Abstract
Chronic hypoxia during gestation suppresses large-conductance Ca2+-activated K+ (BKCa) channel function and impedes uterine arterial adaptation to pregnancy. This study tested the hypothesis that chronic hypoxia has a direct effect in upregulating DNA methyltransferase (DNMT) and epigenetically repressing BKCa channel beta-1 subunit (KCNMB1) expression in uterine arteries. Resistance-sized uterine arteries were isolated from near-term pregnant sheep maintained at ∼300 m above sea level or animals acclimatized to high-altitude (3,801 m) hypoxia for 110 days during gestation. For ex vivo hypoxia treatment, uterine arteries from normoxic animals were treated with 21.0% O2 or 10.5% O2 for 48 h. High-altitude hypoxia significantly upregulated DNMT3b expression and enzyme activity in uterine arteries. Similarly, ex vivo hypoxia treatment upregulated DNMT3b expression and enzyme activity that was blocked by a DNMT inhibitor 5-aza-2'-deoxycytidine (5-Aza). Of importance, 5-Aza inhibited hypoxia-induced hypermethylation of specificity protein (SP) 1 binding site at the KCNMB1 promoter and restored transcription factor binding to the KCNMB1 promoter, resulting in the recovery of KCNMB1 gene expression in uterine arteries. Furthermore, 5-Aza blocked the effect of hypoxia and rescued BKCa channel activity and reversed hypoxia-induced decrease in BKCa channel-mediated relaxations and increase in myogenic tone of uterine arteries. Collectively, these results suggest that chronic hypoxia during gestation upregulates DNMT expression and activity, resulting in hypermethylation and repression of KCNMB1 gene and BKCa channel function, impeding uterine arterial adaptation to pregnancy.
Keywords: hypoxia, DNA methylation, BKCa channel, myogenic tone, uterine arteries
Summary Sentence
Gestational hypoxia promotes hypermethylation and repression of KCNMB1 gene and BKCa channel function in uterine arteries by upregulating DNMT expression and activity, leading to maladaptation of uterine circulation during pregnancy.
Introduction
Uterine vasculature undergoes striking changes during pregnancy to accommodate dramatically increased uterine blood flow to ensure adequate growth of the fetus and well-being of the mother [1–3]. Reduced uterine perfusion is associated with pregnancy complications such as preeclampsia and fetal growth restriction [4–6], leading to increased maternal and fetal morbidity and mortality. Chronic hypoxia during gestation has a profound impact on uterine hemodynamics. Pregnancy at high altitude is accompanied by reduced uteroplacental blood flow, which contributes to an increased incidence of preeclampsia and fetal growth restriction [6, 7]. Thus, gestational hypoxia constitutes a notorious insult to maternal cardiovascular well-being and fetal growth.
The large-conductance Ca2+-activated K+ (BKCa) channel plays a key role in regulating membrane potential and vascular tone [8, 9]. The BKCa channel in vascular smooth muscle cells consists of channel-forming alpha subunits and regulatory beta-1 subunits [10], encoded by KCNMA1 and KCNMB1 genes, respectively. The channel is activated by membrane depolarization and/or an increase in intracellular Ca2+ concentrations [9]. The beta-1 subunit enhances the apparent Ca2+ and voltage sensitivity of the alpha subunit [11], leading to increased BKCa channel activity. Gene deletion has demonstrated the functional importance of the BKCa channel in regulating blood pressure, and mice lacking either alpha or beta-1 subunits are hypertensive [12–14]. The BKCa channel is expressed in both human and ovine uterine arteries [15, 16]. Increased expression of the beta-1 subunit, but not the alpha subunit, and enhanced BKCa channel activity in uterine arteries as the result of steroid hormone actions are credited for reduced uterine vascular tone and increased uterine blood flow during pregnancy [17–19]. However, chronic hypoxia during gestation abrogated pregnancy-induced upregulation of the beta-1 subunit in uterine arteries, leading to elevated uterine vascular tone [20, 21].
The mechanisms underlying gestational hypoxia-induced BKCa channel downregulation in uterine arteries remain elusive. Epigenetic regulation of DNA methylation plays an important role in modulating gene expression patterns and in the pathogenesis of cardiovascular diseases [22, 23]. We recently demonstrated that increased KCNMB1 promoter methylation was associated with a reduction in BKCa channel beta-1 subunit expression and elevated uterine vascular tone in pregnant sheep acclimatized to high-altitude hypoxia [20, 21]. While these studies provided evidence of a correlation of in vivo hypoxia and hypermethylation of KCNMB1 promoter in BKCa channel repression and dysfunction in uterine arteries, it remains undetermined whether hypoxia has a direct effect and whether DNA methylation plays a causal role in the repression of the KCNMB1 gene and BKCa channel function. Herein, we present novel evidence that prolonged hypoxia exerts a direct effect in upregulating DNA methyltransferase (DNMT) and hypermethylation of the KCNMB1 promoter, resulting in KCNMB1 gene repression and BKCa channel dysfunction in uterine arteries in pregnant sheep.
Materials and methods
All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. After tissue collection, animals were killed via intravenous injection of 15 mL T-61 solution (Hoechst-Roussel, Somerville, NJ), according to American Veterinary Medical Association guidelines.
Tissue preparation and treatment
Uterine arteries were harvested from near-term (∼142–145 days of gestation) pregnant sheep (Ovis aries) maintained at ∼300 m above sea level (low altitude, PaO2: ∼102 mm Hg) or exposed to high-altitude hypoxia (3,801 m, PaO2: ∼60 mm Hg) for 110 days (starting from 30 days of gestation) [24, 25]. Animals were anesthetized with intravenous injection of propofol (2 mg/kg) followed by intubation, and anesthesia was maintained on 1.5% to 3.0% isoflurane balanced in O2 throughout the surgery. An incision was made in the abdomen, and the uterus was exposed. The fourth-generation branches of main uterine artery were chosen in the study because they are resistance-sized arteries (∼200 μm) with close characteristics to arterioles in contribution to vascular resistance, and they had been extensively investigated in our previous studies [19–21, 24–26]. Uterine arteries were isolated and removed without stretching and placed into a Krebs solution containing (in mM) 130.0 NaCl, 10.0 HEPES, 6.0 glucose, 4.0 KCl, 4.0 NaHCO3, 1.8 CaCl2, 1.2 MgSO4, 1.18 KH2PO4, and 0.025 EDTA (pH 7.4). To determine the direct effect of hypoxia, uterine arteries were treated ex vivo under normoxic (21.0% O2) or hypoxic (10.5% O2) conditions for 48 h, given a ∼50% decrease in arterial PaO2 observed in high-altitude hypoxic sheep. Uterine arteries were placed in a culture dish containing 5 mL of phenol red-free DMEM supplemented with 1% charcoal-stripped fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin and incubated at 37°C in humidified incubators for 48 h with oxygen levels at either 21.0% O2 for normoxic or 10.5% O2 for hypoxic conditions, as described previously [19, 25, 26].
Measurement of BKCa channel current
Arterial smooth muscle cells were enzymatically dissociated as described previously [19, 20]. Briefly, uterine arteries were mince into small pieces in low Ca2+ HEPES-buffered physiological salt solution (PSS) containing (in mM) 140.0 NaCl, 5.0 KCl, 0.1 CaCl2, 1.2 MgCl2, 10.0 HEPES, and 10.0 glucose (pH 7.4). They were then digested by sequentially incubating the tissues in papain solution containing 1.5 mg/mL papain, 1.5 mg/mL dithiothreitol, and 2.0 mg/mL bovine serum albumin (BSA) for 60 min and collagenase IV solution containing 1.5 mg/mL collagenase IV and 2.0 mg/mL BSA for 120 min. Myocytes were then released in low Ca2+ HEPES-PSS. Only relaxed and spindle-shaped smooth muscle cells were used for recording. Whole-cell K+ currents were recorded using an EPC 10 patch-clamp amplifier with Patchmaster software (HEKA, Lambrecht/Pfalz, Germany) at room temperature as previously described [19, 20]. Briefly, cell suspension drops were placed in a recording chamber, and adherent cells were continuously superfused with HEPES-buffered PSS containing 1.8 in mM Ca2+. Micropipettes were pulled from borosilicate glass and had resistances of 2 to 5 megaohm (mΩ) when filled with the pipette solution containing (in mM) 140.0 KCl, 1.0 MgCl2, 5.0 Na2ATP, 5.0 EGTA, and 10.0 HEPES (pH 7.2). CaCl2 was added to bring free Ca2+ concentrations to 100 nM as determined using WinMAXC software (Chris Patton, Stanford University). Cells were held at –50 mV, and whole-cell K+ currents were evoked by voltage steps from −60 mV to +80 mV by stepwise 10-mV depolarizing pulses (350-ms duration, 10-s intervals) in the absence and presence of 1 mM BKCa channel blocker tetraethylammonium [19, 20]. BKCa currents, determined as the difference between whole-cell K+ currents in the absence of tetraethylammonium and that in the presence of tetraethylammonium, were normalized to cell capacitance and expressed as picoampere per picofarad (pA/pF).
Relaxation studies
Uterine arteries were separated from surrounding tissues and cut into 2-mm ring segments. Isometric tension was measured in the Krebs solution in a tissue bath system (Radnoti, Monrovia, CA) at 37°C as described previously [25, 27]. Briefly, each ring segment was equilibrated for 60 min and then gradually stretched to the optimal resting tension determined by responses to 3 × 120 mM KCl challenges. Tissues were then precontracted with submaximal concentrations of serotonin that produced ∼70% to 80% of the maximal contraction, followed by additions of the BKCa channel opener NS1619 in a cumulative manner.
Measurement of pressure-dependent tone
Pressure-dependent tone of resistance-sized uterine arteries was measured as described previously [19, 24, 25]. Briefly, the arterial segments (diameter ∼150 μm) were mounted and pressurized in an organ chamber (Living Systems Instruments, Burlington, VT). The intraluminal pressure was controlled by a servo system to set transmural pressures, and arterial diameter was recorded using the SoftEdge Acquisition Subsystem (IonOptix LLC, Milton, MA). After the equilibration period, the intraluminal pressure was increased in a stepwise manner from 10 to 100 mmHg in 10-mmHg increments, and each pressure was maintained for 5 min to allow vessel diameter to stabilize before the measurement. The passive pressure–diameter relationship was conducted in Ca2+-free PSS containing 3.0 mM of EGTA to determine the maximum passive diameter. The following formula was used to calculate the percentage of pressure-dependent tone at each pressure step: % tone = (D1 − D2)/D1 × 100, where D1 is the passive diameter in Ca2+-free PSS (0 Ca2+ with 3.0 mM of EGTA) and D2 is the active diameter with normal PSS in the presence of extracellular Ca2+.
Measurement of DNA methyltransferase activity
Nuclear extracts were prepared from uterine arteries using the EpiQuik Nuclear Extraction Kit (Epigentek, Farmingdale, NY). DNMT activity assay was performed using a colorimetric EpiQuik DNMT activity assay kit (Epigentek) following the manufacture's instruction, as described previously [28]. Briefly, nuclear extract was incubated with S-adenosylmethionine and a universal proprietary DNMT substrate in the DNMT assay buffer at 37°C for 2 h. The blank contained only S-adenosylmethionine and substrate without nuclear extract, whereas the positive control contained S-adenosylmethionine and substrate with the purified DNMT enzyme preparation containing both maintenance and de novo DNMTs supplied in the kit. After the incubation, the capture antibody and detection antibody were added in sequence, followed by incubation with developing solution for 10 min at room temperature. Signal was measured by a microplate reader at 450 nm.
Real-time reverse transcription polymerase chain reaction
Total RNA was isolated using TRIzol reagent (Invitrogen, CA) and subjected to reverse transcription with iScript cDNA Synthesis system (Bio-Rad, Hercules, CA). The abundance of KCNMB1 mRNA was measured with real-time polymerase chain reaction (PCR) using iQ SYBR Green Supermix (Bio-Rad), as described previously [21]. Primers used were 5΄-CTGTACCACACGGAGGACACT-3΄ (forward) and 5΄-GTAGAGGCGCTGGAATAGGAC-3΄ (reverse). Real-time PCR was performed in a final volume of 25 μL, and each PCR reaction mixture consisted of 500 nM of primers and iQ SYBR Green Supermix containing 0.625 U hot-start Taq polymerase; 400 μM each of dATP, dCTP, dGTP, and dTTP; 100 mM KCl; 16.6 mM ammonium sulfate; 40 mM Tris-HCl; 6 mM MgSO4; SYBR Green I; and 20 nM fluorescing and stabilizers. The following protocol was used for real-time PCR: 95°C for 5 min, followed by 45 cycles of 95°C for 30 s and annealing for 30 s at 52°C and for 45 s at 72°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference, and serial dilutions of the positive control were performed on each plate to create a standard curve for the quantification. PCR was performed in triplicate, and threshold cycle numbers were averaged for each sample.
Western immunoblotting
Protein abundance of the BKCa channel beta-1 subunit and DNMTs in uterine arteries was measured as described previously [19, 20, 28]. Briefly, tissues were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 10 mM EDTA, 0.1% Tween 20, 0.1% beta-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin, pH 7.4 followed by centrifugation at 4°C for 10 min at 10,000 g, and the supernatants were collected. Samples with equal proteins were loaded onto 7.5% polyacrylamide gel with 0.1% sodium dodecyl sulfate and were separated by electrophoresis at 100 V for 2 h. Proteins were then transferred onto nitrocellulose membranes. After blocking nonspecific binding sites by a Tris-buffered saline solution containing 5% dry milk, membranes were incubated with primary antibodies against the BKCa channel beta-1 subunit (Santa Cruz Biotechnology, Santa Cruz, CA), DNMT1 (Cell Signaling Technology, Danvers, MA), DNMT3a (Cell Signaling Technology), and DNMT3b (Novus Biologicals, Littleton, CO). After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to hyperfilm. Results were quantified with the Kodak electrophoresis documentation and analysis system and Kodak ID image analysis software (Kodak, Rochester, NY). The target protein abundance was normalized to the abundance of beta-actin as a protein loading control.
Quantitative methylation-specific polymerase chain reaction
Genomic DNA was isolated from uterine arteries using a GenElute Mammalian Genomic DNA Mini-Prep kit (Sigma-Aldrich, St. Louis, MO), denatured with 2 N NaOH at 42°C for 15 min and treated with sodium bisulfite at 55°C for 16 h, as previously described [21]. DNA was purified with a Wizard DNA clean-up system (Promega) and dissolved in 120 μL of H2O. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR using specific primers designed to amplify the regions of interest with unmethylated CpG dinucleotides or methylated CpG dinucleotides (CmG), respectively. Real-time methylation-specific PCR was performed using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad). Data of methylated CpG were normalized to that of unmethylated CpG, and were presented as the percentage of methylation at the region of interest (methylated CpG/methylated CpG+unmethylated CpG × 100).
Chromatin immunoprecipitation
Chromatin extracts were prepared from uterine arteries. Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP-IT Express kit (Active Motif), as previously described [21]. Briefly, tissues were exposed to 1% formaldehyde for 10 min to cross-link and maintain DNA/protein interactions. The reactions were stopped with glycine, tissues were washed, and chromatin was isolated and sheared into medium fragments (100–1000 bp) using a sonicator. ChIP reactions were performed using ERα or Sp1 antibodies to precipitate the transcription factor/DNA complex. Primers flanking the Sp1–380 binding site at the KCNMB1 promoter were 5΄-GTCAAAGGCTGAGGGTTTTG-3΄ (forward) and 5΄-GGAGGAGGAGTGGAAGCTCT-3΄ (reverse). PCR amplification products were visualized on 2% agarose gel stained with ethidium bromide. For quantitative real-time PCR amplification, 45 cycles of real-time PCR were performed with 3-min initial denaturation followed by 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad). Data were normalized to input chromatin that had not been going through immunoprecipitation process. The negative control determined by a normal IgG provided in the kit was subtracted. The results were further normalized and presented as percentage of normoxia control.
Pharmacological tools
Current mediated by BKCa channels was determined using BKCa channel blocker tetraethylammonium (Sigma-Aldrich). We have previously demonstrated that tetraethylammonium at 1 mM was just as effective as 100 nM iberiotoxin (Sigma-Aldrich) in inhibiting BKCa channel activity in uterine arteries [19]. The functional roles of BKCa channels were probed using BKCa channel opener NS1619 (Tocris, Bristol, UK) [29, 30]. The specificity of NS1619 on BKCa channels in uterine arteries has been demonstrated previously using iberiotoxin [31]. 5-Aza (Sigma-Aldrich), a DNMT inhibitor, has been extensively used as a pharmacological tool to investigate DNA methylation [32]. Our previous studies demonstrated that 5-Aza at 10 μM effectively ablated norepinephrine-stimulated DNMT activity and hypoxia-induced NR3C1 promoter methylation in rat heart [28, 33]. Therefore, the same concentration of 5-Aza was used in this study.
Statistical analysis
Data were expressed as means ± SEM obtained from the number of experimental animals given. Concentration–response curves were analyzed by computer-assisted nonlinear regression to fit the data using GraphPad Prism (GraphPad Software, San Diego, CA). Differences were evaluated for statistical significance (P < 0.05) by ANOVA or t test where appropriate, and the concentration–response relationship was analyzed with repeated measure ANOVA.
Results
High-altitude acclimatization increased expression and activity of DNA methyltransferases in uterine arteries
We previously demonstrated that high-altitude acclimatization resulted in hypermethylation of the KCNMB1 promoter in uterine arteries of pregnant sheep [21]. Given that DNA methylation is mediated by DNMTs, we determined the expression and activity of DNMTs in uterine arteries from pregnant sheep residing at low altitude and high altitude. As shown in Figure 1A, high-altitude acclimatization significantly increased protein abundance of DNMT3b, but not DNMT1 and DNMT3a, in uterine arteries. Accordingly, uterine arterial DNMT activity was significantly increased in high-altitude sheep as compared to low altitude animals (Figure 1B).
Figure 1.
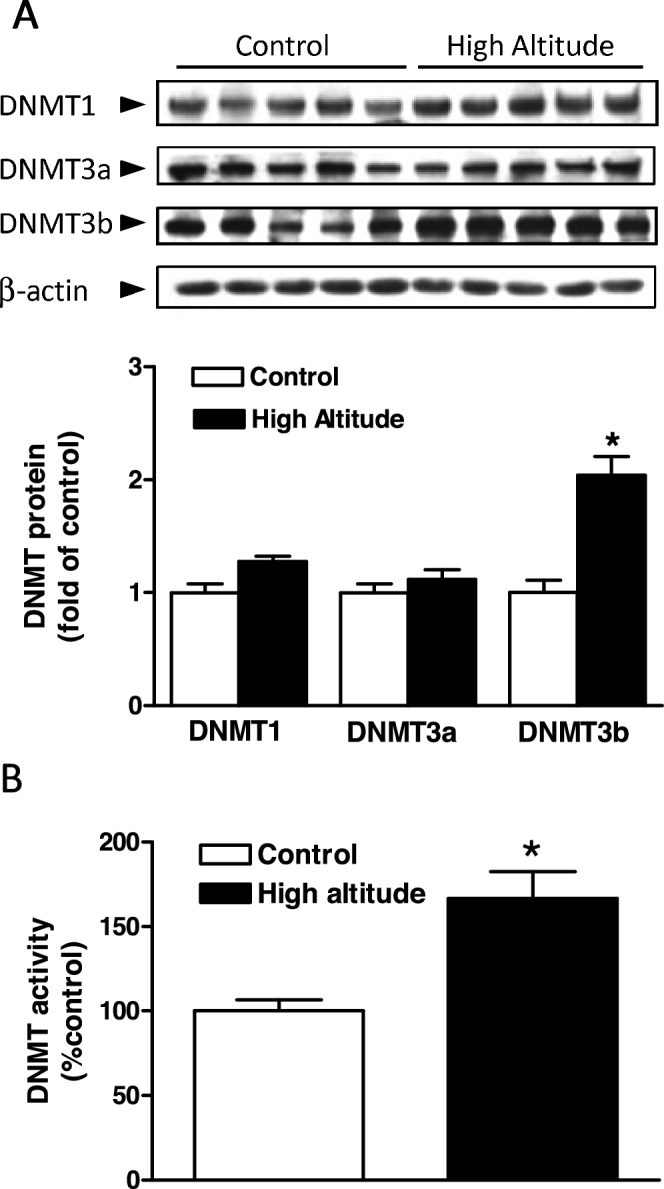
High-altitude hypoxia increased DNMT expression and activity in uterine arteries. Uterine arteries were isolated from low-altitude (control) and high-altitude pregnant sheep. (A) DNMTs protein abundance determined with western blot. (B) DNMT activity determined with a DNMT activity assay kit. Data are means ± SEM from five animals of each group. *P < 0.05, high altitude vs. control.
Ex vivo hypoxic treatment increased expression and activity of DNA methyltransferases in uterine arteries
We then determined the direct effect of hypoxia on expression and activity of DNMTs in uterine arteries. Uterine arteries isolated from low-altitude pregnant sheep were treated with normoxia (21.0% O2) or hypoxia (10.5% O2) for 48 h in the absence or presence of a DNMT inhibitor 5-Aza. In a pattern similar to high-altitude acclimatization, ex vivo hypoxic treatment selectively increased protein abundance of DNMT3b in uterine arteries (Figure 2A). Prolonged hypoxia also significantly increased DNMT activity in uterine arteries. Whereas 5-Aza did not alter DNMT activity under normoxia, it ablated hypoxia-induced increase in DNMT activity (Figure 2B).
Figure 2.
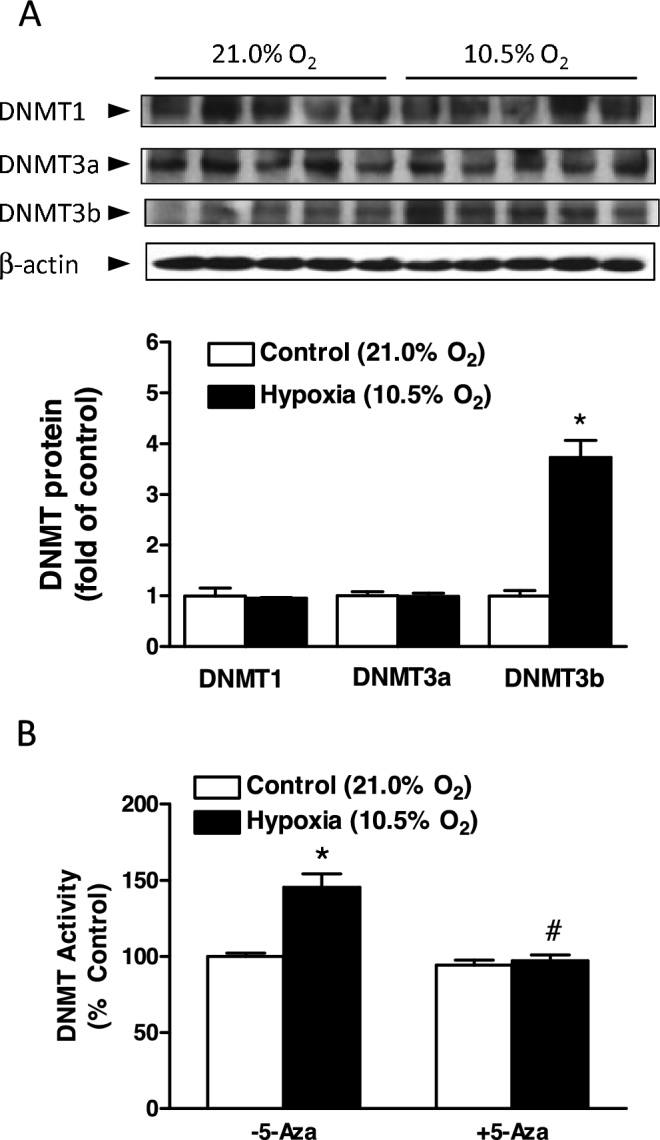
Ex vivo hypoxic treatment increased DNMT expression and activity in uterine arteries. Uterine arteries were isolated from low-altitude pregnant animals and were treated ex vivo with 21.0% O2 (control) and 10.5% O2 (hypoxia) for 48 h in the absence or presence of 5-Aza (10.0 μM). (A) DNMTs protein abundance determined with western blot. (B) DNMT activity determined with a DNMT activity assay kit. Data are mean ± SEM from five animals of each group. *P < 0.05, hypoxia vs. control. #P < 0.05, +5-Aza vs. –5-Aza.
5-Aza blocked hypoxia-induced hypermethylation of the KCNMB1 promoter in uterine arteries
The Sp1 binding site (Sp1–380) at the KCNMB1 promoter is functionally important in the regulation of KCNMB1 promoter activity and is hypermethylated in uterine arteries from pregnant sheep acclimatized to long-term high-altitude hypoxia [21]. We subsequently examined whether hypoxia-induced DNMT upregulation attributed to the increased DNA methylation of the KCNMB1 promoter in uterine arteries using quantitative methylation-specific PCR. Consistent with the findings in high-altitude animals, ex vivo hypoxic treatment significantly increased methylation levels at the Sp1–380 site in uterine arteries (Figure 3A). This hypermethylation was associated with a significant decrease in the binding of ERα and Sp1 to the KCNMB1 promoter (Figure 3B). Although 5-Aza did not change the methylation status of Sp1–380 and the binding of ERα and Sp1 to the KCNMB1 promoter under normoxia, it significantly reduced Sp1–380 methylation levels and increased the binding of ERα and Sp1 to the KCNMB1 promoter under hypoxia (P < 0.05; Figure 3A and B). Of critical importance, 5-Aza essentially annulled hypoxia-induced hypermethylation at the Sp1–380 site and restored the binding of ERα and Sp1 to the KCNMB1 promoter.
Figure 3.
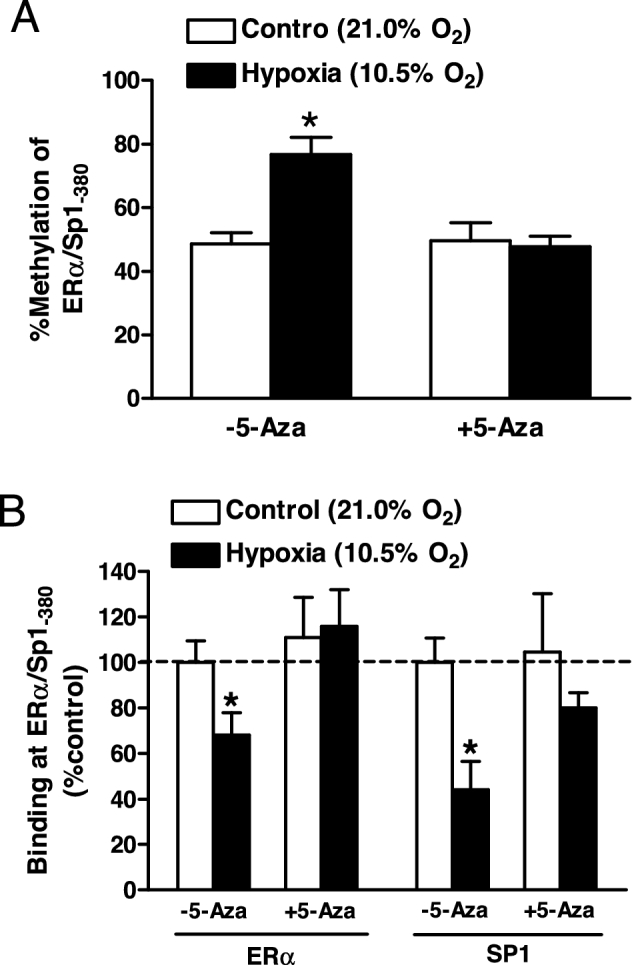
5-Aza blocked hypoxia-induced hypermethylation of KCNMB1 promoter in uterine arteries. Uterine arteries were isolated from low-altitude pregnant animals and were treated ex vivo with 21.0% O2 (control) and 10.5% O2 (hypoxia) for 48 h in the absence or presence of 5-Aza (10.0 μM). (A) Methylation of Sp1-380 binding site at the KCNMB1 promoter determined with quantitative methylation-specific PCR. (B) ERα and Sp1 binding to Sp1–380 site determined with ChIP assays. Data are mean ± SEM from five and six animals for quantitative methylation-specific PCR and ChIP, respectively. *P < 0.05, hypoxia vs. control.
5-Aza rescued KCNMB1 gene expression in uterine arteries
Acclimatization to high altitude reduced BKCa channel beta-1 subunit mRNA and protein abundance in uterine arteries of pregnant sheep [20, 21]. Similarly, ex vivo hypoxia also significantly decreased beta-1 subunit transcripts and protein abundance in uterine arteries (P < 0.05; Figure 4A and B). As hypermethylation is associated with gene silencing [34] and methylated SP1–380 significantly reduced KCNMB1 promoter activity [21], we further investigated the causal role of DNA methylation in hypoxia-induced downregulation of BKCa channel beta-1 subunit in uterine arteries. As shown in Figure 4A and B, 5-Aza significantly increased BKCa channel expression under hypoxic, but not normoxic, conditions (P < 0.05), and hypoxia-induced repression of BKCa channel beta-1 subunit was abrogated by 5-Aza.
Figure 4.
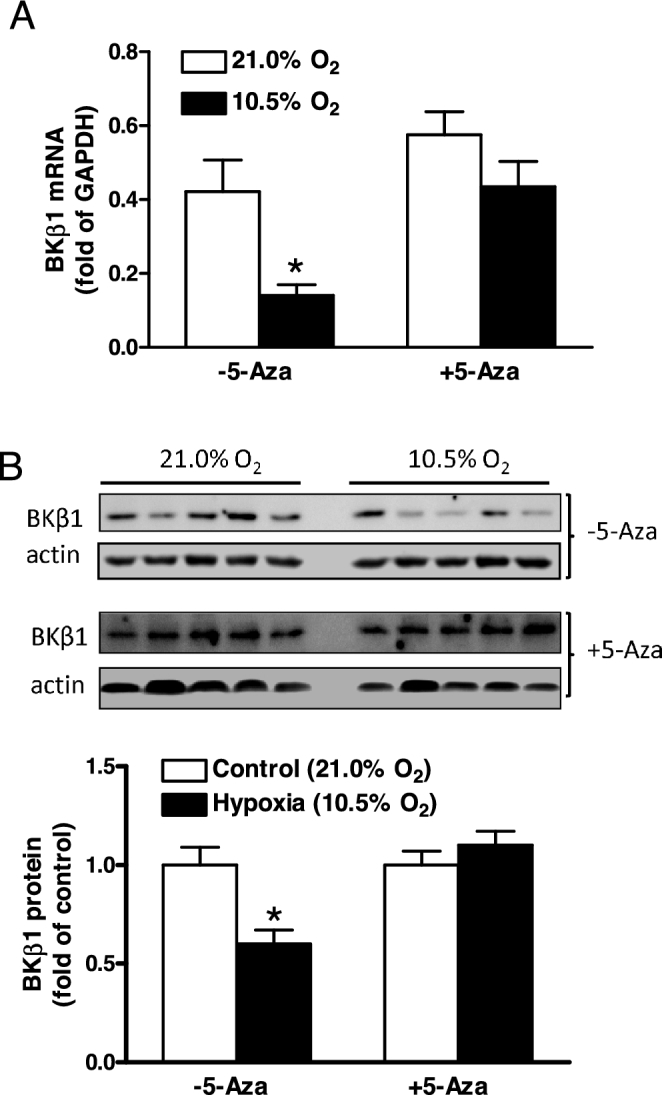
5-Aza restored hypoxia-induced KCNMB1 gene repression in uterine arteries. Uterine arteries were isolated from low-altitude pregnant animals and were treated ex vivo with 21.0% O2 (control) and 10.5% O2 (hypoxia) for 48 h in the absence or presence of 5-Aza (10.0 μM). (A) BKCa channel beta-1 subunit (BKβ1) mRNA abundance determined with real-time RT-PCR. (B) BKCa channel beta-1 subunit (BKβ1) protein abundance determined with western blot. Data are means ± SEM from six and five animals for mRNA and protein measurements, respectively. *P < 0.05, hypoxia vs. control.
5-Aza ablated hypoxia-induced suppression of BKCa channel activity in uterine arteries
Current–voltage curves measure the current–voltage relationship of channel activation. We then determined further a causal role of hypermethylation and repression of KCNMB1 in altering BKCa channel activity in uterine arteries by measuring current amplitude at various membrane potentials. Uterine arteries of pregnant sheep were treated with normoxia (21.0% O2) or hypoxia (10.5% O2) for 48 h in the absence or presence of 5-Aza, and BKCa channel currents were determined in myocytes. In the absence of 5-Aza, the hypoxic treatment significantly decreased BKCa channel current density (e.g., 23.1 ± 2.5 pA/pF vs. 8.6 ± 1.0 pA/pF at +80 mV; P < 0.05; Figure 5A). BKCa channel currents were not significantly altered by 5-Aza under the normoxic condition. However, 5-Aza abrogated the effect of hypoxia (e.g., 25.6 ± 2.5 pA/pF vs. 23.8 ± 0.8 pA/pF at +80 mV; P > 0.05; Figure 5B).
Figure 5.
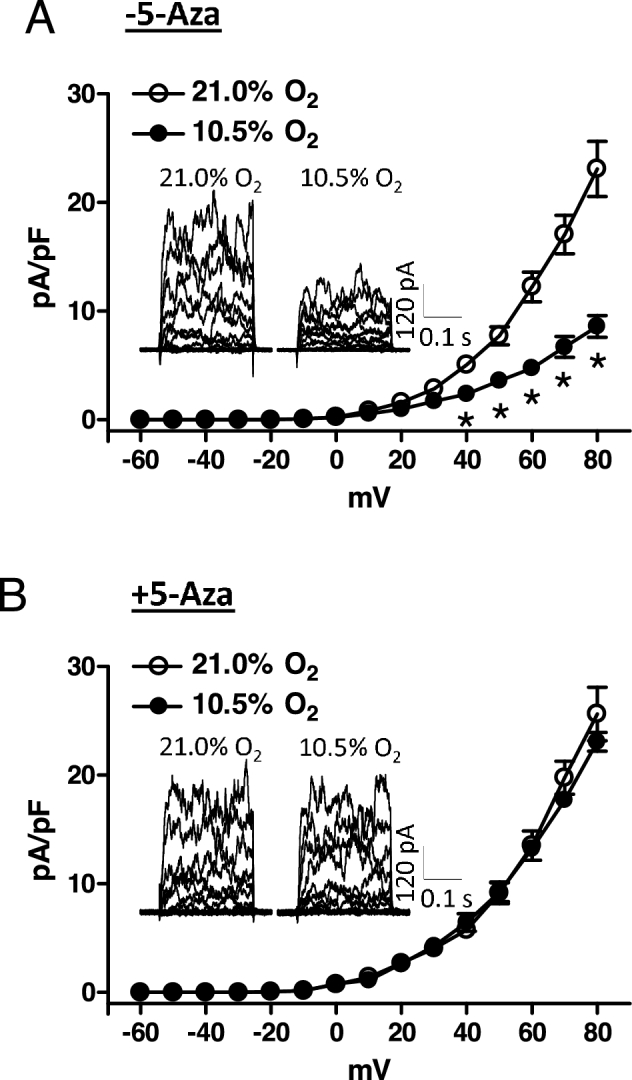
5-Aza recovered hypoxia-induced reduction of BKCa channel activity in uterine arteries. Uterine arteries were isolated from low-altitude pregnant animals and were treated ex vivo with 21.0% O2 and 10.5% O2 for 48 h in the absence (A) or presence (B) of 5-Aza (10.0 μM). Arterial myocytes were freshly isolated from uterine arteries, and BKCa channel current density was determined in the absence and presence of tetraethylammonium (TEA, 1.0 mM) as described in Materials and Methods. Data are mean ± SEM of 7 to 10 cells from five animals of each group. *P < 0.05, 10.5% O2 vs. 21.0% O2.
5-Aza restored BKCa channel-mediated relaxations of uterine arteries
The functional importance of DNA methylation in regulating BKCa channels was further determined in uterine arteries by investigating NS1619-induced relaxations. NS1619 relaxes uterine arteries via activation of the BKCa channel, and high-altitude acclimatization inhibits BKCa channel-mediated relaxations in uterine arteries from pregnant animals [31]. The ex vivo hypoxic treatment provided comparable findings. Under the normoxic condition, NS1619 induced concentration-dependent relaxations of uterine arteries, and the hypoxic treatment significantly decreased NS1619-induced relaxations (Figure 6A). Consistent with aforementioned electrophysiological studies, 5-Aza treatment significantly enhanced NS1619-induced relaxation of uterine arteries cultured under hypoxic, but not normoxic, conditions. As a matter of fact, there were no significant differences in NS1619-induced relaxations between normoxic and hypoxic conditions in the presence of 5-Aza (P > 0.05; Figure 6B).
Figure 6.
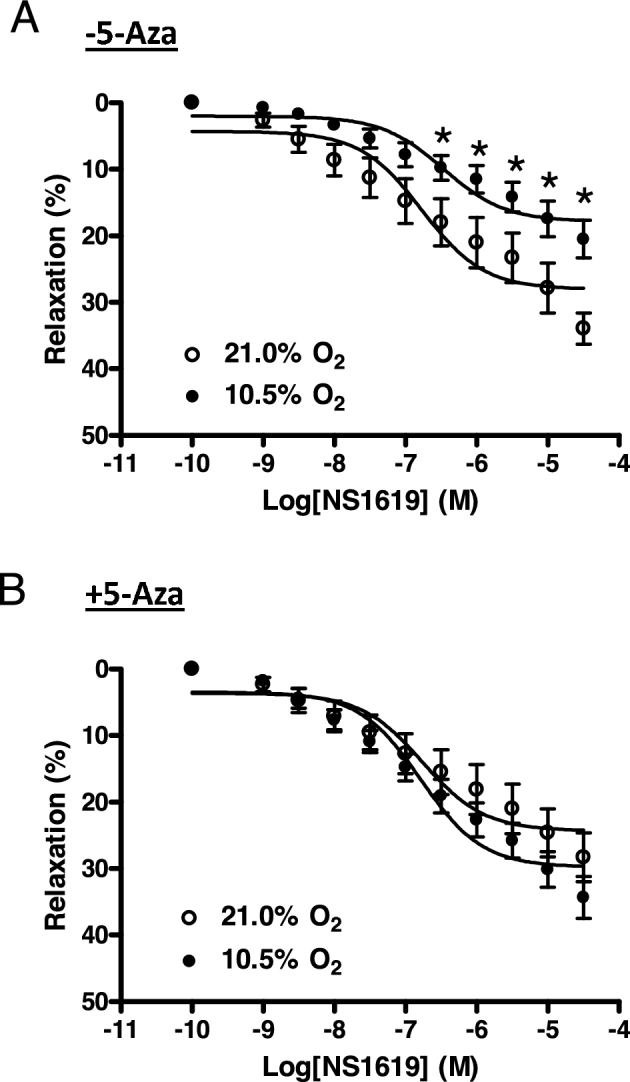
5-Aza reversed hypoxia-induced reduction of BKCa channel-mediated relaxations of uterine arteries. Uterine arteries were isolated from low-altitude pregnant animals and were treated ex vivo with 21.0% O2 and 10.5% O2 for 48 h in the absence (A) or presence (B) of 5-Aza (10.0 μM). Uterine arteries were contracted with serotonin (1 μM) followed by additions of NS1619. Data are mean ± SEM of tissues from six to eight animals of each group. *P < 0.05, 10.5% O2 vs. 21.0% O2.
5-Aza inhibited hypoxia-induced increase in pressure-dependent tone of uterine arteries
The BKCa channel plays a critical role in regulating pressure-dependent myogenic tone in uterine arteries [19, 20]. We then determined the role of DNA methylation in hypoxia-induced increases in pressure-dependent tone of uterine arteries. Uterine vascular myogenic tone developed as intraluminal pressure increased. As shown in Figure 7A, the hypoxic treatment resulted in a significant increase in pressure-dependent tone in uterine arteries in the absence of 5-Aza. 5-Aza did not alter pressure-dependent tone in uterine arteries treated under normoxic condition. However, 5-Aza significantly reduced the tone under hypoxic condition (P < 0.05) and virtually rescinded the hypoxic effect on pressure-dependent tone (Figure 7B).
Figure 7.
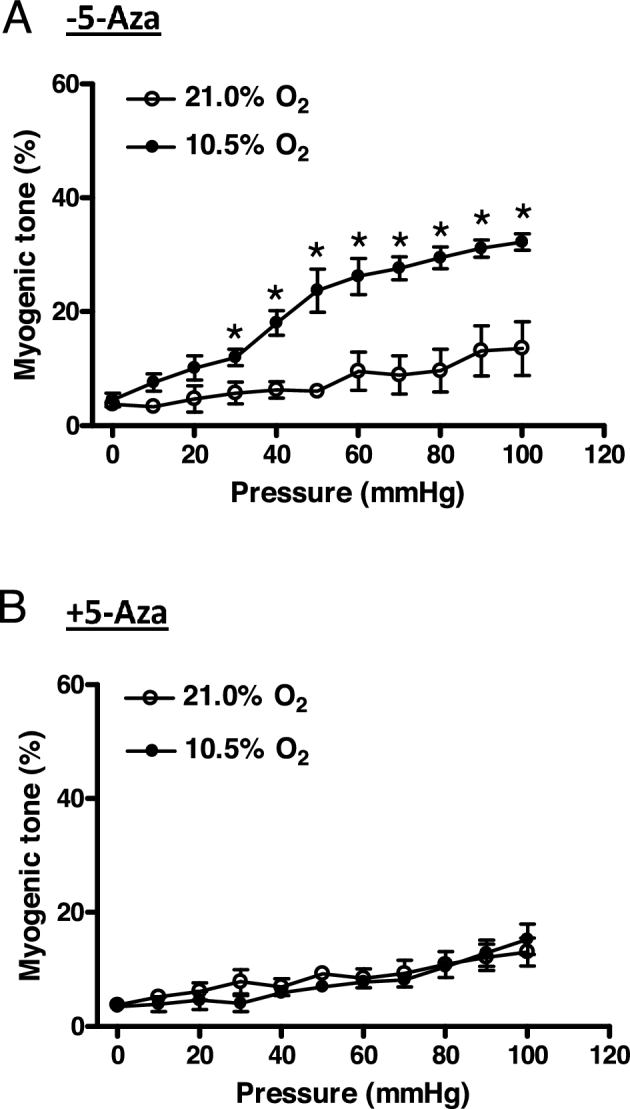
5-Aza inhibited hypoxia-induced increase in pressure-dependent myogenic tone of uterine arteries. Uterine arteries were isolated from low-altitude pregnant animals and were treated ex vivo with 21.0% O2 and 10.5% O2 for 48 h in the absence (A) or presence (B) of 5-Aza (10.0 μM). Pressure-dependent myogenic tone was determined. Data are mean ± SEM of tissues from five animals of each group. *P < 0.05, 10.5% O2 vs. 21.0% O2.
Discussion
Hypoxia is an important regulator of gene expression [26, 35, 36]. Ion channels including the BKCa channel in vascular smooth muscle cells are major targets of hypoxia [9, 37]. Hypoxia exposure reduced BKCa channel beta-1 subunit expression and channel activity in cultured vascular smooth muscle cells [38]. Similarly, our present studies revealed that ex vivo hypoxia also suppressed BKCa channel beta-1 subunit expression in uterine arteries of pregnant sheep, replicating high altitude's effects on this vasculature [20, 21]. Although significant progresses have been made in understanding ion channel dysregulation following hypoxia exposure, the molecular mechanisms controlling their expression under this pathophysiological condition remained enigmatic.
DNA methylation is a primary mechanism for epigenetic influence on gene expression in mammals and plays an important role in the pathogenesis of cardiovascular diseases [39]. An increasing body of evidence implicates an altered DNA methylation profile following chronic hypoxia exposure [26, 40–42]. DNA methylation is catalyzed by the DNMT family that includes DNMT1, DNMT3a, and DNMT3b. The methylation of CpG is established de novo by DNMT3a and DNMT3b and is maintained by DNMT1. In this study, we found that the expression of DNMT3b was selectively increased in uterine arteries of pregnant sheep acclimatized to long-term high-altitude hypoxia, which was associated with elevated enzyme activity. Similarly, high-altitude acclimatization selectively increased DNMT3a in pig lungs [43]. Of importance, ex vivo hypoxic treatment, in a way similar to high-altitude acclimatization, increased the expression and activity of DNMTs in uterine arteries, indicating a direct effect of hypoxia. The increase in DNMT3b expression was much greater following ex vivo hypoxia. However, both high-altitude acclimatization and ex vivo hypoxia produced similar alterations of DNMT activity. The discrepancy might be due to different extents of posttranslational modifications. Hypoxia has also been shown to increase DNMT1 and/or DNMT3b expression in other tissues [44, 45]. The observations of increased DNMT3b expression and enzyme activity in this study highlight a novel mechanism by which hypoxia could heighten DNA methylation in uterine arteries of pregnant sheep acclimatized to long-term high altitude.
In this study, we revealed that ex vivo hypoxic treatment significantly increased methylation levels of Sp1–380 at the KCNMB1 promoter in uterine arteries. Of importance, this hypermethylation was ablated by a DNMT inhibitor 5-Aza. We found that 5-Aza did not alter DNMT activity and methylation status of the Sp1-380 binding site at KCNMB1 promoter under normoxia. Similar results were demonstrated in the heart in which 5-Aza blocked norepinephrine-induced increase of DNMT activity and methylation of protein kinase C epsilon (PKCε) promoter without affecting the basal enzyme activity and promoter methylation [28]. It is not uncommon that some enzyme inhibitors inhibit stimulated-enzyme activity without affecting basal activity. Although it is possible that the procedure of nuclear protein extraction might affect the interaction of 5-Aza with enzymes, the finding that 5-Aza inhibited hypoxia-induced DNMT activity suggests that the inhibition is sustained. This is consistent with the previous studies [28, 46, 47]. Of importance, 5-Aza inhibition of hypoxia-induced DNMT activity is consistent with the inhibition of promoter methylation and reversal of transcription factor binding and KCNMB1 gene expression. Thus, this study established a direct effect of hypoxia in increasing KCNMB1 promoter methylation via elevated DNMT expression and activity in uterine arteries. Increased expression of DNMT1 and DNMT3b in lungs of fawn-hooded rats caused superoxide dismutase-2 (SOD2) downregulation through hypermethylation of the SOD2 promoter in pulmonary arterial smooth muscle cells, leading to the development of pulmonary arterial hypertension [48]. Likewise, chronic hypoxia-induced SOD2 downregulation due to hypermethylation of SOD2 gene was accompanied by enhanced expression of DMNT1 and DNMT3b in carotid bodies of rats [44]. Moreover, hypermethylation-mediated syncytin-1 gene downregulation in preeclamptic placenta occurred along with DNMT1 and DNMT3b upregulation [49]. Thus, upregulation of DNMTs is an important mechanism leading to gene hypermethylation in various pathophysiological conditions. It is currently unclear how hypoxia initiates DNMT3b upregulation in uterine arteries. A recent study showed that hypoxia-induced DNMT1 and DNMT3b upregulation is regulated by the hypoxia-inducible transcription factor (HIF) 1-α in human cardiac fibroblast cells [45]. Activation of HIFs is the primary cellular response to hypoxia [50]. Acclimatization to high altitude enhanced HIF-1α expression in uterine arteries of pregnant sheep [27]. It is possible that hypoxia-stimulated HIF-1α upregulation triggered an increase in DNMT3b expression in uterine arteries. Moreover, it appears that hypoxia-induced elevation of oxidative stress is a major determinant in suppressing BKCa channel expression and activity in uterine arteries [51]. Reactive oxygen species were linked to increased PKCε promoter methylation and gene repression in rodent hearts [52]. Thus, oxidative stress may alter the methylation status in uterine arteries following hypoxia exposure. These notions will need further investigation to confirm.
Methylation of a gene promoter is generally associated with gene silencing [34, 53]. Sp1 is an important transcriptional factor participating in the regulation of gene expression [54]. Our previous study revealed that ovine KCNMB1 promoter is a TATA-less promoter with a Sp1 binding site at position –380 (Sp1–380), and this site is essential for the promoter activity [21]. DNA methylation may hinder the binding of Sp1 to its cognate sites [55, 56]. In addition, ERα has been implicated in stimulating transcription through the ERα−Sp1 complex [57]. We showed that ERα could interact with Sp1 to form an ERα–Sp1 complex to activate KCNMB1 expression via binding to the Sp1 site [21]. Likewise, estrogen-stimulated expression of KCNN3, which encodes the small-conductance Ca2+-activated K+ channel KCa2.3 or SK3, involves interactions between ERα and Sp1 [58, 59]. Not surprisingly, the binding of Sp1 and ERα to Sp1–380 of the KCNMB1 promoter in uterine arteries was suppressed by hypoxia as a result of hypermethylation at this site. Consequently, KCNMB1 expression was significantly decreased at both mRNA and protein levels. Our findings demonstrate a novel mechanism that methylation-dependent disruption of Sp1/ERα binding instigates KCNMB1 repression. DNA hypermethylation also causes repression of other channels including Kv1.3 and Cav3.1 [60, 61]. Of importance, the inhibition of DNMTs by 5-Aza restored the binding of Sp1 and ERα to Sp1-380 and recovered KCNMB1 expression in uterine arteries. Thus, our observations provide a causative mechanism of hypoxia-induced hypermethylation in BKCa channel beta-1 subunit downregulation in uterine arteries. We recently demonstrated that hypoxia-induced ERα hypermethylation resulted in ESR1 repression, which was associated with the ablation of estrogen-mediated upregulation of BKCa channel activity and downregulation of pressure-dependent myogenic tone of uterine arteries [62]. It is conceivable that increased expression and activity of DNMT3b could also contribute to the hypoxia-induced ERα hypermethylation. These findings are consistent with the previous study showing an important role of estrogen in the regulation of BKCa channel activity and uterine blood flow in pregnant sheep [17].
The BKCa channel plays a key role in regulating uterine vascular tone during pregnancy [19]. Therefore, reduced BKCa channel beta-1 subunit expression has important functional consequences. This downregulation could decrease beta-1 subunit abundance on membrane surface of vascular smooth muscle cells, leading to diminished BKCa channel activity. The resultant membrane depolarization in turn promotes Ca2+ influx via opening voltage-gated Ca2+ channels, which is the primary mechanism to trigger contraction of vascular smooth muscle cells. Consequently, prolonged hypoxia impaired BKCa channel-mediated vasorelaxation and increased uterine vascular tone. The reversal of hypoxia-impaired BKCa channel function by 5-Aza strongly supports the notion that heightened methylation of KCNMB1 promoter contributes to elevated uterine vascular tone during gestational hypoxia. Consistently, uterine blood flow was decreased in human acclimatized to high altitude [63, 64]. An inadequate uterine and placental perfusion was evidenced by the increased angiogenesis in human and ovine placenta [65–67]. The insufficient blood supplies to both uterus and placenta would lead to an increased occurrence of preeclampsia and fetal growth restriction [6, 7]. Hence, hypermethylation of the KCNMB1 promoter highlights a novel mechanism whereby gestational hypoxia exerts its role in the maladaptation of uterine circulation, and this epigenetic modification could function as a link between the environmental factor O2 and pregnancy outcomes. Indeed, DNA methylation is increasingly being acknowledged as a key factor in the pathogenesis of various cardiovascular diseases, including hypertension and atherosclerosis [39, 68, 69]. In addition, evidence of DNA methylation in the pathogenesis of pregnancy complications is emerging. For example, expression of endothelial nitric oxide synthase was reduced in human endothelial cells isolated from umbilical veins of growth restricted fetuses compared with cells from control pregnancies, and this downregulation was due to DNMT1-mediated hypermethylation of the NOS3 promoter [70]. Nitric oxide (NO) plays an important role in steroid hormone-mediated adaptation of uterine blood flow in pregnancy [15, 71, 72]. It has been demonstrated that BKCa channel activity is increased by NO and endothelium-derived hyperpolarization [73, 74]. It is possible that hypoxia-mediated methylation may decrease NOS3, contributing to the regulation of BKCa channel activity in uterine arteries.
Gestational hypoxia reduces uterine blood flow and increases the frequency of preeclampsia and fetal growth restriction, and BKCa channel dysfunction in uterine arteries plays a critical role in the occurrence of aberrant uterine circulation. This study provides evidence that chronic hypoxia impairs BKCa channel-mediated function by hypermethylation-mediated BKCa channel beta-1 subunit downregulation in uterine arteries, promoting uterine vascular dysfunction and aberrant uterine circulation. In addition, this study presents a novel finding that constraining methylation ameliorates adverse impacts of chronic hypoxia and improves BKCa channel expression and function in uterine arteries, which could in turn mend uterine perfusion impaired by gestational hypoxia. These findings provide new insights into therapeutic approaches for pregnancy complications associated with gestational hypoxia including preeclampsia and fetal growth restriction.
Acknowledgments
Author contributions: XQH, DX, SY, and LZ conceived and designed experiments. XQH, MC, CD, DX, XH conducted the experiments and analyzed data. XQH and LZ interpreted data and wrote the manuscript, and CD and DX participated in manuscript preparation. LZ provided support for the studies. All authors have read and approved submission of the manuscript.
Conflict of interest: The authors declare no conflicts of interest.
References
- 1. Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol, 1977, 232:H231–H235. [DOI] [PubMed] [Google Scholar]
- 2. Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol, 1992, 80:1000–1006. [PubMed] [Google Scholar]
- 3. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda), 2009, 24:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lang U, Baker RS, Braems G, Zygmunt M, Kunzel W, Clark KE. Uterine blood flow—a determinant of fetal growth. Eur J Obstet Gynecol Reprod Biol, 2003, 110, (Suppl 1):S5–S61. [DOI] [PubMed] [Google Scholar]
- 5. Lambert G, Brichant JF, Hartstein G, Bonhomme V, Dewandre PY. Preeclampsia: an update. Acta Anaesthesiol Belg, 2014, 65:137–149. [PubMed] [Google Scholar]
- 6. Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, Julian CG, Wilson MJ, Bigham AW, Shriver MD, Honigman B, Vargas E et al. , High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol, 2011, 300:R1221–R1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore LG, Charles SM, Julian CG. Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol, 2011, 178:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett, 2010, 584:2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu XQ, Zhang L. Function and regulation of large conductance Ca2+-activated K+ channel in vascular smooth muscle cells. Drug Discov Today, 2012, 17:974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanaka Y, Meera P, Song M, Knaus HG, Toro L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes. J Physiol, 1997, 502, (Pt 3):545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol, 2000, 116:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature, 2000, 407:870–876. [DOI] [PubMed] [Google Scholar]
- 13. Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca2+ spark/STOC coupling and elevated blood pressure. Circ Res, 2000, 87:E53–E60. [DOI] [PubMed] [Google Scholar]
- 14. Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P et al. , Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation, 2005, 112:60–68. [DOI] [PubMed] [Google Scholar]
- 15. Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol, 2000, 279:H319–H328. [DOI] [PubMed] [Google Scholar]
- 16. Rosenfeld CR, Word RA, DeSpain K, Liu XT. Large conductance Ca2+-activated K+ channels contribute to vascular function in nonpregnant human uterine arteries. Reprod Sci, 2008, 15:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E2β-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol, 2001, 281:H422–H431. [DOI] [PubMed] [Google Scholar]
- 18. Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol, 2009, 296:H1878–H1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension, 2011, 58:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson SM, Zhang L. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance Ca2+-activated K+ channel activity in uterine arteries. Hypertension, 2012, 60:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen M, Dasgupta C, Xiong F, Zhang L. Epigenetic upregulation of large-conductance Ca2+-activated K+ channel expression in uterine vascular adaptation to pregnancy. Hypertension, 2014, 64:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim GH, Ryan JJ, Marsboom G, Archer SL. Epigenetic mechanisms of pulmonary hypertension. Pulm Circ, 2011, 1:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirodkar AV, Marsden PA. Epigenetics in cardiovascular disease. Curr Opin Cardiol, 2011, 26:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol, 2009, 296:H1840–H1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension, 2010, 56:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dasgupta C, Chen M, Zhang H, Yang S, Zhang L. Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-α gene in ovine uterine arteries via heightened promoter methylation. Hypertension, 2012, 60:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiao D, Hu XQ, Huang X, Zhou J, Wilson SM, Yang S, Zhang L. Chronic hypoxia during gestation enhances uterine arterial myogenic tone via heightened oxidative stress. PLoS One, 2013, 8:e73731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao D, Dasgupta C, Chen M, Zhang K, Buchholz J, Xu Z, Zhang L. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res, 2014, 101:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olesen SP, Munch E, Moldt P, Drejer J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur J Pharmacol, 1994, 251:53–59. [DOI] [PubMed] [Google Scholar]
- 30. Holland M, Langton PD, Standen NB, Boyle JP. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br J Pharmacol, 1996, 117:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhu R, Hu XQ, Xiao D, Yang S, Wilson SM, Longo LD, Zhang L. Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension, 2013, 62:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int J Cancer, 2008, 123:8–13. [DOI] [PubMed] [Google Scholar]
- 33. Xiong F, Lin T, Song M, Ma Q, Martinez SR, Lv J, MataGreenwood E, Xiao D, Xu Z, Zhang L. Antenatal hypoxia induces epigenetic repression of glucocorticoid receptor and promotes ischemic-sensitive phenotype in the developing heart. J Mol Cell Cardiol, 2016, 91:160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bird AP., CpG-rich islands and the function of DNA methylation. Nature, 1986, 321:209–213. [DOI] [PubMed] [Google Scholar]
- 35. Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, Elton TS. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. J Appl Physiol, 1994, 77:1451–1459. [DOI] [PubMed] [Google Scholar]
- 36. Ferreiro CR, Chagas AC, Carvalho MH, Dantas AP, Jatene MB, Bento De Souza LC, Lemos Da Luz P. Influence of hypoxia on nitric oxide synthase activity and gene expression in children with congenital heart disease: a novel pathophysiological adaptive mechanism. Circulation, 2001, 103:2272–2276. [DOI] [PubMed] [Google Scholar]
- 37. Shimoda LA, Polak J. Hypoxia. 4. Hypoxia and ion channel function. Am J Physiol Cell Physiol, 2011, 300:C951–C967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Navarro-Antolin J, Levitsky KL, Calderon E, Ordonez A, Lopez-Barneo J. Decreased expression of maxi-K+ channel β1-subunit and altered vasoregulation in hypoxia. Circulation, 2005, 112:1309–1315. [DOI] [PubMed] [Google Scholar]
- 39. Baccarelli A, Rienstra M, Benjamin EJ. Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ Cardiovasc Genet, 2010, 3:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res, 2010, 107:365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robinson CM, Neary R, Levendale A, Watson CJ, Baugh JA. Hypoxia-induced DNA hypermethylation in human pulmonary fibroblasts is associated with Thy-1 promoter methylation and the development of a pro-fibrotic phenotype. Respir Res, 2012, 13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen M, Xiong F, Zhang L. Promoter methylation of Egr-1 site contributes to fetal hypoxia-mediated PKCε gene repression in the developing heart. Am J Physiol Regul Integr Comp Physiol, 2013, 304:R683–R689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ban DM, Zhang B, Wang ZX, Zhang H, Wu CX. Differential gene expression of epigenetic modifying enzymes between Tibet pig and Yorkshire in high and low altitudes. Genet Mol Res, 2015, 14:3274–3280. [DOI] [PubMed] [Google Scholar]
- 44. Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP et al. , Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci USA, 2012, 109:2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watson CJ, Collier P, Tea I, Neary R, Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann A, Sharaf O, Baugh JA. Hypoxia-induced epigenetic modifications are associated with cardiac tissue fibrosis and the development of a myofibroblast-like phenotype. Hum Mol Genet, 2014, 23:2176–2188. [DOI] [PubMed] [Google Scholar]
- 46. Mishra AK, Abrahamsson A, Dabrosin C. Fulvestrant inhibits growth of triple negative breast cancer and synergizes with tamoxifen in ERα positive breast cancer by up-regulation of ERbeta. Oncotarget, 2016, 7:56876–56888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neveu WA, Mills ST, Staitieh BS, Sueblinvong V. TGF-β1 epigenetically modifies Thy-1 expression in primary lung fibroblasts. Am J Physiol Cell Physiol, 2015, 309:C616–C626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation, 2010, 121:2661–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhuang XW, Li J, Brost BC, Xia XY, Chen HB, Wang CX, Jiang SW. Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr Pharm Des, 2014, 20:1796–1802. [DOI] [PubMed] [Google Scholar]
- 50. Semenza GL., Hypoxia-inducible factors in physiology and medicine. Cell, 2012, 148:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu XQ, Huang X, Xiao D, Zhang L. Direct effect of chronic hypoxia in suppressing large conductance Ca2+-activated K+ channel activity in ovine uterine arteries via increasing oxidative stress. J Physiol, 2016, 594:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiong F, Xiao D, Zhang L. Norepinephrine causes epigenetic repression of PKCε gene in rodent hearts by activating Nox1-dependent reactive oxygen species production. FASEB J, 2012, 26:2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science, 2001, 293:1068–1070. [DOI] [PubMed] [Google Scholar]
- 54. Li L, Davie JR. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann Anat, 2010, 192:275–283. [DOI] [PubMed] [Google Scholar]
- 55. Douet V, Heller MB, Le Saux O. DNA methylation and Sp1 binding determine the tissue-specific transcriptional activity of the mouse Abcc6 promoter. Biochem Biophys Res Commun, 2007, 354:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li D, Da L, Tang H, Li T, Zhao M. CpG methylation plays a vital role in determining tissue- and cell-specific expression of the human cell-death-inducing DFF45-like effector A gene through the regulation of Sp1/Sp3 binding. Nucleic Acids Res, 2008, 36:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Safe S., Transcriptional activation of genes by 17β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm, 2001, 62:231–252. [DOI] [PubMed] [Google Scholar]
- 58. Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun, 2003, 303:660–668. [DOI] [PubMed] [Google Scholar]
- 59. Pierce SL, England SK. SK3 channel expression during pregnancy is regulated through estrogen and Sp factor-mediated transcriptional control of the KCNN3 gene. Am J Physiol Endocrinol Metab, 2010, 299:E640–E646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toyota M, Ho C, Ohe-Toyota M, Baylin SB, Issa JP. Inactivation of CACNA1G, a T-type calcium channel gene, by aberrant methylation of its 5΄ CpG island in human tumors. Cancer Res, 1999, 59:4535–4541. [PubMed] [Google Scholar]
- 61. Brevet M, Fucks D, Chatelain D, Regimbeau JM, Delcenserie R, Sevestre H, Ouadid-Ahidouch H. Deregulation of 2 potassium channels in pancreas adenocarcinomas: implication of KV1.3 gene promoter methylation. Pancreas, 2009, 38:649–654. [DOI] [PubMed] [Google Scholar]
- 62. Chen M, Xiao D, Hu XQ, Dasgupta C, Yang S, Zhang L. Hypoxia represses ER-α expression and inhibits estrogen-induced regulation of Ca2+-activated K+ channel activity and myogenic tone in ovine uterine arteries: causal role of DNA methylation. Hypertension, 2015, 66:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol (1985), 1995, 79:15–22. [DOI] [PubMed] [Google Scholar]
- 64. Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol (1985), 1995, 79:7–14. [DOI] [PubMed] [Google Scholar]
- 65. Zamudio S., The placenta at high altitude. High Alt Med Biol, 2003, 4:171–191. [DOI] [PubMed] [Google Scholar]
- 66. Krebs C, Longo LD, Leiser R. Term ovine placental vasculature: comparison of sea level and high altitude conditions by corrosion cast and histomorphometry. Placenta, 1997, 18:43–51. [DOI] [PubMed] [Google Scholar]
- 67. Parraguez VH, Atlagich M, Diaz R, Cepeda R, Gonzalez C, De los Reyes M, Bruzzone ME, Behn C, Raggi LA. Ovine placenta at high altitudes: comparison of animals with different times of adaptation to hypoxic environment. Anim Reprod Sci, 2006, 95:151–157. [DOI] [PubMed] [Google Scholar]
- 68. Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, Carletto A, Pattini P, Corrocher R, Olivieri O. Epigenetic control of 11β-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis, 2008, 199:323–327. [DOI] [PubMed] [Google Scholar]
- 69. Kim M, Long TI, Arakawa K, Wang R, Yu MC, Laird PW. DNA methylation as a biomarker for cardiovascular disease risk. PLoS One, 2010, 5:e9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Krause BJ, Costello PM, Munoz-Urrutia E, Lillycrop KA, Hanson MA, Casanello P. Role of DNA methyltransferase 1 on the altered eNOS expression in human umbilical endothelium from intrauterine growth restricted fetuses. Epigenetics, 2013, 8:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest, 1996, 98:2158–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rosenfeld CR, Roy T. Prolonged uterine artery nitric oxide synthase inhibition modestly alters basal uteroplacental vasodilation in the last third of ovine pregnancy. Am J Physiol Heart Circ Physiol, 2014, 307:H1196–H1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol, 1998, 124:1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kiraly I, Pataricza J, Bajory Z, Simonsen U, Varro A, Papp JG, Pajor L, Kun A. Involvement of large-conductance Ca2+ -activated K+ channels in both nitric oxide and endothelium-derived hyperpolarization-type relaxation in human penile small arteries. Basic Clin Pharmacol Toxicol, 2013, 113:19–24. [DOI] [PubMed] [Google Scholar]


