single embryo may be due to the effect being local and undetectable under the conditions of this study.
Keywords: bovine, embryo-maternal interaction, microarray, oviduct, RNA-Seq
Introduction
Following ovulation, the bovine oocyte undergoes fertilization, and the resulting embryo spends the first 3–4 days of life in the oviduct, during which time it undergoes, morphologically, the first mitotic cell divisions and, transcriptionally, embryonic genome activation (EGA; at the 8- to 16-cell stage) [1]. The developing embryo then enters the uterus, where it soon forms a blastocyst, hatches from the zona pellucida, and forms an ovoid and then a tubular structure before undergoing elongation to form a filamentous conceptus that initiates implantation around Day 19 postconception.
Clear evidence indicates a two-way interaction between the uterus and developing conceptus. For example, it has been well demonstrated that circulating progesterone directly regulates uterine gene expression that in turn drives conceptus elongation [2, 3]. Until maternal recognition of pregnancy, the temporal changes that occur in the endometrial transcriptome are similar between pregnant and cyclic heifers [4]. However, by Day 15 [5] to Day 16 [4], the first transcriptomic responses of the endometrium to the embryo can be detected. These changes are predominantly, but perhaps not exclusively, due to conceptus secretion of interferon tau; however, other conceptus-derived factors, such as prostaglandins, may also be involved [5, 6]. Indeed, not only does the endometrium respond to the conceptus, the response elicited is related to the type of conceptus present (e.g., in vivo, in vitro fertilization [IVF], or cloned) and the likely developmental outcome [7, 8].
Despite this demonstration of an interaction between the developing conceptus and the uterine endometrium in early pregnancy, the evidence for reciprocal crosstalk during the transit of the early embryo through the oviduct is less clear. On the one hand, very convincing evidence suggests a positive influence of the oviduct on the quality of the early embryo. For example, short-term culture of in vitro-produced bovine zygotes in the oviducts of cattle [9, 10], sheep [11, 12], or even mice [13] has been shown to improve embryo quality as measured in terms of morphology, gene expression, cryotolerance, and pregnancy rate after transfer. In contrast, relatively little evidence exists for a reciprocal effect of the early embryo on the oviduct. Limited data demonstrate an effect of gametes [14–17] or embryos [18, 19] on the oviduct transcriptome and secretory proteome. All of these studies come from litter-bearing species (mice and swine), in which any embryo-induced alterations likely are amplified by the multiple embryos present. Tangible evidence for the existence of reciprocal embryo-oviduct interaction comes from the investigation of differential transport of fertilized and unfertilized eggs in the oviduct in the mare. It has been suggested that the fertilized horse embryo produces prostaglandin E2 that favors its oviductal transport to the uterus [20, 21], whereas nonfertilized oocytes are retained in the oviduct [22].
We hypothesized that the early bovine embryo elicits an oviductal response during its transit through the oviduct that may contribute to its subsequent development. To test this hypothesis, the effect of the presence of single or multiple embryos on the transcriptional response of the epithelial cells of the oviduct was investigated.
Materials and Methods
All experimental procedures involving animals were licensed by the Department of Health and Children, Ireland. Protocols were in accord with the Cruelty to Animals Act (Ireland 1897) and the European Community Directive 86/609/EC and were sanctioned by University College Dublin's Institutional Animal Research Ethics Committee.
Experiment 1
The aim of experiment 1 was to examine the effect of the presence of a single embryo (vs. an unfertilized oocyte) on the transcriptome of the epithelial cells of the oviductal isthmus (see Fig. 1A for experimental design). For the duration of the experiment, all animals were housed indoors on a slatted floor and were fed a diet consisting of grass and maize silage supplemented with a standard beef ration. The estrous cycles of crossbred beef heifers (n = 19, predominantly Charolais and Limousin cross; age, 23.0 ± 0.7 mo [mean ± SEM]; weight, 583.3 ± 12.4 kg) were synchronized using a 7-day Controlled Internal Drug Release (CIDR; 1.38 g of progesterone; Pfizer) insert combined with a dose of 0.02 mg of a gonadotropin-releasing hormone (GnRH) agonist (buserelin; Receptal; Intervet) and administration of 15 mg of a prostaglandin F2α analog (Prosolvin; Intervet) on the day before CIDR removal. Heifers were observed for signs of estrus four times per day commencing 30 h after CIDR withdrawal, and only those recorded in standing estrus (Day 0; n = 17) were used. Heifers were randomly allocated to one of two groups: cyclic group (nonbred, n = 6) or pregnant group (artificially inseminated 12 and 24 h after first standing in estrus with frozen-thawed semen from a bull of proven fertility, n = 11).
Fig. 1.
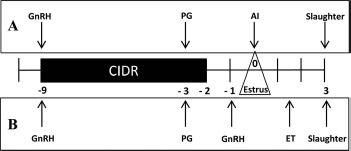
Experimental design. The estrous cycles of crossbred beef heifers were synchronized using a 7-day progesterone (P4)-releasing intravaginal device (CIDR; 1.38 g of P4) with administration of a prostaglandin F2α analog (PG) the day before CIDR removal. A) Experiment 1. Heifers (n = 19) were allocated to one of two groups: cyclic (nonbred) or pregnant (artificially inseminated at standing estrus). All heifers were slaughtered on Day 3 after estrus. B) Experiment 2. To ensure the ovulation, 0.02 mg of a GnRH agonist (buserelin) was administered 32 h after CIDR removal. Heifers (n = 17) were allocated to one of two groups: cyclic, sham transfer in both oviducts or pregnant with 50 in vitro-produced presumptive zygotes transferred in the ipsilateral oviduct and a sham transfer carried out in the contralateral oviduct. All transfers were carried out endoscopically on Day 1.5, and all animals were slaughtered on Day 3 after estrus. ET, endoscopic transfer.
Sample collection.
Animals were slaughtered in a commercial abattoir 3 days (74.6 ± 1.3 h) after the start of standing estrus. Following slaughter, the reproductive tract was removed, sealed in a plastic bag, transported to the laboratory on ice, and processed. The oviduct ipsilateral to the corpus luteum was trimmed free of tissue and divided in half to separate ampulla and isthmus regions. The ampulla and isthmus were then separately flushed with 500 μl of PBS. The presence and location (ampulla vs. isthmus) of an unfertilized oocyte or an embryo was verified under a microscope. In all cases, the oocyte/embryo was located in the isthmus. After flushing, each isthmus section was opened longitudinally and gently scraped with a blade to recover epithelial cells. The cells obtained were snap-frozen and stored at −80°C. Epithelial cells from the oviductal isthmus of five heifers with a confirmed nonfertilized oocyte (cyclic) and five heifers with an 8-cell stage embryo (pregnant) were used for gene expression analysis.
RNA extraction and microarray hybridization.
Total RNA was extracted from oviductal isthmus epithelial cell samples of individual heifers by the TRIzol method per the manufacturer's instructions (Invitrogen). Following on-column DNase digestion and RNA cleanup (Qiagen), both the quality and quantity of the RNA were determined using the Agilent Bioanalyzer (Agilent Technologies) and the NanoDrop 1000 (Thermo Fischer Scientific, Inc.), respectively. All samples had an RNA Integrity Number of greater than 8.0. Transcriptomic analysis was carried out using the Bovine Gene ST 1.0 microarray (Affymetrix). A total of 150 ng of RNA was used for reverse transcription with the Ambion WT Expression Kit (Life Technologies), whereas the rest was stored for microarray validation analysis by quantitative real-time PCR (qPCR). All samples were processed with the appropriate amount of Poly-A RNA controls from the Affymetrix GeneChip Poly-A RNA Control Kit as specified within the Ambion user's manual. Purified cDNA (5.5 μg) was fragmented and labeled using the GeneChip WT Terminal Labeling kit (Affymetrix), and fragmentation was verified using the Agilent 2100 Bioanalyzer. Hybridization was performed according to the Affymetrix user's manual. Briefly, fragmented, biotin-labeled cDNA was hybridized to the Affymetrix Bovine Gene ST 1.0 microarray as described within the Encore Biotin Module user's guide appendix. Samples were hybridized for 16 h at 45°C in a GeneChip Hybridization Oven 640 (Affymetrix) while rotating at 45 rpm. Microarrays were processed using the Affymetrix GeneChip Fluidic Station 450. Staining was carried out with streptavidin-conjugated phycoerythrin (SAPE), followed by amplification with a biotinylated anti-streptavidin antibody and by a second round of SAPE before scanning using a GeneChip Scanner 3000 (Affymetrix) and GeneChip Command Console software (Affymetrix).
Microarray data analysis.
For microarray results, the raw signal intensities were read into R and preprocessed using RMA implemented in the affy package [23] of the Bioconductor project [24]. Arrays were analyzed using probe set remappings from Molecular & Behavioral Neuroscience, Institute, University of Michigan [25]. Lists of differentially expressed genes (DEGs) were determined by the Limma package [26] employing linear modeling and an empirical Bayes framework to shrink the variance of measurements on each probe set. A modified t-test was then carried out, and all P-values were adjusted for multiple testing using the Benjamini and Hochberg false-discovery rate method.
Experiment 2
The experimental design is shown in Figure 1B. Crossbred beef heifers (n = 17, predominantly Charolais and Limousin cross; age, 22.5 ± 1.2 mo; weight, 571.8 ± 10.6 kg) were managed and synchronized as described for experiment 1. To ensure that ovulation had occurred at the time of embryo transfer, 0.02 mg of a GnRH agonist (buserelin) was administered 32 h after CIDR removal. Heifers were observed for signs of estrus four times per day commencing 30 h after CIDR withdrawal, and all were seen in standing estrus (Day 0; n = 17). Heifers were randomly allocated to one of two groups, cyclic or pregnant. On Day 1.5 after the onset of estrus, 50 presumptive zygotes were transferred endoscopically into the oviduct ipsilateral to the corpus luteum in the pregnant group (n = 9). A sham transfer (media only) was carried out into the ipsilateral oviduct of heifers in the cyclic group (n = 8).
In vitro embryo production, transfer, and tissue collection.
Unless otherwise stated, all chemicals were purchased from Sigma Chemical Co. The techniques for producing embryos in vitro have been described previously [12]. Immature cumulus-oocyte complexes (COCs) were obtained by aspirating follicles from bovine ovaries collected at a commercial abattoir. The COCs were matured for 24 h in TCM-199 supplemented with 10% (v/v) fetal calf serum (FCS) and 10 ng/ml of epidermal growth factor at 39°C under an atmosphere of 5% CO2 in air with maximum humidity. Frozen-thawed, Percoll-separated bull sperm at a concentration of 1 × 106 spermatozoa/ml was used to inseminate the matured COCs. Gametes were coincubated at 39°C under an atmosphere of 5% CO2 in air with maximum humidity. At approximately 20 h postinsemination, presumptive zygotes were denuded, divided into groups of 50, and held in 500 μl of synthetic oviductal fluid (SOF) supplemented with 5% FCS until transfer. As a control, a representative number of embryos was left in the laboratory to confirm normal embryo development.
Fifty presumptive zygotes in a volume of approximately 50 μl were transferred per recipient to the oviduct ipsilateral to the corpus luteum on Day 1.5 (36.7 ± 2.6 h) after standing estrus as described previously [27–29]. Briefly, recipients were restrained and received epidural anesthesia (5 ml of adrenacaine; Norbrook). The tip of a bitubular endoscopic set (Storz) containing the endoscope and the transfer system was placed in the peritoneal cavity via the fornix of the vagina. After passive air movement into the cavity, the reproductive organs were assessed regarding suitability for embryo transfer. The transfer system consisted of a 1-ml syringe embedded into a manual dosimeter (IVFETflex.com) and connected to a perfusion tube (catalog no. 701908; Braun). A fire-polished and curved, 50-μl glass capillary (Brand) was attached to the end of the perfusion tube. Embryos were loaded in SOF supplemented with 5% FCS into the tip of the glass capillary and transferred via the infundibulum into the ampulla.
Animals were slaughtered in a commercial abattoir 3 days (79.4 ± 3.3 h) after the start of standing estrus. Following slaughter, the reproductive tract was removed, sealed in a plastic bag, transported to the laboratory on ice, and processed immediately as described for experiment 1. In addition, the uterine horn of pregnant heifers ipsilateral to the corpus luteum was flushed with 20 ml of PBS to recover any embryos that had potentially entered the uterus. After flushing, the isthmus section of the ipsilateral oviduct was opened longitudinally and gently scraped with a blade to recover the epithelial cells that were stored as described for experiment 1. Samples from five heifers from the cyclic (sham transfer) group and five heifers from which a large number (>20) of embryos were recovered were used for RNA-Seq.
RNA extraction and RNA-Seq.
Total RNA was isolated from oviductal isthmus epithelial samples as described above. Libraries were prepared and sequenced by Global Biologics. One microgram of total RNA was used as input for the Illumina TruSeq Stranded mRNA HT library construction procedure. Oligo-dT magnetic beads were used to enrich mRNA before fragmentation and primer hybridization for cDNA synthesis. The resulting cDNA was prepared for sequencing by 3′ adenylation, adapter ligation, and PCR amplification (98°C for 30 sec; 14 cycles of 98°C for 10 sec, 60°C for 30 sec, 72°C for 30 sec; and 72°C for 5 min). Library validation was performed using a Fragment Analyzer (Advanced Analytical) with HiSens NGS reagents followed by quantitation using the Qubit HS DNA Assay (Life Technologies) and qPCR library kit for Illumina platforms (Kappa Biosystems, Inc.). Libraries were pooled and sequenced as 50-bp, paired-end sequences on the Illumina HiSeq 2500 platform.
Analysis of RNA-Seq data was completed using CLC Genomics Server 6.0 and CLC Genomics Workbench 7.0.4 (CLC bio). Reads were quality trimmed (error probability, 0.001) with default parameters, and 13 bp were removed from the 5′ end of each read. The sequences were mapped to the Bos taurus genome (UMD 3.1) with Ensembl version 75 annotations requiring paired mapping and using reads per kilobase of transcript per million reads for expression values. Quality control was assessed with a boxplot of square root-transformed expression values verifying that all samples displayed similar distributions. Differential expression of genes was tested using the Empirical analysis of Digital Gene Expression [30] method in CLC Genomics Workbench incorporated from the edgeR Bioconductor package [31]. Genes were filtered (P < 0.05, fold-change ≥ 2) and log2 transformed, and sample clustering was confirmed by principal component analysis.
Gene ontology analysis.
The list of the top DEGs (P < 0.05, fold-change ≥ 2) obtained from RNA-Seq analysis was used to carried out Gene Ontology (GO) analysis using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) [32, 33] to achieve meaningful overrepresented data. From the output obtained by DAVID, GO FAT terms were used instead of GO ALL because the FAT category filters out the very broad GO terms based on a measured specificity of each term to yield more specific terms. Using these data, biological processes, molecular function, cellular component, and KEGG pathways were analyzed.
Quantitative real-time PCR.
Validation of the RNA-Seq results was performed by qPCR analysis for 10 of the top up- and down-regulated genes selected from the list of DEGs between pregnant and cyclic heifers. Total RNA (1000 ng) from the samples used for microarray analysis was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit following the manufacturer's instruction (Applied Biosystems). All primers were designed using Primer-BLAST software (www.ncbi.nlm.nih.gov/tools/primersblast/) to span exon-exon boundaries when possible. All qPCR reactions were carried out in duplicate on the Rotorgene 6000 Real-Time Cycler (Corbett Research) by adding 5 ng of each sample to the PCR mix (GoTaq qPCR Master Mix; Promega Corporation) containing the specific primers selected to amplify serine/threonine kinase 32A (STK32A); solute carrier family 26, member 3 (SLC26A3); keratocan (KERA); pyroglutamylated RFamide peptide receptor (QRFPR); multiple C2 domains, transmembrane 1 (MCTP1); superoxide dismutase 3, extracellular (SOD3); proline/arginine-rich end leucine-rich repeat protein (PRELP): vesicle amine transport protein 1 homolog (T. californica)-like (VAT1L): suppressor of cytokine signaling 3 (SOCS3); and chemokine (C-C motif) ligand 20 (CCL20). Primer sequences and the approximate sizes of the amplified fragments of all transcripts are shown in Supplemental Table S1 (Supplemental Data are available online at www.biolreprod.org). Cycling conditions were 94°C for 3 min followed by 35 cycles of 94°C for 15 sec, 56°C for 30 sec, and 72°C for 10 sec, with 10 sec of fluorescence acquisition. Each pair of primers was tested to achieve efficiencies close to one, and then the comparative cycle threshold (CT) method was used to quantify expression levels as described by Schmittgen and Livak [34]. To avoid primer dimer artifacts, fluorescence was acquired in each cycle at a temperature higher than the melting temperature of primer dimers (specific for each product, 76–86°C). Then, the threshold cycle or the cycle during the log-linear phase of the reaction at which fluorescence increased above background was determined for each sample. The ΔCT value was determined by subtracting the endogenous control (an average of H2AZ and ACTB) CT value for each sample from each gene CT value of the sample. Calculation of ΔΔCT involved using the highest sample ΔCT value (i.e., the sample with the lowest target expression) as a constant to subtract from all other ΔCT sample values. Fold-changes in the relative gene expression of the target were determined using the equation 2–ΔΔCT. Data obtained were analyzed using the SigmaStat (Jandel Scientific) software package. Student t-test was performed to study the differences in expression values between pregnant and cyclic heifers.
To validate the findings of experiment 1, in which no DEGs were detected in the isthmus epithelium by microarray analysis in the presence of a single embryo, the abundance of the same transcripts were assessed in those tissues by qPCR.
Results
Experiment 1
Oocyte and embryo recovery.
Of the 19 animals synchronized, 17 exhibited standing estrus (89.5%) and were used for the experiment. In the inseminated group, 8 of the 11 heifers (72.7%) yielded an embryo, which in all cases was located in the isthmus of the oviduct ipsilateral to the corpus luteum. Of the eight embryos recovered, one was at the 4-cell stage and five at the 8-cell stage, whereas embryos recovered from two animals were between the 8- and 16-cell stages of development. In the cyclic group, an unfertilized oocyte was found in the ipsilateral isthmus in all six heifers (100%).
Oviduct gene expression.
Correspondence analysis revealed that the overall transcriptional profile was similar in both groups, with little separation of the pregnant and cyclic heifers (Fig. 2). This was confirmed by the absence of DEGs between the ipsilateral oviductal isthmus of pregnant and cyclic heifers. Thus, under the conditions of this study, the presence of a single 8-cell embryo did not affect the transcriptome of the oviduct.
Fig. 2.
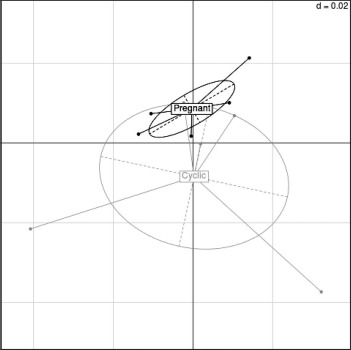
Correspondence analysis demonstrating the source of greatest variation in the oviduct transcriptional profile in experiment 1. Each dot represents all the transcripts expressed on one microarray representing one tissue site from one animal.
Experiment 2
Based on the outcome of experiment 1, a second experiment was conducted to test the hypothesis that the presence of multiple embryos would amplify the effect of any putative embryo-derived signal on the transcriptome of the epithelial cells of the oviductal isthmus. In addition, RNA-Seq was used as this method is more sensitive than microarray analysis [35].
Embryo recovery.
Of the eight heifers in the cyclic group, one did not have a functional corpus luteum when the samples were recovered at slaughter; this heifer was removed from the experiment. Embryos were recovered from eight of the nine heifers that underwent embryo transfer. Of the 450 transferred embryos, 181 (40.2%) were recovered. The majority (78.5%) of the embryos were found in the ipsilateral isthmus portion of the oviduct, although some embryos were located in the ampulla (16.0%) and, in one heifer, even in the uterus (5.5%). In relation to the developmental stage, most recovered embryos were at the 8-cell stage (60.8%); however, small numbers of 1-cell (13.2%) and 2- to 4-cell (26.0%) embryos were also found. Heifers selected for RNA-Seq analysis were those that yielded a large number (>20) of embryos.
A representative number of embryos were not transferred and were maintained in the laboratory to ensure normal development occurred. Cleavage and blastocyst rate were within the normal range (88.4% and 43.1%, respectively).
Oviduct gene expression determined by RNA-Seq and GO analysis.
RNA-Seq analysis revealed a total of 24 595 expressed transcripts in the isthmus (Supplemental Table S2). In contrast to experiment 1, correspondence analysis revealed a clear separation between pregnant and cyclic heifers (i.e., the overall transcriptional profile was different between groups) (Fig. 3). The comparison between pregnant and cyclic heifers revealed 278 DEGs (P < 0.05, fold-change ≥ 2) (Supplemental Table S3). Of these DEGs, 123 and 155 were up- and down-regulated, respectively, in the pregnant group. A list of the top 10 up- and down-regulated genes is shown in Figure 4.
Fig. 3.
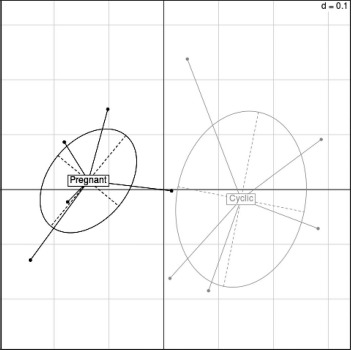
Correspondence analysis demonstrating the source of greatest variation in the oviduct transcriptional profile in experiment 2. Each dot represents all the transcripts expressed on one RNA-Seq representing one tissue site from one animal.
Fig. 4.
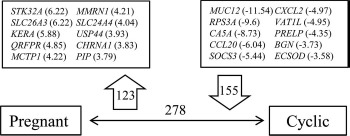
Illustration of DEGs between pregnant and cyclic heifers showing the top-10 up- and down-regulated genes in pregnant animals (123 and 155 genes, respectively) (P < 0.05, fold-change > 2). Values in parentheses indicate the fold-change for each gene. Complete gene list can be found in Supplemental Table S1.
Analysis of the GO terms associated with the list of DEGs showed that in pregnant heifers, two biological processes (containing a minimum of two genes) were overrepresented in the up-regulated genes, whereas 73 GO biological process terms were associated with the down-regulated genes identified in pregnant heifers (Supplemental Tables S4.1 and S4.2, respectively). From the biological processes in the up-regulated genes, only cell division (three genes) and cytokinesis (two genes) were overrepresented (Supplemental Table S4.1), whereas among the down-regulated genes, the most abundant biological processes were those related to immune function: immune response (eight genes), defense response (seven genes), inflammatory response (five genes), adaptive immune response (nine genes), immune system development (four genes), positive regulation of immune system process (four genes), immunoglobulin mediated immune response (three genes), B-cell mediated immunity (three genes), lymphocyte-mediated immunity (three genes), acute inflammatory response (three genes), leukocyte-mediated immunity (three genes), and regulation of phagocytosis (two genes) (Supplemental Table S4.2). In the molecular function category, binding of nucleotides (18 genes), purines (16 genes), ribonucleotides (16 genes), and ATP (13 genes) were overrepresented in the up-regulated genes (Supplemental Table S4.3), whereas cytoskeletal protein binding (five genes), exopeptidase activity (four genes), endopeptidase (four genes) and peptidase (four genes) inhibitor activity were overrepresented in the down-regulated genes (Supplemental Table S4.4). In relation to the cellular component, the up-regulated genes were expressed mainly in the chromosome (four genes) and in the cell surface (three genes) (Supplemental Table 4.5), whereas the extracellular region (22 genes) and plasma membrane (19 genes) were overrepresented among the down-regulated genes (Supplemental Table S4.6).
The KEGG pathway analysis revealed six pathways among the up-regulated genes in pregnant heifers represented by vascular smooth muscle contraction, focal adhesion, regulation of actin cytoskeleton, TGF-beta signaling pathway, oocyte meiosis, and axon guidance (Supplemental Table S4.7), whereas four pathways were present among the down-regulated genes, with the pathway of complement and coagulation cascades being the most represented (Supplemental Table S4.8).
Quantitative real-time PCR validation.
The expression pattern of eight genes (four up-regulated [SLC26A3, MCTP1, STK32A, and KERA] and four down-regulated [SOCS3, VAT1L, PRE-LP, and SOD3] in pregnant heifers) was assessed by qPCR and was consistent with the results from the RNA-Seq analysis. The expression of the other two genes tested, QRFPR and CCL20, was not significantly different between pregnant and cyclic heifers, although the direction of the change in expression was consistent with that observed in the RNA-Seq (Fig. 5).
Fig. 5.
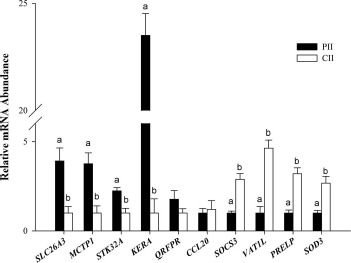
Quantitative real-time PCR analysis of selected genes for RNA-Seq validation (experiment 2). Across 20 comparisons (10 genes × 2 heifer groups [pregnant vs. cyclic]), the expression pattern of the selected genes obtained by qPCR was consistent with the results from the RNA-Seq analysis in all but two cases (QRFPR and CCL20). For each transcript, bars with different superscripts differ significantly (P < 0.05). PII, pregnant ipsilateral isthmus; CII, cyclic ipsilateral isthmus.
Consistent with the microarray results from experiment 1, the expression of none of the above genes (apart from KERA) was affected by the presence of a single embryo (Fig. 6).
Fig. 6.
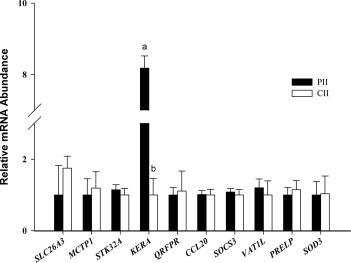
Quantitative real-time PCR analysis of differentially expressed transcripts from experiment 2 in tissues from experiment 1. Across 20 comparisons (10 genes × 2 heifer groups [pregnant vs. cyclic]), the expression pattern of the selected genes was as expected, with no differences between groups in all but one case (KERA). For each transcript, bars with different superscripts differ significantly (P < 0.01). PII, pregnant ipsilateral isthmus; CII, cyclic ipsilateral isthmus.
Discussion
The main findings of the present study were 1) that the presence of a single 8-cell embryo in the isthmus of the oviduct ipsilateral to the corpus luteum failed to elicit a detectable response in terms of a change in the oviductal transcriptome and 2) that the presence of multiple embryos in the oviduct resulted in altered expression of 278 genes in the oviduct epithelium.
Relatively few reports have been published on the transcriptome of the bovine oviduct in vivo. Using a combination of subtracted cDNA libraries and cDNA array hybridization, Bauersachs et al. [36] described the differential expression of 35 genes between the ipsilateral versus contralateral oviducts in cyclic heifers on Day 3.5. The same group subsequently described differential gene expression in bovine oviduct epithelial cells at estrus and diestrus (Day 12) [37], although the physiological relevance of this observation is questionable given that the embryo leaves the oviduct by Day 4 in cattle. Others have described embryo-induced alterations in the gene expression profile of bovine oviduct epithelial cells in vitro following culture with or without embryos [38, 39]. These results are certainly interesting, but they invariably involve the culture of groups rather than single embryos and typically examine gene expression in the oviduct cells after culture of embryos for 7–8 days to the blastocyst stage. As above, the physiological interpretation of such data is difficult given that the bovine embryo leaves the oviduct around Day 4 after estrus and, thus, that the oviduct in vivo is normally not exposed to a blastocyst-stage embryo.
Early embryonic development is probably the most critical period of mammalian development. In this short time, various morphological and biochemical changes occur and are affected by the embryo's environment. Among these changes, the bovine embryo at the 8-cell stage (Day 3 after estrus in vivo or IVF) switches from using the mRNA derived from the maternal genome to that resulting from EGA [1]. EGA is considered to be the most critical event for viability during early development [40] and is associated with early differentiation events, successful embryo implantation, and fetal development [41]. In a very comprehensive study, Gad et al. [10] found that changing culture conditions from in vivo to in vitro, or vice versa, around the time of EGA critically influenced the gene expression patterns of the resulting blastocysts. Similarly, we have shown that bovine embryos show temporal sensitivity to the culture environment after fertilization, which is manifested in terms of the quality of the blastocysts produced [42].
In line with the temporal changes that occur in oviduct morphology and the composition of the oviductal fluid [43–45], the energy requirements of the developing embryo change as it develops from a 1-cell zygote through the early cleavage divisions to form a multicellular blastocyst. In general, embryos throughout pre-elongation development are reliant on oxidative phosphorylation via oxidation of pyruvate and amino acids to generate ATP for embryo development; however, a switch to an increased contribution of glycolysis occurs during compaction and blastulation [46].
In experiment 1, microarray technology was used as we have previously employed this technology to demonstrate embryo-induced changes in the uterine transcriptome [47]. Furthermore, the small number of studies that have reported effects of gametes/embryos on oviduct gene expression also used microarrays [14, 17, 19]. Thus, it was reasonable to hypothesize that we should be able to detect differences in the bovine oviduct transcriptome due to the presence of an embryo, should they exist.
One could argue that by collecting the oviducts on Day 3, approximately 48 h after the last insemination, any observed effects could be attributable to the presence of spermatozoa and embryo (rather than an embryo alone). Indeed, others have reported sperm-induced changes in the oviduct transcriptome and secretory proteome [14–17]. However, this is a somewhat moot point as we failed to detect any differences between pregnant and cyclic heifers that could be ascribed to the sperm, embryo, or a combination of both. Furthermore, the apparent discordance of our observations with the aforementioned published data regarding sperm-induced effects on the oviduct likely reflect the major differences in several aspects between these studies. First, the time at which the oviduct cells were collected was very different in our study and compared to the others. For example, Fazeli et al. [14] collected samples from mice 6 h after mating, and Georgiou et al. [16] collected samples from pigs 24 h after artificial insemination. Furthermore, artificial insemination in pigs involves very high concentrations of sperm and a very large volume (in that study, 3 billion sperm were inseminated in a volume of 100 ml). This is in contrast to the situation in cows, where approximately 15 million sperm are inseminated in a volume of 250 μl (0.25 ml). In the study by Georgiou et al. [15], sow oviducts were recovered at slaughter and incubated in vitro for 18 h after infusion of 1 ml of PBS with or without 1 million sperm. This is clearly not comparable with our study. Finally, in the study by Almiñana et al. [17], sows were laparoscopically inseminated with X- or Y-sorted sperm (300 000 spermatozoa/100 μl), and the samples were collected 24 h later.
Under the conditions of experiment 1, we failed to detect an effect of the presence of an 8-cell embryo on the transcriptome of the oviduct. This is in contrast to observations in mice [18] and pigs [19], where embryo-induced changes in gene expression in the oviduct have been reported. However, these are litter-bearing species in which the observed changes may be a consequence of an amplification effect induced by the presence of multiple embryos. Furthermore, the physiological effect of a single cow embryo in an oviduct that is approximately 20 cm in length is not comparable to the situation in mice, where multiple embryos are located in an oviduct measuring perhaps 1–2 cm, or even in swine, where approximately 10 embryos may be located in each oviduct. However, the possibility that the embryo, measuring approximately 120 μm in diameter, could have a local effect on the oviduct epithelium at the precise point where it is located cannot be dismissed. For these reasons, experiment 2 was carried out using a previously validated model of multiple embryo transfer [3, 48]. Fifty embryos were transferred per heifer in an attempt to amplify and detect any putative oviductal response to the embryos. Whereas the presence of 50 embryos in the oviduct clearly is not what normally occurs in cattle, we and others have used this model to successfully demonstrate that the oviduct has a positive influence on embryo quality [9, 10, 49]. Furthermore, several groups including ours have used the ewe oviduct as a means of culturing bovine embryos to the blastocyst stage to produce high-quality embryos for transfer [50]. We are not aware of any evidence that culture of multiple embryos is deleterious for the embryo or the reproductive tract.
Following multiple embryo transfer, the comparison between pregnant and cyclic heifers resulted in 278 DEGs (P < 0.05, fold-change ≥ 2). Interestingly, GO analysis revealed that most of the biological processes overrepresented among the down-regulated genes in pregnant heifers were involved in the immune system. This is consistent with data from pigs showing that most of the transcripts differentially expressed in pregnant sows were down-regulated in the uterine horn in response to blastocysts when compared to oocytes, many of which were related to the immune system [19].
The down-regulated DEGs found in pregnant heifers were related with the complement system, inflammation, or major histocompatibility complex (MHC). The complement system “complements” the capacity of antibodies and phagocytic cells to clear pathogens from the organism. Thus, it has been proposed that complement inhibition is a requirement for successful pregnancy [51] given that the activation of this system has been involved in acute rejection of transplants [52]. In the present study, the gene that codes for C3 was down-regulated in pregnant heifers. C3 plays a central role in the activation of the complement system. Georgiou et al. [16] reported that gene and protein expression of C3 was up-regulated by the sow oviduct in response to spermatozoa and down-regulated in response to the oocytes. The data from the current study suggest that the presence of the embryo is associated with a decreased expression of C3, which could be responsible for avoiding the acute rejection of the embryo by the oviduct.
Inflammation is one of the first reactions against a foreign body by the immune system. Some genes that were down-regulated in pregnant heifers related with inflammation were NFKB2, CXCL2, and CCL20. Whereas NFKB2 is a central activator of genes involved in inflammation, CXCL2 is an inflammatory chemokine. In human ovarian and endometrial cancer cells, knockdown of NFκB has resulted in a reduced expression of CXCL1 and CXCL2 [53]. CCL20 is a chemokine that is primarily chemotactic for dendritic cells and specific lymphocyte subsets [54]. It has been proven that such chemokines can be secreted by neutrophils that play an important role in inflammatory responses [54]. Therefore, our results suggest that inflammatory response is decreased in pregnant heifers.
The MHC classes I and II are molecules that bind peptide antigens and present them to T cells [55]. In our study, two genes related with the MHC were down-regulated in pregnant heifers, CD74 and TAPBP. MHC class II is responsible for exogenous antigen presentation to CD4+ T cells, and the invariant chain (CD74) plays a key role in this process [56]. In mice, the absence of CD74 causes a significant reduction in both class II-restricted antigen presentation and expression of class II molecules at the cell surface [55]. Moreover, an effective response to infectious organisms requires efficient assembly of both MHC class I and class II complexes with pathogen-derived peptides [57]. In a mutant cell line that lacks TAPBP expression, this interaction does not occur, and MHC class I assembly and subsequent cell surface expression is impaired [58]. Therefore, in our study, the embryo antigen presentation may be altered in the maternal oviduct.
Given the outcome of experiment 1 and the fact that RNA-Seq is more sensitive than microarrays, we opted to use RNA-Seq for the second experiment. We acknowledge that the use of different technologies may present challenges in comparing the two experiments. Whether we would have detected differences in experiment 1 had we used RNA-Seq is debatable, mainly because of the large “embryo to oviduct tissue ratio” and the likelihood that any effects of the embryo on the oviduct would be very local in nature. However, as stated above, given that the few studies in the literature that have reported such effects used microarrays suggests that if an effect was present, we should have detected it. The results of the qPCR validation support this notion in that apart from one highly abundant transcript (KERA), none of the DEGs from experiment 2 were differentially expressed in the tissues from experiment 1. To our knowledge, no data on keratocan in the oviduct have been published, and its putative function there is unclear. Keratocan is a keratan sulfate proteoglycan of the extracellular matrix, originally isolated from bovine cornea. The gene is widely expressed in various embryonic mesenchymal tissues, but during development of the whole organism, expression is lost from most tissues except the cornea [59].
Considering all the information above, our findings are the first, to our knowledge, to report a possible signal coming from bovine embryos as early as Day 3 that down-regulates certain genes involved in the immune system, suggesting that the embryo could be able to avoid the maternal immune response by decreasing inflammation, antigen presentation, and action of the complement system. Further studies are needed to define the function of the maternal immune system during passage of the embryo through the oviduct and if this contributes to maternal-embryo tolerance.
In conclusion, evidence for “traffic” from the oviduct to the developing embryo is unequivocal. The presence of multiple embryos in the oviduct resulted in the detection of DEGs in the oviductal isthmus, suggesting a reciprocal crosstalk and that the embryo may be more than just a passive structure on its journey to the uterus. Failure to detect changes in the oviduct transcriptome in the presence of a single embryo may be due to the fact that any effect is very local and would not be detected using these methods.
Supplementary Material
Acknowledgment
The authors thank Mary Wade and Pat Duffy for excellent technical support as well as all of the postgraduate students who helped in various ways.
References
- 1. Memili E, First NL.. Zygotic and embryonic gene expression in cow: a review of timing and mechanisms of early gene expression as compared with other species. Zygote 2000; 8:87–96. [DOI] [PubMed] [Google Scholar]
- 2. Forde N, Carter F, Fair T, Crowe MA, Evans AC, Spencer TE, Bazer FW, McBride R, Boland MP, O'Gaora P, Lonergan P, Roche JF.. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol Reprod 2009; 81:784–794. [DOI] [PubMed] [Google Scholar]
- 3. Clemente M, de La Fuente J, Fair T, Al Naib A, Gutierrez-Adan A, Roche JF, Rizos D, Lonergan P.. Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 2009; 138:507–517. [DOI] [PubMed] [Google Scholar]
- 4. Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, Okumu LA, McGettigan PA, Mehta JP, McBride R, O'Gaora P, Roche JF et al. . Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod 2011; 85:144–156. [DOI] [PubMed] [Google Scholar]
- 5. Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Buttner M, Meyer HH, Spencer TE, Minten M, Sax G, Winter G, Wolf E.. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod 2012; 86:46. [DOI] [PubMed] [Google Scholar]
- 6. Spencer TE, Forde N, Dorniak P, Hansen TR, Romero JJ, Lonergan P.. Conceptus-derived prostaglandins regulate gene expression in the endometrium prior to pregnancy recognition in ruminants. Reproduction 2013; 146:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansouri-Attia N, Sandra O, Aubert J, Degrelle S, Everts RE, Giraud-Delville C, Heyman Y, Galio L, Hue I, Yang X, Tian XC, Lewin HA et al. . Endometrium as an early sensor of in vitro embryo manipulation technologies. Proc Natl Acad Sci U S A 2009; 106:5687–5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauersachs S, Ulbrich SE, Zakhartchenko V, Minten M, Reichenbach M, Reichenbach HD, Blum H, Spencer TE, Wolf E.. The endometrium responds differently to cloned versus fertilized embryos. Proc Natl Acad Sci U S A 2009; 106:5681–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tesfaye D, Lonergan P, Hoelker M, Rings F, Nganvongpanit K, Havlicek V, Besenfelder U, Jennen D, Tholen E, Schellander K.. Suppression of connexin 43 and E-cadherin transcripts in in vitro derived bovine embryos following culture in vitro or in vivo in the homologous bovine oviduct. Mol Reprod Dev 2007; 74:978–988. [DOI] [PubMed] [Google Scholar]
- 10. Gad A, Hoelker M, Besenfelder U, Havlicek V, Cinar U, Rings F, Held E, Dufort I, Sirard MA, Schellander K, Tesfaye D.. Molecular mechanisms and pathways involved in bovine embryonic genome activation and their regulation by alternative in vivo and in vitro culture conditions. Biol Reprod 2012; 87:100. [DOI] [PubMed] [Google Scholar]
- 11. Enright BP, Lonergan P, Dinnyes A, Fair T, Ward FA, Yang X, Boland MP.. Culture of in vitro produced bovine zygotes in vitro vs in vivo: implications for early embryo development and quality. Theriogenology 2000; 54:659–673. [DOI] [PubMed] [Google Scholar]
- 12. Rizos D, Ward F, Duffy P, Boland MP, Lonergan P.. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61:234–248. [DOI] [PubMed] [Google Scholar]
- 13. Rizos D, Pintado B, de la Fuente J, Lonergan P, Gutiérrez-Adán A.. Development and pattern of mRNA relative abundance of bovine embryos cultured in the isolated mouse oviduct in organ culture. Mol Reprod Dev 2007; 74:716–723. [DOI] [PubMed] [Google Scholar]
- 14. Fazeli A, Affara NA, Hubank M, Holt WV.. Sperm-induced modification of the oviductal gene expression profile after natural insemination in mice. Biol Reprod 2004; 71:60–65. [DOI] [PubMed] [Google Scholar]
- 15. Georgiou AS, Sostaric E, Wong CH, Snijders AP, Wright PC, Moore HD, Fazeli A.. Gametes alter the oviductal secretory proteome. Mol Cell Proteomics 2005; 4:1785–1796. [DOI] [PubMed] [Google Scholar]
- 16. Georgiou AS, Snijders AP, Sostaric E, Aflatoonian R, Vazquez JL, Vazquez JM, Roca J, Martinez EA, Wright PC, Fazeli A.. Modulation of the oviductal environment by gametes. J Proteome Res 2007; 6:4656–4666. [DOI] [PubMed] [Google Scholar]
- 17. Almiñana C, Caballero I, Heath PR, Maleki-Dizaji S, Parrilla I, Cuello C, Gil MA, Vazquez JL, Vazquez JM, Roca J, Martinez EA, Holt WV et al. . The battle of the sexes starts in the oviduct: modulation of oviductal transcriptome by X and Y-bearing spermatozoa. BMC Genomics 2014; 15:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee KF, Yao YQ, Kwok KL, Xu JS, Yeung WS.. Early developing embryos affect the gene expression patterns in the mouse oviduct. Biochem Biophys Res Commun 2002; 292:564–570. [DOI] [PubMed] [Google Scholar]
- 19. Almiñana C, Heath PR, Wilkinson S, Sanchez-Osorio J, Cuello C, Parrilla I, Gil MA, Vazquez JL, Vazquez JM, Roca J, Martinez EA, Fazeli A.. Early developing pig embryos mediate their own environment in the maternal tract. PLOS ONE 2012; 7:e33625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber JA, Freeman DA, Vanderwall DK, Woods GL.. Prostaglandin E2 hastens oviductal transport of equine embryos. Biol Reprod 1991; 45:544–546. [DOI] [PubMed] [Google Scholar]
- 21. Weber JA, Freeman DA, Vanderwall DK, Woods GL.. Prostaglandin E2 secretion by oviductal transport-stage equine embryos. Biol Reprod 1991; 45:540–543. [DOI] [PubMed] [Google Scholar]
- 22. Van Niekerk CH, Gerneke WH.. Persistence and parthenogenetic cleavage of tubal ova in the mare. Onderstepoort J Vet Res 1966; 33:195–232. [PubMed] [Google Scholar]
- 23. Gautier L, Cope L, Bolstad BM. Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004; 20:307–315. [DOI] [PubMed] [Google Scholar]
- 24. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T et al. . Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 2004; 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, Watson SJ, Meng F.. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 2005; 33:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smyth GK. Limma: linear models for microarray data.:Gentleman R, Cary V, Huber W, Irizarry R, Dudoit S. (eds.),Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York:Springer; 2005:397–420. [Google Scholar]
- 27. Havlicek V, Wetscher F, Huber T, Brem G, Mueller M, Besenfelder U.. In vivo culture of IVM/IVF embryos in bovine oviducts by transvaginal endoscopy. J Vet Med A Physiol Pathol Clin Med 2005; 52:94–98. [DOI] [PubMed] [Google Scholar]
- 28. Rizos D, Carter F, Besenfelder U, Havlicek V, Lonergan P.. Contribution of the female reproductive tract to low fertility in postpartum lactating dairy cows. J Dairy Sci 2010; 93:1022–1029. [DOI] [PubMed] [Google Scholar]
- 29. Maillo V, Rizos D, Besenfelder U, Havlicek V, Kelly AK, Garrett M, Lonergan P.. Influence of lactation on metabolic characteristics and embryo development in postpartum Holstein dairy cows. J Dairy Sci 2012; 95:3865–3876. [DOI] [PubMed] [Google Scholar]
- 30. Robinson MD, Smyth GK.. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 2008; 9:321–332. [DOI] [PubMed] [Google Scholar]
- 31. Robinson MD, McCarthy DJ. Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang DW, Sherman BT, Lempicki RA.. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57. [DOI] [PubMed] [Google Scholar]
- 33. Huang DW, Sherman BT, Lempicki RA.. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schmittgen TD, Livak KJ.. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 35. Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y.. RNA-Seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 2008; 18:1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bauersachs S, Blum H, Mallok S, Wenigerkind H, Rief S, Prelle K, Wolf E.. Regulation of ipsilateral and contralateral bovine oviduct epithelial cell function in the postovulation period: a transcriptomics approach. Biol Reprod 2003; 68:1170–1177. [DOI] [PubMed] [Google Scholar]
- 37. Bauersachs S, Rehfeld S, Ulbrich SE, Mallok S, Prelle K, Wenigerkind H, Einspanier R, Blum H, Wolf E.. Monitoring gene expression changes in bovine oviduct epithelial cells during the estrous cycle. J Mol Endocrinol 2004; 32:449–466. [DOI] [PubMed] [Google Scholar]
- 38. Cordova A, Perreau C, Uzbekova S, Ponsart C, Locatelli Y, Mermillod P.. Development rate and gene expression of IVP bovine embryos cocultured with bovine oviduct epithelial cells at early or late stage of preimplantation development. Theriogenology 2014; 81:1163–1173. [DOI] [PubMed] [Google Scholar]
- 39. Schmaltz-Panneau B, Cordova A, Dhorne-Pollet S, Hennequet-Antier C, Uzbekova S, Martinot E, Doret S, Martin P, Mermillod P, Locatelli Y.. Early bovine embryos regulate oviduct epithelial cell gene expression during in vitro co-culture. Anim Reprod Sci 2014; 149:103–116. [DOI] [PubMed] [Google Scholar]
- 40. Meirelles FV, Caetano AR, Watanabe YF, Ripamonte P, Carambula SF, Merighe GK, Garcia SM.. Genome activation and developmental block in bovine embryos. Anim Reprod Sci 2004; 82-83:13–20. [DOI] [PubMed] [Google Scholar]
- 41. Niemann H, Wrenzycki C.. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology 2000; 53:21–34. [DOI] [PubMed] [Google Scholar]
- 42. Lonergan P, Rizos D, Gutierrez-Adán A, Moreira PM, Pintado B, de la Fuente J, Boland MP.. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod 2003; 69:1424–1431. [DOI] [PubMed] [Google Scholar]
- 43. Hugentobler SA, Diskin MG, Leese HJ, Humpherson PG, Watson T, Sreenan JM, Morris DG.. Amino acids in oviduct and uterine fluid and blood plasma during the estrous cycle in the bovine. Mol Reprod Dev 2007; 74:445–454. [DOI] [PubMed] [Google Scholar]
- 44. Hugentobler SA, Morris DG, Sreenan JM, Diskin MG.. Ion concentrations in oviduct and uterine fluid and blood serum during the estrous cycle in the bovine. Theriogenology 2007; 68:538–548. [DOI] [PubMed] [Google Scholar]
- 45. Hugentobler SA, Humpherson PG, Leese HJ, Sreenan JM, Morris DG.. Energy substrates in bovine oviduct and uterine fluid and blood plasma during the estrous cycle. Mol Reprod Dev 2008; 75:496–503. [DOI] [PubMed] [Google Scholar]
- 46. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ.. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil 1996; 106:299–306. [DOI] [PubMed] [Google Scholar]
- 47. Forde N, Carter F, Fair T, Crowe MA, Evans ACO, Spencer TE, Bazer FW, O'Gaora P, McBride R, Boland MP, Lonergan P, Roche JF.. Effect of pregnancy and progesterone on gene expression in the uterine endometrium of cattle. Biol Reprod 2009; 78:60–61. [DOI] [PubMed] [Google Scholar]
- 48. Forde N, Beltman ME, Duffy GB, Duffy P, Mehta JP, O'Gaora P, Roche JF, Lonergan P, Crowe MA.. Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol Reprod 2011; 84:266–278. [DOI] [PubMed] [Google Scholar]
- 49. Carter F, Rings F, Mamo S, Holker M, Kuzmany A, Besenfelder U, Havlicek V, Mehta JP, Tesfaye D, Schellander K, Lonergan P.. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol Reprod 2010; 83:707–719. [DOI] [PubMed] [Google Scholar]
- 50. Lazzari G, Colleoni S, Lagutina I, Crotti G, Turini P, Tessaro I, Brunetti D, Duchi R, Galli C.. Short-term and long-term effects of embryo culture in the surrogate sheep oviduct versus in vitro culture for different domestic species. Theriogenology 2010; 73:748–757. [DOI] [PubMed] [Google Scholar]
- 51. Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunol Invest 2008; 37:645–659. [DOI] [PubMed] [Google Scholar]
- 52. Wasowska BA, Lee CY, Halushka MK, Baldwin WM III. . New concepts of complement in allorecognition and graft rejection. Cell Immunol 2007; 248:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kavandi L, Collier MA, Nguyen H, Syed V.. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell Biochem 2012; 113:3143–3152. [DOI] [PubMed] [Google Scholar]
- 54. Scapini P, Laudanna C, Pinardi C, Allavena P, Mantovani A, Sozzani S, Cassatella MA.. Neutrophils produce biologically active macrophage inflammatory protein-3alpha (MIP-3alpha)/CCL20 and MIP-3beta/CCL19. Eur J Immunol 2001; 31:1981–1988. [DOI] [PubMed] [Google Scholar]
- 55. Elliott EA, Drake JR, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell RA.. The invariant chain is required for intracellular transport and function of major histocompatibility complex class II molecules. J Exp Med 1994; 179:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Geneve L, Menard C, Labrecque N, Thibodeau J.. The p35 human invariant chain in transgenic mice restores mature B cells in the absence of endogenous CD74. Int Immunol 2012; 24:645–660. [DOI] [PubMed] [Google Scholar]
- 57. Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P.. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science 1997; 277:1306–1309. [DOI] [PubMed] [Google Scholar]
- 58. Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P.. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 1996; 5:103–114. [DOI] [PubMed] [Google Scholar]
- 59. Chakravarti S. Focus on molecules: keratocan (KERA). Exp Eye Res 2006; 82:183–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


