Abstract
STUDY QUESTION
Are anti-Müllerian hormone (AMH) levels assessed in women aged 32–44 associated with risk of incident early natural menopause?
SUMMARY ANSWER
We observed strong, significant associations between lower AMH levels and higher risk of early menopause.
WHAT IS KNOWN ALREADY
The ability to predict risk early menopause, defined as menopause before age 45, prior to fertility decline would improve options for family planning and cardiovascular disease prevention. Though AMH is an established marker of menopause timing in older reproductive-aged women, whether AMH is associated with risk of early menopause has not been evaluated.
STUDY DESIGN, SIZE, DURATION
We assessed these relations in a nested case–control study within the prospective Nurses’ Health Study II cohort. Premenopausal blood samples were collected in 1996–1999. Participants were followed until 2011 for early natural menopause, with follow-up rates >94%.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Early menopause cases (n = 327) were women reporting natural menopause between blood collection and age 45. Controls (n = 491) experienced menopause after age 45 and included 327 cases matched to controls on the basis of age at blood draw (±4 months) and other factors. AMH levels up to 12 years before early menopause were assayed in 2016.
MAIN RESULTS AND THE ROLE OF CHANCE
In multivariable conditional logistic regression models adjusting for matching factors, body mass index, smoking, parity, oral contraceptive use, and other factors, each 0.10 ng/ml decrease in AMH was associated with a 14% higher risk of early menopause (95% confidence interval (CI) 1.10 to 1.18; P < 0.001). In polynomial regression models including linear and quadratic terms for AMH, odds ratios for early menopause for women with AMH levels of 1.5, 1.0 and 0.5 ng/ml compared to 2.0 ng/ml were 2.6, 7.5 and 23 (all P < 0.001). Significant associations were observed irrespective of smoking status, adiposity, infertility history and menstrual cycle characteristics. Furthermore, models assessing the predictive ability of AMH showed high concordance, and C-statistics were high, ranging from 0.68 (age ≤35) to 0.93 (age 42).
LIMITATIONS, REASONS FOR CAUTION
Our population was relatively homogenous with respect to race/ethnicity. Further work in more ethnically diverse populations is needed.
WIDE IMPLICATION OF THE FINDINGS
To our knowledge, this is the first prospective study to evaluate whether AMH levels are associated with early menopause. These findings support the utility of AMH as a clinical marker of early menopause in otherwise healthy women.
STUDY FUNDING/COMPETING INTEREST(S)
This project was supported by UM1CA176726, R01CA67262, and R01HD078517 from the U.S. Department of Health and Human Services, National Institutes of Health. No competing interests declared.
Keywords: early menopause, age at menopause, prospective studies, anti-Müllerian hormone, epidemiology, reproductive decline
Introduction
The prevalence of early natural menopause in Western populations is high, with up to 10% of women experiencing the cessation of ovarian function before the age of 45. As women increasingly delay childbearing into their older reproductive years, the consequence of early menopause for fertility and family planning are substantial (Broekmans et al., 2009), as once fertility has begun to decline, options for fertility preservation are limited (Panay and Fenton, 2008). Furthermore, the hormonal changes associated with the early cessation of ovarian function may increase risk of cardiovascular disease, osteoporosis, cognitive decline and other chronic diseases (Shuster et al., 2010; Bleil et al., 2013; Muka et al., 2016). The ability to predict early menopause before the onset of decline in fertility would allow women to make more informed decisions on the timing of childbearing and to consider options for treatment.
Anti-Müllerian hormone (AMH), a glycoprotein produced by granulosa cells of primary follicles (Visser et al., 2012), has been established as a marker of time to menopause in older reproductive-aged women. AMH levels are strongly correlated with the size of the antral follicle pool, at least in older premenopausal women (van Rooij et al., 2005; Ledger, 2010; Rosen et al., 2012; Steiner, 2013) and population-based studies have consistently found AMH level to be a better predictor of time to menopause than age and other reproductive hormones (Sowers et al., 2008; van Disseldorp et al., 2008; Tehrani et al., 2009, 2011; Broer et al., 2011; Dólleman et al., 2013; Freeman et al., 2012a, 2012b; Depmann et al., 2016). However, to our knowledge, whether AMH could be clinically relevant as a marker of risk of early menopause has not been evaluated, as few population-based studies conducted to date have had sufficient numbers of women experiencing early natural menopause to assess this relation. Whether AMH may be a marker of early menopause in healthy, symptom-free women across a range of premenopausal ages remains unknown (Depmann et al., 2016).
We have evaluated prospectively whether AMH levels are associated with risk of early menopause among a subset of participants in the Nurses’ Health Study II (NHS2).
Materials and Methods
Study population
The NHS2 is a prospective study of 116,429 US female registered nurses who responded to a mailed questionnaire in 1989. Participants were 25–42 years old at baseline and provided information on the medical history and health-related behaviors such as oral contraceptives, menstrual and pregnancy history, and smoking status. Cohort members have completed questionnaires every two years to update information on risk factors and to identify new diagnoses of disease, with a cumulative response rate of 94%. The study protocol was approved by the Institutional Review Board at Brigham and Women’s Hospital.
NHS2 blood cohort
Between 1996 and 1999, members of the NHS2 who had not been diagnosed with cancer were invited to provide self-collected blood samples. Women who were premenopausal, not using menopausal hormone therapy (HT), oral contraceptives (OCs) or other hormones, and who had not been pregnant in the past 6 months were asked to collect two timed blood samples during the same menstrual cycle. These included a follicular phase sample (Day 3–5) and a luteal phase sample (7–9 days before the anticipated start date of next menses). Women with irregular cycles were asked to collect their luteal phase samples 22 days after last menses. Premenopausal women unwilling to collect timed samples or those currently using HT or OCs were asked to provide a single untimed sample. The actual start date of next menses was then confirmed by postcard, allowing us to confirm cycle phase. Upon receipt, samples were centrifuged, separated into blood components, and archived at –130°C or colder in continuously monitored liquid nitrogen freezers. Participants providing a blood sample did not differ from the main NHS2 cohort in terms of mean BMI (26 versus 26 kg/m2) and parity (1.9 versus 1.9 children), proportion ever smoking (34% versus 36%) and ever using OCs (86% versus 88%), and other factors (Tworoger et al., 2006). Samples were received from 29,611 women, of whom ~23,000 were premenopausal. For our prospective analysis of early menopause, participation was limited to premenopausal women providing blood samples before age 45 (~15,000; median age ~42 yrs).
Assessment of menopause timing
Beginning in 1989, NHS members were asked if their menstrual periods had ceased permanently, with response options of: (1) No, premenopausal; (2) Yes, no menstrual periods; (3) Yes, had menopause but now have periods induced by hormones; and (4) Not sure (e.g. started hormones prior to cessation of periods). Women reporting that their periods had stopped were then asked: (1) at what age did your periods cease (open response) and (2) for what reason did your periods cease, with response options of surgery, radiation or chemotherapy, or natural. All women were then asked about use of menopausal HT including their timing and the type used. These questions have been reported on each biennial questionnaire.
A small number of women reported being postmenopausal on one questionnaire and then subsequently reported being premenopausal. For these women, we defined age at menopause as age after which periods were absent for 12 months or more, and then confirmed that this status persisted for at least three consecutive questionnaires.
Incident case identification and control selection
We first excluded women with diagnoses of cardiovascular disease or cancer (other than non-melanoma skin cancer) before blood collection. From among women who were premenopausal at the time of blood collection, we identified incident cases of early menopause as women who reported natural menopause between the date of receipt of their blood sample and age 45, through the end of follow-up in June, 2011 (n = 327). We then defined two sets of controls. First, cases were matched 1:1 with control women who were: (1) premenopausal at the time of blood collection; (2) reported menopause ≥ age 47; and (3) did not report hysterectomy or oophorectomy before age 47 (n = 327 controls). Because AMH levels are strongly correlated with age, cases and controls were matched by age at the time of blood collection (±4 months), as well as by fasting status, time of day, season of blood collection, and sample type (luteal phase or random timing). Secondly, to maximize the generalizability of our sample, we selected additional controls who experienced menopause ≥ age 45, preferentially selecting women with natural menopause at ages 45 and 46 to ensure that the control group included women on the earlier end of the normal range of menopause age (n = 164), for a total of 491 controls (see Supplementary Fig. S1).
AMH measurement
AMH was measured at Children’s Hospital, Boston Massachusetts, by an ultra-sensitive ELISA assay from ANSH Labs (picoAMH; Webster, TX, USA). The assay employs the quantitative sandwich enzyme immunoassay technique. The day-to-day variabilities of the assay at concentrations of 0.023, 0.087 and 0.373 ng/ml are 5.8%, 3.2% and 4.3%, respectively. The coefficient of variation from samples from a blinded plasma pool assayed alongside our analytic samples was 8.6%.
Covariate assessment
At the time of blood collection, women provided information on current weight, menstrual cycle regularity and change in cycle characteristics as compared to when they were in their 20s, exogenous hormone use, physical activity participation, alcohol use, smoking status, and medication use. Information on demographic and other behavioral factors was obtained from NHS2 biennial questionnaires. At baseline (1989), women provided information on race, ethnicity, height, age at menarche, and the number of years until their menstrual cycles became regular after menarche. Smoking status, number of cigarettes smoked per day, pregnancy history, and OC use were measured every 2 years. Cumulative breast feeding history was measured in 1993 and 1997 from all cohort members, and in 2003 from women reporting pregnancies after 1997. For all time varying factors, we used covariate data measured closest in time to each individual’s date of blood collection (1995, 1997 or 1999).
Statistical analysis
We compared characteristics of cases and controls at the time of blood collection using age-adjusted generalized linear models. We compared AMH levels (ng/ml) in cases (n = 327) and all controls (n = 491) graphically, plotting geometric mean and 95% confidence intervals by age at blood collection. We then compared AMH levels in 327 cases and 327 matched controls using conditional logistic regression, estimating odds ratios (OR) and 95% confidence intervals (CI). AMH levels were evaluated both continuously and as quartiles. Because initial results suggested non-linear relations of AMH levels with early menopause risk, we additionally used polynomial regression models including AMH levels with both linear and quadratic terms (i.e. AMH and AMH2). Because of the low incidence of early menopause, ORs were approximations of relative risk.
In addition to minimally adjusted models (i.e. adjusted only for matching factors), we built two sets of multivariable (MV) models. MV1 included demographic and behavioral factors (race/ethnicity, smoking, BMI, physical activity and alcohol use). MV2 further adjusted for reproductive factors (age at menarche, number of years until cycles became regular, parity, breast feeding, duration of OC use, and exogenous hormone use). No covariate met statistical criteria for confounding, as the inclusion of each factor individually and all factors simultaneously did not alter the regression coefficient for the AMH-early menopause association by > 5%.
We then evaluated whether the relation of AMH levels and early menopause risk varied by BMI, smoking status, menstrual cycle characteristics, and infertility history. To maximize our power for these comparisons, we used unconditional logistic regression and included all controls (n = 491), adjusting for matching factors as well as other covariates. Effect modification was assessed with likelihood ratio tests comparing nested models with and without multiplicative interaction terms.
To evaluate the robustness of associations, we conducted sensitivity analyses excluding women with untimed blood samples (n = 73 cases and 107 controls excluded), women reporting exogenous hormone use at the time of blood collection (n = 13 cases and 11 controls excluded) and women reporting a clinician-made diagnosis of PCOS during follow-up (n = 27 cases and 26 controls excluded).
Finally, we assessed the predictive ability of AMH levels for incidence of early menopause using receiver operator characteristic (ROC) curve analysis (Pepe et al., 2008). Because of the strong relations between age and AMH, these analyses were performed stratifying by age at blood collection. Strata for ages 33 and 34 were not included due to small sample sizes and impact on precision. Predictive ability was evaluated using area under the curve (AUC). All hypothesis tests were two-sided, with alpha set at 0.05. Statistical analyses were conducted with SAS version 9 (Cary, NC).
Results
Characteristics comparing early menopause cases (n = 327) with matched controls (n = 327) and all controls (i.e. matched + unmatched; n = 491) are shown in Table I. For both comparisons, cases were similar to controls in terms of mean age, BMI, age at menarche, parity, OC use and physical activity. Cases reported shorter mean interval between menarche and onset of menstrual cycle regularity and shorter duration of breast feeding than controls. Cases and controls differed significantly by race, ethnicity, smoking status and pack years of smoking. Cases were more likely to report a history of infertility and current exogenous hormone use than matched controls.
Table I.
Age-adjusted characteristics of early menopause cases and controls at the time of blood collection, Nurses’ Health Study 2, 1996–1999.
| Characteristic | Cases (n = 327) | Matched Controls (n = 327) | All Controls (n = 491) | ||
|---|---|---|---|---|---|
| mean (SE) | mean (SE) | P | mean (SE) | P | |
| Age at blood collection (yrs)a | 40.2 (2.8) | 40.2 (2.8) | 0.94 | 40.3 (2.7) | 0.54 |
| Body mass index (kg/m2) | 25.3 (0.3) | 25.0 (0.3) | 0.45 | 25.0 (0.2) | 0.38 |
| Age at menarche (yrs) | 12.4 (0.08) | 12.3 (0.07) | 0.58 | 12.4 (0.06) | 0.97 |
| Years until cycle became regular (yrs)b | 1.4 (0.08) | 1.7 (0.09) | 0.01 | 1.6 (0.07) | 0.05 |
| Pack years of cigarette smokingc | 13.0 (0.8) | 10.7 (0.8) | 0.07 | 10.7 (0.7) | 0.03 |
| Parity (number of pregnancies ≥6 mo)d | 2.3 (0.06) | 2.4 (0.06) | 0.33 | 2.4 (0.05) | 0.31 |
| Duration of exclusive breast feeding (mo)d | 5.1 (0.4) | 6.9 (0.4) | 0.002 | 6.7 (0.3) | 0.002 |
| Duration of oral contraceptive use (mo) | 49.8 (3.1) | 51.3 (3.0) | 0.72 | 52.5 (2.5) | 0.50 |
| n (%) | n (%) | P | n (%) | P | |
| White racee | 312 (95.4) | 323 (98.8) | 0.01 | 479 (97.5) | 0.09 |
| Non-Hispanic ethnicitye | 317 (96.9) | 325 (99.4) | 0.04 | 487 (99.2) | 0.02 |
| Current smoker | 46 (14.0) | 30 (9.2) | 0.05 | 47 (9.6) | 0.05 |
| Former smoker | 59 (18.0) | 80 (24.5) | 0.04 | 122 (24.9) | 0.02 |
| Nulliparous | 76 (23.2) | 64 (19.6) | 0.37 | 102 (20.8) | 0.37 |
| Physical activity >1 time/week | 133 (40.7) | 137 (41.9) | 0.75 | 217 (44.2) | 0.32 |
| Alcohol intake ≥1 drink/day | 18 (5.5) | 29 (8.9) | 0.10 | 45 (9.2) | 0.06 |
| History of infertility | 87 (26.6) | 59 (18.0) | 0.009 | 120 (24.4) | 0.48 |
| Current exogenous hormone use | 13 (4.0) | 3 (0.9) | 0.01 | 11 (2.2) | 0.12 |
aMatching factor; standard deviation shown for age rather than standard error.
bAmong women whose cycles ever became regular (n = 757).
cLimited to ever smokers.
dLimited to parous women.
eParticipants are asked to best describe their race, with response options of White; Black or African American; Asian; American Indian/Alaskan Native; Native Hawaiian or Pacific Islander; Other. Respondents can select multiple options. Participants are also asked if they consider themselves to be Spanish/Hispanic/Latina (yes/no).
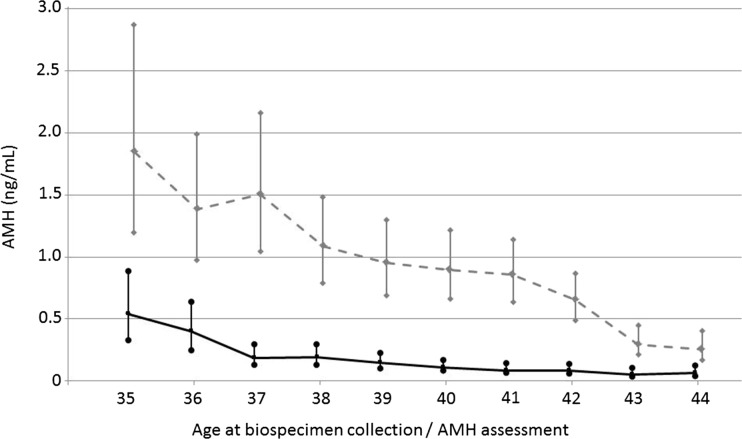
The mean AMH level was significantly lower in cases (0.40 ng/ml) than matched controls (1.9 ng/ml; P < 0.001) and all controls (1.7 ng/ml; P < 0.001). As shown in Fig. 1, geometric mean AMH levels were consistently lower in cases than controls at all ages.
Figure 1.
Age (at blood draw)-specific anti-Müllerian hormone (AMH) levels among early menopause cases (n = 327, solid line) and controls (n = 491, dotted line); geometric means and 95% confidence intervals. Nurses’ Health Study II, 1996–2011.
In conditional logistic regression models, we observed a strong and significant association of decreasing AMH levels and risk of early menopause (Table II). In minimally adjusted models, each 0.10 ng/ml lower AMH was associated with a 14% higher risk of early menopause (OR = 1.14; 95% CI = 1.10–1.17; P < 0.001). Results from models adjusted for demographic and behavioral factors (MV1) and additionally for reproductive factors (MV2) were very similar. The inclusion of a quadratic term (AMH2) significantly improved model fit compared to the model with a continuous term alone, consistent with a curvilinear relation (Table II). Results from polynomial models show estimates for coefficients and standard errors for both the linear and squared AMH terms, as well as model-based OR for early menopause for a range of AMH values. In MV2, compared to an AMH level of 2.0 ng/ml, the calculated ORs for early menopause associated with AMH levels of 1.5, 1.0 and 0.5 ng/ml were 2.6, 7.5 and 23, respectively.
Table II.
Odds ratios (OR), 95% confidence intervals (CI), regression coefficient and standard error estimates (SE) for early menopause by level of anti-Müllerian hormone (AMH) in early menopause cases (n = 327) and matched controls (n = 327), Nurses' Health Study II, 1996–2011.
| Simple conditionala | MV1b | MV2c | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| AMH (per 0.10 ng/ml lower) | 1.14 (1.10–1.17) | 1.13 (1.10–1.17) | 1.14 (1.10–1.18) |
| Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | |
| AMH (per 0.10 ng/ml lower) | 0.223 (0.03)d | 0.219 (0.03)d | 0.244 (0.03)d |
| AMH*AMH | 0.0013 (0.0002)d | 0.0013 (0.0002)d | 0.0014 (0.0002)d |
| AMH (ng/ml) | OR (95% CI)e | OR (95% CI)e | OR (95% CI)e |
| 2.00 | Ref | Ref | Ref |
| 1.50 | 2.4 (2.0–3.0) | 2.4 (1.9–3.0) | 2.6 (2.0–3.4) |
| 1.00 | 6.3 (4.0–9.8) | 6.1 (3.9–9.5) | 7.5 (4.4–13) |
| 0.50 | 17 (8.8–34) | 16 (8.2–33) | 23 (10–52) |
| 0.20 | 33 (14–76) | 31 (13–72) | 46 (17–127) |
| 0.10 | 41 (17–100) | 38 (16–94) | 59 (20–171) |
| 0.05 | 46 (18–114) | 43 (17–108) | 66 (22–200) |
aSimple conditional model: Adjusted only for matching factors: age at blood collection, fasting status, time of day of blood collection, season, sample type (luteal phase vs. randomly timed).
bMV1: Additionally adjusted for race (white, other); ethnicity (Hispanic, non-Hispanic); smoking status at blood collection (current, not current); pack-years of smoking (continuous); BMI at blood collection (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2); physical activity at blood collection (<1, 1, 2–3, ≥4 times per week); and alcohol use at blood collection (none; <1, 1, >1 drink per day).
cMV2: additionally adjusted for age at menarche (≤11, 12, 13, 14, ≥15 years); years until cycles became regular (<1, 1–2, 3–4, ≥5, never); parity (0, 1–2, 3–4, ≥5 full-term pregnancies); duration of exclusive breast feeding (continuous); infertility history (yes, no); duration of oral contraceptive use as of blood collection (none, 1–23, 24–71, 72–119, ≥120 months); exogenous hormone use at blood collection (yes, no).
dP < 0.001.
eOR and 95% CI for early menopause comparing range of AMH values to the referent of 2.0 ng/ml, based on coefficient estimates for AMH + AMH*AMH.
In analyses stratified by participant characteristics at blood draw, lower AMH levels were significantly associated with early menopause risk in all subgroups (Table III), though we observed evidence of effect measure modification. Associations were slightly stronger for women reporting changes in menstrual cycle regularity since their 20s (OR for 1.0 versus 2.0 ng/ml = 7.3) than for women reporting no change (OR = 6.3; P interaction <0.001). ORs were higher for ever-smokers (OR for 1.0 versus 2.0 ng/ml = 12) than for never-smokers (OR = 5.2; P interaction = 0.006). Results were also stronger among women with BMI ≥25 versus <25 kg/m2 (P interaction = 0.004), and varied somewhat by history of infertility (P = 0.002).
Table III.
Coefficients and standard error estimates (SE), odds ratios (OR) and 95% confidence intervals (CI) for early menopause by level of anti-Müllerian hormone (AMH) among cases (n = 327) and all controls (n = 491), stratified by potential effect modifiers, Nurses' Health Study II, 1996–2011.
| No change in menstrual cyclea | Change in menstrual cyclea | ||
|---|---|---|---|
| n = 119 cases versus 251 controls | n = 147 versus 167 | ||
| Coefficient (SE)b | Coefficient (SE)b | P intc | |
| AMH (per 0.10 ng/ml lower) | 0.216 (0.03)d | 0.308 (0.04)d | <0.001 |
| AMH*AMH | 0.0011 (0.0002)d | 0.0036 (0.0007)d | |
| AMH (ng/ml) | OR (95% CI)e | OR (95% CI)e | |
| 2.00 | Ref | Ref | |
| 1.50 | 2.4 (1.9–3.1) | 2.5 (2.0–3.1) | |
| 1.00 | 6.3 (3.9–10) | 7.3 (4.4–12) | |
| 0.50 | 17 (8.1–36) | 26 (11–59) | |
| Never smoker | Ever smoker | ||
| n = 222 vs. 322 | n = 105 vs. 169 | ||
| Coefficient (SE)b | Coefficient (SE)b | Pintc | |
| AMH (per 0.10 ng/ml lower) | 0.199 (0.02)d | 0.386 (0.06)d | 0.006 |
| AMH*AMH | 0.0012 (0.0001)d | 0.0046 (0.0009)d | |
| AMH (ng/ml) | OR (95% CI)e | OR (95% CI)e | |
| 2.00 | Ref | Ref | |
| 1.50 | 2.2 (1.9–2.6) | 3.1 (2.2–4.3) | |
| 1.00 | 5.2 (3.6–7.4) | 12 (5.7–25) | |
| 0.50 | 13 (7.4–22) | 58 (17–197) | |
| BMI < 25 kg/m2 | BMI ≥ 25 kg/m2 | ||
| n = 204 vs. 306 | n = 123 vs. 185 | ||
| Coefficient (SE)b | Coefficient (SE)b | Pintc | |
| AMH (per 0.10 ng/ml lower) | 0.226 (0.03)d | 0.271 (0.04)d | 0.004 |
| AMH*AMH | 0.0012 (0.0002)d | 0.0026 (0.0005)d | |
| AMH (ng/ml) | OR (95% CI)e | OR (95% CI)e | |
| 2.00 | Ref | Ref | |
| 1.50 | 2.5 (2.0–3.1) | 2.5 (1.9–3.1) | |
| 1.00 | 6.7 (4.4–10) | 6.8 (4.1–12) | |
| 0.50 | 19 (9.9–37) | 22 (9.5–50) | |
| No history of infertility | History of infertility | ||
| n = 240 vs. 371 | n = 87 vs. 120 | ||
| Coefficient (SE)b | Coefficient (SE)b | Pintc | |
| AMH (per 0.10 ng/ml lower) | 0.234 (0.02)d | 0.277 (0.05)d | 0.002 |
| AMH*AMH | 0.0012 (0.0002)d | 0.0033 (0.0009)f | |
| AMH (ng/ml) | OR (95% CI)e | OR (95% CI)e | |
| 2.00 | Ref | Ref | |
| 1.50 | 2.6 (2.1–3.1) | 2.2 (1.7–2.9) | |
| 1.00 | 7.2 (4.8–11) | 6.0 (3.3–10.6) | |
| 0.50 | 21 (11–39) | 19 (7.1–49) |
aParticipant report whether menstrual cycle length, regularity and pattern has changed since they were in their 20s; excludes women using exogenous hormones.
bUnconditional logistic regression modeling, adjusted for matching factors: age (months), fasting status, time of day of blood collection, season and analytic batch
cP values for interaction from likelihood ratio test comparing models with and without interaction terms.
dP < 0.001.
eOR and 95% CI for early menopause comparing range of AMH values to the referent of 2.0 ng/ml, based on coefficient estimates for AMH + AMH*AMH.
fP < 0.01.
Results from sensitivity analyses excluding the small number of women using exogenous hormones, women not reporting PCOS, and women with untimed blood samples were all highly consistent with the main analysis (results not shown).
In assessment of predictive ability using ROC curve analyses stratified by age at AMH measurement, the AUCs ranged from 0.68 for women age 35 to 0.93 for women aged 42 (Table IV).
Table IV.
Results of age-specific receiver operating characteristic curve analyses of anti-Müllerian hormone (AMH) for prediction of early menopause, stratified by age at AMH measurementa; area under the curve (AUC)b and AMH (ng/ml), sensitivity (Se), and specificity (Sp) at Youden’s indexc, Nurses’ Health Study 2, 1996–2011.
| Statistics at maximum discriminating cut point (J, Youden’s Index) | ||||||
|---|---|---|---|---|---|---|
| Age | N | Cases | AUC (95% CI) | AMH | Se | Sp |
| 35 | 26 | 11 | 67.9 (51.9, 83.9) | 1.038 | 63.6 | 73.3 |
| 36 | 50 | 22 | 84.4 (75.9, 92.9) | 0.623 | 68.2 | 89.3 |
| 37 | 68 | 27 | 77.0 (64.3, 89.7) | 0.616 | 63.0 | 82.9 |
| 38 | 72 | 32 | 91.2 (87.0, 95.4) | 0.659 | 84.4 | 82.5 |
| 39 | 84 | 31 | 84.7 (76.4, 93.0) | 0.510 | 83.9 | 75.5 |
| 40 | 87 | 32 | 86.0 (78.5, 93.5) | 0.716 | 96.9 | 69.1 |
| 41 | 90 | 36 | 88.6 (82.7, 94.5) | 0.444 | 88.9 | 79.6 |
| 42 | 114 | 46 | 92.7 (89.4, 96.0) | 0.228 | 84.8 | 89.7 |
| 43 | 106 | 40 | 88.0 (81.7, 94.3) | 0.210 | 90.0 | 78.8 |
| 44 | 111 | 45 | 84.7 (76.3, 83.1) | 0.088 | 86.7 | 77.3 |
aAnalysis restricted to 809 women ages 35–44, due to small numbers of participants in age 34 and 34 strata.
b95% confidence interval for AUC also provided; standard error for AUC calculated under normal approximation using the method of Hanley and McNeil (1982).
cYouden’s index calculated as the maximum of Se + Sp.
Discussion
To our knowledge, this is the first prospective study to evaluate whether lower AMH levels are associated with risk of early menopause among healthy women. In analyses adjusting for age, parity, infertility and other factors, we observed strong, significant associations between lower AMH levels and higher risk of natural menopause prior to age 45. Significant associations of AMH and early menopause risk were observed in various subgroups of women, including women with and without a history of infertility, smokers and non-smokers. Furthermore, AMH was strongly related to risk both among women reporting menstrual cycle irregularity and among those reporting regular cycles.
Previous studies of AMH and menopause timing have been unable to address whether AMH is related to risk of early menopause due to small sample sizes, older participant age at baseline AMH measurement, and the relatively low prevalence of early menopause (Depmann et al., 2016). For example, Depmann and colleagues (2016) evaluated AMH and age at menopause among 216 normally cycling women, with mean age 41.6 years at baseline. Over an average of 14.8 years, 81 women reached menopause, 31 were in transition and 43 maintained regular cycles. Results demonstrated that, beginning at the age of 45, AMH levels significantly predicted menopause onset independent of age. Though this finding was consistent with previous studies, the authors note that their results and those from all other analyses conducted to date (Dólleman et al., 2013; Sowers et al., 2008; Broer et al., 2011; Tehrani et al., 2011; Freeman et al., 2012a, 2012b), underscore that AMH currently has ‘limited potential for the prediction of the extreme ages at menopause’ (p. 1585). Furthermore, because prior studies have been unable to evaluate whether AMH predicts risk of early menopause, Depmann and colleagues concluded that there ‘is currently no ground for AMH-based menopause prediction in the day-to-day clinical practice’ (p. 1585). Results from our study directly fill this substantial gap in knowledge regarding AMH’s clinical utility to predict early menopause.
Depmann et al. (2016) demonstrated that AMH levels were most strongly predictive of menopause timing among women aged 20–43 with regular cycles, suggesting that AMH may have more limited utility among women with irregular cycles and other potential markers of declining fertility. In our study, associations of AMH levels and early menopause varied modestly by participant characteristics but were consistently strong and significant regardless of age-related changes in cycle characteristics, infertility history, smoking status and body weight. The persistence of associations across strata further supports the utility of AMH to predict early menopause among healthy women as well as women with other indicators of early fertility decline and higher cardiovascular risk.
Our study has several limitations warranting consideration. Though premenopausal, participants in our analyses were already aged 35–44 years at the time of blood collection. Thus, we were unable to evaluate whether AMH levels in even younger reproductive aged women are associated with early menopause. Similarly, in the NHS2 blood cohort, very few women provided a blood sample and then reported menopause before age 40 (n = 16 cases); our statistical power was thus too low to evaluate risk of premature ovarian insufficiency. Additional prospective studies of young adult women will be necessary to answer these important questions.
Our participants self-reported onset of menopause, which may be a source of misclassification. We attempted to limit the potential impact in several ways. First, at the time of blood collection, all participants provided information on menstrual cycle characteristics and confirmed that they were premenopausal. Second, menopause status was queried prospectively every 2 years between blood collection and the end of follow-up in 2011. This method for assessing menopause timing has been well-validated in a similar prospective study of US nurses (Colditz et al., 1987) Third, in our main analyses, cases were compared to matched controls with menopause at age 47 or later to limit the potential misclassification of cases as controls or vice-versa. As results comparing cases with matched controls were highly similar to those with all controls, potential misclassification of cases as controls appears to have had minimal effects on ORs. Because AMH levels were measured in blood samples collected prior to assessment of menopause timing in our prospective study, we would expect any misclassification of menopause age to attenuate study results rather than exaggerate them.
In summary, we observed strong inverse associations of AMH levels and risk of early natural menopause in our prospective study. Our findings support the utility of AMH as a clinical marker of early menopause risk among healthy women both with and without established risk factors for early reproductive decline.
Supplementary Material
Authors’ roles
Conception and design of study: ERBJ, JEM, BWW; analysis and interpretation of data: ERBJ, JEM, SEH, BAR, ACPS, AHE, AZS, BWW; drafting of manuscript or revising critically for important intellectual content: ERBJ, JEM, SEH, BAR, ACPS, AZS, AHE, BWW; and final approval of version to be published: ERBJ, JEM, SEH, BAR, ACPS, AZS, AHE, BWW.
Funding
This project was supported by UM1CA176726, R01CA67262 and R01HD078517 from the U.S. Department of Health and Human Services, National Institutes of Health.
Conflict of interest
No competing interests declared.
References
- Bleil ME, Gregorich SE, McConnell D, Rosen MP, Cedars MI. Does accelerated reproductive aging underlie premenopausal risk for cardiovascular disease? Menopause 2013;20:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009;30:465–493. [DOI] [PubMed] [Google Scholar]
- Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC et al. . Anti-Müllerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 2011;96:2532–2539. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–325. [DOI] [PubMed] [Google Scholar]
- Depmann M, Eijkemans MJC, Broer SL, Scheffer GJ, van Rooij IA, Lavens JS, Broekmans FJ. Does anti-Mullerian hormone predict menopause in the general population? Results of a prospective ongoing cohort study. Human Reprod 2016;31:1579–1587. [DOI] [PubMed] [Google Scholar]
- Dólleman M, Faddy MJ, van Disseldorp J, van der Schouw YT, Messow CM, Leader B, Peeters PH, McConnachie A, Nelson SM, Broekmans FJ. The relationship between anti-Mullerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab 2013;98:1946–1953. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of anti-Müllerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril 2012. b;98:1254–9.e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Müllerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 2012. a;97:1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JA, NcNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;148:29–36. [DOI] [PubMed] [Google Scholar]
- Ledger WL. Clinical utility of measurement of anti-Mullerian hormone in reproductive endocrinology. J Clin Endocrinol Metab 2010;95:5144–5154. [DOI] [PubMed] [Google Scholar]
- Muka T, Oliver-Williams C, Kunutsor S, Lavans JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767–777. [DOI] [PubMed] [Google Scholar]
- Panay N, Fenton A. Premature ovarian failure: a growing concern. Climacteric 2008;11:1–3. [DOI] [PubMed] [Google Scholar]
- Pepe MS, Feng Z, Gu JW. Comments on ‘Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al., Statistics in Medicine. (DOI: 10.1002/sim.2929). Stat Med 2008;27:173–181. [DOI] [PubMed] [Google Scholar]
- Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, Cedars MI. A characterization of the relationship of ovarian reserve markers with age. Fertil Steril 2012;97:238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolf JF Jr. Anti-Müllerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 2008;93:3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ. Biomarkers of ovarian reserve as predictors of reproductive potential. Semin Reprod Med 2013;31:437–442. [DOI] [PubMed] [Google Scholar]
- Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum anti-Müllerian hormone concentration. Menopause 2011;18:766–770. [DOI] [PubMed] [Google Scholar]
- Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of anti-Müllerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause 2009;16:797–802. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res 2006;66:2476–2482. [DOI] [PubMed] [Google Scholar]
- van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. Relationship of serum anti-Müllerian hormone concentration to age at menopause. J Clin Endocrinol Metab 2008;93:2129–2134. [DOI] [PubMed] [Google Scholar]
- van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Telde ER. Serum anti-Müllerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 2005;83:979–987. [DOI] [PubMed] [Google Scholar]
- Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012;8:331–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.