We thank Dr. Bliwise and agree with the need for clarity regarding normative values, across the life span, for the percentage of nighttime sleep spent in slow wave sleep (%SWS). To further investigate the apparent discrepancy in %SWS between these two samples, we accessed publically available data (provided by the National Sleep Research Resource, see http://sleepdata.org ) from both the Sleep Heart Health Study (SHHS; Redline et al., 1998 ) and Osteoporotic Fractures in Men Sleep Study (MrOS).
The SHHS results referred to were based on a restricted sample which excluded participants who had “exposures or conditions likely to have large effects on sleep architecture [except sleep disordered breathing]” ( Redline et al., 2004 ). This exclusion supported the original report’s aim of assessing associations of sleep disordered breathing, age, sex, and ethnicity with sleep architecture, independent of potential confounders/mediators. However, to clarify normative values of %SWS by age in these samples of older men (regardless of etiology), excluding participants with factors affecting sleep architecture is less appropriate. Although the original SHHS report noted that there were no differences in sleep staging between participants included and excluded, estimates in the entirety of available data (vs the restricted SHHS report sample) will differ.
In addition, as noted by Dr. Bliwise, the SHHS report applied covariate adjustments as part of their analytic strategy (to assess the independence of multiple correlates of sleep architecture stages). However, adding adjustments could obscure prevalent age variability (i.e., by estimating the expected %SWS of men in different age groups as if they were equivalent on the selected covariates). Finally, the SHHS report applied a −log(−log( P + 0.001)) transformation where P was the proportion of sleep time spent in slow wave stages. We therefore examined the effect of this transformation on the estimated %SWS, without covariate adjustment, by age among all men at least 67 years old (which was the lowest age in MrOS) in SHHS ( n = 1,075 and MrOS ( n = 2,872).
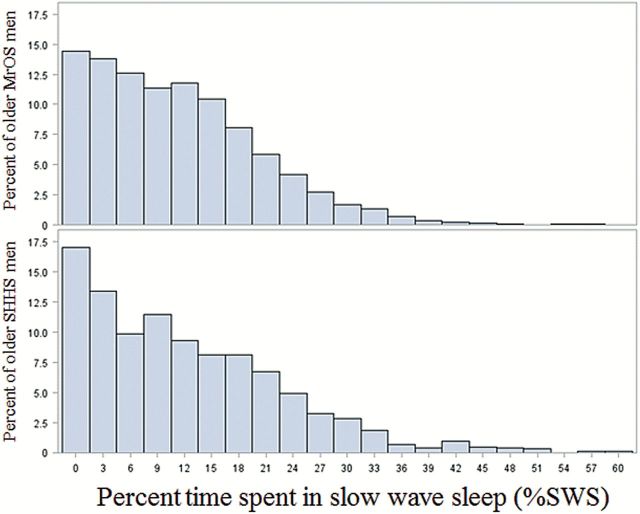
The original distributions ( Figure 1 ) broadly overlapp and are both skewed left with a long tail that encompasses a significant proportion of both samples. Applying the −log(−log( P + 0.001)) transformation lowers estimated average %SWS by normalizing the distributions, effectively lessening the influence of the distribution’s tail (of men with greater %SWS). Given these distributional observations, to make valid statistical inferences regarding the effects of age (in quartiles) and study on %SWS, we evaluated predicted means and 95% confidence intervals with this transformation applied in both studies ( Table 1 ). There were no differences in the percentage of time spent in SWS between men in SHHS and MrOS ( F value = 0.16, p = .69). There was a significant effect of age quartile ( F value = 3.42, d f = 3, p = .02), and this effect was consistent across studies (interaction of study and age quartile, F value = 0.96, p = .41).
Figure 1.
Histograms illustrating broad overlap in the distributions of percentage of nighttime sleep spent in slow wave sleep among older men in Sleep Heart Health Study and Osteoporotic Fractures in Men Sleep Study
Table 1.
Predicted Means (95% CIs) for %SWS Among Older (≥67 years) Men, by Age Quartile in MrOS and SHHS
| Age (years) | MrOS | SHHS | Combined | |||
|---|---|---|---|---|---|---|
| Mean | (95% CI) | Mean | (95% CI) | Mean | (95% CI) | |
| ≤71 | 9.29 | (8.55–10.07) | 9.60 | (8.53–10.74) | 9.44 | (8.82–10.09) |
| 72–75 | 9.00 | (8.32–9.71) | 8.02 | (6.83–9.31) | 8.50 | (7.84–9.19) |
| 76–80 | 8.44 | (7.80–9.11) | 8.96 | (7.61–10.45) | 8.70 | (7.99–9.43) |
| >80 | 7.92 | (7.26–8.62) | 7.52 | (5.89–9.40) | 7.72 | (6.87–8.63) |
Notes. %SWS = percentage of nighttime sleep spent in slow wave sleep; CI = confidence interval; MrOS = Osteoporotic Fractures in Men Sleep Study; SHSS = Sleep Heart Health Study.
%SWS was examined after −log(−log( P + 0.001)) transformation and is presented after back transformation to facilitate interpretation.
Because unadjusted or adjusted predicted means (even with measures of spread) are not the best distributional descriptors for a skewed continuous variable, we also present the 5th, 25th, 50th (median), 75th, and 95th percentile for %SWS across age groups ( Table 2 ). Regardless of how these distributions are examined, the distribution of %SWS by age is consistent across studies, with vastly more variability within than across age groups or studies. Thus, the pronounced difference in %SWS across samples that Dr. Bliwise identified is likely the result of the SHHS report applying a transformation, exclusion criteria and adjustments (as was appropriate to achieve their aims), while the MrOS report provided unadjusted means and standard deviations for descriptive purposes.
Table 2.
Percentiles of %SWS Among Older (≥67 years) Men by Age Quartile in MrOS and SHHS
| Age (years) | N | 5th | 25th | 50th | 75th | 95th |
|---|---|---|---|---|---|---|
| MrOS | ||||||
| ≤71 | 663 | 0.45 | 5.06 | 10.30 | 16.70 | 27.60 |
| 72–75 | 749 | 0.25 | 4.02 | 10.60 | 16.70 | 27.70 |
| 76–79 | 785 | 0.16 | 3.38 | 9.59 | 16.90 | 28.10 |
| >80 | 675 | 0.14 | 2.86 | 9.10 | 16.20 | 29.40 |
| Overall | 2872 | 0.21 | 3.72 | 9.89 | 16.70 | 28.20 |
| SHHS | ||||||
| ≤71 | 432 | 0.00 | 4.06 | 10.23 | 19.54 | 32.44 |
| 72–75 | 276 | 0.00 | 2.39 | 10.08 | 17.65 | 30.74 |
| 76–79 | 240 | 0.00 | 3.21 | 10.48 | 18.24 | 30.72 |
| >80 | 127 | 0.00 | 1.68 | 7.88 | 16.91 | 37.99 |
| Overall | 1075 | 0.00 | 3.21 | 10.01 | 18.81 | 32.04 |
| Overall | ||||||
| ≤71 | 1095 | 0.20 | 4.66 | 10.30 | 17.78 | 28.92 |
| 72–75 | 1025 | 0.13 | 3.68 | 10.50 | 16.80 | 28.40 |
| 76–79 | 1025 | 0.14 | 3.31 | 9.66 | 17.10 | 29.40 |
| >80 | 802 | 0.13 | 2.57 | 8.88 | 16.50 | 29.90 |
| Overall | 3947 | 0.14 | 3.52 | 9.93 | 17.00 | 29.20 |
Note. MrOS = Osteoporotic Fractures in Men Sleep Study; SHSS = Sleep Heart Health Study.
Perhaps most importantly, this exercise clearly show that %SWS among older men is highly variable. Distributions widely overlap such that it would be perfectly reasonable to observe two men born 10 years apart to have the same %SWS. Although age quartile and %SWS were significantly and consistently associated across studies, the overall correlation between age expressed continuously and %SWS is very weak (Spearman r = −.06). Therefore, among older men, %SWS is highly variable, but age explains only a small portion of this variability. This is consistent with the meta-analytic observation that age-related changes in %SWS are difficult to ascertain after adulthood (i.e., see Figure 1c in Ohayon, Carskadon, Guilleminault, & Vitiello, 2004 ), likely due to substantial variability within samples of same aged older adults. The determinants of %SWS among older men are likely specific disease processes related to biological aging rather than chronological age itself. Recent literature suggests that atrophy and amyloid deposition are associated with %SWS and that these factors together predict memory function ( Mander et al., 2013 , 2015 ). Future longitudinal research is needed to establish temporality in relations between brain structural pathology, %SWS, and common mental diseases of aging.
Funding
S. F. Smagula is supported by the grant T32 MH019986, and this work is supported through the National Sleep Research Resource (R24 HL11447) and the MrOS Sleep Study (R01 HL071194).
References
- Mander B. A. Marks S. M. Vogel J. W. Rao V. Lu B. Saletin J. M. , … Walker M. P . ( 2015. ). [beta]-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation . Nature Neuroscience , 18 , 1051 – 1057 . doi: 10.1038/nn.4035http://www.nature.com/neuro/journal/v18/n7/abs/nn.4035.html#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander B. A. Rao V. Lu B. Saletin J. M. Lindquist J. R. Ancoli-Israel S. , … Walker M. P . ( 2013. ). Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging . Nature Neuroscience , 16 , 357 – 364 . doi: 10.1038/nn.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M. M. Carskadon M. A. Guilleminault C. , & Vitiello M. V . ( 2004. ). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan . Sleep , 27 , 1255 – 1273 . [DOI] [PubMed] [Google Scholar]
- Redline S. Kirchner H. L. Quan S. F. Gottlieb D. J. Kapur V. , & Newman A . ( 2004. ). The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture . Archives of Internal Medicine , 164 , 406 – 418 . doi: 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- Redline S. Sanders M. H. Lind B. K. Quan S. F. Iber C. Gottlieb D. J. , … Kiley J. P.;. Sleep Heart Health Research Group . ( 1998. ). Methods for obtaining and analyzing unattended polysomnography data for a multicenter study . Sleep , 21 , 759 – 767 . [PubMed] [Google Scholar]