Abstract
Angiogenesis in the ovary occurs rapidly as the ovarian follicle transforms into a mature corpus luteum. Granulosa cells produce vascular endothelial growth factor A (VEGFA) in response to the ovulatory gonadotropin surge. VEGFA is established as a key mediator of angiogenesis in the primate ovulatory follicle. To determine if additional VEGF family members may be involved in angiogenesis within the ovulatory follicle, cynomolgus monkeys (Macaca fascicularis) received gonadotropins to stimulate multiple follicular development, and human chorionic gonadotropin (hCG) substituted for the luteinizing hormone surge to initiate ovulatory events. Granulosa cells of monkey ovulatory follicles contained mRNA and protein for VEGFC and VEGFD before and after hCG administration. VEGFC and VEGFD were detected in monkey follicular fluid and granulosa cell-conditioned culture media, suggesting that granulosa cells of ovulatory follicles secrete both VEGFC and VEGFD. To determine if these VEGF family members can stimulate angiogenic events, monkey ovarian microvascular endothelial cells (mOMECs) were obtained from monkey ovulatory follicles and treated in vitro with VEGFC and VEGFD. Angiogenic events are mediated via three VEGF receptors; mOMECs express all three VEGF receptors in vivo and in vitro. Exposure of mOMECs to VEGFC increased phosphorylation of AKT, while VEGFD treatment increased phosphorylation of both AKT and CREB. VEGFC and VEGFD increased mOMEC migration and the formation of endothelial cell sprouts in vitro. However, only VEGFD increased mOMEC proliferation. These findings suggest that VEGFC and VEGFD may work in conjunction with VEGFA to stimulate early events in angiogenesis of the primate ovulatory follicle.
Keywords: endothelial cell, ovulation, macaque, ovary, vascular endothelial growth factor, FIGF
Summary Sentence
VEGFC and VEGFD produced by granulosa cells of the ovulatory follicle may contribute to follicular angiogenesis and ovulation.
Introduction
Angiogenesis is the formation of new blood vessels from existing blood vessels [1]. Similarly, lymphangiogenesis is the formation of new lymphatic vessels from existing lymphatic vessels [2]. Both of these circulatory systems consist of vessels lined with specialized endothelial cells, and formation of new vessels is stimulated by factors such as hypoxia and members of the vascular endothelial growth factor (VEGF) family [3]. Angiogenesis within the ovulatory follicle is underway at the time of ovulation [4,5]. Disruption of angiogenesis by introduction of antiangiogenic agents into the ovulatory follicle can prevent follicle rupture and oocyte release [6–12]. Both angiogenesis and lymphangiogenesis occur during the formation of the corpus luteum, and both vessel systems are essential for normal luteal function [13–17]. However, a role for developing lymphatics during the process of ovulation has not been rigorously addressed.
Members of the VEGF family are key paracrine regulators of both angiogenesis and lymphangiogenesis. VEGF family members interact with VEGF receptors (VEGFRs). VEGFA, acting via FLT1 (also known as VEGFR1) and KDR (also known as VEGFR2), is most often associated with angiogenesis [18]. VEGFC and VEGFD (also known as C-fos induced growth factor (FIGF)), acting via FLT4 (also known as VEGFR3), are often associated with lymphangiogenesis [2]. However, recent studies have demonstrated roles for VEGFC, VEGFD, and FLT4 in angiogenesis [19,20]. Similarly, VEGFC and VEGFD have been reported to act via KDR to promote angiogenesis and lymphangiogenesis [21]. It is becoming clear that vascular endothelial cells and lymphatic endothelial cells may respond via many of the same ligands, receptors, and intracellular signals for development and maintenance of these two distinct vessel systems.
Expression of VEGF family members and VEGFRs has been reported in the ovulatory follicle. VEGFA is produced by granulosa cells of ovulatory follicles, and levels increase soon after exposure to an ovulatory gonadotropin stimulus [22–29]. FLT1 and KDR have been detected in ovulatory follicles, with protein localized to the theca layer [25,27,30]. Analysis of gene array data indicates that the ovulatory gonadotropin surge regulates expression of many angiogenic factors, including both ligands and receptors, in human and monkey ovulatory follicles [31,32]. VEGFC mRNA levels in human luteinized granulosa cells were reduced by gonadotropin exposure in a cell culture model which may be more representative of the function of the corpus luteum than the ovarian follicle [26]. Little else is known regarding expression of VEGFC, VEGFD, and their receptors in the context of the ovulatory follicle or the development of lymphatics as the follicle transitions to become the corpus luteum.
These studies were undertaken to determine if VEGFC and VEGFD are produced by the primate ovulatory follicle. Macaque ovarian tissues and primary endothelial cells of macaque ovarian origin were utilized to determine if VEGFC and VEGFD may play a role in angiogenesis or lymphangiogenesis as the follicle transforms into the corpus luteum.
Methods
Animals
Ovarian cells and tissues were obtained from adult female cynomolgus monkeys (Macaca fascicularis) at Eastern Virginia Medical School (Norfolk, VA). All animal protocols were approved by the Eastern Virginia Medical School Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Briefly, adult females with normal menstrual cycles were maintained as previously described [33]. Blood samples were obtained either consciously or under ketamine chemical restraint (5–10 mg/kg body weight). Serum steroids were determined using the Immulite 1000 immunoassay system (Siemens Medical Diagnostics Solutions, Malvern, PA). Aseptic surgeries were performed by laparotomy or laparoscopy in a dedicated surgical suite with appropriate anesthesia (1%–2% isoflurane gas vaporized in 100% oxygen) and comprehensive monitoring as previously described, except that atropine was omitted [34]. Postoperative pain control was provided by buprenorphine and ketoprofen.
Ovarian cells and tissues
An ovarian stimulation model was used to obtain ovaries with multiple ovulatory follicles [33]. Briefly, monkeys received 90 IU of recombinant human follicle-stimulating hormone (FSH, Merck) for 6–8 days, followed by 90 IU of FSH plus 60 IU of recombinant human luteinizing hormone (LH , Serono) for 2 days to stimulate the growth of multiple follicles. Animals also received a GnRH antagonist (0.5 mg/kg Antide (Serono) or 0.03 mg/kg Ganirelix (Merck)) daily to prevent an endogenous LH surge. Adequate follicular development was monitored by ultrasonography and rising serum estradiol. Follicular aspiration was performed during aseptic surgery before (0 h) or up to 36 h after administration of 1000 IU recombinant human chorionic gonadotropin (r-hCG, Serono). At aspiration, each follicle larger than 4 mm in diameter was pierced with a 22-gauge needle, and the contents of all aspirated follicles were pooled. Whole ovaries were also obtained from monkeys experiencing ovarian stimulation. These ovaries were bisected, maintaining at least two follicles greater than 4 mm in diameter on each piece. Ovarian pieces were either fixed in 10% formalin and embedded in paraffin or frozen in liquid propane and stored at –80°C until sectioned. Additional ovaries obtained 36 h after hCG were used to isolate monkey ovarian microvascular endothelial cells (mOMECs).
Whole ovaries and luteal tissues were also obtained from monkeys experiencing natural menstrual cycles. Serum samples obtained once daily beginning at mid-follicular phase were assayed for estradiol and progesterone. Serum LH (Endocrine Technology Support Core, Oregon National Primate Research Center, Beaverton, OR) was also determined when ovaries were collected ±2 days of ovulation. For luteal tissues, day 1 of the luteal phase is defined as the first day of low serum estradiol following the midcycle estradiol peak; serum progesterone is elevated above 1 ng/ml by luteal day 2 [35]. Ovaries with ovulatory follicles or corpora lutea were fixed in 10% formalin and embedded in paraffin or frozen in liquid propane and stored at –80°C until sectioned.
Monkey granulosa cells
Monkey granulosa cells and oocytes were pelleted from the follicular aspirates by centrifugation at 300 × g. The resulting supernatant (follicular fluid) was stored at –80°C pending analysis. Oocytes were manually removed from the pelleted cells, and a granulosa cell-enriched population of the remaining cells was obtained by the Percoll gradient centrifugation [36]. Granulosa cells were either used for cell culture or frozen in liquid nitrogen and stored at –80°C.
Granulosa cells were plated on fibronectin-coated cultureware in chemically defined, serum-free DMEM-Ham's F12 medium containing supplements including human low-density lipoprotein and treated with hCG (20 IU/ml; Sigma-Aldrich, St. Louis, MO) as previously described [36]. Spent culture media was stored at –20°C.
Monkey ovarian microvascular endothelial cells isolation and culture
Isolation and culture of mOMECs was previously detailed [4]. Briefly, follicles of stimulated ovaries were bisected, and the cells lining the interior of each follicle were collected and subjected to collagenase dispersal. Endothelial cells were enriched using CD31 Dynabeads (Invitrogen, Rockville, MD) following the manufacturer's protocol, plated on fibronectin coated tissue cultureware, and maintained in EGM-2MV (EGM2) media (Lonza). This media is optimized for culture of microvascular endothelial cells and contains serum as well as growth factors including VEGFA, hydrocortisone, fibroblast growth factor 2, insulin-like growth factor 1, and epidermal growth factor. A second Dynabead isolation was performed at the second passage, resulting in a proliferating primary cell population of greater than 95% endothelial cells as previously described [4]. Additional bead isolations were performed every fifth passage. Many experiments were performed using basal media (EMB-2, Lonza, Allendale, NJ), which lacks serum and growth factors listed above. Optimal doses of VEGFA, VEGFC, and VEGFD were determined by treating cells with a range of doses and assessment of migration (assay described below; data not shown). Experiments were performed on passages 4–9.
RNA isolation, amplification, and quantitative PCR
Total RNA was prepared using Trizol reagent (Invitrogen), treated with DNase, and reverse transcribed as previously described [37]. Quantitative PCR (qPCR) was performed using a Roche LightCycler and FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Indianapolis, IN). The strategy for design of species-specific primers and reaction conditions for quantitative detection of cynomolgus macaque mRNAs was previously described [36]. Amplification of monkey cDNA used primers for VEGFC (forward: CCAGCTGTGTGACCGTGC; reverse: GATCATGAGGATCTGCATCCG; Accession# XM_005556349), VEGFD (forward: GAAACATGCGTGGAGG; reverse: GTCGGCAAGCACTTAC; Accession# XM_005593026), and ACTB (forward: ATCCGCAAAGACCTGT; reverse: GTCCGCTAGAAGCAT; Accession# AY765990). PCR products were sequenced to confirm amplicon identity. At least 5 log dilutions of the sequenced PCR product were included in each assay and used to generate a standard curve. No amplification was observed when cDNA was omitted. For each sample, the content of the mRNA of interest and ACTB mRNA was determined in independent assays. All data are expressed as the ratio of mRNA of interest to ACTB.
Western blotting
Preparation of cell lysates and western blotting was performed as previously described [38]. For some experiments, spent media from hCG-stimulated cultures of monkey granulosa cells obtained after ovarian stimulation [39] and follicular fluid from monkey follicular aspirates were concentrated by centrifugation using Amicon centrifugal filter units (Millipore, Billerica, MA) with 100,000 kDa and 10,000 kDa cutoffs, retaining proteins sized 10,000–100,000 kDa in a small volume. Cell lysates, media, and follicular fluid were separated on polyacrylamide Tris-HCl gels (Bio-Rad, Richmond, CA), transferred to a polyvinylidene fluoride membrane (Immobilon; Millipore), and probed using antibodies against VEGFC (1 μg/ml, GeneTex, Irvine, CA), VEGFD (1 μg/ml; GeneTex), AKT (1:1000; Cell Signaling, Danvers, MA), p-AKT (1:1000; Cell Signaling), CREB (1:1000; Cell Signaling), p-CREB (1;1000; Cell Signaling), MAPK3/1 (1:2000; Cell Signaling), p-MAPK3/1 (1:1000; Cell Signaling), PI3Kinase p85 alpha (1:750, Abcam, Cambridge, MA), or pan-actin (1:1000; Millipore). Membranes were incubated with anti-rabbit or anti-mouse AP-conjugated antibody (1:10,000 dilution; Applied Biosystems, Foster City, CA) and protein bands visualized with Tropix CDP-Star (Applied Biosystems).
Immunofluorescent detection of proteins in ovarian tissues and monkey ovarian microvascular endothelial cells
Frozen tissue sections were fixed in 10% formalin for 20 min, and immunodetection proceeded essentially as previously described [4], using anti-human lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1; 10 μg/ml; R&D Systems, Minneapolis, MN) and anti-human von Willebrand Factor (VWF; 7.75 μg/ml; Dako, Glostrup, Denmark); secondary chicken anti-goat (1:1000 dilution; 594 nm) and goat anti-rabbit (1:1000 dilution; 488 nm) antibodies were obtained from Molecular Probes (ThermoFisher, Pittsburgh, PA). Incubation with primary antibodies directed against human VEGFC (5 μg/ml; GeneTex) and VEGFD (5 μg/ml; GeneTex) was followed by visualization with goat anti-rabbit secondary antibody (1:1000 dilution; 488 nm).
Monkey OMECs cultured on chamber slides were fixed in 10% formalin for 20 min, and immunostaining proceeded as previously described [4] using primary antibodies directed against FLT1 (5 μg/ml; R&D systems), KDR (4 μg/ml; Santa Cruz, Dallas, TX), or FLT4 (1:50 dilution; Millipore), followed by incubation with either chicken anti-goat, goat anti-rabbit, or goat anti-mouse secondary antibody (1:400; Molecular Probes/ThermoFisher; 488 nm). Immunodetection of FLT1, KDR, and FLT4 was also performed on frozen sections of monkey ovaries containing large preovulatory follicles as described for mOMECs. For colocalization of each VEGFR with VWF, incubation with primary and secondary antibodies was performed as described above for detection of a single VEGFR, followed by incubation with a direct-labeled VWF antibody as previously described [4].
All cells and tissue sections were counterstained with DAPI (0.5 μg/ml; ThermoFisher) and coverslipped. Images were obtained using an Olympus BX41 microscope fitted with a DP70 digital camera and associated software. Omission of the primary antibody served as a negative control.
Migration assay
Migration of mOMECs was assessed as previously detailed [4]. Briefly, mOMECs were plated on porous cell culture well inserts (8 μm pore size, Falcon, Corning, NY) in basal media. Media in the well consisted of basal media with or without the addition of recombinant human VEGFA165 (VEGFA; 5 ng/ml; R&D Systems), VEGFC (20 ng/ml; R&D Systems), or VEGFD (20 ng/ml; R&D Systems); addition of basal medium and EGM2 served as negative and positive controls, respectively. Optimal concentrations of VEGF family members were determined in preliminary experiments (Supplemental Figure S1), and findings were similar to published ED50 values for each VEGF family member [40,41]. Cells were incubated for 24 h, and then cells remaining on the inside of the insert were removed with a cotton swab. Optimal incubation time was determined as previously described [4]. The inserts were fixed in 70% ethanol and stained with hematoxylin and eosin. Four areas of each insert were photographed as described above, and the number of migrated cells was counted for each image.
Proliferation assay
Monkey OMECs were grown to 60% confluence, switched to basal medium overnight, then to basal media with the addition of recombinant human VEGFA, VEGFC, or VEGFD as described for migration assay above. The addition of basal medium or EGM2 served as negative or positive controls, respectively. After 24 h, cells were fixed and immunostained using an antibody directed against Ki67 (0.35 μg/ml, Dako) as previously described [4]. Omission of the primary antibody served as a negative control. For each mOMEC line, four images were obtained for each treatment using an Olympus BX41 microscope fitted with a DP70 digital camera and associated software. Positive and negative nuclei were counted; the percentage of Ki67 positive nuclei was determined for each treatment.
Sprouting assay
Cells were added to Cytodex microcarrier beads (500–1000 cells per bead; GE Healthcare, Little Chalfont, UK) and embedded in a fibrin matrix as previously described [4,42]. Basal media with or without the addition of VEGFA, VEGFC, or VEGFD as described for migration assay above was added on top of matrixes; EGM2 media was used as a positive control. For each well, five areas were photographed 24 h after initiation of cultures using an Olympus CK40 microscope, Infinity Lite camera, and associated software (Lumenera, Nepean, ON, Canada). Beads with sprouts that were entirely within the frame were used to generate sprout counts and measurements. The number of sprouts per bead was determined, and an average number of sprouts/bead was determined for each treatment group.
Data analysis
Data were assessed for heterogeneity of variance by the Bartlett test. Data were log or square root transformed when the Bartlett test yielded P < 0.05; transformed data were subjected to the Bartlett test to confirm that P > 0.05. All data sets were assessed by ANOVA (without or with repeated measures), followed by the Duncan multiple range test (StatPak version 4.12 software; Northwest Analytical, Portland, OR). Significance was assumed at P < 0.05. Data are expressed as mean ± SEM.
Results
Vascular endothelial growth factor C production by monkey granulosa cells
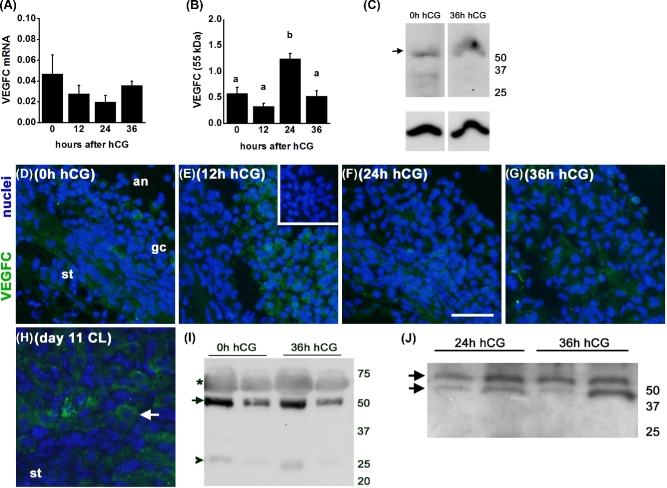
VEGFC mRNA and protein were expressed by granulosa cells obtained from monkey ovulatory follicles before (0 h) and 12, 24, or 36 h after administration of an ovulatory dose of hCG. VEGFC mRNA was detected in all samples examined, and mRNA levels were not altered by exposure to hCG in vivo (Figure 1A). VEGFC protein was detected as multiple bands by western blot, with the largest and most prominent size protein seen at 55 kDa and a faint band inconsistently observed at 25 kDa. Granulosa cell content of the 55 kDa form of VEGFC was low 0–12 h after hCG, peaked 24 h after hCG, and was low again 36 h after hCG; other bands were inconsistently detected (Figure 1B and C).
Figure 1.
VEGFC production and release by monkey granulosa cells. (A) VEGFC mRNA is expressed by granulosa cells before (0 h) and at 12, 24, and 36 h after administration of an ovulatory dose of hCG. VEGFC mRNA levels were expressed relative to ACTB mRNA; n = 3–5 animals/group. ANOVA showed no differences between groups. (B) VEGFC 55 kDa protein (relative to pan-actin) in lysates of granulosa cells collected before and after hCG were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05; n = 3–4 animals/group. (C) Representative VEGFC (top) and pan-actin (bottom) detection in lysates of granulosa cells obtained after ovarian stimulation and before (0 h) or 36 h after hCG administration. VEGFC is detected as a major band at 55 kDa, with inconsistent detection of smaller size proteins; pan-actin was detected as a single band. (D–G) VEGFC immunodetection (green) in granulosa cells (gc) of ovarian tissues obtained before (D, 0 h) and 12 (E), 24 (F), and 36 (G) h after hCG. (H) VEGFC immunodetection (arrow) in large luteal cells of a day 11 corpus luteum (CL) served as a positive control. For panels D–G, images are oriented with stroma (st) in lower left and antrum (an) in upper right. Nuclei are blue. All images are representative of n = 3–4 animals/group. All images are at same magnification; bar in image F = 20 μm. (I) VEGFC detection in follicular fluid obtained 0 and 36 h after hCG administration as 55 kDa (arrow) and 25 kDa (arrowhead) forms; faint, broad band at about 65 kDa is likely albumin (asterisk). Representative of n = 3 animals/group. (J) VEGFC detection in spent media from cultures of monkey granulosa cells obtained 24 and 36 h after hCG administration in vivo. VEGFC resolved as a doublet at approximately 50–55 kDa (arrows); representative of n = 3 animals/group. For panels C, I, and J, positions of molecular weight standards (kDa) are shown at right.
VEGFC protein was detected by immunofluorescence in granulosa cells of ovulatory follicles obtained both before and after exposure to hCG (Figure 1D–G). Staining was also occasionally observed in the stroma surrounding ovulatory follicles, which contains theca cells, fibroblasts, and other cell types along with connective tissue. VEGFC staining in large luteal cells of the monkey corpus luteum served as a positive control; luteal stroma was essentially devoid of staining (Figure 1H).
VEGFC protein is released by granulosa cells. VEGFC protein was detected in monkey follicular fluid, with a strong band observed at 55 kDa and a very faint band present at 25 kDa (Figure 1I). There were no apparent differences in the sizes or quantity of VEGFC proteins in follicular fluid obtained before (0 h) and 36 h after hCG administration. VEGFC was also detected as a doublet at 50–55 kDa in spent media from cultures of monkey granulosa cells, again with no apparent difference between granulosa cells collected 24 and 36 h after hCG administration (Figure 1J).
Vascular endothelial growth factor D production by monkey granulosa cells
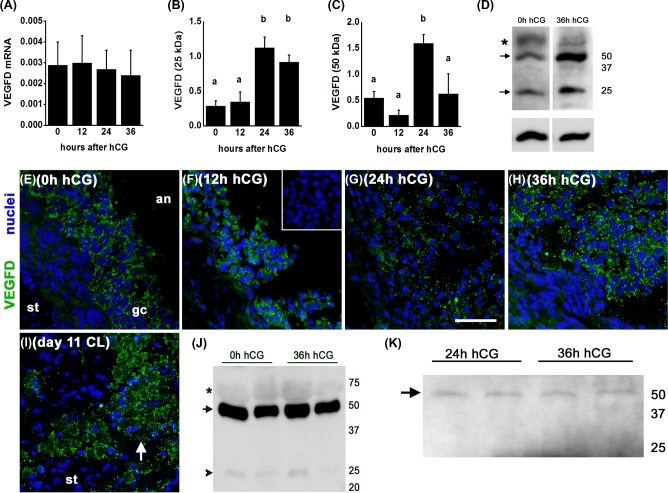
VEGFD mRNA and protein were also expressed by granulosa cells obtained from monkey ovulatory follicles before (0 h) and 12, 24, or 36 h after administration of an ovulatory dose of hCG. VEGFD mRNA was detected in all samples examined, and mRNA levels were not altered by exposure to hCG in vivo (Figure 2A). VEGFD was detected as multiple bands by western blot, with the most prominent proteins seen at 50 and 25 kDa. Levels of the 25 kDa form of VEGFD were low 0–12 h after hCG, peaked 24 h after hCG, and remained elevated 36 h after hCG administration (Figure 2B and D). Levels of the 50 kDa form of VEGFD were low 0–12 h after hCG, peaked 24 h after hCG, and were low again 36 h after hCG administration (Figure 2C and D).
Figure 2.
VEGFD production and release by monkey granulosa cells. (A) VEGFD mRNA is expressed by granulosa cells before (0 h) and at 12, 24, and 36 h after administration of an ovulatory dose of hCG. VEGFD mRNA levels were expressed relative to ACTB mRNA; n = 4–5 animals/group. ANOVA showed no differences between groups. (B and C) VEGFD 25 and 50 kDa proteins (relative to pan-actin) in lysates of granulosa cells collected before and after hCG were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05; n = 3–4 animals/group. (D) Representative VEGFD (top) and pan-actin (bottom) detection in lysates of granulosa cells obtained after ovarian stimulation and before (0 h) or 36 h after hCG administration. VEGFD is detected as a band at 25 and 55 kDa (arrows), with broad band at 65 kDa likely representing albumen; pan-actin was detected as a single band. (E and H) VEGFD immunodetection (green) in granulosa cells (gc) of ovarian tissues obtained before (E, 0 h) and 12 (F), 24 (G), and 36 (H) h after hCG. (I) VEGFD immunodetection in a day 11 corpus luteum (CL) served as a positive control. For panels E–H, images are oriented with stroma (st) in lower left and antrum (an) in upper right. Nuclei are blue. All images are representative of n = 4–5 animals/group. All images are at same magnification; bar in panel G = 20 μm. (J) VEGFD detection in follicular fluid obtained before (0 h) and 36 h after hCG administration as 50 kDa (arrow) and 25 kDa (arrowhead) forms; faint, broad band at about 65 kDa is likely albumin (asterisk). Representative of n = 3 animals/group. (K) VEGFD detection in spent media from cultures of monkey granulosa cells obtained 24 and 36 h after hCG administration in vivo. VEGFD resolved as a single band of 50 kDa (arrow), representative of n = 3 animals/group. For panels D, J, and K, positions of molecular weight standards (kDa) are shown at right.
VEGFD protein was detected by immunofluorescence in granulosa cells of ovulatory follicles obtained 0–36 h after hCG; VEGFD detection appeared to be restricted to the cytoplasm and was punctate in appearance (Figure 2E–H). Staining was not observed in the stroma surrounding ovulatory follicles. VEGFD staining in the monkey corpus luteum served as a positive control; luteal stroma was essentially devoid of staining (Figure 2I).
VEGFD protein is released by granulosa cells. VEGFD protein was detected in monkey follicular fluid, with a strong band observed at 50 kDa and a faint band present at 25 kDa (Figure 2J). There were no apparent differences in the sizes or quantity of VEGFD proteins in follicular fluid obtained before (0 h) and 36 h after hCG administration. VEGFD (50 kDa) was also detected in spent media from cultures of monkey granulosa cells, with no apparent difference between granulosa cells collected 24 and 36 h after hCG administration (Figure 2K).
Von Willebrand Factor and LYVE1 localization in monkey ovulatory follicles
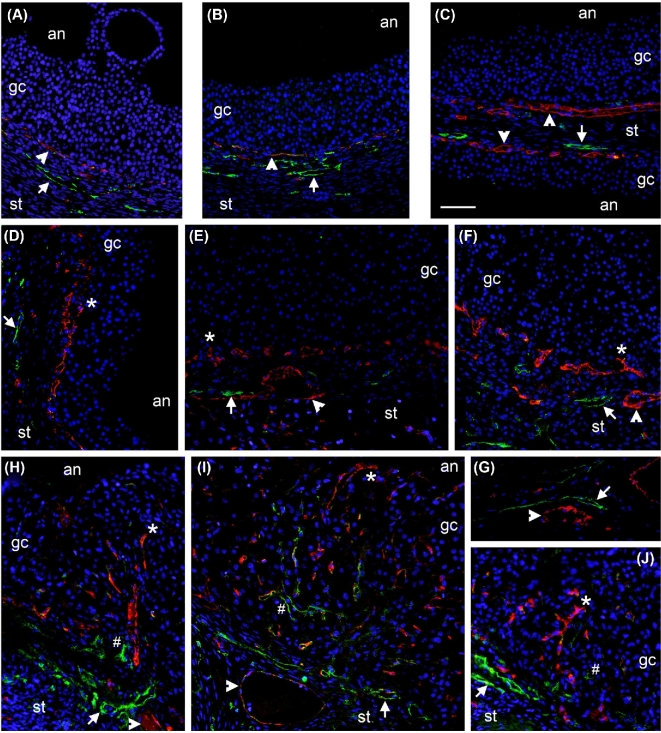
Previous studies by our laboratory demonstrated that angiogenesis of the monkey ovulatory follicle is initiated by the ovulatory gonadotropin surge, with vascular endothelial cells present among the granulosa cells of ovulatory follicles 24–36 h after the gonadotropin surge and prior to ovulation [4]. To determine if lymphangiogenesis is also stimulated in response to the ovulatory gonadotropin surge, ovarian tissues were dual immunostained for the vascular endothelial cell protein VWF and the lymphatic endothelial cell protein LYVE1. Consistent with our previous report, vascular endothelial cells were observed in the perifollicular stroma but not within the granulosa cell layer of the ovulatory follicle before administration of an ovulatory dose of hCG (Figure 3A–C). Ovulatory follicles obtained 36 h after hCG contained VWF+ cells among the granulosa cells (Figure 3D–F). LYVE1 immunodetection was also performed using these ovarian tissues. Before hCG, LYVE1 immunodetection was restricted to the ovarian stroma (Figure 3A–C). LYVE1 immunodetection remained restricted to the ovarian stroma and was never observed within the granulosa cell of follicles collected 36 h after hCG, or just before the expected time of ovulation (Figure 3D–F). In ovaries collected during natural menstrual cycles after ovulation, both VWF and LYVE1 immunostaining were evident within the luteinizing granulosa cell layer (Figure 3H–J). Overall within the granulosa cell layer, LYVE1 staining was observed much less frequently than was VWF staining, and LYVE1 staining was only observed in areas where VWF staining was also observed. In areas where both VWF and LYVE1 staining were observed, VWF+ cells were consistently located closer to the antrum of the follicle when compared to the location of LYVE1+ cells. Immunodetection of VWF and LYVE1 in the medullary region of the ovary showed the presence of vessels lined with vascular endothelial cells (VWF+) or lymphatic endothelial cells (LYVE1+) (Figure 3G). Individual cells showing staining for both VWF and LYVE1 were very rare.
Figure 3.
Angiogenesis, but not lymphangiogenesis, precedes ovulation in primate follicles. Immunofluorescent detection of vascular endothelial cells (VWF, red), lymphatic endothelial cells (LYVE1, green), and cell nuclei (DAPI, blue) was performed in monkey ovarian tissues collected before hCG (A–C, 0 h hCG) or 36 h after hCG (D–F) but prior to ovulation. Image in panel C shows two adjacent follicles with intervening stroma (st). Ovaries were also collected during natural menstrual cycles 2 days after peak serum LH and visual observation of an ovulatory stigmata at the time of collection (H–J). In all panels, arrowheads indicate VWF+ cells in the perifollicular stroma, arrows indicate LYVE1+ cells in the perifollicular stroma, asterisks (*) indicate VWF+ cells among granulosa/granulosa-lutein cells, and hash signs (#) indicate LYVE1+ cells among granulosa/granulosa-lutein cells. (G) Detection of vascular endothelial cells (VWF, red, arrowhead) and lymphatic endothelial cells (LYVE1, green, arrow) in large vessels of the ovarian medulla served as a positive control. Ovarian stroma (st), granulosa/granulosa-lutein cells (gc), and follicle antrum (an) are indicated. Images are representative of n = 3–4 animals/group and are at the same magnification; bar in panel C = 50 μm.
Vascular endothelial growth factors C and D activate vascular endothelial growth factor receptor signaling in monkey ovarian microvascular endothelial cells
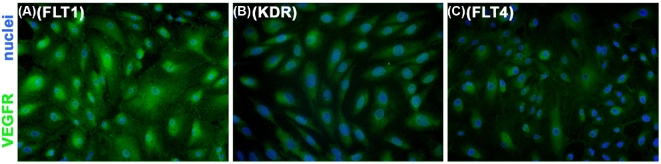
To determine if VEGFC and VEGFD act directly at ovarian endothelial cells, proliferating primary cultures of mOMECs [4] were immunostained for FLT1, KDR, and FLT4 (Figure 4A–C). All three VEGFRs were detected. VEGFR proteins appeared to be located in the cytoplasm and/or plasma membrane and not in the nucleus, consistent with typical location of these receptors. Immunofluorescent detection of FLT1, KDR, and FLT4 also colocalized with immunodetection of VWF in sections of monkey ovaries containing large ovulatory follicles (Supplemental Figure S2), demonstrating that follicular endothelial cells express FLT1, KDR, and FLT4 in vivo and in vitro.
Figure 4.
Monkey OMECs express VEGFRs in vitro. Immunodetection of FLT1 (A), KDR (B), and FLT4 (C) in cultured mOMECs shows each VEGFR in green; nuclei are blue. Representative of four mOMEC lines.
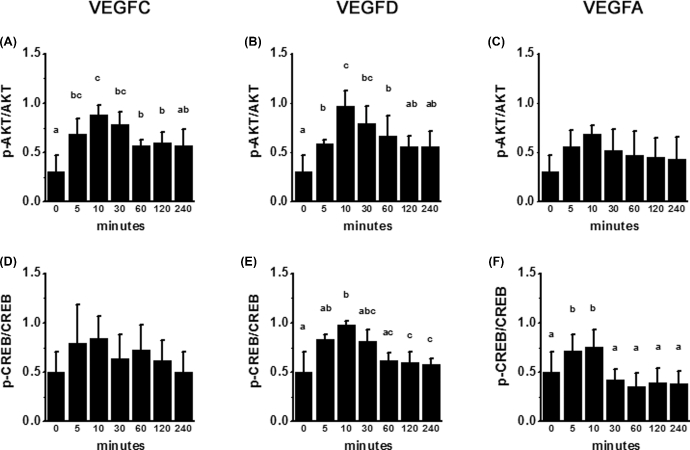
To further examine VEGFR-mediated signaling in mOMECs, cultures were treated with either VEGFC or VEGFD for times ranging from 5 min to 4 h. Cells were then harvested for the assessment of phosphorylation of key signal transduction mediators previously reported to be regulated by VEGFR activation (reviewed in [18]). Treatment with VEGFC or VEGFD resulted in a transient increase in phosphorylation of AKT (Figure 5A and B). Treatment with VEGFD, but not VEGFC, resulted in a transient increase in CREB phosphorylation (Figure 5D and E). In these experiments, treatment of additional cultures with VEGFA increased phosphorylation of CREB but not AKT and served as a positive control (Figure 5C and F). Treatment with VEGFC, VEGFD, or VEGFA did not significantly alter phosphorylation of MAPK3/1; these treatments also did not alter total PI3Kp85 protein (Supplemental Figure S3).
Figure 5.
VEGFR activation via VEGFC and VEGFD. Monkey OMECs were treated in culture for 5–240 min with VEGFC (A, D), VEGFD (B, E), or VEGFA (C, F); cells were harvested for western blot detection of total AKT, p-AKT, total CREB, p-CREB, and pan-actin. Ratios of phosphorylated to total protein for p-AKT/AKT (A–C) and p-CREB/CREB (D–F) were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05, n = 3 mOMEC lines/group.
Vascular endothelial growth factors C and D stimulate angiogenic events
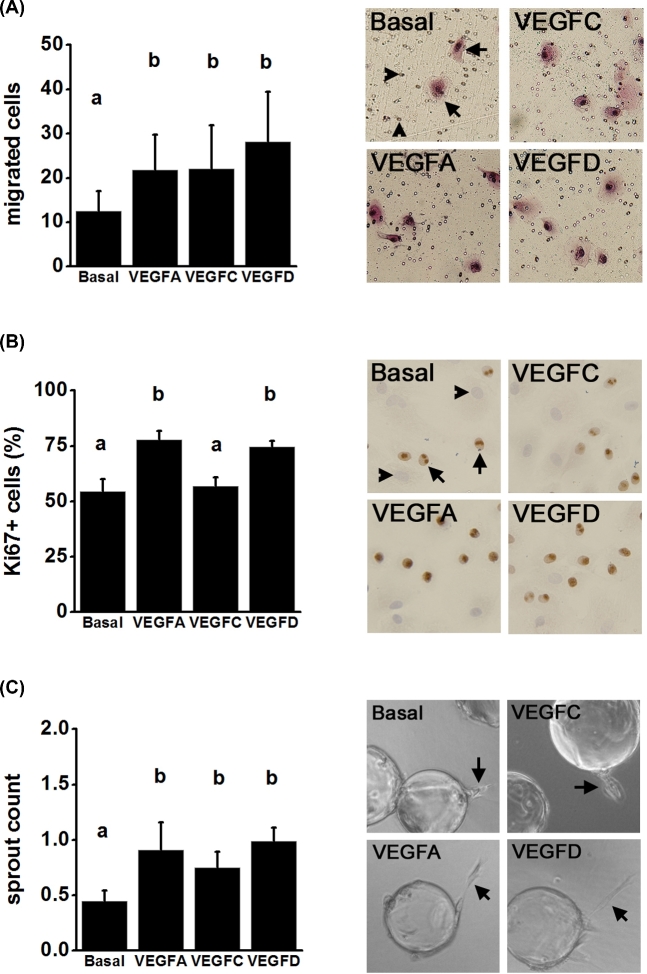
The earliest events in the formation of a new capillary include endothelial cell migration, proliferation, and formation of capillary sprouts [1]. VEGFC and VEGFD each stimulated mOMEC migration in vitro (Figure 6A). VEGFD, but not VEGFC, increased mOMEC proliferation above the levels seen in basal cultures (Figure 6B). Finally, mOMECs were treated with VEGFC or VEGFD in an in vitro model of endothelial cell sprout formation. After 1 day in vitro, cultures treated with VEGFD had a higher number of sprouts when compared to cultures treated with basal medium; VEGFC did not increase sprout formation (Supplemental Figure S4). Cultures treated with VEGFC or VEGFD for 2 days each had a higher number of sprouts than were seen in cultures treated with basal medium only (Figure 6C). VEGFA stimulated migration, proliferation, and sprout formation as anticipated and served as a positive control in these experiments (Figure 6A–C).
Figure 6.
VEGF family members promote angiogenic events in vitro. (A) Migration of mOMECs through a porous membrane in response to treatment for 24 h with basal medium alone or with the addition of VEGFA, VEGFC, or VEGFD. Representative images of migrated cells after each treatment. In basal image, arrows indicate migrated cells (pink); arrowheads indicate pores. (B) Proliferation of mOMECs as assessed by Ki67 immunodetection and expressed as percentage of total cells which were Ki67+. Representative images for each treatment are shown; examples of nuclei which are Ki67+ (brown, arrows) and Ki76– (blue, arrowheads) are indicated in basal image. (C) Sprout formation by mOMECs after culture with each treatment for 2 days. Representative images show beads with mOMECs forming sprouts (arrows) on day 2 in vitro for each treatment. For each graph, data were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05; n = 4 mOMEC lines/group.
Discussion
VEGFC and VEGFD are often associated with lymphangiogenesis [2], but these VEGF family members have been implicated in angiogenesis as well [1]. We have previously shown that vascular endothelial cells enter the granulosa cell layer of monkey and human ovulatory follicles prior to ovulation [4,5]. In this study, lymphatic endothelial cells were not present in the luteinizing granulosa cell layer prior to follicle rupture and oocyte release. In contrast, both vascular endothelial cells and lymphatic endothelial cells were present in the young primate corpus luteum ([16] and this study). While both angiogenesis and lymphangiogenesis are regulated by many of the same signaling pathways and can occur concurrently, angiogenesis has been reported to proceed in advance of lymphangiogenesis [43]. Our findings support the conclusion that angiogenesis is underway before ovulation in the primate follicle, while lymphatic vessels remain relatively quiescent in the ovarian stroma until after ovulation. Therefore, experiments were undertaken to determine if VEGFC and VEGFD may be important for the formation of new vascular capillary networks within the ovulatory follicle.
Both VEGFC and VEGFD are produced and secreted by cells of the primate ovulatory follicle. VEGFC and VEGFD mRNA and protein were expressed by monkey granulosa cells before and after hCG administration. Immunostaining did not consistently detect VEGFC and VEGFD in other follicular cell types, although VEGFC may be present in stroma surrounding ovulatory follicles. VEGFC and VEGFD protein levels in granulosa cells increased after the ovulatory gonadotropin stimulus and reached peak levels prior to ovulation. These proteins were also detected in spent granulosa cell culture media and follicular fluid obtained during the ovulatory interval, indicating that granulosa cells secrete VEGFC and VEGFD consistent with a role in ovulatory events. VEGFC mRNA and protein have previously been detected in human and monkey granulosa luteal cells [16,26]. The present report is the first to demonstrate VEGFC and VEGFD production by follicular granulosa cells of any mammalian species. We also report for the first time that VEGFC and VEGFD production by the follicle may be responsive to the ovulatory gonadotropin surge.
Detection of multiple sizes of VEGFC and VEGFD proteins in monkey ovulatory follicles is consistent with current understanding of the processing of these proteins in mammalian cells. Monkey granulosa cells, follicular fluid, and spent cell culture media contained primarily larger forms of VEGFC (55 kDa) and VEGFD (50 kDa); smaller forms (25 kDa) of each protein were also occasionally present. Multiple forms of VEGFC and VEGFD are produced by posttranslational processing. This is notably different from the production of multiple forms of VEGFA which arise from differential mRNA splicing [44]. VEGFC and VEGFD are each synthesized as a single preproprotein containing an N-terminal propeptide, a VEGF homology domain, and a cysteine-rich C-terminal propeptide [45,46]. These preproproteins have a predicted molecular mass of 50–60 kDa; further processing yields smaller (20–30 kDa) forms. Monomer (20–30 kDa), dimer (45–60 kDa), and preproprotein (50–60 kDa) forms can be secreted; preproprotein forms can be processed into monomers after secretion through the activity of plasmin and other proteases present in the ovulatory follicle [45–47]. In this study, all proteins were resolved with reducing gels, so dimers would not be detected. Smaller protein forms of VEGFC and VEGFD contain the VEGF homology domain, which interacts with VEGFRs. In the monkey follicle, larger forms of VEGFC and VEGFD predominated in granulosa cells, follicular fluid, and cell culture media. Additional processing may take place in the extracellular space near endothelial cells to convert larger forms of VEGFC and VEGFD to smaller forms that are more potent ligands for VEGFRs and lead to phosphorylation of the tyrosine kinase component of the receptor's intracellular domain [45,48,49].
Endothelial cells of monkey ovulatory follicles expressed all three VEGFRs both in vivo and in vitro. In mOMECs, VEGFC treatment led to phosphorylation of AKT, while VEGFD treatment led to phosphorylation of both CREB and AKT. Interestingly, VEGFA activated only CREB. VEGFC and VEGFD have previously been shown to activate both KDR and FLT4 in cell lines transfected with these receptors [45,48]. While VEGFA activates FLT1 and KDR but not FLT4, activation of FLT1 by VEGFC or VEGFD is unlikely [49]. KDR is commonly referred to as the universal VEGFR since many aspects of angiogenesis can be mediated via this receptor and its downstream signaling pathways [1]. Signaling pathways activated by ligand binding to KDR include AKT, CREB, and MAPK [49,50]. In contrast, FLT4 is most often linked to the AKT signaling pathway [49]. In mOMECs, the activation of CREB by VEGFD and VEGFA suggests that this signaling pathway is mediated via KDR; VEGFC did not appear to activate KDR under the conditions used for these studies. The activation of AKT by VEGFC and VEGFD (but not VEGFA) indicates that AKT phosphorylation may be simulated via FLT4 in mOMECs. Interestingly, no VEGF family member increased MAPK3/1 phosphorylation in mOMECs. MAPK-mediated signaling has been associated with the activation of both KDR and FLT4 in embryonic angiogenesis [49]. The ability of VEGFD to activate multiple signal transduction pathways likely explains why VEGFD was more effective than VEGFC to regulate angiogenic events in vitro in our studies.
Both VEGFC and VEGFD-stimulated events were associated with angiogenesis in vitro. VEGFD promoted mOMEC migration, proliferation, and sprout formation. VEGFC increased migration and had a modest but significant effect on sprout formation; VEGFC did not stimulate mOMEC proliferation. Previous studies using vascular endothelial cells from other origins have shown that VEGFC effectively promotes migration but requires very high concentrations to stimulate cell proliferation [45,51]. VEGFC has also been previously shown to induce new sprouts from established vessels [52,53]. VEGFD has previously been shown to increase proliferation, migration, and capillary-like sprouting at the concentrations used in this study [40,48]. Overall, our findings are consistent with the concept that VEGFD is able to stimulate many aspects of angiogenesis via the activation of both KDR and FLT4, while VEGFC action through FLT4 promotes some, but not all, aspects of angiogenesis.
VEGFC and VEGFD may be important paracrine mediators of angiogenesis in the primate ovulatory follicle and may complement the established role for VEGFA in follicular angiogenesis. The studies presented here suggest that VEGFD may be a more potent stimulator of follicular angiogenesis than VEGFC. One unanswered question is whether VEGFC and VEGFD are present in the follicle at sufficient quantities to impact angiogenesis associated with ovulation. In this study, higher concentrations of VEGFC and VEGFD (20 ng/ml) were utilized to stimulate endothelial cell functions when compared to VEGFA (5 ng/ml). Quantitative assays for monkey VEGFC and VEGFD are not currently available. In addition, bioavailability of VEGF family members may be modulated in vivo by binding to extracellular matrix or to soluble forms of VEGFRs [54–56]. Cells involved in active angiogenesis and lymphangiogenesis may compete for ligands and further reduce the local availability of VEGF family members [43]. In summary, it would be difficult, if not impossible, to accurately quantify the pool of bioactive VEGF family members available to interact with VEGFRs on endothelial cells in a specific region of the follicle. The presence of additional vascular growth regulators and dimerization of VEGFRs with each other or coreceptors adds additional levels of complexity [1]. Targeted studies will be needed to determine if VEGFC and VEGFD support VEGFA and other regulators of angiogenesis to stimulate new blood vessel formation during the process of primate ovulation. Finally, these studies do not discount a role for VEGFC and VEGFD produced by follicular cells to support the development of lymphatics, which are essential to the proper function of the corpus luteum.
Supplementary Material
Supplementary data are available at BIOLRE online.
Supplemental Figure 1. Dose-ranging test for VEGF family members in mOMEC migration. Numbers of migrated cells in vitro in response to VEGFA (0.1–100 ng/ml), VEGFC (2–200 ng/ml), and VEGFD (2–200 ng/ml) were determined. For each treatment, migrated cells are expressed relative to migrated cells in response to basal media (set equal to 1.0 and indicated by horizontal line).
Supplemental Figure 2. Ovarian endothelial cells express VEGFRs in vivo. Immunodetection of VWF (A, C, E; orange) colocalizes with detection of a single VEGFR (FLT1 (B), KDR (D), FLT4 (F); green) in ovarian tissue obtained after 36 h hCG. For each VEGFR, dual staining was performed with VWF, and the tissue was photographed separately for visualization of VWF and the individual VEGFR. Arrows indicate endothelial cells branching into granulosa cell (gc) layer. st = ovarian stroma. Representative of n = 3.
Supplemental Figure 3. Monkey OMEC MAPK3/1 phosphorylation and PI3Kp85 levels are not altered by treatment with VEGFC or VEGFD in vitro. Pan-actin detection confirmed similar loading. Representative of n = 3 mOMEC lines.
Supplemental Figure 4. Sprouting by mOMECs in vitro is increased by VEGFD on day 1 of culture. Treatment with VEGFC did not increase sprout number on day 1; VEGFA increased sprout formation and served as a positive control. Data were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05; n = 4 mOMEC lines/group.
Supplemental Table S1. Source and dilution of antibodies used.
Acknowledgments
The authors would like to thank Ms. Sarah Pisani for technical assistance. Recombinant human FSH and Ganirelix were generously provided by Merck & Co., INC, (Kenilworth, NJ). Serono Reproductive Biology Institute (Rockland, MA) kindly provided recombinant human LH and Antide.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplemental Figure 1. Dose-ranging test for VEGF family members in mOMEC migration. Numbers of migrated cells in vitro in response to VEGFA (0.1–100 ng/ml), VEGFC (2–200 ng/ml), and VEGFD (2–200 ng/ml) were determined. For each treatment, migrated cells are expressed relative to migrated cells in response to basal media (set equal to 1.0 and indicated by horizontal line).
Supplemental Figure 2. Ovarian endothelial cells express VEGFRs in vivo. Immunodetection of VWF (A, C, E; orange) colocalizes with detection of a single VEGFR (FLT1 (B), KDR (D), FLT4 (F); green) in ovarian tissue obtained after 36 h hCG. For each VEGFR, dual staining was performed with VWF, and the tissue was photographed separately for visualization of VWF and the individual VEGFR. Arrows indicate endothelial cells branching into granulosa cell (gc) layer. st = ovarian stroma. Representative of n = 3.
Supplemental Figure 3. Monkey OMEC MAPK3/1 phosphorylation and PI3Kp85 levels are not altered by treatment with VEGFC or VEGFD in vitro. Pan-actin detection confirmed similar loading. Representative of n = 3 mOMEC lines.
Supplemental Figure 4. Sprouting by mOMECs in vitro is increased by VEGFD on day 1 of culture. Treatment with VEGFC did not increase sprout number on day 1; VEGFA increased sprout formation and served as a positive control. Data were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05; n = 4 mOMEC lines/group.
Supplemental Table S1. Source and dilution of antibodies used.
References
- 1. Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev, 2011, 12:551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Secker GA, Harvey NL. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev Dyn, 2015, 244:323–331. [DOI] [PubMed] [Google Scholar]
- 3. Morfoisse F, Renaud E, Hantelys F, Prats AC, Garmy-Susini B. Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis. Mol Cell Oncol, 2015, 2:e1024821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trau HA, Davis JS, Duffy DM. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod, 2015, 92:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trau HA, Brannstrom M, Curry TEJ, Duffy DM. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum Reprod, 2016, 31:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor trap R1R2. Endocrinology, 2002, 143:2797–2807. [DOI] [PubMed] [Google Scholar]
- 7. Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod, 2002, 67:1305–1312. [DOI] [PubMed] [Google Scholar]
- 8. Fraser HM, Hastings JM, Allen D, Morris KD, Rudge JS, Wiegand SJ. Inhibition of delta-like ligand 4 induces luteal hypervascularization followed by functional and structural luteolysis in the primate ovary. Endocrinology, 2012, 153:1972–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hazzard TM, Rohan RM, Molskness TA, Fanton JW, D’Amato RJ, Stouffer RL. Injection of anti-angiogenic agents into the macaque preovulatory follicle: disruption of corpus luteum development and function. Endocrine, 2002, 17:199–206. [DOI] [PubMed] [Google Scholar]
- 10. Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception, 2005, 71:239–248. [DOI] [PubMed] [Google Scholar]
- 11. Garside SA, Henkin J, Morris KD, Norvell SM, Thomas FH, Fraser HM. A thrombospondin-mimetic peptide, ABT-898, suppresses angiogenesis and promotes follicular atresia in pre- and early-antral follicles in vivo. Endocrinology, 2010, 151:5905–5915. [DOI] [PubMed] [Google Scholar]
- 12. Gomez R, Simon C, Remohi J, Pellicer A. Vascular endothelial growth factor receptor-2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology, 2002, 143:4339–4348. [DOI] [PubMed] [Google Scholar]
- 13. Fraser HM., Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol, 2006, 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown HM, Robker RL, Russell DL. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology, 2010, 151:5446–5455. [DOI] [PubMed] [Google Scholar]
- 15. Berisha B, Schilffarth S, Kenngott R, Sinowatz F, Meyer HH, Schams D. Expression of lymphangiogenic vascular endothelial growth factor family members in bovine corpus luteum. Anat Histol Embryol, 2013, 42:292–303. [DOI] [PubMed] [Google Scholar]
- 16. Xu F, Stouffer RL. Existence of the lymphatic system in the primate corpus luteum. Lymphat Res Biol, 2009, 7:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rutkowski JM, Ihm JE, Lee ST, Kilarski WW, Greenwood VI, Pasquier MC, Quazzola A, Trono D, Hubbell JA, Swartz MA. VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy. Am J Pathol, 2013, 183:1596–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol, 2006, 7:359–371. [DOI] [PubMed] [Google Scholar]
- 19. Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res, 2007, 67:593–599. [DOI] [PubMed] [Google Scholar]
- 20. Anisimov A, Alitalo A, Korpisalo P, Soronen J, Kaijalainen S, Leppanen VM, Jeltsch M, Yla-Herttuala S, Alitalo K. Activated forms of VEGF-C and VEGF-D provide improved vascular function in skeletal muscle. Circ Res, 2009, 104:1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmokel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J, 2007, 21:1003–1012. [DOI] [PubMed] [Google Scholar]
- 22. Christenson LK, Stouffer RL. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J Clin Endocrinol Metab, 1997, 82:2135–2142. [DOI] [PubMed] [Google Scholar]
- 23. Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod, 1999, 5:1115–1121. [DOI] [PubMed] [Google Scholar]
- 24. Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Regulation of vascular endothelial growth factor-A and its soluble receptor sFlt-1 by luteinizing hormone in vivo: implication for ovarian follicle angiogenesis. Fertil Steril, 2008, 89:922–926. [DOI] [PubMed] [Google Scholar]
- 25. Chowdhury MWH, Scaramuzzi RJ, Wheeler-Jones CPD, Khalid M. The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology, 2010, 73:856–872. [DOI] [PubMed] [Google Scholar]
- 26. Laitinen M, Ristimaki A, Honkasalo M, Narko K, Paavonen K, Ritvos O. Differential hormonal regulation of vascular endothelial growth factors VEGF, VEGF-B, and VEGF-C messenger ribonucleic acid levels in cultured human granulosa-luteal cells. Endocrinology, 1997, 138:4748–4756. [DOI] [PubMed] [Google Scholar]
- 27. Berisha B, Steffl M, Welter H, Kliem H, Meyer HH, Schams D, Amselgruber W. Effect of the luteinising hormone surge on regulation of vascular endothelial growth factor and extracellular matrix-degrading proteinases and their inhibitors in bovine follicles. Reprod Fertil Dev, 2008, 20:258–268. [DOI] [PubMed] [Google Scholar]
- 28. Babitha V, Panda RP, Yadav VP, Chouhan VS, Dangi SS, Khan FA, Singh G, Bag S, Taru Sharma G, Silvia WJ, Sarkar M. Amount of mRNA and localization of vascular endothelial growth factor and its receptors in the ovarian follicle during estrous cycle of water buffalo (Bubalus bubalis). Anim Reprod Sci, 2013, 137:163–176. [DOI] [PubMed] [Google Scholar]
- 29. Koos RD., Increased expression of vascular endothelial growth/ permeability factory in the rat ovary following an ovulatory stimulus: potential roles in follicle rupture. Biol Reprod, 1995, 52: 1426–1435. [DOI] [PubMed] [Google Scholar]
- 30. Otani N, Minami S, Yamoto M, Shikone T, Otani H, Nishiyama R, Otani T, Nakano R. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab, 1999, 84:3845–3851. [DOI] [PubMed] [Google Scholar]
- 31. Wissing ML, Kristensen SG, Anderson CY, Mikkelsen AL, Host T, Borup R, Grondahl ML. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod, 2014, 29:997–1010. [DOI] [PubMed] [Google Scholar]
- 32. Xu F, Stouffer RL, Muller J, Hennebold JD, Wright JW, Bahar A, Leder G, Peters M, Thorne M, Sims M, Wintermantel T, Lindenthal B. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod, 2011, 17:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seachord CL, VandeVoort CA, Duffy DM. Adipose-differentiation related protein: a gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod, 2005, 72:1305–1314. [DOI] [PubMed] [Google Scholar]
- 34. Duffy DM, Chaffin CL, Stouffer RL. Expression of estrogen receptor α and β in the rhesus monkey corpus luteum during the menstrual cycle: Regulation by luteinizing hormone and progesterone. Endocrinology, 2000, 141:1711–1717. [DOI] [PubMed] [Google Scholar]
- 35. Duffy DM, Stouffer RL. Progesterone receptor messenger ribonucleic acid in the primate corpus luteum during the menstrual cycle: Possible regulation by progesterone. Endocrinology, 1995, 136:1869–1876. [DOI] [PubMed] [Google Scholar]
- 36. Markosyan N, Dozier BL, Lattanzio FA, Duffy DM. Primate granulosa cell response via prostaglandin E2 receptors increases late in the periovulatory interval. Biol Reprod, 2006, 75:868–876. [DOI] [PubMed] [Google Scholar]
- 37. Duffy DM, Seachord CL, Dozier BL. An ovulatory gonadotropin stimulus increases cytosolic phospholipase A2 (cPLA2) expression and activity in granulosa cells of primate periovulatory follicles. J Clin Endocrinol Metab, 2005, 90:5858–5865. [DOI] [PubMed] [Google Scholar]
- 38. Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: Implications for control of primate ovulation by PGE2. J Clin Endocrinol Metab, 2005, 90:1021–1027. [DOI] [PubMed] [Google Scholar]
- 39. Markosyan N, Duffy DM. Prostaglandin E2 acts via multiple receptors to regulate plasminogen-dependent proteolysis in the primate periovulatory follicle. Endocrinology, 2009, 150:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, Bussolino F, Oliviero S. c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci USA, 1999, 96:9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA, 1998, 95:14389–14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakatsu MN, Davis J, Hughes CC. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp, 2007, 3:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakao S, Zandi S, Hata Y, Kawahara S, Arita R, Schering A, Sun D, Melhorn MI, Ito Y, Lara-Castillo N, Ishibashi T, Hafezi-Moghadam A. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: an endogenous trapping mechanism links lymph- and angiogenesis. Blood, 2011, 117:1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics?, Nat Rev Cancer, 2008, 8:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J, 1997, 16:3898–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stacker SA, Stenvers K, Caesar C, Vitali A, Domagala T, Nice E, Roufail S, Simpson RJ, Moritz R, Karpanen T, Alitalo K, Achen MG. Biosynthesis of vascular endothelial growth factor-D involves proteolytic processing which generates non-covalent homodimers. J Biol Chem, 1999, 274:32127–32136. [DOI] [PubMed] [Google Scholar]
- 47. Curry TE Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med, 2006, 24:228–241. [DOI] [PubMed] [Google Scholar]
- 48. Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA, 1998, 95:548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Claesson-Welsh L., VEGF receptor signal transduction—a brief update. Vascul Pharmacol, 2016, 86:14–17. [DOI] [PubMed] [Google Scholar]
- 50. Kang Z, Zhu H, Luan H, Han F, Jiang W. Curculigoside A induces angiogenesis through VCAM-1/Egr-3/CREB/VEGF signaling pathway. Neuroscience, 2014, 267:232–240. [DOI] [PubMed] [Google Scholar]
- 51. Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu JS, Isner JM. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol, 1998, 153:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA, 1995, 92:3566–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development, 1996, 122:3829–3837. [DOI] [PubMed] [Google Scholar]
- 54. Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev, 2002, 16:2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Singh N, Tiem M, Watkins R, Cho YK, Wang Y, Olsen T, Uehara H, Mamalis C, Luo L, Oakey Z, Ambati BK. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood, 2013, 121:4242–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG, Chappell J, Wilting J et al. , Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med, 2009, 15:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOLRE online.
Supplemental Figure 1. Dose-ranging test for VEGF family members in mOMEC migration. Numbers of migrated cells in vitro in response to VEGFA (0.1–100 ng/ml), VEGFC (2–200 ng/ml), and VEGFD (2–200 ng/ml) were determined. For each treatment, migrated cells are expressed relative to migrated cells in response to basal media (set equal to 1.0 and indicated by horizontal line).
Supplemental Figure 2. Ovarian endothelial cells express VEGFRs in vivo. Immunodetection of VWF (A, C, E; orange) colocalizes with detection of a single VEGFR (FLT1 (B), KDR (D), FLT4 (F); green) in ovarian tissue obtained after 36 h hCG. For each VEGFR, dual staining was performed with VWF, and the tissue was photographed separately for visualization of VWF and the individual VEGFR. Arrows indicate endothelial cells branching into granulosa cell (gc) layer. st = ovarian stroma. Representative of n = 3.
Supplemental Figure 3. Monkey OMEC MAPK3/1 phosphorylation and PI3Kp85 levels are not altered by treatment with VEGFC or VEGFD in vitro. Pan-actin detection confirmed similar loading. Representative of n = 3 mOMEC lines.
Supplemental Figure 4. Sprouting by mOMECs in vitro is increased by VEGFD on day 1 of culture. Treatment with VEGFC did not increase sprout number on day 1; VEGFA increased sprout formation and served as a positive control. Data were assessed by ANOVA and the Duncan test; groups with no common letters are different, P < 0.05; n = 4 mOMEC lines/group.
Supplemental Table S1. Source and dilution of antibodies used.