Abstract
It has been shown that adverse obstetrical outcomes such as pre-eclampsia and intrauterine growth retardation correlate with maternal infection. In this study, we investigated mechanisms involved in infection-associated abnormalities in cytotrophoblast function. Primary human first trimester cytotrophoblast cells were isolated and treated with lipopolysaccharide (LPS). Levels of the cytokines and chemokines were measured and cytotrophoblast invasion was investigated. In addition, first trimester decidual macrophages were isolated and treated with the conditioned medium from LPS-treated cytotrophoblast cells, and macrophage migration was assessed. Coculturing decidual macrophages with cytotrophoblast cells was conducted to investigate macrophage costimulatory molecule and receptor expression and intracellular cytokine production. We found that LPS exposure increased cytotrophoblast production of pro-inflammatory cytokines tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-6, and chemokines IL-8, macrophage inflammatory protein (MIP)-1alpha, and CXCL12 in a dose-dependent manner. In addition, LPS decreased cytotrophoblast invasion, and its effect was Toll-like receptor 4 (TLR4)-dependent and partly TNF-alpha-dependent. Conditioned medium from LPS-stimulated cytotrophoblast cells increased decidual macrophage migration and this effect was partly TLR4-dependent. Furthermore, coculturing decidual macrophages with LPS-exposed cytotrophoblast cells up-regulated macrophage CD80 and CD86 expression and intracellular TNF-alpha and IL-12p40 production, while down-regulating macrophage CD206 and CD209 expression and intracellular IL-10 secretion. LPS-stimulated macrophages also inhibited cytotrophoblast invasion. In conclusion, our results indicate that LPS increases the production of a subset of proinflammatory cytokines and chemokines by human first trimester cytotrophoblast cells, decreases cytotrophoblast invasion, and alters the cross talk between cytotrophoblast cells and decidual macrophages.
Keywords: cytotrophoblast, decidual macrophages, infection, lipopolysaccharide, Toll-like receptor 4
Introduction
The placenta constitutes a physical and immunological barrier against invading pathogens. A tightly balanced immune response at the maternal-fetal interface is essential to allow protection from local pathogen invasion and blood-borne pathogens and their toxins while avoiding overly robust inflammation that might adversely affect the pregnancy. Severe infection or maternal hyperresponsivity to less severe infectious insults could result in miscarriage or less devastating placental damage that could result in other adverse pregnancy outcomes.
During human pregnancy, there are two trophoblast cell types that form the direct fetal contribution to the maternal-fetal interface. One is syncytiotrophoblast, while the other is extravillous trophoblast (EVT) cells [1]. The syncytiotrophoblast is initially in contact with maternal blood sinuses (venous lakes) as early as 15 days after fertilization [2] and later in pregnancy when maternal blood flow into the intervillous space greatly increases. EVT cells are a highly migratory cell population that invades the maternal decidua and inner third of the myometrium and remodels the uterine spiral arteries [3]. Decidual EVT cells are in direct contact with the immune cells populating the uterine decidua; those invading the maternal spiral arteries could be directly exposed to circulating pathogens and pathogenic toxins. Both cell types express Toll-like receptors (TLRs), including TLR4 [4, 5]. TLRs are an important group of pattern recognition receptors [6]. Because TLRs have been identified in both syncytiotrophoblast and EVT cells, they may enable these cells to recognize pathogens through these receptors and induce immune responses. While TLRs at the maternal-fetal interface may play an important role in generating an immune response against invading pathogens, they could also contribute to several pregnancy pathologies associated with placental dysfunction, including pre-eclampsia and intrauterine growth retardation (IUGR) [7]. It has been demonstrated that placental expression of TLR2, TLR3, TLR4, and TLR9 is increased in pregnancies complicated by pre-eclampsia, which may indicate an association between innate immune response and pre-eclampsia [8]. In addition, it has been reported that maternal immune system activation via TLR3 during pregnancy causes pre-eclampsia-like symptoms in rats [9] and in mice [10]. Likewise, TLR4 activation induces preterm delivery, fetal death, and IUGR in mice [11]. Using a mouse model, Girardi et al. [12] have demonstrated that pregnancies complicated by miscarriage or IUGR are characterized by complement activation, inflammatory infiltrates in the placenta, and defective placental development.
Invading fetal-derived trophoblast cells interact with immune cells at the maternal-fetal interface within the human maternal decidua. Macrophages are the second most abundant leukocytes in the decidua throughout pregnancy, accounting for 20%–30% of the total decidual leukocytes [13]. Macrophages can be classified into two major subtypes termed classically activated (M1) macrophages and alternatively activated (M2) macrophages [14, 15]. M1 macrophages exhibit the capacity to kill intracellular microbes, express costimulatory molecules, secrete proinflammatory molecules such as IL-12, IL-23, and reactive oxygen species, and skew T cell responses toward a Th1 phenotype [14-16]. In contrast, M2 macrophages express mannose and scavenger receptors, produce anti-inflammatory cytokines including IL-10 and TGF-β, participate in tissue remodeling, maintain tissue homeostasis, and direct Th2 responses [17, 18]. Although the exact roles of decidual macrophages are not fully defined, studies have demonstrated that these cells are involved in a variety of processes, including remodeling of uterine arteries, regulation of trophoblast implantation, immune modulation, promotion of immune tolerance to the semi-allogeneic fetus, and initiation of parturition [19-22].
While human decidual macrophages are certainly important to the maintenance of pregnancy, an excess of macrophages in the decidua induces EVT cell apoptosis and limits their invasion of spiral arteries [23]. Deficient trophoblast invasion is associated with several severe complications of pregnancy, including pre-eclampsia and IUGR [24-26]. Pre-eclampsia is a syndrome of heterogenous origin characterized by insufficient EVT invasion and aberrant remodeling of the uterine spiral arteries. It has been shown that primiparity, chronic hypertension, diabetes mellitus, renal disease, obesity, previous pre-eclampsia, and multifetal gestation are associated with pre-eclampsia [27]. However, maternal infections such as periodontitis and gingivitis have also been related to an increased risk of pre-eclampsia [28, 29]. In mice, Listeria monocytogenes infection during early gestation leads to decidual cell death, tissue disintegration, and resorption of the developing embryo [30], and we hypothesize that maternal infection in humans could have related adverse effects on placental development and function that may lead to adverse obstetrical outcomes. This may be responsible for a subset of women exhibiting obstetrical disorders characterized by poor placentation, particularly those with more severe infections or overly robust responses to infection. We specifically hypothesize that TLR signaling could be a potential link between the innate immune system and the defective trophoblast invasion and function detected in the placentae of some women with pre-eclampsia. The present study was performed to elucidate the effects of the Gram-negative bacterial endotoxin lipopolysaccharide (LPS), a specific ligand of TLR4, on cytotrophoblast function, including the production of cytokines and chemokines, cytotrophoblast invasion, and cytotrophoblast-associated changes in the migration and phenotypes of decidual macrophages.
Materials and Methods
Ethical Approval
The study was approved by the Medical Ethics Committee of Guangzhou First People's Hospital, Guangzhou Medical University. First trimester cytotrophoblast cells and macrophages were isolated from placentas and deciduae obtained during elective first trimester (6–10 wk) terminations of pregnancy performed in the Department of Obstetrics and Gynecology, Guangzhou First People's Hospital, Guangzhou Medical University. Written informed consent was obtained from the study participants prior to their enrollment.
Isolation of First Trimester Cytotrophoblast Cells
We have successfully isolated cytotrophoblast cells from human term placentas [31]. Here, we isolated cytotrophoblast cells from human first trimester placentas using similar methods with minor modifications. Briefly, villous tissues were dissected free of membranes, rinsed, and minced in phosphate-buffered saline (PBS) (Life Technologies). The villous samples were digested three times in a digestion enzyme medium containing 1 mg/ml Dispase II (Life Technologies) and 0.1 mg/ml DNase I (Roche) at 37°C for 15 min each cycle. Released cells were then purified on a discontinuous Percoll gradient (GE Healthcare) and centrifuged at 730 × g for 20 min at 4°C. The layer between the 45% and 35% Percoll aliquots containing cytotrophoblast cells (density: 1.050–1.060 g/ml) were collected. Collected cells were further immunopurified by eliminating CD45RB-positive cells of myeloid origin using a phycoerythrin (PE)-conjugated anti-CD45RB antibody (Ab) (1:10; Miltenyi Biotec) and anti-PE-microbeads (1:5; Miltenyi Biotec), and depleting fibroblasts using anti-fibroblast microbeads (1:5; Miltenyi Biotec) according to the manufacturer's instructions.
Isolation of First Trimester Decidual Macrophages
A detailed description of the procedure used to isolate decidual macrophages has been reported elsewhere [32]. In brief, decidual tissues from first trimester pregnancy were collected, washed in PBS, and cut into small pieces. Minced decidual tissue was digested three times in PBS containing 1 mg/ml Dispase II and 0.1 mg/ml DNase I at 37°C for 20 min each cycle. Released cells were separated from undigested tissue pieces by filtering through a 40-μm pore nylon mesh. Mononuclear cells were enriched via centrifugation over Ficoll-Hypaque (GE Healthcare) at 800 × g for 20 min at 20°C. The CD14+ macrophage subpopulation was purified by positive selection using anti-CD14 microbeads (20 μl; Miltenyi Biotech) according to the manufacturer's protocol. The purity of isolated CD14+ macrophages was more than 95% as determined by flow cytometry (data not shown).
Enzyme-Linked Immunosorbent Assay
Isolated cytotrophoblast cells (5 × 105 cells/ml) were treated with PBS or serially diluted Escherichia coli LPS (O111:B4) (1, 10, 100, or 1000 ng/ml) (Sigma-Aldrich). The LPS serotype O111:B4 was selected because this pathogenic enteric E. coli serotype induces endotoxemia [33]. In selected experiments, cytotrophoblast cells (5 × 105 cells/ml) were pretreated with anti-TLR4 Ab (1 μg/ml) or PBS for 2 h before the addition of 100 ng/ml LPS. Twenty-four hours later, culture supernatants were collected and stored at −80°C for batched cytokine determination. Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-4, IL-6, IL-10, IL-12p70, IL-8, macrophage inflammatory protein (MIP)-1α, and CXCL12 levels were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). All the assays were conducted according to the manufacturer's instructions.
Matrigel Invasion Assay
Cytotrophoblast invasion was evaluated in a Matrigel-coated transwell system with 8-μm pore size polyethylene terephthalate membranes (BD Biosciences) as previously described [34] with minor modifications. In brief, 2 × 105 cytotrophoblasts in 200 μl of Dulbecco-modified Eagle medium with 10% fetal bovine serum (Life Technologies) were seeded in the upper chamber of a 24-well plate. In order to investigate the role of TLR4, TNF-α, IL-1β, and IL-6 in LPS-induced decreases in trophoblast invasion, LPS (100 ng/ml) in the presence of anti-TLR4 Ab (1 μg/ml), anti-TNF-α Ab (1 μg/ml), anti-IL-1β Ab (1 μg/ml), or anti-IL-6 Ab (1 μg/ml) in 600 μl medium was placed in the lower chamber. In selected experiments, decidual macrophages treated with PBS, LPS (100 ng/ml), or neutralizing Abs against TNF-α (1 μg/ml) or IL-12 (1 μg/ml) in the presence of LPS were placed in the lower chamber. Medium alone in the lower chamber served as a negative control. Cells were incubated at 37°C for 24 h. Noninvading cells were carefully swabbed off the upper surface of the membrane. The membranes were stained using crystal violet (Sigma-Aldrich) and mounted onto glass slides. Stained cells were counted at a magnification of 200× under a Leica DMIL microscope (Leica Microsystems). The invasion index was defined as the number of invading cells in the experimental group divided by that of the negative control group. The assay was carried out in triplicate and repeated three times independently. All of the neutralizing Abs were purchased from BioLegend.
Preparation of Cytotrophoblast-Conditioned Medium
Isolated primary cytotrophoblast cells (1 × 106/ml) were culture in Dulbecco-modified Eagle medium with 10% fetal bovine serum in the presence of 100 ng/ml LPS at 37°C. After 48 h, the cell culture supernatants were collected as LPS-stimulated cytotrophoblast conditioned medium (CM) and stored at −80°C before use. Conditioned medium prepared from cytotrophoblast cells in the absence of LPS served as control CM.
Migration Assay
We evaluated the migration of decidual macrophages toward cytotrophoblast CM using an 8-μm membrane insert system (Corning). Isolated decidual macrophages (2 × 105) were seeded in the upper chamber of a 24-well plate. A variety of concentrations of cytotrophoblast CM were added to the lower chamber. In addition, 20% control CM, 20% cytotrophoblast CM with or without anti-TLR4 Ab (1 μg/ml), and LPS (100 ng/ml) with or without anti-TLR4 Ab (1 μg/ml) were added to the lower chamber. Basal culture medium in the lower chamber served as a negative control. After 24 h, cells in the lower chamber were collected and labeled with fluorescein isothiocyanate-conjugated anti-CD14 Ab (BioLegend). The numbers of CD14+ cells were calculated using flow cytometry. The results were expressed as fold change of the numbers of CD14+ cells relative to those isolated from basal culture medium controls. The assay was carried out in triplicate and repeated three times independently.
Cell Coculture Study
For cocultures, a 0.4-μm membrane insert system (Corning) was used. Cytotrophoblast cells (2 × 105) treated with LPS (100 ng/ml) or PBS were seeded in the upper chamber of a 24-well plate, while macrophages (2 × 105) were cultured in the lower chamber. This culture system prevented macrophages from direct contact with cytotrophoblast cells and facilitated harvesting macrophages without cytotrophoblast cell contamination. After 24 h, macrophages were collected from the lower chamber and analyzed for surface expression of CD80, CD86, CD206, and CD209, and intracellular production of TNF-α, IL-12p40, and IL-10 using flow cytometry.
Flow Cytometry
The fluorescence-conjugated Abs and their isotype controls used in this study are summarized in Table 1. Aliquots of 106 cytotrophoblast cells in 50 μl PBS were incubated with fluorescence-conjugated Abs against CD45 and CD163 for 30 min at 4°C. After washing twice with PBS, cells were fixed in a fixation buffer (BioLegend). For intracellular cytokine staining, cells were resuspended in a permeabilization wash buffer (BioLegend) and incubated with fluorescence-conjugated Abs against vimentin and cytokeratin-7. Alternatively, macrophages were incubated with fluorescence-conjugated Abs against CD80, CD86, CD206, and CD209 for 30 min at 4°C. After washing twice with PBS, cells were fixed and permeabilized, and incubated with fluorescence-conjugated Abs against TNF-α, IL-12p40, and IL-10. Isotype controls were established using matched fluorescence-labeled isotype control Abs and equivalent immunostaining conditions. Immunostained cells were analyzed on a FACSCanto flow cytometer (BD Biosciences) using FACSDiva software (BD Biosciences). The expression levels of cytokeratin-7, vimentin, CD45, and CD163 on purified trophoblast cells were measured. Macrophage surface expression of CD80, CD86, CD206, and CD209, and intracellular production of TNF-α, IL-12p40, and IL-10 were analyzed.
Table 1.
Antibodies used in the flow cytometry.
| Antibody | Fluorochrome | Titer | Isotype | Manufacturer |
|---|---|---|---|---|
| Anti-CD45 Ab | Allophycocyanin (APC)/Cy7 | 1:5 | Mouse IgG1, κ | BioLegend |
| Anti-CD163 Ab | PE/Cy7 | 1:10 | Mouse IgG1, κ | BioLegend |
| Anti-vimentin Ab | Alexa Fluor 488 | 1:10 | Mouse IgG1, κ | BD Biosciences |
| Anti-cytokeratin-7 Ab | Alexa Fluor 568 | 1:25 | Rabbit IgG | Abcam |
| Anti-CD80 Ab | Fluorescein isothiocyanate (FITC) | 1:5 | Mouse IgG1, κ | BioLegend |
| Anti-CD86 Ab | Peridinin chlorophyll (PerCp)/Cy5.5 | 1:5 | Mouse IgG2b, κ | BioLegend |
| Anti-CD206 | APC/Cy7 | 1:5 | Mouse IgG1, κ | BioLegend |
| Anti-CD209 Ab | PE/Cy7 | 1:5 | Mouse IgG2a, κ | BioLegend |
| Anti-TNF-α Ab | APC | 1:5 | Mouse IgG1, κ | BioLegend |
| Anti-IL-12p40 Ab | APC | 1:5 | Mouse IgG1, κ | BioLegend |
| Anti-IL-10 Ab | PE | 1:5 | Rat IgG2a, κ | BioLegend |
Statistical Analysis
All statistical analyses were performed using SPSS 19.0 software (IBM). Data were analyzed using one-way analysis of variance (ANOVA) with Bonferroni post hoc testing when the variances were homogeneous or with Tamhane T2 post hoc testing when the variances were not homogeneous, and Student t-test. Results were expressed as mean ± SD. A P < 0.05 was considered significant.
Results
Purity of Isolated Cytotrophoblast Cells
The percentage of cytokeratin-7-positive cytotrophoblast cells exceeded 98% (Fig. 1A). Contaminating mesenchymal cells, leukocytes, and Hofbauer cells made up less than 2% of purified cytotrophoblast cells as assessed by the expression of vimentin, CD45, and CD163, respectively (Fig. 1B–D).
Fig. 1.
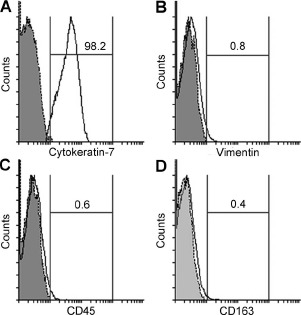
Purity of isolated villous cytotrophoblast cells. The expression levels of cytokeratin-7 (A), vimentin (B), CD45 (C), and CD163 (D) on purified cytotrophoblast cells were analyzed using flow cytometry. Gray-shaded histogram: isotype-matched negative control. Black line: specific Ab expression. Numbers indicate the percentages of particular Ab-positive cells among isolated cells. The depicted result is representative of four independent experiments.
Effects of LPS on Cytotrophoblast Cytokine Secretion
Lipopolysaccharide treatment significantly increased the release of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 into the cytotrophoblast culture supernatants in a dose-dependent manner (P < 0.01) (Fig. 2A–C). However, LPS exposure did not affect cytotrophoblast production of IL-12p70, IL-4, or IL-10 (Fig. 2D–F). Additionally, the levels of TNF-α, IL-1β, and IL-6 in the cytotrophoblast culture supernatants were significantly decreased when LPS-stimulated cytotrophoblast cells were pretreated with neutralizing anti-TLR4 Ab (P < 0.01) (Fig. 3).
Fig. 2.
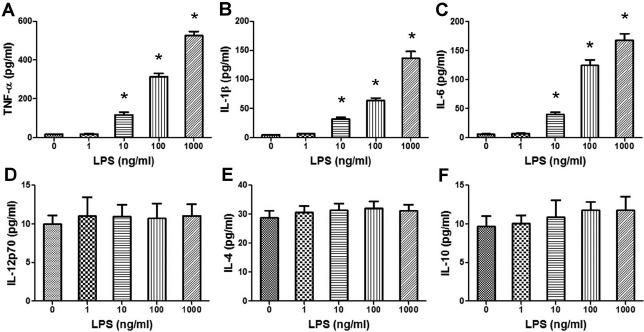
Effects of LPS on the levels of cytokines secreted into cytotrophoblast supernatants. Isolated cytotrophoblast cells (5 × 105 cells/ml) were treated with PBS or serially diluted LPS (1, 10, 100, or 1000 ng/ml) for 24 h. The levels of TNF-α (A), IL-1β (B), IL-6 (C), IL-12p70 (D), IL-4 (E), and IL-10 (F) in the culture supernatants were assessed using ELISA. Data are presented as mean ± SD of four independent experiments. *P < 0.01 versus the control group.
Fig. 3.
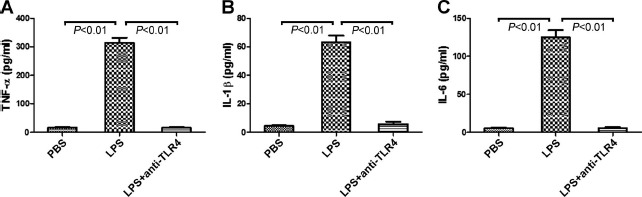
Effects of anti-TLR4 Ab on cytotrophoblast proinflammatory cytokine production following LPS exposure. Isolated cytotrophoblast cells were pretreated with anti-TLR4 Ab for 2 h before the addition of PBS or 100 ng/ml LPS for 24 h. The levels of TNF-α (A), IL-1β (B), and IL-6 (C) in the culture supernatants were measured using ELISA. Data are presented as mean ± SD of four independent experiments.
Effects of LPS on Cytotrophoblast Chemokine Production
The production of chemokines IL-8, MIP-1α, and CXCL12 in the cytotrophoblast culture supernatants upon LPS treatment was markedly up-regulated compared with controls (P < 0.01), and this effect was dose-dependent (Fig. 4).
Fig. 4.
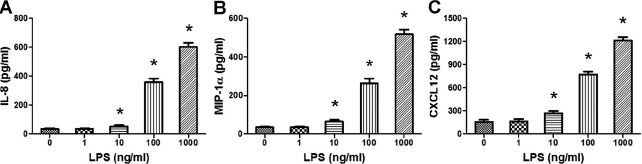
Effects of LPS on cytotrophoblast chemokine secretion. Isolated cytotrophoblast cells (5 × 105 cells/ml) were treated with PBS or serially diluted LPS (1, 10, 100, or 1000 ng/ml) for 24 h. The levels of IL-8 (A), MIP-1α (B), and CXCL12 (C) in the culture supernatants were assessed using ELISA. Data are presented as mean ± SD of four independent experiments. *P < 0.01 versus the control group.
Effects of LPS on Early Cytotrophoblast Invasion
As shown in Figure 5, LPS exposure dramatically decreased cytotrophoblast invasion (P < 0.01). In order to explore a possible role for TLR4 signaling in infection-induced alterations in cytotrophoblast invasion, we pretreated LPS-activated cytotrophoblast cells with neutralizing anti-TLR4 Ab. In addition, because LPS treatment significantly increased cytotrophoblast TNF-α, IL-1β, and IL-6 production, we used neutralizing Abs against TNF-α, IL-1β, and IL-6 to investigate whether these specific proinflammatory cytokines play a role in LPS-mediated decreases in cytotrophoblast invasion. Upon pretreatment with anti-TLR4 Ab, primary cytotrophoblast cell invasion was markedly increased when compared to LPS-exposed cytotrophoblast cells without pretreatment with anti-TLR4 Ab (P < 0.01). The invasion of anti-TNF-α Ab pretreated, LPS-exposed cytotrophoblast cells was also significantly increased compared with LPS-stimulated cytotrophoblast cells without anti-TNF-α Ab pretreatment (P < 0.01) but was still lower than medium-treated control cytotrophoblast cells (P < 0.01). Pretreatment with Abs against IL-1β and IL-6 had no significant effects on cytotrophoblast invasion compared with LPS-exposed cytotrophoblast cells without the pretreatment.
Fig. 5.
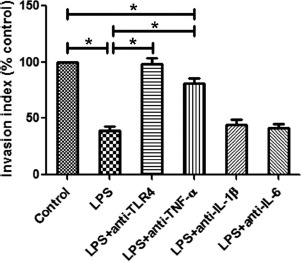
Comparison of cytotrophoblast invasion following LPS treatment. The invasion of primary cytotrophoblast cells maintained under a variety of 24 h exposures was evaluated by Matrigel invasion assay. The invasion indices of human primary cytotrophoblast cells treated with 100 ng/ml LPS and neutralizing Abs against TLR4, TNF-α, IL-1β, or IL-6 in the presence of LPS were normalized to that of the control. Data are presented as mean ± SD of three independent experiments; *P < 0.01.
Comparison of Decidual Macrophage Migration
As shown in Figure 6A, different doses of CM from LPS-stimulated cytotrophoblasts significantly promoted decidual macrophage migration (P < 0.01), and the macrophage migration reached its highest when these cells were treated with 20% cytotrophoblast CM. We therefore chose 20% cytotrophoblast CM as the chemoattractant in subsequent migration experiments. As shown in Figure 6B, in comparison to control CM, CM from cytotrophoblasts exposed to LPS markedly increased decidual macrophage migration (P < 0.01). The migration of decidual macrophages treated with cytotrophoblast CM in the presence of anti-TLR4 Ab was notably lower than cells treated with cytotrophoblast CM in the absence of anti-TLR4 Ab (P < 0.01) but was still higher than cells treated with control CM (P < 0.05). In addition, we used LPS alone as a positive control. We found that macrophages treated with LPS markedly up-regulated macrophage migration (P < 0.01). As expected, pretreating macrophages with anti-TLR4 Ab abrogated the effect of LPS (P < 0.01), indicating that LPS-mediated macrophage migration is fully TLR4-dependent (Fig. 6B).
Fig. 6.
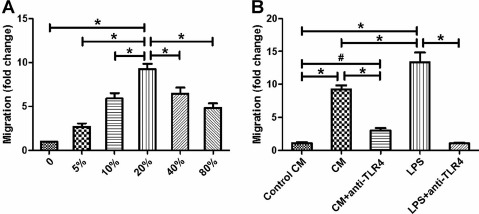
Comparison of decidual macrophage migration. The migration of decidual macrophages toward cytotrophoblast CM was investigated using an 8-μm membrane insert system. Isolated decidual macrophages (2 × 105) were seeded in the upper chamber. Different concentrations of cytotrophoblast CM (A), 20% control CM, 20% cytotrophoblast CM with or without anti-TLR4 Ab (1 μg/ml), and LPS (100 ng/ml) with or without anti-TLR4 Ab (1 μg/ml) (B) were added to the lower chamber. After 24 h, macrophage migration was investigated as fold change of the numbers of CD14+ cells relative to those in basal culture medium of controls using flow cytometry. Data are presented as mean ± SD of three independent experiments; #P < 0.05 and *P < 0.01.
Comparison of Receptor Expression on Decidual Macrophages
To study the effects of the cross talk between cytotrophoblasts and decidual macrophages on the phenotypes of decidual macrophages, decidual macrophages were indirectly cocultured with cytotrophoblast cells using a 0.4-μm membrane insert system. The expression of the costimulatory molecules CD80 and CD86, the mannose receptor CD206, and the scavenger receptor CD209 on decidual macrophages was analyzed. Coculture with LPS-treated cytotrophoblast cells significantly increased the expression of CD80 and CD86 but decreased the expression of CD206 and CD209 on decidual macrophages compared with macrophages cocultured with PBS-treated cytotrophoblast cell controls (P < 0.01) (Fig. 7).
Fig. 7.
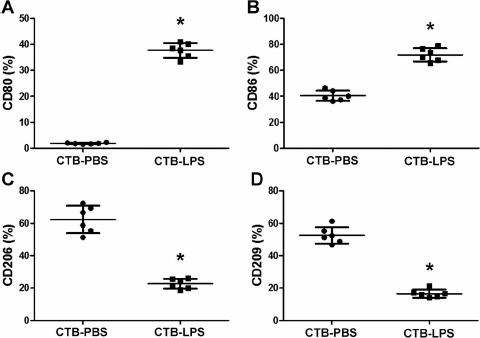
Comparison of the expression of receptors on decidual macrophages. Decidual macrophages (2 × 105) were cocultured with cytotrophoblast cells (2 × 105) treated with PBS or LPS (100 ng/ml) in a 0.4-μm membrane insert system for 24 h. The expression levels of CD80 (A), CD86 (B), CD206 (C), and CD209 (D) on these macrophages were analyzed using flow cytometry. Data are presented as mean ± SD of six independent experiments. CTB, cytotrophoblast. *P < 0.01 versus PBS-treated cytotrophoblast cells.
Comparison of Cytotrophoblast Cell-Induced Intracellular Cytokine Production in Decidual Macrophages
The production of intracellular TNF-α and IL-12p40 in decidual macrophages cocultured with LPS-exposed cytotrophoblast cells was markedly increased compared with macrophages cocultured with PBS-treated cytotrophoblast cells (P < 0.01) (Fig. 8, A and B). However, the production of IL-10 was notably decreased in macrophages cocultured with LPS-stimulated cytotrophoblast cells compared with controls (P < 0.01) (Fig. 8C).
Fig. 8.
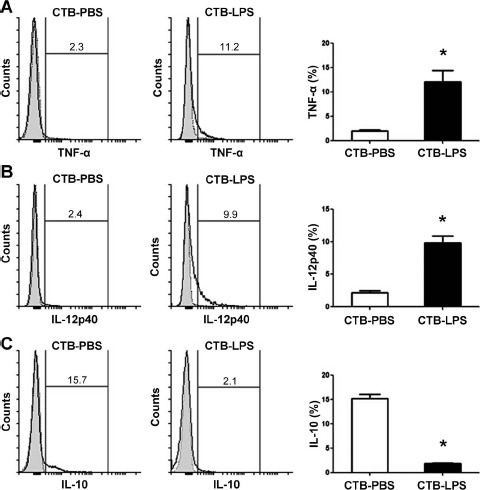
Comparison of intracellular cytokine production in decidual macrophages. Decidual macrophages (2 × 105) were cocultured with cytotrophoblast cells (2 × 105) treated with PBS or LPS (100 ng/ml) in a 0.4-μm membrane insert system for 24 h. The intracellular production of TNF-α (A), IL-12p40 (B), and IL-10 (C) in macrophages was analyzed using flow cytometry. Gray-shaded histogram: isotype-matched negative control. Black line: specific Ab expression. Numbers indicate the percentages of particular Ab-positive cells among macrophages. Data are presented as mean ± SD of six independent experiments. CTB, cytotrophoblast. *P < 0.01 compared to PBS-treated cytotrophoblast cells.
Effects of LPS-Treated Decidual Macrophages on Cytotrophoblast Invasion
As shown in Figure 9, while PBS-treated decidual macrophages had no effects on cytotrophoblast invasion, LPS-stimulated decidual macrophages significantly decreased trophoblast invasion compared with PBS-treated decidual macrophages (P < 0.01). Because LPS treatment markedly increased the production of TNF-α and IL-12 in decidual macrophages, we used neutralizing Abs against TNF-α and IL-12 to investigate whether these cytokines play a role in the effects of LPS-exposed decidual macrophages on cytotrophoblast invasion. LPS-stimulated decidual macrophages pretreated with Abs against TNF-α and IL-12 significantly up-regulated cytotrophoblast invasion compared with LPS-stimulated decidual macrophages without pretreatment (P < 0.01 and P < 0.05, respectively) (Fig. 9).
Fig. 9.
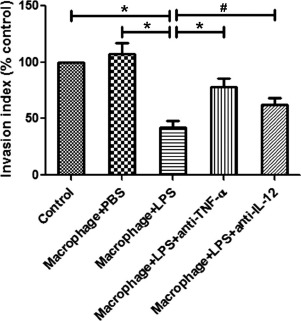
Comparison of cytotrophoblast invasion toward decidual macrophages. The invasion of primary cytotrophoblast cells maintained under a variety of 24 h exposures was evaluated by Matrigel invasion assay. The invasion indices of human primary cytotrophoblast cells toward decidual macrophages treated with PBS, LPS (100 ng/ml), or LPS plus neutralizing Abs against TNF-α or IL-12 were normalized to that of the control. Data are presented as mean ± SD of three independent experiments; *P < 0.01 and #P < 0.05.
Discussion
We utilized primary first trimester villous cytotrophoblast cells for our study because utilization of these cells may reflect the initial steps in the invasive differentiation process, that is, detachment of trophoblast cells from villous basement membranes [35]. In addition, approximately 40% of isolated villous cytotrophoblast cells from human first trimester placenta will differentiate into EVT cells [36], and villous cytotrophoblast cells are widely used to study trophoblast invasiveness [35]. Release of proinflammatory cytokines is associated with the pathophysiology of pre-eclampsia [37, 38] and IUGR [11, 39]. Our data demonstrate that LPS induces the secretion of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 in primary first trimester cytotrophoblast cells but has no effect on the production of IL-12p70, IL-4, and IL-10. Pretreatment with an anti-TLR4 Ab abrogated the production of TNF-α, IL-1β, and IL-6 by LPS-stimulated cytotrophoblasts, suggesting that LPS-induced inflammatory cytokine production by cytotrophoblasts is completely TLR4-dependent. Previous studies have demonstrated that LPS treatment enhances TNF-α expression in primary trophoblast cells [40] and syncytiotrophoblast [41] from term human placentas as well as IL-6 production in first trimester EVT cells [42]. Our findings were in agreement with these studies even though different placental cell types were investigated.
We also found that LPS exposure caused a dose-dependent increase in the secretion of the chemokines IL-8, MIP-1α, and CXCL12. IL-8 is a potent neutrophil chemoattractant [43], and it has been reported that MIP-1α plays a major role in the recruitment of leukocytes to the sites of infection [44]. In addition, the secretion of CXCL12 by EVT cells within the decidua and spiral arteries induces migration of decidual natural killer cells [45]. Chemokines mediate the migration of immune cells to sites of infection and inflammation and are important in the activation of immune cells at these sites. Recruitment of maternal immune cells plays an important role in controlling infection. However, proinflammatory cytokines produced by maternal immune cells result in inhibition of trophoblast migration and are directly cytotoxic to invading trophoblasts [46]. In addition, activation of maternal immune cells also leads to destruction of the villous architecture and induces trophoblast apoptosis [47].
We also demonstrated that activation of the innate immune response resulted in decreased first trimester cytotrophoblast cell invasion in vitro, confirming prior reports showing that LPS exposure leads to a decrease in invasion of first trimester EVT cells [42]. Previous studies have demonstrated that coculture of immortalized HTR-8/SVneo human trophoblast cells with LPS-stimulated macrophages decreases trophoblast cell invasion [48, 49]. In the present study, the LPS-induced decrease in cytotrophoblast invasion was completely abrogated by Ab-mediated TLR4 blockade, suggesting that the effects of LPS on primary cytotrophoblast cell invasion are largely controlled by TLR4-mediated inflammatory signaling pathways. Moreover, anti-TNF-α Ab significantly up-regulated the invasion of LPS-treated primary villous cytotrophoblast cells, suggesting that TNF-α secreted by cytotrophoblast cells upon LPS treatment down-regulates cytotrophoblast invasion. This is in agreement with a previous report showing that TNF-α, both alone or in combination with interferon (IFN)-γ, inhibits primary EVT cell invasion [50]. Notably, the addition of a TNF-α-neutralizing Ab to LPS-treated cytotrophoblast cells did not fully abrogate decreases in cytotrophoblast invasion in our model, indicating that other factors may be involved in the regulation of cytotrophoblast invasion. Although CXCL12 has been found to inhibit trophoblast invasion in other studies [51], the effect of CXCL12 secreted by cytotrophoblast cells upon LPS stimulation in our investigations may be outweighed by the effects of increases in the production of TNF-α.
Substantial numbers of macrophages are closely associated with invasive trophoblast cells in vivo [52]. Based on the observation that LPS-stimulated cytotrophoblast cells produce the chemokines IL-8, MIP-1α, and CXCL12 that can attract leukocytes into sites of inflammation, we hypothesized that cytotrophoblast cells stimulated by LPS could modify the migration of decidual macrophages. Therefore, we performed migration studies using an 8-μm membrane insert system. Treatment with CM from LPS-exposed cytotrophoblast cells significantly increased the migration of decidual macrophages, while pretreatment with anti-TLR4 Ab partly abrogated the effects of cytotrophoblast CM, suggesting that the effects of cytotrophoblast CM on macrophage migration are only partially TLR4-dependent. Other LPS-stimulated soluble molecules in cytotrophoblast CM must also play a role in macrophage migration.
In order to dissect the potential mechanisms underlying inflammation-induced decreases in cytotrophoblast invasion, we investigated whether LPS-exposed cytotrophoblast cells altered the activation state of decidual macrophages. Macrophages display a high degree of plasticity and their activation states and functions are determined by the conditions within their surrounding microenvironment [53]. In normal pregnancy, decidual macrophages possess properties associated with M2 macrophages and are characterized by expression of CD14, HLA-DR, the mannose receptor CD206, and the scavenger receptor CD209, as well as production of anti-inflammatory cytokines such as IL-10, TGF-β, and IL-13 [20, 54, 55]. In our study, decidual macrophages exposed in a transwell system to LPS-stimulated cytotrophoblast cells transitioned from a suppressive M2 phenotype characterized by the expression of CD206 and CD209 and production of intracellular IL-10 to a proinflammatory M1 profile exhibiting expression of costimulatory molecules CD80 and CD86 as well as production of the proinflammatory cytokine TNF-α and IL-12p40. In addition, we demonstrated that LPS-activated macrophages inhibited the invasion of cytotrophoblast cells, and this effect was TNF-α and IL-12 dependent. Our study is in accordance with the prior study showing that TNF-α secreted by activated macrophages decreases trophoblast invasion in vitro [49]. Successful pregnancy requires the activation state of decidual macrophages to be strictly regulated. Inappropriate polarization of decidual macrophages is associated with inadequate remodeling of uterine vessels and pre-eclampsia [23, 56].
In summary, our results indicate that stimulation of isolated human first trimester primary cytotrophoblast cells with LPS leads to the production of a subset of proinflammatory cytokines and chemokines as well as decreased trophoblast invasion, processes that could contribute to adverse pregnancy outcomes. In addition, LPS-stimulated cytotrophoblast cells promote the migration of decidual macrophages and drive these cells from an anti-inflammatory M2 phenotype toward a pro-inflammatory M1 polarization.
References
- 1. Sargent IL, Borzychowski AM, Redman CW.. NK cells and human pregnancy–an inflammatory view. Trends Immunol 2006; 27:399–404. [DOI] [PubMed] [Google Scholar]
- 2. Hamilton WJ, Boyd JD.. Development of the human placenta in the first three months of gestation. J Anat 1960; 94:297–328. [PMC free article] [PubMed] [Google Scholar]
- 3. Wallace AE, Fraser R, Cartwright JE.. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update 2012; 18:458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Y, Krikun G, Abrahams VM, Mor G, Guller S.. Cell type-specific expression and function of toll-like receptors 2 and 4 in human placenta: implications in fetal infection. Placenta 2007; 28:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G.. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol 2004; 173:4286–4296. [DOI] [PubMed] [Google Scholar]
- 6. Kawai T, Akira S.. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010; 11:373–384. [DOI] [PubMed] [Google Scholar]
- 7. Riley JK, Nelson DM.. Toll-like receptors in pregnancy disorders and placental dysfunction. Clin Rev Allergy Immunol 2010; 39:185–193. [DOI] [PubMed] [Google Scholar]
- 8. Pineda A, Verdin-Terán SL, Camacho A, Moreno-Fierros L.. Expression of toll-like receptor TLR-2, TLR-3, TLR-4 and TLR-9 is increased in placentas from patients with preeclampsia. Arch Med Res 2011; 42:382–391. [DOI] [PubMed] [Google Scholar]
- 9. Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM.. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens 2009; 22:1314–1319. [DOI] [PubMed] [Google Scholar]
- 10. Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM.. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension 2011; 58:489–496. [DOI] [PubMed] [Google Scholar]
- 11. Zhao M, Chen YH, Dong XT, Zhou J, Chen X, Wang H, Wu SX, Xia MZ, Zhang C, Xu DX.. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS One 2013; 8:e82713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE.. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med 2006; 203:2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bartmann C, Segerer SE, Rieger L, Kapp M, Sütterlin M, Kämmerer U.. Quantification of the predominant immune cell populations in decidua throughout human pregnancy. Am J Reprod Immunol 2014; 71:109–119. [DOI] [PubMed] [Google Scholar]
- 14. Mills CD, Ley K.. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun 2014; 6:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosser DM, Edwards JP.. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown MB, von Chamier M, Allam AB, Reyes L.. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol 2014; 5:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3:23–35. [DOI] [PubMed] [Google Scholar]
- 18. Gordon S, Martinez FO.. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593–604. [DOI] [PubMed] [Google Scholar]
- 19. Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL.. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 2009; 174:1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagamatsu T, Schust DJ.. The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci 2010; 17:209–218. [DOI] [PubMed] [Google Scholar]
- 21. Co EC, Gormley M, Kapidzic M, Rosen DB, Scott MA, Stolp HA, McMaster M, Lanier LL, Bárcena A, Fisher SJ.. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol Reprod 2013; 88:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL.. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 2012; 86:39. [DOI] [PubMed] [Google Scholar]
- 23. Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B.. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest 2001; 81:1143–1152. [DOI] [PubMed] [Google Scholar]
- 24. Ji L, Brkić J, Liu M, Fu G, Peng C, Wang YL.. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Aspects Med 2013; 34:981–1023. [DOI] [PubMed] [Google Scholar]
- 25. Cudihy D, Lee RV.. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol 2009; 29:576–582. [DOI] [PubMed] [Google Scholar]
- 26. O'Tierney-Ginn PF, Lash GE.. Beyond pregnancy: modulation of trophoblast invasion and its consequences for fetal growth and long-term children's health. J Reprod Immunol 2014; 104–105:37–42. [DOI] [PubMed] [Google Scholar]
- 27. Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol 2013; 94:247–257. [DOI] [PubMed] [Google Scholar]
- 28. Cota LO, Guimarães AN, Costa JE, Lorentz TC, Costa FO.. Association between maternal periodontitis and an increased risk of preeclampsia. J Periodontol 2006; 77:2063–2069. [DOI] [PubMed] [Google Scholar]
- 29. Chaparro A, Blanlot C, Ramírez V, Sanz A, Quintero A, Inostroza C, Bittner M, Navarro M, Illanes SE.. Porphyromonas gingivalis, Treponema denticola and toll-like receptor 2 are associated with hypertensive disorders in placental tissue: a case-control study. J Periodontal Res 2013; 48:802–809. [DOI] [PubMed] [Google Scholar]
- 30. Guleria I, Pollard JW.. Aberrant macrophage and neutrophil population dynamics and impaired Th1 response to Listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect Immun 2001; 69:1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Schust DJ.. Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod Biol Endocrinol 2015; 13:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fock V, Mairhofer M, Otti GR, Hiden U, Spittler A, Zeisler H, Fiala C, Knöfler M, Pollheimer J.. Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol 2013; 191:3734–3743. [DOI] [PubMed] [Google Scholar]
- 33. Akarsu ES, Mamuk S.. Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats. Am J Physiol Regul Integr Comp Physiol 2007; 292:R1846–R1850. [DOI] [PubMed] [Google Scholar]
- 34. Du MR, Guo PF, Piao HL, Wang SC, Sun C, Jin LP, Tao Y, Li YH, Zhang D, Zhu R, Fu Q, Li DJ.. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol 2014; 192:1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knöfler M. Critical growth factors and signalling pathways controlling human trophoblast invasion. Int J Dev Biol 2010; 54:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cervar-Zivkovic M, Stern C.. Trophoblast isolation and culture.HH Kay, DM Nelson, Wang Y. (eds.),The Placenta: From Development to Disease.England:Wiley-Blackwell;2011:155–162. [Google Scholar]
- 37. Xie C, Yao MZ, Liu JB, Xiong LK.. A meta-analysis of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 in preeclampsia. Cytokine 2011; 56:550–559. [DOI] [PubMed] [Google Scholar]
- 38. Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW.. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol 2013; 70:412–427. [DOI] [PubMed] [Google Scholar]
- 39. Xu DX, Chen YH, Wang H, Zhao L, Wang JP, Wei W.. Tumor necrosis factor alpha partially contributes to lipopolysaccharide-induced intra-uterine fetal growth restriction and skeletal development retardation in mice. Toxicol Lett 2006; 163:20–29. [DOI] [PubMed] [Google Scholar]
- 40. Torricelli M, Voltolini C, Bloise E, Biliotti G, Giovannelli A, De Bonis M, Imperatore A, Petraglia F.. Urocortin increases IL-4 and IL-10 secretion and reverses LPS-induced TNF-alpha release from human trophoblast primary cells. Am J Reprod Immunol 2009; 62:224–231. [DOI] [PubMed] [Google Scholar]
- 41. Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JR, Bocking AD.. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol 2009; 200:532.e1–532.e8. [DOI] [PubMed] [Google Scholar]
- 42. Anton L, Brown AG, Parry S, Elovitz MA.. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: possible mechanisms of first trimester placental dysfunction. Hum Reprod 2012; 27:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Russo RC, Garcia CC, Teixeira MM, Amaral FA.. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol 2014; 10:593–619. [DOI] [PubMed] [Google Scholar]
- 44. Griffith JW, Sokol CL, Luster AD.. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014; 32:659–702. [DOI] [PubMed] [Google Scholar]
- 45. Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O.. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood 2003; 102:1569–1577. [DOI] [PubMed] [Google Scholar]
- 46. Ahn H, Park J, Gilman-Sachs A, Kwak-Kim J.. Immunologic characteristics of preeclampsia, a comprehensive review. Am J Reprod Immunol 2011; 65:377–394. [DOI] [PubMed] [Google Scholar]
- 47. Kim CJ, Romero R, Chaemsaithong P, Kim JS.. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015; 213:S53–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Renaud SJ, Macdonald-Goodfellow SK, Graham CH.. Coordinated regulation of human trophoblast invasiveness by macrophages and interleukin 10. Biol Reprod 2007; 76:448–454. [DOI] [PubMed] [Google Scholar]
- 49. Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH.. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod 2005; 73:237–243. [DOI] [PubMed] [Google Scholar]
- 50. Otun HA, Lash GE, Innes BA, Bulmer JN, Naruse K, Hannon T, Searle RF, Robson SC.. Effect of tumour necrosis factor-α in combination with interferon-γ on first trimester extravillous trophoblast invasion. J Reprod Immunol 2011; 88:1–11. [DOI] [PubMed] [Google Scholar]
- 51. Warner JA, Zwezdaryk KJ, Day B, Sullivan DE, Pridjian G, Morris CA.. Human cytomegalovirus infection inhibits CXCL12- mediated migration and invasion of human extravillous cytotrophoblasts. Virol J 2012; 9:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Helige C, Ahammer H, Moser G, Hammer A, Dohr G, Huppertz B, Sedlmayr P.. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: a quantitative evaluation. Hum Reprod 2014; 29:8–17. [DOI] [PubMed] [Google Scholar]
- 53. Gordon S, Plüddemann A, Martinez Estrada F.. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 2014; 262:36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gustafsson C, Mjösberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J.. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS One 2008; 3:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J.. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol 2011; 187:3671–3682. [DOI] [PubMed] [Google Scholar]
- 56. Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schröder W, Kaufmann P, Rath W.. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta 1999; 20:229–233. [DOI] [PubMed] [Google Scholar]


